Abstract
Advance directives (ADs) refer to a category of documents that enable individuals to state their preferences for future healthcare decisions in the event of incapacitation. The purpose of the study was to examine differences in psychosocial characteristics and AD completion rates in persons newly diagnosed with cancer. This sample comes from data collected for a psychosocial registry for patients with cancer. The following psychosocial data were collected: Functional Assessment of Cancer Therapy-General, Profile of Mood States-short form, and the Functional Assessment of Chronic Illness Therapy-Spiritual Well-being Scale. The sample included 312 persons who had been diagnosed with cancer within the previous 5 months. There were no statistically significant differences between completion of an AD and the measured psychosocial characteristics at baseline (mean, 4.2 months); however, at 3 months, patients who reported higher quality of life (Functional Assessment of Cancer Therapy-General) were associated with lower AD completion rates and persons with greater mood disturbances had higher rates of AD completion. Our data suggest the need to develop strategies that would facilitate discussion of ADs for patients entering the cancer care continuum.
Keywords: Advance directive, Cancer, Psychosocial cancer registry, Psychosocial characteristics
Persons who are newly diagnosed with cancer are faced with multiple decisions and adjustments related to treatment, the impact of serious illness on their lives, and ultimately, the ability to maintain their quality of life (QOL). Continual advances in cancer treatment have provided hope of survival for patients and families that may have not been expected in the past.1 Despite these advances, individual response to cancer treatment varies greatly, and certain patients will not survive. In fact, 25% of all deaths in the United States are cancer related.2 Because the psychological and physical burden of the cancer and its treatment may impact decisional capacity, it is important for persons with cancer to discuss their preference and treatment wishes with a proxy.
Currently, upon the loss of cognitive capacity to communicate preferences, decisions regarding healthcare can be incorporated in advance directives (ADs). Advance directives are legal documents that include the living will or durable power of attorney for healthcare which are recognized and authorized in the United States by the Patient Self-determination Act.3 The living will specifies preferences for treatments and interventions and allows people to refuse life-sustaining treatment, and the durable power of attorney for healthcare appoints a proxy to make decisions for the patient based on the patient’s wishes in the event of incapacitation.
Literature Review
Several studies have investigated the use of ADs in patients with cancer. Lamont and Siegler4 interviewed 111 hospitalized patients with cancer to determine whether patients had ADs. Of these patients, half were admitted for elective chemotherapy and half were admitted for an acute medical problem. Thirty-three percent had 1 of the completed documents and 32% had both; thus, 65% had at least 1 document. Patients who were older and white and whose goal of chemotherapy was to extend life rather than palliate symptoms were more likely to complete ADs. Duration of diagnosis (median, 239 days) and estimated prognosis were not found to be predictors of AD completion. An emergent admission for an acute pulmonary process was the only significant predictor of the patient’s desire to discuss his/her advance care planning preferences. Of those with completed ADs (1 or both), only 9% discussed their preferences with their oncologist; however, 23% wished they would have discussed their preferences with their oncologist.
Ganti et al5 retrospectively investigated the presence of written ADs in patients who underwent hematopoietic stem-cell transplantation (HSCT). Of 299 patients, 42% (n = 127) had copies of ADs in their charts, and 58% did not have ADs (n = 172). Of those who had ADs present in their medical record, 22% (n = 28) had completed the document before their cancer diagnosis, 69% (n = 87) completed the document after the diagnosis but before the HSCT, and 9% (n = 12) completed documents after HSCT. Age was the only factor that was associated with AD completion, with persons older than 40 years being more likely to have an AD before HSCT. There were no differences found between sex, level of education, stage of disease (early, intermediate, and advanced), or use of spiritual coping and having an AD.
Kish et al6 found ADs documented in 236 charts (27%) of 872 patients with cancer who were admitted to an intensive care unit (ICU) in a cancer hospital. Malignancies were classified as solid tumors, leukemia, and lymphoma/myeloma. Advance directives were more common in older, white patients with hematologic cancers. Patients who had relapsing disease had ADs more frequently than did those who were in remission or who were newly diagnosed. Advance directives were found in 16% of patients who were newly diagnosed with cancer.
