Abstract
Introduction
Developing in vitro models for studying cell biology and cell physiology is of great importance to the fields of biotechnology, cancer research, drug discovery, toxicity testing, as well as the emerging fields of tissue engineering and regenerative medicine. Traditional two dimensional (2D) methods of mammalian cell culture have several limitations and it is increasingly recognized that cells grown in a three dimensional (3D) environment more closely represent normal cellular function due to the increased cell-to-cell interactions, and by mimicking the in vivo architecture of natural organs and tissues.
Areas Covered
In this review, we discuss the methods to form 3D multi-cellular spheroids, the advantages and limitations of these methods, and assays used to characterize the function of spheroids. The use of spheroids has led to many advances in basic cell sciences, including understanding cancer cell interactions, creating models for drug discovery and cancer metastasis, and they are being investigated as basic units for engineering tissue constructs. As so, this review will focus on contributions made to each of these fields using spheroid models.
Expert Opinion
Multi-cellular spheroids are rich in biological content and mimic better the in vivo environment than 2D cell culture. New technologies to form and analyze spheroids are rapidly increasing their adoption and expanding their applications.
1. Introduction
The culture of mammalian cells in vitro has led to numerous conceptual advances in cell biology and to the understanding of the formation and function of tissues, organs, as well as diseased states such as cancer. Most of these findings were elucidated using traditional 2D culture techniques, but biology is clearly a complex 3D system. 2D cell culture techniques do not faithfully replicate all of the mechanical and biochemical signals present in vivo1. In 2D techniques, cell-to-plastic interactions prevail rather than the crucial cell-to-cell and cell-toextracellular matrix (ECM) interactions that form the basis for normal cell function2. Additionally, the plastic Petri dish is an unnaturally stiff substrate compared to the softer mechanical environment that cells experience in vivo which is well known to alter cell function.
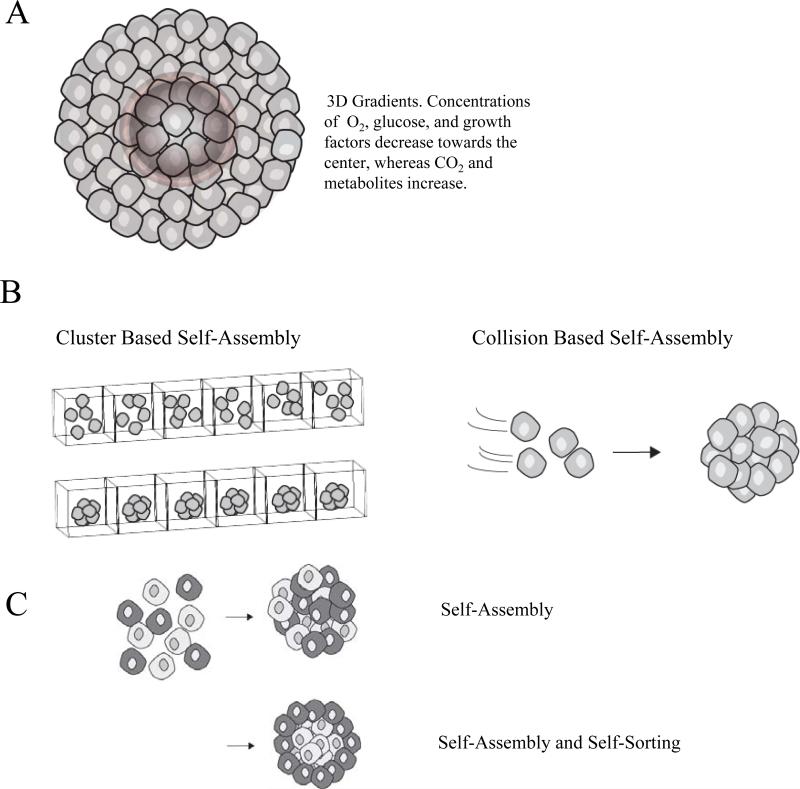
In the absence of an attachment surface or scaffold, cells will aggregate and undergo the process of self-assembly. During self-assembly, mono-dispersed cells form 3D microtissues called multicellular spheroids (MCSs). Self-assembly mimics natural processes that occur during embryogenesis, morphogenesis and organogenesis3-5 (Figure 1). Spheroids mimic the architectural and functional characteristics of native tissue such as cardiomyocyte spheroids that beat with heart-like rhythm, hepatocyte spheroids that have liver-like functionality, as well as human endothelial cells vascularizing fibroblast microtissues6-9.
Figure 1.
(A) The concentric organization of proliferating and necrotic cells in multicellular spheroids is influenced by the molecular gradients of soluble factors which are established by convection and diffusion within the spheroid microenvironment. (B) There are two main categories of spheroid bio-fabrication, cluster based and collision based self-assembly. In cluster based self-assembly, monodispersed cells are segregated into compartments, settle, and aggregate to form spheroids. In collision based self-assembly, suspended cells collide to form spheroids. (C) When certain mixed cell populations self-assemble, the specific pattern of segregation that occurs is referred to as self-sorting.
