Abstract
Purpose
Curcumin has been shown to have potent anticancer activities like inhibition of cell proliferation, induction of apoptosis, and suppression of angiogenesis. Transforming growth factor-β (TGF-β) signaling plays a complex role in tumor suppression and promotion depending on the tumor type and stage. However, the effect of curcumin on TGF-β signaling in cancer cells and the role of TGF-β signaling in curcumin-induced anticancer activities have not been determined. Here, we investigate the role of curcumin on TGF-β signaling, and whether TGF-β signaling is involved in the antitumor activities of curcumin.
Methods
Human non-small cell lung cancer (NSCLC) cell lines, ACC-LC-176 (without TGF-β signaling), H358, and A549 (with TGF-β signaling) were treated with curcumin to determine cell growth, apoptosis, and tumorigenicity. Antitumor activities of curcumin were determined using these cell lines and an in vivo mouse model. We also tested the effect of curcumin on TGF-β/Smad signaling by western blotting and by luciferase assays.
Results
Curcumin inhibited cell growth and induced apoptosis of all three NSCLC cell lines in vitro and in vivo. It significantly reduced subcutaneous tumor growth by these three cell lines irrespective of TGF-β signaling status. Curcumin inhibited TGF-β-induced Smad2/3 phosphorylation and transcription in H358 and A549 cells, but not in ACC-LC-176 cells.
Conclusions
Curcumin reduces tumorigenicity of human lung cancer cells in vitro and in vivo by inhibiting cell proliferation and promoting apoptosis. These results suggest that TGF-β signaling is not directly involved in curcumin-mediated growth inhibition, induction of apoptosis, and inhibition of tumorigenicity.
Keywords: Curcumin, NSCLC, TGF-β, Smad, Tumorigenicity, Apoptosis
Introduction
Lung cancer is the leading cause of cancer-related morbidity and mortality worldwide among both men and women (Siegel et al. 2012). Despite the advancement in the understanding and treatment, the overall 5-year survival for all patients diagnosed with lung cancer remains less than 15 %, a rate that has barely changed over the last 30 years. Clearly more efforts are needed to improve our understanding of lung cancer biology in order to improve treatment and prevention strategies. Lung cancer is sometimes sensitive to either chemotherapy or radiation therapy, depending on types and stages. Despite recent advances in therapy of lung cancer, side effects of treatment and multidrug resistance remain the main obstacle of lung cancer treatment. Exploring novel agents with minimum side effects and maximum sensitivity attracts attention for lung cancer research.
Curcumin, an active component of the spice turmeric derived from Curcuma longa, has been used extensively as a coloring and flavoring agent of food in Asian countries. It is used in traditional medicine and is known to have antioxidant, anti-inflammatory, antifibrotic, and anticancer activities. Curcumin has been documented to suppress cancer cell proliferation and to promote apoptosis in a variety of tumor cells (Radhakrishna Pillai et al. 2004; Su et al. 2010; Yang et al. 2012b; Shishodia et al. 2007). This compound is also reported to inhibit migration and invasion of human lung cancer cells (Lin et al. 2009). It has been reported to have low toxicity to mammals at high doses (Samaha et al. 1997; Carroll et al. 2011; Cheng et al. 2001). These advantages make curcumin a strong candidate as a novel anticancer agent, either alone or in combination with other chemotherapeutic drugs. A phase I clinical trial documented that curcumin in combination with docetaxel-based chemotherapy improved biological and clinical outcomes in patients with advanced breast cancer (Bayet-Robert et al. 2010). Recently, a phase II clinical trial of curcumin was reported to prevent the development of colorectal neoplasia (Carroll et al. 2011). Modulation of multiple target genes, including Akt, mitogen-activated protein kinases (MAPK), nuclear factor-κB (NF-κB), matrix metalloproteinase (MMP), tumor necrosis factor (TNF), signal transducer and activator of transcription 3 (STAT3), and cell cycle regulatory proteins, has been implicated in the anticancer activity of curcumin (Lin et al. 2009; Thangapazham et al. 2006; Yang et al. 2012a; Kim et al. 2005). Therefore, it is important to study the mechanism of function of curcumin in further detail due to its excellent potential for therapeutic intervention of lung cancer.
Transforming growth factor-β signaling pathway plays a pivotal but complex role in tumor development and progression depending on tumor types and stages. TGF-β signaling regulates various biological processes including cell growth, differentiation, angiogenesis, apoptosis, and extracellular matrix remodeling (Massague 1998). Alterations in TGF-β signaling are linked to a variety of human diseases including cancer, inflammation, and tissue fibrosis. Ligand binding to TGF-β receptors initiates Smad2/3/4 complex formation and translocation to the nucleus (Smad pathway) to control gene expression. TGF-β signaling can also activate MAPK pathways (non-Smad pathway) in concert with other growth factors (Derynck and Zhang 2003). Previous studies from our laboratory provided evidence for TGF-β as a lung cancer suppressor (Anumanthan et al. 2005; Halder et al. 2011), and Smad pathway plays a critical role in this tumor suppressor function of TGF-β (Samanta et al. 2012). Several growth factors and exogenous small molecule inhibitors function as tumor suppressors or promoters through cross-talking with the TGF-β signaling pathway. Curcumin, as a tumor suppressor, was reported to regulate TGF-β signaling cascade in neonatal lung fibroblast (Sakurai et al. 2011), renal cells (Gaedeke et al. 2004), keloid fibroblasts (Hsu et al. 2010), and scleroderma fibroblasts (Song et al. 2011). However, to our knowledge, nothing is known about the effect of curcumin on TGF-β signaling in lung cancer cells of epithelial origin and whether TGF-β-induced tumor suppressor function is involved in curcumin-mediated antitumor activities.