Previous studies have focused on demographic variables and AD completion in hospitalized patients with varied time intervals from the cancer diagnosis. Only 1 study addressed spiritual coping and ADs and no studies have investigated psychosocial issues that impact QOL in persons who are newly diagnosed with cancer and AD completion. Because facilitating advance care planning is an important responsibility for nurses, it would be helpful to have a better understanding of factors that might be associated with reluctance to complete ADs. The purpose of this article is to examine the differences in psychosocial characteristics of outpatients with or without ADs who are newly diagnosed with cancer.
Methods
Design
This was a secondary analysis of data collected for a psychosocial registry for persons with cancer. The registry project was designed to obtain longitudinal descriptive data for QOL and symptom research. Registries are used to collect data on incidence, prevalence, treatment, and prevention of disease and to measure QOL.7 Cancer registries emerged with the organization of the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute in 1973. The SEER registry includes data on patient demographics, tumor site, morphology, first treatment course, and vital statistics data. As opposed to the SEER registry and other disease-specific registries, a psychosocial data registry contains both physiological and patient-reported data. A registry can be used to identify potential participants for additional data collection, thus decreasing the time involved in subject recruitment. As in this study, a registry can also provide researchers with large samples in which to study a specific phenomenon.
Sample and Setting
A convenience sample was drawn from the outpatient cancer clinic of a Midwestern National Cancer Institute–designated Comprehensive Cancer Center. To be eligible for the study, individuals should meet the following inclusion criteria: older than 18 years (defined as an adult in the state of Ohio), ability to comprehend the English language (to establish informed consent and read or hear the interview questions), a new diagnosis of cancer, and receiving ongoing care at the cancer center. Exclusion criteria included cognitive impairment and immediate referral for a bone marrow/stem cell transplant because much of this type of treatment is conducted as an inpatient and this study focused on an outpatient patient population.
Procedure
Approval for this study was obtained from the hospital’s institutional review board. Patients meeting eligibility criteria were invited for voluntary participation in the registry. Informed consent was obtained by trained research nurses. Interrater reliability was established and maintained at greater than 90% agreement for all questionnaires. Patients were enrolled from July 2005 through December 2007. Data were collected at 2 intervals: at enrollment and 3 months after enrollment. At enrollment, a patient was considered to have an AD through self-report of a living will or durable power of attorney for healthcare or both. At the 3-month interval, patients were randomized to face-to-face interview or chart review to determine the presence of an AD. Randomization was conducted at 3 months to determine if the same data were obtainable from computerized charts and face-to-face interviews. All patients were asked if they had an AD at enrollment, and data on ADs were again collected 3 months later even if they reported having an AD at the time of enrollment. Demographic and clinical data were gathered at enrollment. In addition, the following data were collected at both enrollment and at 3 months: the Eastern Cooperative Oncology Group (ECOG) scale, Functional Assessment of Cancer Therapy-General (FACT-G), Functional Assessment of Chronic Illness Therapy-Spiritual Well-being Scale, and Profile of Mood States (POMS) short form. Based on patient preference, data were collected via face-to-face interviews, mail survey (postage-paid return envelopes were provided), self-administration while at the cancer center, or via telephone interview.
Instruments
EASTERN COOPERATIVE ONCOLOGY GROUP SCALE
The ECOG scale was used to measure performance status. There are 6 possible ECOG scores, ranging from 0 (fully active, able to carry on all predisease performance without restriction) to 5 (dead). The ECOG scale is used by healthcare providers and researchers to assess how a patient’s disease is progressing, assess how the disease affects activities of daily living, and determine how one might tolerate a particular treatment regimen.8
CHARLSON INDEX
The Charlson is a weighted comorbidity score based on the number and severity of medical conditions. The Charlson Index of Comorbidity predicts 1-year mortality from selected comorbidities by assigning weights to 19 conditions including cardiovascular, liver, lung, and connective tissue diseases; diabetes; hematological and solid tumor cancers; and AIDS.9 A score of 1, 2, 3, or 6 is assigned for each comorbid condition, and a higher total score is predictive of higher mortality rates. The potential range of scores is 0 to 37. The patient’s primary condition for which he/she is seeking treatment (ie, cancer in this study) is not counted in the Charlson score. The Charlson was designed and validated to predict medical outcomes.10 Although Charlson and colleagues10 originally used it to predict 1-year mortality, it has been validated among multiple acute care patient populations as a generic measure of severity of illness because of its prognostic capability and simplicity.11–13
FUNCTIONAL ASSESSMENT OF CANCER THERAPY-GENERAL
Quality of life was measured using the FACT-G. The questionnaire consists of 27 Likert-scale items with 4 subscales (physical well-being, social/family well-being, emotional well-being, and functional well-being). A higher score, both on the subscales and the total score, indicates a higher QOL (range of total score, 0–108). This can be administered in an interview and self-report formats. Reliability and validity have been established,14 with Cronbach α values for the subscales ranging from .71 to .96.