The in vivo-like microenvironment that the spheroid creates is the result of numerous factors including the formation of molecular gradients. Gradients of soluble components in the cell culture medium (e.g., nutrients, oxygen, growth factors) as well as those produced by cells (e.g. metabolites, paracrine factors) are established due to the barriers to diffusion imposed by the spheroid, as well as the rates of consumption and production of these factors by the cells10. In addition to gradients, spheroids create an in vivo-like microenvironment by forming more complex cell-to-cell interactions and cell-to-ECM adhesions. These serve to simultaneously deliver additional signals including mechanical forces, biochemical signals and electrical coupling that can influence cell shape, motility, proliferation, and differentiation as well as gene expression1, 11.
In this article, we do not review the entire field of 3D cell culture. Excellent comprehensive reviews and books can be found elsewhere. Much of this work is focused on culturing cells attached to and cast in various natural and synthetic scaffolds. Instead, we focus our review on multi-cellular spheroids formed without an attachment to a scaffold or gel. Cell-to-cell adhesion is one of the key driving forces of their formation. In most cases, these structures are termed spheroids due to their approximate spherical shape. However, other shapes are now possible, hence the term multi-cellular microtissues or simply microtissues. Regardless of how they are formed, spheroids and microtissues are all characterized by a very high density of cells, a density that more closely approximates that of normal organs or tissues. We discuss the techniques to form microtissues, their advantages and disadvantages, as well as uses of microtissues in basic biology, cancer research, tissue engineering and drug discovery.
2. Methods
2.1 Techniques to Form Spheroids and Microtissues
2.1.1 Pellet Culture
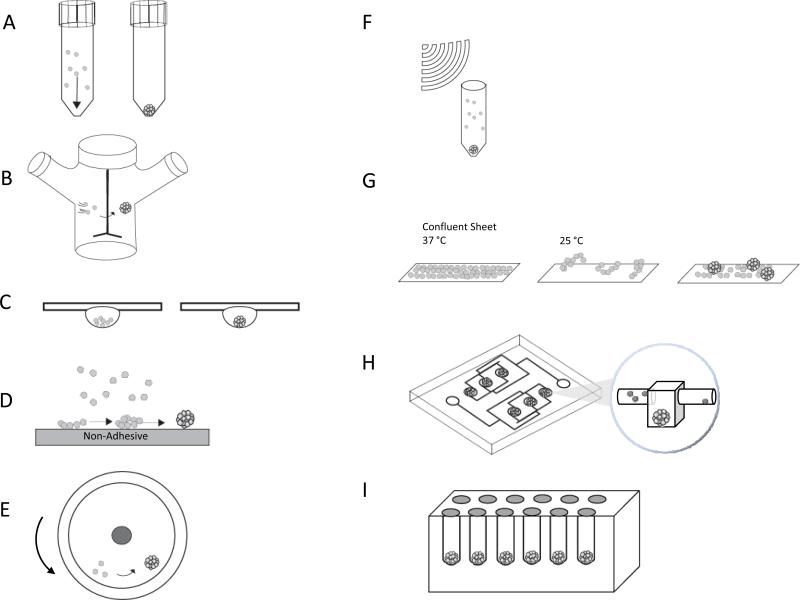
The pellet culture technique uses centrifugal force to concentrate cells to the bottom of a tube maximizing the opportunity for cell-to-cell adhesions to form (Figure 2A). To form spheroids, a cell suspension in a tube with a conical bottom is centrifuged at a speed of 500g for 5 minutes12. To optimize this process, the cell suspension may be incubated on a shaker for one hour prior to centrifugation. Due to the ease of formation, pellet culture is often used to create spheroids to study chondrogenesis, and bone formation, and for the differentiation of mesenchymal stem cells12-16. While a large number of cells can aggregate rapidly, a disadvantage is the shear stress from centrifugation that can damage cells. This method is used to form spheroids of fairly large diameter which can create a low oxygen concentration in the center. For chondrocytes, differentiation is stimulated by low oxygen, but for other cells types, hypoxia can cause necrosis in the spheroid core 17, 18. Additionally, while this method is simple and rapid to perform, it has not been scaled up for mass production of spheroids, nor can the cells be visualized as they aggregate.
Figure 2.
Methods of spheroid bio-fabrication (A) Pellet Culture (B) Spinner Culture (C) Hanging Drop (D) Liquid Overlay (E) Rotating Wall Vessel (F) External Force (G) Cell Sheets (H) Microfluidics (I) Nonadhesive Hydrogel Micro-molds
2.1.2 Spinner Culture
The spinner culture method creates spheroids by preventing cells in suspension from settling and by promoting cell-to-cell collisions via constant stirring (Figure 2B)19, 20. To form spheroids, spinner flasks are seeded with a uniform, well mixed single cell suspension and the fluid environment and mass transfer in the flask is controlled by convective forces generated by an impeller or magnetic stir bar. Maintaining a constant rotation speed is critical. An agitation speed that is too slow will allow the spheroids to settle, but one that is too high will cause cell damage due to fluid shear stress. One advantage is that the fluid movement aids mass transport in and out of the spheroids. Spinner culture has been used with a variety of primary cells, cell lines, and mixtures of two different cell types to form heterotypic spheroids7, 21-23. Spinner culture can be scaled up to produce large number of spheroids. By decreasing the agitation speed and allowing the cells to settle, culture conditions can be controlled through media changes and addition of drugs and growth factors. Due to shear forces, this method may not be useful for cells with low cohesiveness, cells that are sensitive to shear forces, or for adherent cells that may undergo apoptosis while in suspension. Due to constant mixing, cells cannot be visualized as they aggregate.