In this study, we have investigated the role of curcumin on cell proliferation, apoptosis, and tumorigenicity in lung cancer cells. Our results have demonstrated that curcumin inhibits cell proliferation, induces apoptosis, and suppresses tumorigenicity in human NSCLC cells in vitro and in vivo and that TGF-β signaling has no role in these functions of curcumin. We also have observed that curcumin inhibits TGF-β-induced Smad2 and Smad3 phosphorylation and transcription in TGF-β-sensitive lung cancer cell lines. This evidence indicates that TGF-β signaling is not directly involved in curcumin-mediated effects on NSCLC cells.
Materials and methods
Cell culture and animals
Human NSCLC cell lines ACC-LC-176, H358, and A549 were maintained in RPMI containing 7 % fetal bovine serum as described previously (Halder et al. 2011). Female athymic nude mice (6 weeks old) were used for in vivo tumor xenograft studies. All animal work was performed in accordance with Institutional Animal Care and Use Committee and state and federal guidelines for the humane treatment and care of laboratory animals.
Reagents and antibodies
TGF-β1 was purchased from R&D Systems (Minneapolis, MN, USA). Curcumin was obtained from Sigma (Sigma Bio-chemicals). Anti phospho-Smad2, Smad2, phospho-Smad3, Smad3, Smad4, PARP (Cell Signaling), and β-actin (Sigma Bio-chemicals) were used for western blot analyses.
Cell counting assay
Cells (3,000/well) were split into 12-well plates and cultured for overnight. Cells were then treated with different concentrations of curcumin (5, 10, and 20 μM) for 5 days. Media with indicated concentration of curcumin were replaced every 2 days. Treatment with DMSO was used as placebo control. Cells were counted every 24 h, and the average cell numbers from triplicate wells were plotted. These experiments were repeated three times.
Flow cytometric analysis for cell apoptosis
Cells (1 × 105/well) were split into 6-well plates and cultured for overnight, and then treated with different concentrations of curcumin (5, 10, 20, and 40 μM) for 24 h. Cells were harvested for FITC Annexin V and propidium iodide (PI) staining, following the protocol of the FITC Annexin V Detection Kit (BD Pharmingen). Briefly, cells were washed twice with cold phosphate-buffered saline (PBS) and resuspended in 100 μl 1× binding buffer. Cells were then stained with 5 μl FITC Annexin V and 5 μl PI for 15 min in the dark. Then, 400 μl 1× binding buffer was added, and cells were analyzed by flow cytometry within 1 h. Cells that stain positive with Annexin V are undergoing apoptosis, while cells that stain positive with both Annexin V and PI are either in the late stages of apoptosis or necrosis.
Soft agarose tumorigenicity assay
One milliliter semisolid 0.8 % sea plaque agarose was plated in each well of 6-well plates, and let it solidified for at least an hour. Then, ACC-LC-176, H358, and A549 cells (2.0 × 104) were suspended in 1 ml of 0.4 % agarose containing 7 % FBS and plated on the top of the above agarose. After 48 h, cells were treated with media containing different concentrations of curcumin. The media with curcumin were replaced every other day. Picture of colonies was taken by phase-contrast microscope 12 days after cell splitting. Colonies grown on the soft agarose were counted using GEL COUNT™ software.
Luciferase reporter assay
ACC-LC-176, H358, and A549 cells (20,000/well) were cultured in 12-well plates for 24 h, and then transiently transfected with p3TP-Lux, (GAGA)9 MLP-Luc, and CMV-βgal reporter plasmids for 5 h. Cells were pretreated with curcumin of indicated concentration for 2 h, and 5 ng/ml TGF-β was added thereafter for 22 h. Cell lysates were used to measure luciferase and β-gal activities, and normalized luciferase activity was presented.
In vivo tumorigenicity assay
ACC-LC-176, H358, and A549 cells were harvested and suspended in PBS. 100 μl cell suspension (3 × 106 cells/mouse) was subcutaneously injected into the right flank of each athymic nude mice. Mice were randomly divided into control (n = 5) and curcumin-treated (n = 5) groups. Tumor volume was monitored and measured with a slide calipers. Curcumin treatment was started as soon as the tumor had reached to a minimum accurately measurable size. Curcumin at 50 mg/kg was injected i.p. every third day. DMSO was injected i.p. as placebo control. Tumor volume was measured every third day to follow the tumor growth. Mice were weighed every third day to evaluate drug toxicity. Tumor volume was calculated with the formula: V = L × W 2/2, where L is length and W is width of a tumor. Tumors were also weighed at the end of the experiment. This experiment was repeated twice.