PROFILE OF MOOD SATES-SHORT FORM
The 30-item POMS-short form was used to measure mood state. The instrument has a 5-point Likert scale and 6 sub-scales (tension/anxiety, depression, anger, vigor, fatigue, and confusion). Theoretical range for the subscales is 0 to 20. A total mood disturbance score (range, 20–100) is obtained by summing the 6 subscales. Reliability and validity have been established.15,16 Higher scores, with the exception of the vigor subscale, are indicative of greater mood disturbance.17
FUNCTIONAL ASSESSMENT OF CHRONIC ILLNESS THERAPY-SPIRITUAL WELL-BEING SCALE
Spirituality was measured using the 12-item Functional Assessment of Chronic Illness Therapy-Spiritual Well-being Scale. A 5-point Likert scale (‘‘not at all’’ to ‘‘very much’’) produces 2 subscales and a total score. A higher score is consistent with greater spirituality. Cronbach α for the total scale and 2 subscales ranged from .81 to .88.18
Results
Data were entered and analyzed using SPSS 15.0 software. Descriptive statistics and χ2 and t tests were used to compare the demographic and psychosocial characteristics of patients with or without an AD. Logistic regression was used to determine predictors of having an AD.
A total of 381 patients were interviewed at baseline (enrollment), which occurred within 5 months (mean, 4.2 months) of their diagnosis of cancer, and of these patients, 180 patients were interviewed again 3 months after the baseline interview. The refusal rate was 25% (n = 130). The reasons for refusal included the following categories: not interested, overwhelmed, too sick/ weak, not comfortable with the Health Insurance Portability and Accountability Act portion of the consent, and passive refusals. The rate of attrition from baseline to the second data collection interval at 3 months was 9%; 5 patients dropped out of the study and 18 died. Of the 5 patients who dropped out, 3 were too ill to complete the interview, 1 was overwhelmed, and 1 gave no reason. Most data at baseline were collected via face-to-face interviews (n = 188; 60%); others were completed via mail, self-report while at the cancer center, and via telephone. At time 2, the participants were randomized to 2 data collection groups; 50% of the participants received interviews, and 50% of the data were collected via chart review. Thus, at time 2 (n = 113), the number of analyzed participants decreased because we were unable to obtain complete AD data from chart review.
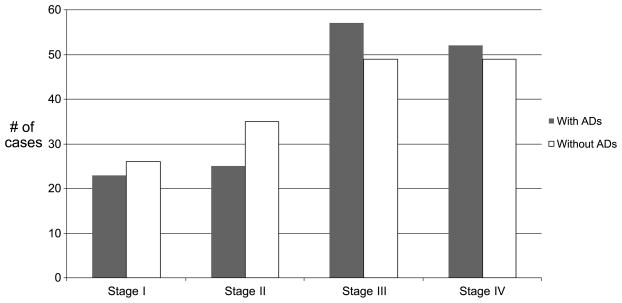
At the time of enrollment, 49% (n = 185) had ADs (living will or durable power of healthcare), and 53% had completed ADs by the 3-month interview (Table 1). Categories of cancer diagnoses included hematologic, lung, gynecologic, breast, head and neck, colorectal, gastrointestinal, and other (Table 2). The other characteristics of the sample at the baseline interview are described in Table 3. There were more women than men without ADs. Race was categorized into white and African American because there were only 5 persons in the ‘‘other’’ category, which included Hispanic and Asian persons. Marital status was categorized into married or not married, which included separated, divorced, widowed, never married, single, or unknown. Employment status was categorized into employed and unemployed, which included those who were retired, disabled, and not employed. The breakdown of cancer stages was as follows: stage I, n = 39; stage II, n = 60; stage III, n = 89; and stage IV, n = 101 (Figure 1). Twenty-seven patients had unspecified cancer stages but were classified as either localized or advanced. Those patients who were classified as localized (n = 10) were placed in stage I, and those classified as advanced (n = 17) were placed in stage IV. Similarly, the breakdown of ECOG status were the following: ECOG 0, n = 138; ECOG I, n = 165; ECOG II, n = 40; and ECOG III, n = 15. No patients were categorized as ECOG IV. Patients who reported a history of cancer had a remote diagnosis for which they were no longer receiving treatment.