2.1.3 Hanging Drop
The hanging drop method relies on gravity-enforced self-assembly to produce spheroids24. To make spheroids, small volumes (20-30 μL) of a cell suspension are pipetted onto the inside lid of a tissue culture plate. The lid is inverted, and the drops stay attached to the lid due to surface tension. Gravity causes the cells to settle and concentrate at the bottom of the drop, and a single spheroid is formed24, 25 (Figure 2C). A variety of cell types have formed spheroids using this method including both primary cells as well as cell lines7. Different cell types can be co-cultured to form heterotypic spheroids. Spheroid size and cellular composition is controlled by adjusting the cell density in each drop. Advances into high throughput production of spheroids using the hanging drop method have been established, producing up to 384 spheroids in a single array26. However, with this method, it is difficult to track these spheroids during formation and it is nontrivial to exchange media or add drugs.
2.1.4 Liquid Overlay
The liquid overlay method inhibits the attachment of cells to tissue culture plates and promotes cell-cell aggregation. In this method, a cell suspension is seeded onto flat tissue culture dishes made of low-adhesive surfaces such as agarose27, 28, and poly (2-hydroxethyl methacrylate) (pHEMA)29 (Figure 2D). The plates are rocked and with a small amount of shaking, the cells aggregate into spheroids. Both primary cells and cell lines form spheroids using this method. While the method is easy, the spheroids are often heterogeneous in both size and shape. This technique, when performed in 96 well plates, allows for monitoring of individual spheroid formation and growth.
2.1.5 Rotating Wall Vessel
The rotating wall vessel creates a microgravity environment that maintains cells in suspension and allows cells to aggregate into spheroids. A cell suspension in a rotating wall vessel is slowly rotated about an x-axis maintaining the cells in continuous free fall (Figure 2E). Initially, rotation is very slow (~15 rpms), but as spheroids begin to form and the mass of the aggregates increase, rotation is increased to keep the aggregates in suspension (~25 rpms)30. Spheroids from both primary cells as well as a variety of cell lines have been formed using this method. Heterotypic spheroids can be formed by co-culture of different cell types. Long term culture is possible and culture conditions can be controlled by perfusion which is useful for controlling differentiation31. The method produces aggregates in a low shear environment. Although the yield is high, there can be variability in spheroid size. While some advances in tracking the aggregates have been made32, it remains difficult to monitor the assembly of spheroids in real-time.
2.1.6 External Force
The external force method uses any force to concentrate mono-dispersed cells into a high density that facilitates cell aggregation. External forces used are electric fields, magnetic force, and ultrasound (Figure 2F). For electric fields, positive dielectrophoresis in a low conductivity iso-osmotic solution is used to concentrate cells33. For magnetic fields, cells are incubated with magnetic cationic liposomes (MCLs) containing a magnetite core (Fe3O4)34, 35. After endocytosis, the cells can be formed into specific patterns using magnetic fields. For ultrasound, an ultrasound standing wave trap is used to concentrate cells and facilitate their aggregation36. Cell adhesion is often very nonspecific and it can be difficult to control spheroid size. The physiological changes to the cells caused by these external forces are not well characterized.
2.1.7 Cell Sheets
In addition to spheroids, multi-cellular cell sheets have been produced by culturing cells on a polymer, such as poly (N-isopropylacrylamide) (PNIPAAm), which is thermo-responsive. changing its hydrophilicity and hydrophobicity with a change in temperature37. Lowering the temperature to 20ºC for one hour causes the polymer to change from hydrophobic to hydrophilic, thus causing the release of a contiguous sheet of cells. To form spheroids, small cell sheets that have been released can be further incubated on a non-adhesive surface where they will compact and form spheroids (Figure 2G). The cell sheet method has been used with hepatocytes37, endothelial cells38, cardiomyocytes39, 40, epithelial cells, and mesenchymal stem cells for use in cartilage engineering41. Additionally, co-culture of multiple cell types has been achieved by creating layers of cell sheets from different cell types, and then allowing the multi-layered sheet to assemble into a spheroid38, 42.
2.1.8 Micro-fluidics
When using micro-fluidics, cells are flowed through a micro-channel network into micro-chambers where they are partitioned and exposed to micro-rotational flow that causes cells to aggregate43 (Figure 2H). The method works for primary cells, cell lines, and co-culture of multiple cell types44, 45. Micro-fluidic devices allow for high throughput production of size controlled spheroids for high throughput analysis, as many of these platforms are equipped with biosensors for real-time imaging and monitoring of the system46, 47. Additionally, the perfusion system allows for tight control of the fluid shear stresses and the concentration of soluble factors surrounding the spheroids47, 48.