Immunohistochemical analysis
Immunohistochemical analysis was performed in the Immunohistochemistry Core of the Medical College of Vanderbilt University. Tissues from subcutaneous tumors were fixed in 10 % formalin. Immunostaining for hematoxylin and eosin (H&E), Ki67, and cleaved caspase 3 was performed on 5-μm-thick formalin-fixed paraffin-embedded sections. Hearts and livers were stained with H&E to observe the cell morphology and evaluate any toxicity associated with drug treatment.
Western blot analyses
ACC-LC-176, H358, and A549 cells were treated with 5 ng/ml TGF-β in the presence or absence of curcumin (10 and 20 μM) for 24 h, or with curcumin alone of indicated concentration (5, 10, 20, and 40 μM) for 24 h, or with 10 μM curcumin for different time points (6, 12, 24, and 48 h). Cells or tumor tissues were solubilized in mammalian lysis buffer as described previously (Halder et al. 2005). Protein lysates from either cells or tumor tissues were used for western blot analysis.
Statistical analysis
All data are presented as mean ± SD and analyzed using Student’s t test. Statistical tests were performed using SPSS 15.0 software or GraphPad Prism 5.0 for windows. P values less than 0.05 (P < 0.05) were considered statistically significant.
Results
Curcumin reduces cell proliferation and induces apoptosis of NSCLC cells
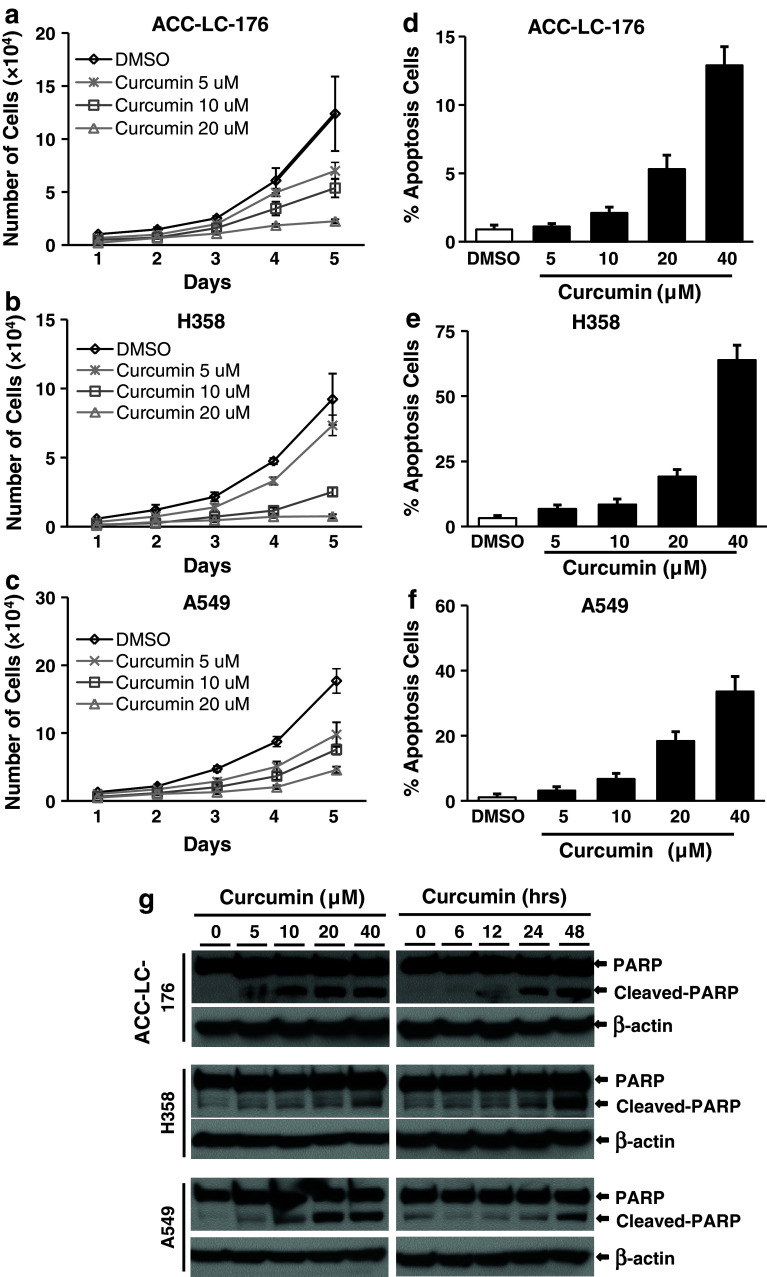
Inhibition of cell proliferation and induction in apoptosis contribute to the antitumor activity of curcumin (Radhakrishna Pillai et al. 2004; Shishodia et al. 2007; Su et al. 2010; Yang et al. 2012b). Two of the most important tumor suppressor effects of TGF-β are its ability to inhibit proliferation and induce apoptosis of many cell types. To determine whether TGF-β has any role in the anticancer effects of curcumin, we used ACC-LC-176 cell line (without TGF-β signaling) due to the loss of TβRII expression (Halder et al. 2011) and H358 and A549 cell lines (with TGF-β signaling) for cell counting assays and flow cytometric analyses (PI and Annexin V staining). Cells were treated with increasing concentrations of curcumin for 24 h and then counted. Curcumin inhibited the growth of ACC-LC-176 (Fig. 1a), H358 (1b), and A549 cells (1c) in a dose-dependent manner. In an attempt to test whether curcumin has any differential role on apoptosis in these cell lines, we performed a quantitative determination of apoptosis by FACS analyses. We observed that the percentage of Annexin V positive cells (apoptotic cells) significantly increased in a dose-dependent manner in all three lung cancer cell lines (Fig. 1d–f). We next verified the effect of curcumin on the induction in apoptosis by measuring the cleaved PARP in these cell lines. The degradation of nuclear PARP was increased by curcumin treatment in both concentration- and time-dependent manners in all three cell lines (Fig. 1g). Taken together, these results suggest that curcumin inhibits cell proliferation and induces apoptosis in NSCLC cells and that TGF-β signaling has no role on these curcumin-mediated functions in vitro.