Table 1.
Advance Directive Use at Baseline and in 3 Months
| Advance Directive Use | Yes |
No |
|---|---|---|
| No. (%) | No. (%) | |
| At enrollment | 185 (49) | 196 (51) |
| At 3 mo | 96 (53) | 84 (47) |
Table 2.
Advance Directives and Type of Cancer
| Type of Cancer | With Advance Directives (n = 184) | Without Advance Directives (n = 194) |
|---|---|---|
| Hematologic | 34 | 35 |
| Lung | 27 | 37 |
| Gynecologic | 16 | 26 |
| Breast | 18 | 24 |
| Head and neck | 17 | 24 |
| Colorectal | 24 | 13 |
| Other | 48 | 35 |
Table 3.
Relationships Between Patient Characteristics and Advance Directives (ADs) (n = 381)
| Variable | With ADs (n = 185) |
Without ADs (n = 196) |
t/χ2 | P |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Sex | 5.56 | .018 | ||
| Female | 92 (50) | 121 (62) | ||
| Male | 93 (50) | 75 (38) | ||
| Race | 30.53 | .000 | ||
| White | 161 (90) | 126 (66) | ||
| African American | 18 (10) | 65 (34) | ||
| Marital status | 10.99 | .001 | ||
| Married | 134 (72) | 110 (56) | ||
| Not married | 51 (28) | 86 (44) | ||
| Level of education | 16.89 | .001 | ||
| Less than high school | 5 (3) | 21 (11) | ||
| High school | 83 (46) | 97 (52) | ||
| College | 61 (34) | 55 (29) | ||
| Postcollege | 30 (17) | 14 (8) | ||
| Employment status | 0.259 | .611 | ||
| Employed | 70 (39) | 78 (41) | ||
| Not employed | 110 (61) | 110 (59) | ||
| Income | 14.69 | .001 | ||
| ≤$20,000 | 24 (14) | 52 (28) | ||
| $21,000–$49,999 | 55 (32) | 67 (35) | ||
| ≥$50,000 | 95 (54) | 70 (37) | ||
| Cancer stage | 2.12 | .145 | ||
| I and II | 48 (31) | 61 (38) | ||
| III and IV | 109 (69) | 98 (62) | ||
| History of cancer | 11.69 | .001 | ||
| Yes | 35 (19) | 14 (7) | ||
| No | 147 (81) | 178 (93) | ||
| Performance status (Eastern Cooperative Oncology Group) | 0.006 | .937 | ||
| 0 and 1 | 147 (84) | 156 (85) | ||
| 2 and 3 | 27 (16) | 28 (15) | ||
| Age, mean (SD), y | 63.4 (11.4) | 55.9 (12.3) | 6.18 | .000 |
Figure 1.
Cancer stage.
There were statistically significant differences in age, sex, race, marital status, level of education, income, and history of cancer between those who had ADs and those who did not. Patients who were older, white, and married and with a high school or college education, income greater than $50,000, and a history of cancer were more likely to have completed ADs than were those who did not. Furthermore, there were more women than men without ADs. A greater proportion of patients with stage III and IV cancer and ECOG status 0 and 1 had ADs than did those with stage I and II cancer and ECOG status 2 and 3, but these were not statistically significant.
Independent t tests were run to determine differences in comorbid illness and psychosocial characteristics between those with ADs and those without within 5 months of diagnosis (or at their baseline interview) (Table 4) and again in 3 months (Table 5). The Charlson comorbidity index scores (calculated only at baseline) were nearly the same for those with and without ADs. At enrollment, there were no statistically significant differences between completion of an AD and the measured psychosocial characteristics. At 3 months, there were statistically significant differences in FACT-G scores (P = .036) and POMS-total mood disturbance scores (P = .053) between those who had ADs and those who did not. Patients with ADs reported a lower QOL and more mood disturbances than did those without ADs.
Table 4.