2.1.9 Micro-molded Nonadhesive Hydrogels
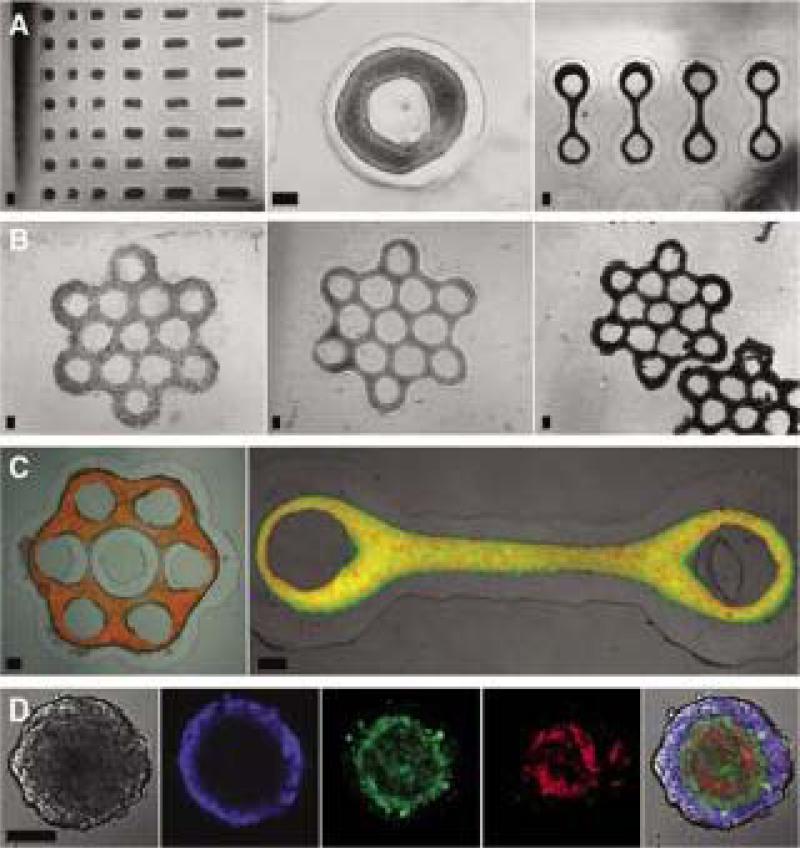
Micro-molded nonadhesive hydrogels have been used to form spheroids as well as microtissues with different shapes49, 50. This method uses computer aided design software and rapid prototyping to form micro-molds that contain an array of cylindrical pegs with rounded tops. Nonadhesive hydrogels (agarose or polyacrylamide) are then cast from these micro-molds (Figure 2I). After the micro-molded gels are seeded, the cells settle to the bottom of the recesses, are unable to attach to the nonadhesive hydrogel, and cell-to-cell interactions drive the formation of a single spheroid in each recess. This technology has been used to form spheroids using many different cell types including primary cells and cell lines from a variety of tissues including, skin, brain, ovary, liver, heart, and breast9, 49, 51-53. The method can be scaled up to create up to 822 spheroids in a single mold, with homogenous shape, size, and cell composition. These factors are easily controlled by adjusting the number of cells, and the ratio of the cell mixture seeded onto the micro-molded hydrogel. Cells can be imaged as they self-assemble and it's easy to change media and add drugs, antibodies, or growth factors. In addition to spheroids, micro-mold designs have been used to direct the self-assembly of cells to more complex shapes such as rods, toroids, or honeycombs50 (Figure 3A-E).
Figure 3.
Micro-molded hydrogels can be used to direct the self-assembly of a number of shapes including rodes, toroids, loop-ended dogbones, and honeycombs. (A) Microtissues with complex geometries were self-assembled from various cell types: rat hepatoma (H35) rods (left), MCF-7 toroid (middle), and H35 loop-ended dog bones (right). (B) MCF-7 cells seeded into honeycomb recesses (left), formed a branched microtissue by 1 day (middle), and compacted slightly but retained the honeycomb shape for at least 3 days after removal from the mold (right). Mixed-cell populations produced multilayered microtissues with controlled shape. (C) Images of microtissues with normal human fibroblasts (NHF) (red) cores coated by an outer layer of H35 (green, left) or HUVEC (green, right). (D) Confocal microscopy images of self-sorting of three cell types within a 2-day-old tritypic spheroid. (E) The eight orbital honeycomb of NHG (6×106 cells) was stained with a live/dead assay after 24 hours to show viability. Scale bars are 200 μm (panels A-C), 100 μm (panel D), and 1800 μm (panel E). Figure permissions Biotechniques, and Biofabrication.
2.2 Methods for Working with Spheroids
2.2.1 Imaging
3D spheroids are more challenging to image than thin flat cells grown on conventional 2D cultures, but they offer new opportunities for more high content biological information. The spherical structure of the spheroid with its radial symmetry provides opportunities for mapping cell functions to these gradient and 3D microenvironments.