Fig. 1.
Curcumin inhibits cell growth and induces apoptosis of NSCLC cells. NSCLC cell lines ACC-LC-176 (a), H358 (b), and A549 (c) were treated with curcumin with indicated concentrations for 5 days. Cell counting was performed every 24 h using cell counting hemocytometer after treatment (a–c). The percentage of apoptotic cells was analyzed by flow cytometry (d–f) after 24-h treatment. Data were presented as the mean ± SD of triplicate determinations. g NSCLC cells ACC-LC-176, H358, and A549 were treated with curcumin as indicated for 24 h (left panel), or with 10 μM curcumin for different time points (6, 12, 24, 48 h; right panel). Protein lysates from treated cells were used for analyzing the level of PARP and cleaved PARP by western blot analyses
Curcumin inhibits tumorigenicity of NSCLC cells in vitro and in vivo
Very little studies have been reported about the effects of curcumin on tumorigenicity of lung cancer cells in vivo. We herein examined the role of curcumin on tumorigenicity of NSCLC cells both in vitro and in vivo. In the anchorage-independent (soft agarose) assay, curcumin treatment significantly inhibited the colony formation (in both number and size) in all three cell lines in a dose-dependent manner (Fig. 2a, b). Compared to DMSO treatment as control, all three cell lines showed strong colony formation, while H358 cells grew slower than other cells. Low dose of curcumin (1 μM) inhibited the number of colonies by 31.6 % in ACC-LC-176, 21.4 % in H358, and 11.6 % in A549 cells (Fig. 2b). These data suggest that these NSCLC cells are sensitive to curcumin treatment.
Fig. 2.
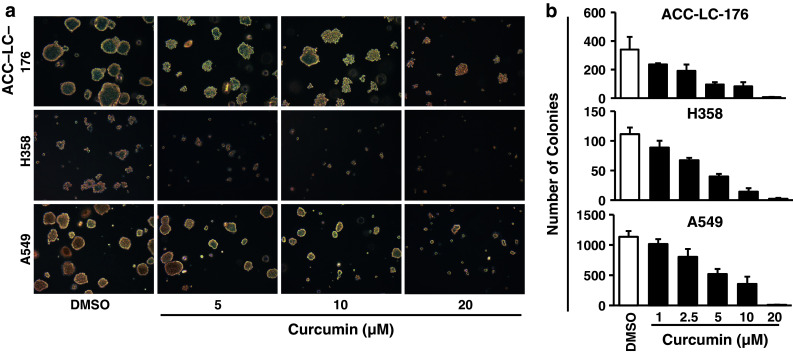
Curcumin inhibits tumorigenicity of NSCLC cells in vitro. a, b ACC-LC-176, H358, and A549 cells growing in semisolid 0.4 % sea plaque agarose were treated with curcumin with indicated concentrations for 10 days. Colonies grown on the soft agarose were counted and analyzed with GEL COUNT™ software. Representative pictures of colonies treated with curcumin for 10 days are shown. Pictures of colonies were taken at ×100 magnification. Data are presented as the mean ± SD from three individual plates
We next determined the effects of curcumin on tumorigenicity of NSCLC cells in vivo using xenograft model. Curcumin treatment (50 mg/kg mouse weight) was started for every 3 days for a total of 3 weeks when subcutaneous tumors were palpable. Tumor growth by all three cell lines was significantly suppressed by curcumin treatment when compared with the control group (Fig. 3). Curcumin decreased the tumor weight to 17.0 % in ACC-LC-176 (Fig. 3a), 27.7 % in H358 (Fig. 3b), and 31.2 % in A549, respectively (Fig. 3c). These results are consistent with the in vitro assay. Taken together, these results indicate that curcumin inhibits tumorigenicity of NSCLC cell lines in vitro and in vivo, regardless of the condition of TGF-β signaling.
Fig. 3.