Relationships Between Psychosocial Characteristics and ADs at Baseline
| Patients With ADs (n = 175a) |
Patients Without ADs (n = 184a) |
CI | t | P | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| Charlson | 0.57 (1.0) | 0.58 (1.2) | −0.250 to 0.218 | −0.133 | .89 |
| FACT-G total score | 82.2 (15.1) | 79.1 (16.8) | −0.347 to 6.54 | 1.77 | .078 |
| FACIT-spirituality | 36.3 (8.9) | 37.3 (8.2) | −2.86 to 0.778 | −1.13 | .261 |
| POMS-depression subscale | 2.4 (2.8) | 3.1 (3.6) | −1.36 to 0.021 | −1.92 | .058 |
| POMS-total mood disturbance score | 12.2 (15.3) | 13.3 (19.5) | −5.25 to 2.39 | −0.734 | .461 |
Abbreviations: AD, advance directive; CI, confidence interval; FACIT, Functional Assessment of Chronic Illness Therapy Spiritual Well-being Scale; FACT-G, Functional Assessment of Cancer Therapy-General; POMS, Profile of Mood States.
Differences in sample sizes are due to missing data. Sample sizes: Charlson, n = 359; FACT-G, n = 335; FACIT, n = 343; POMS-depression, n = 340; POMS-total score, n = 325.
Table 5.
Relationships Between Psychosocial Characteristics and ADs at 3 Months
| Patients With ADs (n = 61a) |
Patients Without ADs (n = 52a) |
CI | t | P | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| FACT-G total score | 80.0 (19.0) | 87.1 (13.2) | −14.15 to −0.498 | −2.13 | .036 |
| FACIT-spirituality | 37.1 (8.4) | 37.5 (8.5) | −3.58 to 2.75 | −0.262 | .794 |
| POMS-depression subscale | 2.7 (3.4) | 2.1 (2.6) | −0.511 to 1.77 | 1.09 | .277 |
| POMS-total mood disturbance score | 12.9 (19.0) | 6.4 (15.3) | −0.088 to 13.08 | 1.96 | .053 |
Abbreviations: AD, advance directive; CI, confidence interval; FACIT, Functional Assessment of Chronic Illness Therapy Spiritual Well-being Scale; FACT-G, Functional Assessment of Cancer Therapy-General; POMS, Profile of Mood States.
Differences in sample sizes are due to missing data. Sample sizes: FACT-G, n = 90; FACIT, n = 113; POMS-depression, n = 111; POMS-total score, n = 109.
After examining relationships using bivariate analyses, a multivariate approach was used. Logistic regression was conducted to determine which independent variables (age, sex, race, marital status, level of education, employment status, income, cancer stage, history of cancer, and performance status) were predictors of having an AD. Data screening indicated that there were no outliers. The model with all of the variables in the equation was statistically significant in predicting an AD (P = .021), and the correct classification for AD and no AD occurred 74% of the time (R2 = 0.364, Nagelkerke test). Of the 10 independent variables, only 4 made a statistically significant contribution to the prediction of having an AD: age (odd ratio [OR], 1.07; 95% confidence interval [CI], 1.03–1.09; P = .000), race (OR, 0.261; 95% CI, 0.16–0.60; P = .001), education (OR, 1.87; 95% CI, 1.24–2.81; P = .003), and history of cancer (OR, 3.09; 95% CI, 1.11–8.58; P = .30).
Discussion
In the psychosocial cancer registry of persons with newly diagnosed cancer, the rates of AD completion were higher than we had expected. One possible explanation is that most patients verbally reported (via face-to-face interview) whether they had a living will or durable power of healthcare. Patients may have overreported completing ADs because they had the intention to do so or because they thought they should have completed an AD but did not. Another potential reason for the high AD completion may be that more healthcare providers are talking to their patients about ADs than in previous years. We did observe a small increase in AD completion 3 months after the enrollment interview, which is likely related to patients having more time to consider ADs after their diagnosis of cancer.
The completion rates for patients in this cancer registry was similar to completion rates reported with patients with hematologic malignancies who were undergoing their first HSCT, despite the differences in the population.5 Besides the difference in the type of cancer diagnoses, the patients in these 2 other studies were different in 2 major ways. First, for patients undergoing HSCT, the median time period from diagnosis was 12 months, and second, HSCT was done with a curative intent, which may be unlike some patients in the registry who were newly diagnosed (average, 4.2 months), with a majority having an advanced stage of cancer and possibly few options for cure. It is unclear why these completion rates would be so similar because it seems that completion rates would be higher among patients who are undergoing HSCT compared with cancer registry patients because they may have had more time to consider ADs in addition to preparing to undergo a surgery with a significant potential for short-term morbidity and mortality. Because the study by Ganti5 assessed the completion of ADs by written documentation and the registry used self-report at baseline, it is possible that actual AD completion was higher for patients who underwent HSCT because ADs were actually verified in the chart.