All of the conventional methods of microscopy have been used to image spheroids. Scanning electron microscopy (SEM) has been used to obtain low and high resolution images of the surface of spheroids. Low resolution images reveal cell size, shape and organization on the surface, whereas high resolution images reveal important biological structures such as pores and micro-villi. Conventional brightfield, and phase contrast microscopy have been used to image the x and y dimensions of spheroids and time lapse has been used to view and quantify the self-assemble of spheroids and microtissues of other shapes50. Spheroids have a significant z dimension and so side view microscopy has been used to image spheroid height54, 55. Due to their thickness and lack of transparency, none of these methods are able to obtain adequate images of the interior of the spheroid.
To view their interior, spheroids have been fixed, sectioned and stained with a variety of conventional dyes (e.g., hematoxylin and eosin) antibodies, small molecules and fluorescent molecules. Images of these sections provide valuable information about a single slice through the interior of a spheroid. In some cases, serial sections of the same spheroid have been used to reconstruct 3D organization. As an alternative to these labor intensive approaches, confocal fluorescence microscopy has been used to obtain thin optical sections of fluorescently stained spheroids. These images can be rendered to create a high resolution 3D reconstruction of the spheroid and it's interior. However, the maximum penetration depth of confocal microscopy is about 50μm. Two photon fluorescence microscopy overcomes this limitation and has been used to obtain optical sections and a high resolution 3D reconstruction of the entire spheroid. Recently, a new microscopy technique, light sheet based fluorescence microscopy (LSFM), has been used to image fluorescently labeled spheroids to produce a high resolution 3D reconstruction1. Our lab recently reported an easy method to use wide field fluorescent images to construct a 3D map of fluorescence on multiple spheroids as well time lapse data55. The method was used to quantify the kinetics of self-sorting that occurs when two different cell types self-assemble.
2.2.2 Assays for Spheroid Growth
Multiple methods have been used to quantify spheroid growth and the response to growth factors and drug treatments. Like, 2D cell culture, the enzymatic disaggregation of spheroids and the direct counting of cells has been used to quantify growth. However, the technique can be tedious and spheroids are difficult to disaggregate. Measuring the x, y dimension of spheroids from brightfield images has been used to quantify spheroid growth. Care must be taken since spheroids may appear to be near perfect spheres in conventional microscopy, when in fact, they are oblate spheroids whose z dimension can be less than their x, y dimensions. Colorimetric dyes, such as the tetrazolium salt WST-1 [2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2, 4-disulfophenyl)-2H-tetrazolium], as well as measuring DNA content or ATP levels have been used to measure growth. To determine where cell proliferation occurs in 3D, spheroids have been sectioned or visualized via confocal microscopy and immuno-stained for standard proliferation markers such as Ki-67 or bromodeoxyuridine labels.
2.2.3 Mathematical Modeling of Spheroids
Mathematical simulations have been developed to understand various aspects of spheroid formation and spheroid function. Self-assembly and the process of self-sorting that occurs when two different cell types segregate as they self-assemble has been modeled by several groups56-58. One such model, the differential adhesion hypothesis (DAH) posits that cell types self-segregate due to differences in cell-to-cell adhesion or apparent surface tension, with those cells of highest cohesion (like-to-like adhesion) sorting to the inside of a spheroid and those cells with lower cohesion sorting to the outside3, 4, 20, 59. Based on finite element computer simulations, the differential interfacial tension hypothesis (DITH) factors in contributions from cytoskeletal components and cell adhesion molecules60. Most recently, self-sorting has been modeled using an order parameter, which is based on a geometrically driven argument to describe the relationship between heterotypical interface length and the system size61.
Mathematical models have also been formulated for cancer biology to describe the concentration gradients and the microenvironments formed in spheroids, as models of avascular tumors. These models propose mechanisms of tumor cell growth, differentiation, proliferation, and apoptosis based on the concentration of oxygen, nutrients, and metabolites at different radial points within the spheroid. Studies have correlated experimental data and simulations with the transcriptional activity of spheroids in hypoxic and normoxic conditions62, 63. Other models explain the mechanism by which cancer cells metastasize and continuum mathematics has been used to understand the mechanisms that control invasive cell behaviors64. Additional models simulate cancer cell invasion by treating cells as individual units since invasion is thought to be created by interactions between single cells and their surrounding matrix64-66.
Mathematical models have been developed for drug transport in spheroids. The diffusion distance of a drug in a monolayer of cells is short compared to in vivo tissues, whereas a drug must penetrate multi-layers of cells before reaching the center of a tumor67. Cells in monolayer do not adequately replicate the cell-to-cell interactions necessary to form well know bio-obstacles to drug transport such as tight junctions68. Lastly, it has been recognized for over 40 years that cancer cells grown in 2D are unusually sensitive to drug treatment than the same cells grown in 3D. Other models based on tumor spheroids have been used to quantify the effects of diffusion barriers in multi-layer tissues such as cellular compaction, gap junctions, and cellular efflux systems69. Advanced modeling predicts the pharmacokinetics of cancer drug candidates while accounting for the cellular determinants that affect these parameters70.