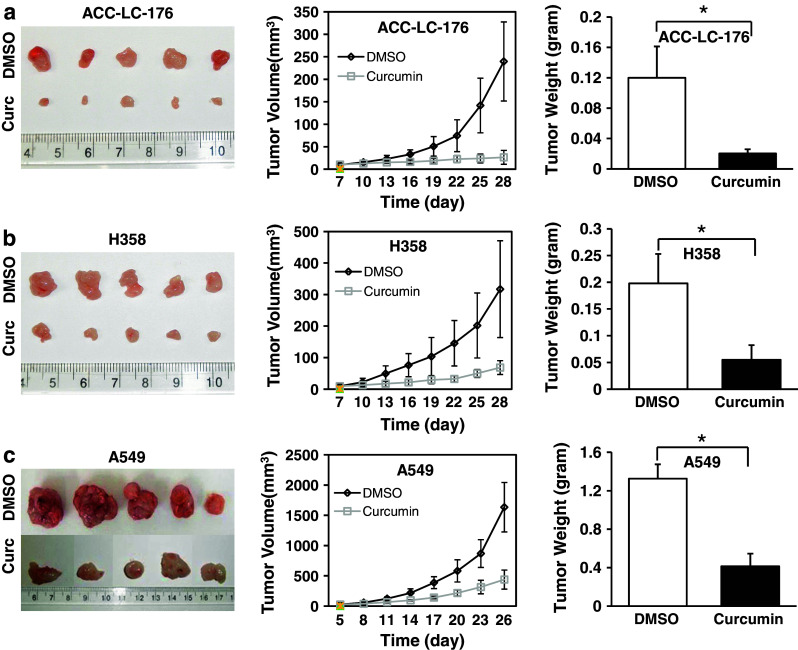
Curcumin inhibits tumorigenicity of NSCLC cells in vivo. NSCLC cells were inoculated at the right flank of athymic nude mice. Mice bearing subcutaneous tumor from NSCLC cell line ACC-LC-176 (a, n = 5 in each group), H358 (b, n = 5 in each group), and A549 (c, n = 5 in each group) were treated with curcumin as soon as the tumors had reached a minimum accurately measurable size. Curcumin (50 mg/kg) or DMSO was administrated i.p. every 3 days. Mice were euthanized at the end of the experiment, and pictures of the tumor mass are presented. Tumor volume was measured every 3 days with a slide calipers from the beginning of drug treatment, and the growth curve was plotted. Tumors were weighed at the end of the experiment. Each plot presents the mean volume ± SD of 5 mice in each group. *P < 0.001
Curcumin with curative dose causes no toxicity to nude mice
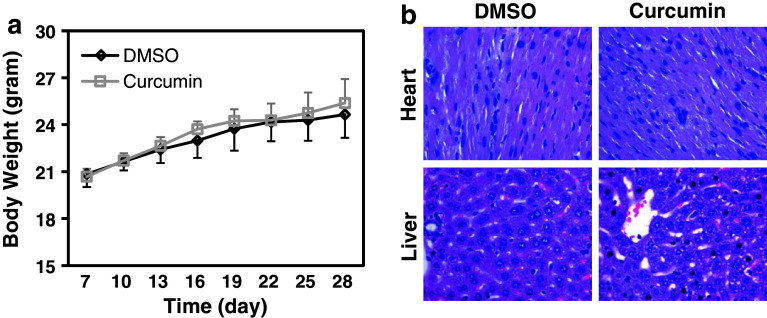
We next tested whether curcumin has any toxic effect on mice with the doses that showed strong antitumor effects. We administered 50 mg/kg of curcumin i.p. to the tumor-bearing nude mice every 3 days for 3 weeks. Curcumin treatment showed little effect on the mice body weight compared to the control group (Fig. 4a). We also examined the cell morphology and tissue structure of liver and heart, which are the main target organs of drug toxicity, by H&E staining. The structure of the liver and the heart, and the morphology of hepatic cells and cardiac cells showed no obvious difference in the curcumin-treated mice compared to the control group (Fig. 4b). These results suggest that curcumin with curative dose has no toxic effect to the animals.
Fig. 4.
No obvious toxicities are present in mice with curcumin treatment. a Mice with or without curcumin treatment were weighed every 3 days from the beginning after inoculation of tumor cells. Each plot represents the mean weight ± SD value of 5 mice in each group at different time point. b Heart and liver were removed at the end of the experiment. Formalin-fixed paraffin-embedded tissue samples were analyzed by performing H&E staining. Pictures are presented at ×400 magnification
Curcumin induces apoptosis and inhibits cell proliferation in NSCLC tumor xenograft model
In order to elucidate the mechanism of the inhibitory effect of curcumin on tumorigenicity, we performed immunohistochemical staining for the tumor xenografts. H&E staining was used to observe the cell morphology. A549 xenograft showed specific tumor morphology in the control group, while curcumin-treated tumors showed some irregular cancer cells with nuclear condensation and shrinkage, which indicates cell apoptosis (Fig. 5c). Among ACC-LC-176, H358, and A549 cells, Ki67 (proliferation marker) expression was significantly down-regulated in tumors with curcumin treatment. On the contrary, the expression of cleaved caspase 3 was increased by curcumin in the xenografts from three cell types, when compared to the control group (Fig. 5a–c). These data provided evidence that curcumin suppresses tumorigenicity in vivo through the inhibition of cell proliferation and induction in apoptosis. These results are also consistent with the data of flow cytometric analyses in vitro (Fig. 1d–f). We further confirmed the apoptosis by verifying the expression of cleaved PARP in cells treated with curcumin. We observed that curcumin induced the cleavage of PARP in a dose-dependent as well as time-dependent manner in three lung cancer cell lines (Fig. 1g).
Fig. 5.