Advance directive completion rates in the cancer registry were higher than those found in hospitalized cancer patients (32%).4 Although patients in each of these samples had varied cancer diagnoses, the median duration since diagnosis for hospitalized patients was 239 days, with elective chemotherapy or an acute medical illness as the 2 major reasons for hospitalization. It seems likely that having a diagnosis of cancer for a longer period of time and being hospitalized either for chemotherapy or an acute problem would influence a person’s desire to complete an AD. Because the hospitalized cancer patients also self-reported AD completion, we were surprised that the rate was not higher. Lastly, the AD completion rate in the registry was much higher than rates for cancer patients in the ICU.6 However, knowing patients’ preferences for treatment is probably most needed the ICU setting. Perhaps, when patients are acutely ill and admitted to an ICU, documentation could be missing even if the patient had an AD.
In our bivariate analysis, our findings that age, race, and marital status impact AD completion was supported in previous studies of persons with or without cancer4–6; however, our finding of more women than men without ADs has not been previously reported. Other demographic variables (level of education, employment status, and income) either were not included or were categorized in different groups in the studies conducted in persons with cancer. In the cancer registry, income was associated with AD completion. Persons who were in the lower income bracket were less likely to report AD completion. This may reflect that they had concerns other than discussing their preferences for treatment or that they had less access to assistance or education about ADs. It was not surprising that persons with a history of cancer were more likely to have an AD than were those without a history of cancer because those with a cancer history had other opportunities to think about and discuss preferences and decisions for healthcare.
With our multivariate analysis, a more parsimonious model emerged. The following variables made a significant contribution to the model: age, race, education, and history of cancer. For every year a person ages, he/she is 1.1 times more likely to have an AD. African American persons were 26% less likely than white persons to have an AD. For every step increase in education, persons were 1.9 times more likely to have an AD, and lastly, persons who had a history of cancer were 3.3 times more likely to have an AD than were persons without a history of cancer.
We did find differences in the perception of QOL and mood between patients with and without ADs at 3 months. Higher FACT-G scores, indicative of a better QOL, were associated with a lower rate of ADs, and higher POMS scores, indicative of more mood disturbances, were associated with higher AD completion. Persons who perceive a higher QOL may be less likely to complete ADs because they might not be concerned about being unable to make decisions at some future date and may be less likely to think of limiting treatments. Persons with greater anxiety, depression, anger, fatigue, or confusion may have felt more concerned about their future ability to make decisions and were thus more inclined to have an AD. In particular, patients who rated themselves as anxious were probably more likely to complete ADs perhaps because they were more anxious about the future. The lack of statistically significant differences in the psychosocial characteristics and AD completion at baseline (enrollment) may be related to the fact that the cancer diagnosis was too recent to affect the measured psychosocial data.
It is surprising that ECOG and cancer stage did not impact AD completion in the cancer registry patients. It would seem that decreased functional ability and more advanced cancer stage would provide a prompt to healthcare providers to discuss preferences for treatment or prompt patients with awareness of ADs to complete them. It could be that regardless of cancer stage and performance status, persons with newly diagnosed cancer and their healthcare providers were hopeful for a curative treatment within the first several months so they were not thinking it would be necessary to know preferences for treatment that were not cure related.
Limitations
One limitation of the data on ADs from the cancer registry is that we do not know if patients had ADs before their new cancer diagnosis, so we were not able to analyze the potential impact of a new diagnosis of cancer and completing ADs. Furthermore, all patients were asked about ADs at the baseline interview and again a time point 2 (in 3 months) regardless if they already reported having an AD, so we could not address whether a period of time after diagnosis was related to AD completion. The last limitation, as discussed earlier, was that AD completion was measured by self-report at baseline, which may be associated with overreporting, and only half of the patients were randomized to self-report at the 3-month interview. The strengths of using the data from the registry include its large sample size and variety of cancer diagnoses.
Implications
Previous studies have shown that persons with cancer want to discuss ADs with their oncologists,4,19 and others found that it was up to the healthcare provider to initiate the discussion about ADs.20 These studies did not report whether persons would have preferred to discuss ADs with their internists, because patients go to oncologists for treatment. Healthcare providers could use the information on the demographic characteristics and ADs found in several studies to reinforce the importance of spending time talking about ADs with those patients who are less likely to complete them. Although it is not feasible to have all patients complete numerous questionnaires to assess psychosocial characteristics, it is important for healthcare providers to ask patients about their perceptions of QOL and about mood disturbances because this information could provide an opportunity for discussing ADs.