3. Applications of Spheroids and Microtissues
3.1 Spheroids as Models for Basic Science
Self-assembly is thought to mimic naturally occurring processes and when two different cell types self-assemble, they often self-sort and compartmentalize to separate regions of the spheroid. This is similar to one cell population spreading over the other during morphogenesis and gastrulation. As so, microtissues produced via self-assembly have been used to elucidate the mechanisms of these phenomena in developmental biology2, 71, 72.
The molecular events controlling self-assembly have been studied using spheroids. Cadherins are critical to self-assembly and varying levels of cadherins can control self-sorting, such that transfected cells with high cadherin levels sort to the center of a spheroid, surrounded by cells with lower cadherin levels3, 20. In addition to cadherins, our lab has shown that connexins (Cx43), another surface adhesion molecule, helps mediate self-assembly51. Connexins form gap junctions that directly couple cells to one another and the adhesive function of gap junctions contributes to self-assembly on par with the adhesive effects of cadherins51. Recently, our lab has also shown that pannexin-1 (Panx1), a surface molecule with topographical similarities to connexins, controls self-assembly, but not via surface adhesion. Panx-1 forms channels that do not dock with other channels. Instead, Panx-1 channels act as pores leaking ATP which activates purinergic receptors (P2X7) causing actin reorganization via elevated calcium levels52. Our lab and others have shown that the cytoskeleton is an active player in self-assembly54, 73, 74. Drug targeting of actomyosin dependent cell tension (Y-27632, a rho kinase inhibitor) slows the rate of self-assembly and alters self-sorting73. Drugs that target myosin-2 (blebbistatin) and the actin network (cytochalasin, Y-27632) block or slow self-assembly of hepatocytes into functional tissue-like structures, and reduce liver-specific activities74-76.
It is becoming increasingly clear that surface adhesion molecules and the cytoskeletal network act in concert to control self-assembly and self-sorting and that the mechanical forces of cell-to-cell interactions play a role in these processes11. Multi-cellular microtissues mimic the in vivo 3D mechano-environment and new designs in microtissues are useful for understanding normal as well as pathologic conditions of mechanobiology. Our lab recently reported an assay to quantify for the first time the forces of self-assembly54. Cells self-assembled a toroid-shaped microtissue that moved up a nonadhesive cone. The mass of the toroid and its rate of movement up the cone were used to calculate cell power, the work expended in the process against the force of gravity. Toroids of fibroblasts exerted tenfold more power than toroids of hepatocytes, 4.3±1.7 pJ/hr and 0.31±0.01 pJ/hr, respectively. Moreover, drug targeting of actomyosin dependent cell tension resulted in a greater than 50% reduction in cell power for both cell types, demonstrating the crucial role of the cytoskeleton. In addition to quantifying the contribution of specific proteins and proteins systems, the toroid-on-cone assay is useful for understanding drug actions and how cell mixtures behave in a 3D mechanical environment54. Recently, the toroid-on-cone assay was used to quantify heterotypic cell mixtures and uncovered a new role for TGF-β177. Another microtissue design used by our lab to investigate 3D cell mechanics is the loop ended dogbone49. The tensile forces of self-assembly in this mechanically constrained design cause fibroblasts to elongate over 30 times their initial diameter. These microtissues break due to these forces, but they can be stabilized by drugs that target the cytoskeleton (Y-27632 and blebbistatin a myosin II inhibitor).
3.2 Spheroids in Cancer Research and Drug Discovery
Tumor spheroids have been used as an in vitro model to mimic the complexity of tumors78. Mixed spheroids have been used to study tumor-stromal cell interactions and the cells in these mixed spheroids have a more in vivo cell shape, architecture, and gene expression profiles79, 80. For example, breast cancer cells co-cultured with fibroblasts showed an invasive phenotype and formed a tissue more similar to primary breast cancer tissue in terms of protein expression in the cancer cells (pancytokeratins, p53, E-cadherin) and the fibroblasts (vimentin) compared 81. Three dimensional tumor-endothelial cell models have been used to measure the angiogenic and metastatic potential of tumor cells and the presence of endothelial cells makes tumor cells more resistant to chemotherapy and radiation82, 83.
One important use of spheroids is as a model for drug discovery and drug transport. Cancer cells are much more drug resistant when grown as spheroids versus monolayer68. For example, the IC50 of paclitaxel and cisplatin in spheroids is 100-fold higher than the IC50 in mono-layers of the same cells. Similar results have been found with antibodies, siRNA, and immunotoxins84-87. This result is, due in part, because of drug penetration, but also due to the cellular phenotype induced by differences in hypoxia, proliferation, cell-to-cell contacts, and gene expression at different locations within a spheroid, much like a tumor88, 89. Tumor spheroids have been used to measure drug penetration in 3D by using fluorescence of anthracyclines such as doxorubicin, or autoradiography of radio-labeled drugs90, 91. 3D models based on human cells have the potential to lead to high-throughput and high-content screening to predict the responsiveness of individual tumors to different therapy regimens.