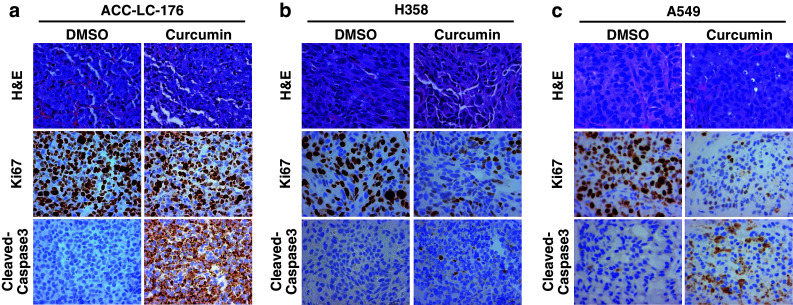
Curcumin inhibits cell proliferation and induces apoptosis in tumor xenografts. a–c Tumor xenografts described in Fig. 3 were used for immunohistochemical staining for H&E, Ki67, and caspase 3. A representative picture from each group is shown. Pictures are shown at ×400 magnification
Curcumin inhibits TGF-β/Smad signaling
Although little is known about the effect of curcumin on TGF-β signaling in fibroblast cells (Gaedeke et al. 2004; Hsu et al. 2010), nothing is known about the role of curcumin on TGF-β signaling in lung cancer cells. In our previous experiments, we have observed that TGF-β signaling has no role in curcumin-induced growth inhibition, apoptosis (Fig. 1), and tumorigenicity (Figs. 2, 3). To determine the potential role of curcumin on downstream TGF-β signaling, we used three lung cancer cell lines, in which ACC-LC-176 cells do not express TβRII and are not responsive to TGF-β, whereas, H358 and A549 cells have all TGF-β signaling components and are responsive to TGF-β. We first verified the phosphorylation of endogenous Smad2 and Smad3 in response to TGF-β by western blot analyses. Exogenous TGF-β induced the phosphorylation of Smad2 and Smad3 in both H358 and A549 cells (Fig. 6b, c), but no effect of TGF-β on Smad2 and Smad3 phosphorylation was observed in ACC-LC-176 cells due to lack of TβRII expression (Fig. 6a). Treatment with curcumin (either 10 or 20 μM) down-regulated TGF-β-induced Smad2 and Smad3 phosphorylation in A549 and H358 cells. However, curcumin had no effect on the basal levels of Smad2, Smad3, and Smad4 in all cell lines (Fig. 6a–c).
Fig. 6.
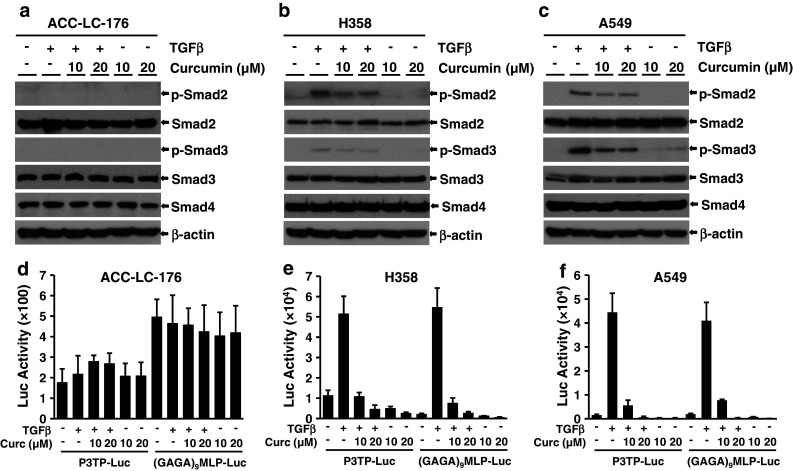
Curcumin inhibits TGF-β/Smad signaling in H358 and A549 cells. Western blot analyses were performed using lysates from ACC-LC-176 (a), H358 (b), and A549 (c) cells treated with TGF-β (5 ng/ml) and 10 or 20 μM curcumin for 22 h. Luciferase reporter assay. ACC-LC-176 (d), H358 (e), and A549 (f) cells were transiently transfected with p3TP-Lux, (GAGA)9 MLP-Luc, and CMV-βgal reporter plasmids, and then pretreated with curcumin of indicated concentrations for 2 h, and 5 ng/ml TGF-β was added thereafter for 22 h. Cell lysates were used to measure luciferase and β-gal activities, and normalized luciferase activities were presented
To test whether the inhibition of Smad2/Smad3 phosphorylation affects downstream transcriptional responses mediated by TGF-β, we performed transient transfection assays using TGF-β-responsive reporters p3TP-Lux and (CAGA)9-MLP-Luc. TGF-β strongly induced reporter activities in H358 and A549 cells, while this effect was antagonized by curcumin in a dose-dependent manner (Fig. 6e, f). On the other hand, TGF-β or curcumin has no effect on these reporter activities in ACC-LC-176 cells (Fig. 6d). These results are consistent with the inhibition of phosphorylation of Smad2 and Smad3 in H358 and A549 cell lines (Fig. 6b, c).
Taken together, curcumin abrogates TGF-β signaling by inhibiting TGF-β-induced Smad2 and Smad3 phosphorylation, and TGF-β signaling does not play any role in the antitumor activities of curcumin.