Discussion of ADs does not need to be addressed by every oncologist, particularly in early stages of treatment in persons with newly diagnosed cancer. However, all healthcare providers who are involved in the care of any patient need to initiate a discussion about their preferences for treatment at some point. This is especially important for patients with potentially life-limiting diseases because waiting until a later stage of cancer narrows the window of opportunity and may limit the time that patients have to think about the difficult topic and discuss it with family. Our data do suggest the need to continue to develop systems and strategies to be more helpful to patients entering the cancer continuum.
Acknowledgments
This study was funded by P20-CA-103736 and Case Western Reserve University Presidential Research Initiative.
References
- 1.Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002;360(9340):1131–1135. doi: 10.1016/S0140-6736(02)11199-8. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Patient Self-determination Act. Omnibus Reconcilation Act Public Law No 101–508 & 4206 (1990).
- 4.Lamont EB, Siegler M. Paradoxes in cancer patients; advance care planning. J Palliat Med. 2000;3(1):27–35. doi: 10.1089/jpm.2000.3.27. [DOI] [PubMed] [Google Scholar]
- 5.Ganti AK, Lass SJ, Vose JM, et al. Outcomes after hematopoietic stem-cell transplantation for hematologic malignancies in patients with or without advance care planning. J Clin Oncol. 2007;25(35):5643–5648. doi: 10.1200/JCO.2007.11.1914. [DOI] [PubMed] [Google Scholar]
- 6.Kish SK, Martin CG, Price KJ. Advance directives in critically ill cancer patients. Crit Care Nurs Clin North Am. 2000;12(3):373–383. [PubMed] [Google Scholar]
- 7.Daly BJ, Douglas SL, Foley H, et al. Psychosocial registry for persons with cancer: a method of facilitating quality of life and symptom research. Psychooncology. 2007;16(4):358–364. doi: 10.1002/pon.1091. [DOI] [PubMed] [Google Scholar]
- 8.Oken M, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 9.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson co-morbidity index for predicting mortality. Med Care. 2005;43(6):607–615. doi: 10.1097/01.mlr.0000163658.65008.ec. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Chin MH, Goldman L. Correlates of early hospital readmission or death in patients with congestive heart failure. Am J Cardiol. 1997;79(12):1640–1644. doi: 10.1016/s0002-9149(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 12.Singh B, Bhaya M, Stern J, et al. Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. Laryngoscope. 1997;107(11):1469–1475. doi: 10.1097/00005537-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Pioro MH, Landefeld CS, Brennan PF, et al. Outcomes-based trial of an inpatient nurse practitioner service for general medical patients. J Eval Clin Pract. 2001;7(1):21–33. doi: 10.1046/j.1365-2753.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 14.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 15.Cordova MJ, Giese-Davis J, Golant M, et al. Mood disturbance in community cancer support groups: the role of emotional suppression and fighting spirit. J Psychosom Res. 2003;55(5):461–467. doi: 10.1016/s0022-3999(03)00510-5. [DOI] [PubMed] [Google Scholar]
- 16.Gill P, Kaur JS, Rummans T, Novotny PJ, Sloan JA. The hospice patient’s primary caregiver: what is their quality of life? J Psychosom Res. 2003;55(5):445–451. doi: 10.1016/s0022-3999(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 17.McNair DM, Lorr M, Droppleman LF. Edits Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 18.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the Functional Assessment of Chronic Illness Therapy-Spiritual Well-being Scale (FACIT-Sp) Ann Behav Med. 2002;24(1):49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 19.Pentz RD, Lenzi R, Holmes F, Khan MM, Verschraegen C. Discussion of the do-not-resuscitate order: a pilot study of patients with refractory cancer. Support Care Cancer. 2002;10(8):573–578. doi: 10.1007/s00520-002-0374-z. [DOI] [PubMed] [Google Scholar]
- 20.Sahm S, Will R, Hommel G. What are cancer patients’ preferences about treatment at the end of life, and who should start talking about it? A comparison with health people and medical staff. Support Care Cancer. 2005;13(4):206–214. doi: 10.1007/s00520-004-0725-z. [DOI] [PubMed] [Google Scholar]