3.3 Spheroids in Tissue Engineering
Spheroids are increasingly being used in tissue engineering for a variety of different organs and tissues92. Spheroids produced from primary rat and mouse cardiomyocytes, as well as a mixed population of cells, beat with a natural rhythm for up to three weeks because the cells are electrically coupled93. Self-assembled constructs of human chondrocytes, as well as pluripotent stem cells differentiated towards a chondrogenic lineage are under consideration for use in tissue engineering94. Compared to cells seeded on a matrix, spheroids of chondrocytes have histological, biochemical, and biomechanical properties more similar to native cartilage95. The mechanical properties of cartilage largely depend on the organization of extracellular matrix, and self-assembled spheroids attain total collagen and sulfated glycosaminoglycans amounts closer to cartilage than those that made with cells on scaffolds16, 96.
In addition to uses in toxicity testing, liver spheroids may have an application in a bioartificial liver device. Hepatocytes cultured in 2D lose differentiated function, but hepatocytes cultured as spheroids retain their polarity, form tight junctions, possess microvilli-lined channels that resemble bile canaliculi and have liver-like function97. Spheroids of the human cell line, HepG2, retained key detoxifying functions required of the liver. Co-culture of primary rat hepatocyte cells with hepatic stellate cells have liver like functionality for over 2 months in culture98. Spheroids of other complex tissues and organs have been formed. Pancreatic epithelial cells have been shown to self-assemble a hollow 3D structure 99, 100. Dissociated cells from the retina self-assemble and self-sort to form distinct cell layers that resemble the retina's architecture complete with photo receptors101.
3.4 Spheroids as Building Blocks
Presently, the biggest challenge to the field of tissue engineering is the in vitro fabrication of large tissue constructs with a high density of living cells, similar to natural organs 102, 103. Nearly all of the clinical successes in tissue engineering are relatively thin tissues (< 2 mm), where the transport of oxygen, nutrients, and metabolic waste critical for cell viability occurs by simple diffusion 102. Likewise, spheroid diameter is limited to 200-400 microns due to the constraints of diffusion. Human umbilical cord endothelial cells (HUVECs) have been used to coat spheroids and the endothelial cells migrate to the core and developed a capillary-like network104. These endothelialized spheroids have been fused to form a larger vascularized tissue that could integrate with the host vascular system after implantation105. Likewise, when human mesenchymal stem cells that were differentiated towards an osteogenic lineage were mixed with HUVECs, they formed a pre-vascular network within 10 days13, 106.
The emerging field of bio-printing and bio-fabrication is seeking to address the issue of large tissue constructs and is using spheroids as building blocks to try and fabricate organs in vitro. Bio-printers, adapted from inkjet printing and rapid prototyping technologies, have been used to fabricate living structures via a layer-by-layer printing of living cells along with an extracellular matrix (ECM) material 107-113. Computer control of the deposition process in the x, y and z dimensions enables the fabrication of complex-shaped living structures, complete with the beginnings of a vascular tree. Spheroids loaded in a cartridge are printed as the “bio-ink” along with an ECM substrate which serves as the “bio-paper”. After printing, spheroids attach to the ECM substrate and fuse with neighboring spheroids to form a contiguous 3D structure111, 112. Control over cell position with a building block is important when building large complex 3D structures108, 114. The relative position of different cell types within spheroids as well as spheroids that have been fused is beginning to be controlled. Likewise, endothelialized spheroids have been used to create microvessels after fusion and the relationship between spheroid size and capillary formation has also been investigated115.
3.5 Microtissues with Complex Shapes as Building Blocks
We and others have investigated the use of building blocks of different shapes for the purpose of bio-fabrication. In addition to spheroids, multi-cellular sheets produced on a thermo-reponsive polymer have been used for numerous cell types including smooth muscle cells and cardiomyocytes116. Layers of cardiac cell sheets have been used to engineer functional 3D tissues that can be transplanted to improve cardiac function after myocardial infarction40. Cell sheets have been wrapped around a mandrel to create a tubular structure and cell sheets have been layered to create a thicker tissue117.
Non-adhesive micro-molds have been used to direct the self-assembly process so that cells form non-spherical multi-cellular microtissues49, 50. Micro-molds have been used to form microtissues in the shape of rods, toroids, loop-ended dogbones, and honeycombs (Figure 3A-E). Rod or cylinder shaped microtissues have also been used in micro-extrusion, a variation of bio-printing. Cylinder shaped microtissues loaded in a cartridge are slowly extruded and sliced into blocks that will round up into spheroids as a method to produce spheroid building blocks.