Discussion
Curcumin, a potent antitumor drug, has been widely studied for several diseases including cancer. The antitumor effect of curcumin in lung cancer has been attributed to the suppression of cell proliferation, induction of mitochondrial pathway-mediated cell apoptosis, inhibition of migration, and invasion through the inhibition of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMP) (Radhakrishna Pillai et al. 2004; Lin et al. 2009; Su et al. 2010). Due to its excellent potential for therapeutic intervention, curcumin has been tested in clinical trials either alone (Carroll et al. 2011) or in combination (Bayet-Robert et al. 2010). Activation of multiple signaling pathways has been implicated in the anticancer activity of curcumin (Lin et al. 2009; Thangapazham et al. 2006; Yang et al. 2012a; Kim et al. 2005). However, nothing is known about the role of curcumin on TGF-β signaling and vice versa in cancer cell lines of epithelial origin.
There is compelling evidence indicating that TGF-β has growth inhibitory and pro-apoptotic effects in epithelial cells and serves as a tumor suppressor. It is widely believed that Smad-dependent pathway is involved in TGF-β tumor suppressor functions. To determine whether TGF-β has any role in the anticancer effects of curcumin, we used ACC-LC-176 cell line [without TGF-β signaling due to lack of TβRII expression (Halder et al. 2011)], and H358 and A549 cell lines (with TGF-β signaling) for in vitro and in vivo studies. This is a good model system to determine the effect of TGF-β-mediated tumor suppressor function on curcumin-mediated regulation of cell proliferation, apoptosis, and tumorigenicity in NSCLC cell lines. Under similar conditions, curcumin inhibits cell proliferation and induces apoptosis in all these NSCLC cell lines (Figs. 1, 2), although H358 and A549 cells are sensitive and ACC-LC-176 cells are resistant to TGF-β. These results are in agreement with the fact that endogenous TGF-β signaling has no role on these curcumin-mediated functions in vitro. Most importantly, in xenograft studies, we observed that curcumin reduces tumorigenicity of these cell lines under the same therapeutic strategy (Fig. 3). There is no significant difference in the effects of curcumin on the tumorigenicity of these cell lines. Our animal study also provides evidence that curcumin with therapeutic dose had no toxicity to the animals, as the animal weights were not affected and the important organs like the liver and the heart of mice were not damaged by the drug (Fig. 4). Immunohistochemical studies suggest that Ki67 (proliferation marker) expression is significantly down-regulated in tumors with curcumin treatment and the expression of cleaved caspase 3 is increased by curcumin in the xenografts from three cell types (Fig. 5). Therefore, curcumin suppresses tumorigenicity of these cell lines by inhibiting cell growth and inducing apoptosis. These in vivo results support above in vitro results, suggesting that TGF-β tumor suppressor functions are not involved in the anticancer effects of curcumin.
TGF-β signaling pathway is critical for the suppression of tumor development and progression (Massague 2008; Padua and Massague 2009). Previous studies from our laboratory have shown that TGF-β exerts tumor suppressor functions in NSCLC cells (Anumanthan et al. 2005; Halder et al. 2011). Stable expression of TβRII in NSCLC cells lacking its expression restores TGF-β-mediated Smad2/3 and Smad4 complex formation, TGF-β responsive gene expression, inhibition of cell proliferation, induction of apoptosis, and finally, decreases tumorigenicity (Anumanthan et al. 2005). In this study, curcumin inhibits TGF-β-induced Samd2/3 phosphorylation and transcriptional activation of TGF-β-sensitive reporters in H358 and A549 cells. In contrast, ACC-LC-176 cells are not responsive to TGF-β, and as a result, curcumin has no effect on TGF-β signaling (Fig. 6). Since curcumin and TGF-β function as tumor suppressor in H358 and A549 cells, it is possible that curcumin might inhibit endogenous TGF-β-induced growth inhibition and apoptosis through abrogation of TGF-β/Smad signaling. If that is the case and if the TGF-β-induced tumor suppressor function were involved in the antitumor activity of curcumin, then one would expect that curcumin-mediated inhibition of cell proliferation, induction of apoptosis, and reduction in tumorigenicity of H358 and A549 cells should be attenuated compared to ACC-LC-176 cells. Interestingly, we do not see any significant difference in the effects of curcumin on these three cell lines. There could be two possibilities for why we do not see any differential effect of curcumin in TGF-β-sensitive and TGF-β-insensitive cell lines: (1) TGF-β/Smad signaling does not contribute to the antitumor effect of curcumin in NSCLC cells and (2) down-regulation of TGF-β/Smad signaling by curcumin is not strong enough to diminish the tumor suppression functions of curcumin.
In conclusion, our study demonstrates that curcumin inhibits cell proliferation, induces cell apoptosis, and suppresses tumorigenicity of NSCLC cells in vitro and in vivo, and these functions of curcumin are independent of the TGF-β/Smad signaling pathway. In addition, these studies paved a way for the investigation into the role of curcumin on other signaling pathways. Our results from this preclinical study suggest that curcumin could be a potential therapeutic agent for both TGF-β-sensitive and TGF-β-resistant lung tumors.
Acknowledgments
This study was supported by R01 CA113519 and NCI SPORE grant in lung cancer (5P50CA90949, project# 4). We thank Dr. Takashi Takahashi (Aichi Cancer Center Research Institute, Nagoya, Japan) for providing us cell line.
Conflict of interest
None.
References
- Anumanthan G, Halder SK, Osada H, Takahashi T, Massion PP, Carbone DP, Datta PK (2005) Restoration of TGF-beta signalling reduces tumorigenicity in human lung cancer cells. Br J Cancer 93(10):1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, Mouret-Reynier MA, Durando X, Barthomeuf C, Chollet P (2010) Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther 9(1):8–14 [DOI] [PubMed] [Google Scholar]
- Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C, Meyskens FL Jr, Brenner DE (2011) Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 4(3):354–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY (2001) Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21(4B):2895–2900 [PubMed] [Google Scholar]
- Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425(6958):577–584 [DOI] [PubMed] [Google Scholar]
- Gaedeke J, Noble NA, Border WA (2004) Curcumin blocks multiple sites of the TGF-beta signaling cascade in renal cells. Kidney Int 66(1):112–120 [DOI] [PubMed] [Google Scholar]
- Halder SK, Beauchamp RD, Datta PK (2005) Smad7 induces tumorigenicity by blocking TGF-beta-induced growth inhibition and apoptosis. Exp Cell Res 307(1):231–246 [DOI] [PubMed] [Google Scholar]
- Halder SK, Cho YJ, Datta A, Anumanthan G, Ham AJ, Carbone DP, Datta PK (2011) Elucidating the mechanism of regulation of transforming growth factor beta type II receptor expression in human lung cancer cell lines. Neoplasia 13(10):912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Chen MJ, Yu YM, Ko SY, Chang CC (2010) Suppression of TGF-beta1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res 302(10):717–724 [DOI] [PubMed] [Google Scholar]
- Kim SY, Jung SH, Kim HS (2005) Curcumin is a potent broad spectrum inhibitor of matrix metalloproteinase gene expression in human astroglioma cells. Biochem Biophys Res Commun 337(2):510–516 [DOI] [PubMed] [Google Scholar]
- Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL, Lin JP, Ma YS, Wu CC, Chung JG (2009) Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and vascular endothelial growth factor (VEGF). Cancer Lett 285(2):127–133 [DOI] [PubMed] [Google Scholar]
- Massague J (1998) TGF-beta signal transduction. Annu Rev Biochem 67:753–791. doi:10.1146/annurev.biochem.67.1.753 [DOI] [PubMed] [Google Scholar]
- Massague J (2008) TGFbeta in cancer. Cell 134(2):215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua D, Massague J (2009) Roles of TGFbeta in metastasis. Cell Res 19(1):89–102 [DOI] [PubMed] [Google Scholar]
- Radhakrishna Pillai G, Srivastava AS, Hassanein TI, Chauhan DP, Carrier E (2004) Induction of apoptosis in human lung cancer cells by curcumin. Cancer Lett 208(2):163–170 [DOI] [PubMed] [Google Scholar]
- Sakurai R, Li Y, Torday JS, Rehan VK (2011) Curcumin augments lung maturation, preventing neonatal lung injury by inhibiting TGF-beta signaling. Am J Physiol Lung Cell Mol Physiol 301(5):L721–L730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS (1997) Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate, and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res 57(7):1301–1305 [PubMed] [Google Scholar]
- Samanta D, Gonzalez AL, Nagathihalli N, Ye F, Carbone DP, Datta PK (2012) Smoking attenuates transforming growth factor-beta-mediated tumor suppression function through downregulation of Smad3 in lung cancer. Cancer Prev Res (Phila) 5(3):453–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S, Chaturvedi MM, Aggarwal BB (2007) Role of curcumin in cancer therapy. Curr Probl Cancer 31(4):243–305 [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A (2012) Cancer statistics. CA Cancer J Clin 62(1):10–29 [DOI] [PubMed] [Google Scholar]
- Song K, Peng S, Sun Z, Li H, Yang R (2011) Curcumin suppresses TGF-beta signaling by inhibition of TGIF degradation in scleroderma fibroblasts. Biochem Biophys Res Commun 411(4):821–825 [DOI] [PubMed] [Google Scholar]
- Su CC, Yang JS, Lu CC, Chiang JH, Wu CL, Lin JJ, Lai KC, Hsia TC, Lu HF, Fan MJ, Chung JG (2010) Curcumin inhibits human lung large cell carcinoma cancer tumour growth in a murine xenograft model. Phytother Res 24(2):189–192 [DOI] [PubMed] [Google Scholar]
- Thangapazham RL, Sharma A, Maheshwari RK (2006) Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J 8(3):E443–E449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CL, Liu YY, Ma YG, Xue YX, Liu DG, Ren Y, Liu XB, Li Y, Li Z (2012a) Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS ONE 7(5):e37960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, Chang CL, Lee HC, Chi CW, Pan JP, Yang WC (2012b) Curcumin induces the apoptosis of human monocytic leukemia THP-1 cells via the activation of JNK/ERK pathways. BMC Complement Altern Med 12:22 [DOI] [PMC free article] [PubMed] [Google Scholar]