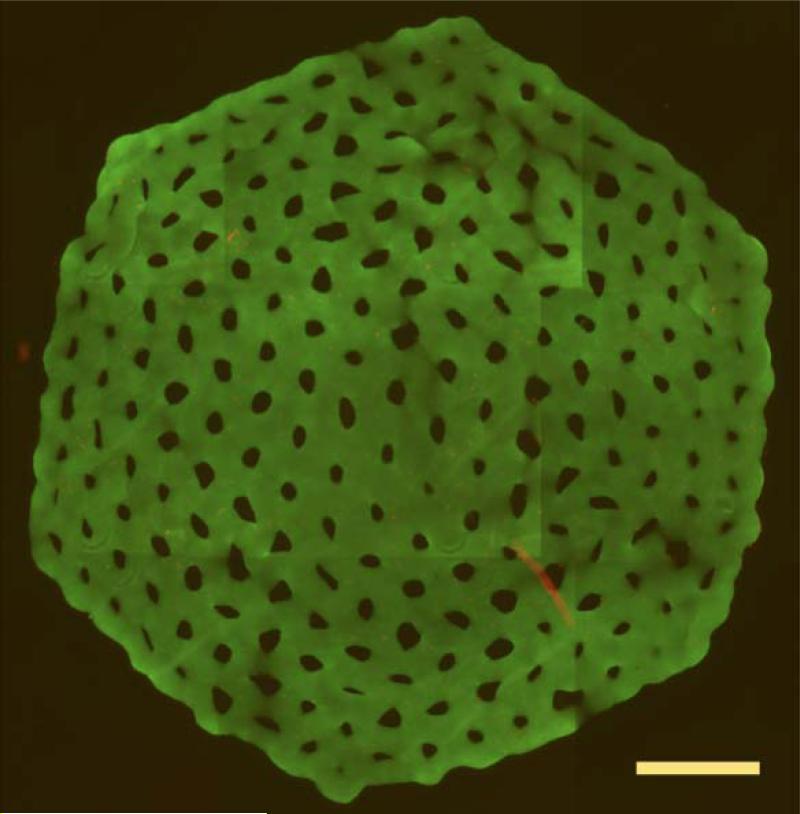
Non-adhesive micro-molds have also been used to produce more complex building blocks in the shape of a toroid49. These structures with high cell densities are interesting because they have a lumen, so cells are located within diffusion distance of a top /bottom surface or the surface of a lumen. Lumen diameter is initially controlled by micro-mold design and when multi-cellular toroids are harvested from the micro-molds, they undergo slow, but predictable changes to their shape and lumen diameter which can be used in their design as a building block118. Moreover, harvested toroids can fuse with one another in a process that is complete within 72 hours and toroids can be used as building units to make a large multi-layered multi-torus structure. In this fused structure, the lumens began to form an interconnected network of open space, mimetic of a vascular network118. Nonadhesive micro-molds have also been used to form a large honeycomb structure (2 cm), a single structure with rings of interconnected cells around lumens119. As a potential building unit, the honeycomb can be significantly larger in the x and y dimensions, while the cross section of the cellular portion of any part of the honeycomb can be kept small, so as to not exceed the critical diffusion distance needed to maintain cell viability.
4. Summary and Conclusions
Multi-cellular spheroids provide many advantages over traditional monolayer culture of cells and have the potential to impact numerous facets of biomedical research. Although challenges exist when working with spheroids, they are promising in vitro models for basic and developmental biology, cancer biology, drug discovery and toxicology studies, as well as tissue engineering. These microtissues have proved to be realistic models of both normal and diseased tissues in vitro. While advances in spheroid formation, imaging, and assays to work with spheroids have been made, there is still a need for optimization and standardization of these protocols and development of more widely adopted endpoint analytical measurements.
5. Expert Opinion
There is little doubt that the biology and biochemistry of spheroids is significantly closer to natural tissues and organs than a thin monolayer of cells grown on a stiff plastic dish. This was first recognized over 40 years ago, when cancer cells grown in 2D were shown to be far more sensitive to radiation and drug treatment than spheroids, a result that more closely mimics the resistance of tumors in vivo. Nevertheless, 2D cell culture rapidly expanded its importance and is now widely adopted in all areas of biological research and drug discovery. The explosion in 2D cell culture was driven by the ease and convenience of culturing cells in 2D and easy methods to nourish, image, manipulate and assay cultured cells. Despite having more relevant biological information, spheroids have lagged behind 2D cell culture, because the methods for culturing spheroids were cumbersome, difficult to control and the assays as well as microscopy techniques to analyze spheroids were equally difficult or nonexistent. New and more convenient methods have been developed for the formation of spheroids. It's now easy to produce large numbers of spheroids of uniform size, control the size of spheroids, produce spheroids of two or more different cell types, produce spheroids from tumor cells as well as normal human cells, and even produce spheroids that are not simple spheres. Multi-cellular toroids, rods and honeycombs are now possible. In addition to being straightforward and convenient, these methods have been adapted to high throughput formats. Moreover, advances in microscopy, fluorescent probes and biochemical assays have made spheroids more amenable to investigation. Thus, it's becoming easier to extract the rich biological content that multi-cellular spheroids can supply.
Spheroids have more biological information because they are a living assembly of cells that signal one another and actively respond to the signals they generate. As the cells aggregate, they move upon each other and pairs of cell types can even self-sort as they self-assemble or cells can form hollow spheres. They maximize cell-to-cell contact, form numerous adhesions between surface adhesion molecules and even form direct cell couplings such as gap junctions. They exert biomechanical forces that change the shape, cytoskeleton and function of the cells. They secrete biochemical signals that alter gene expression and cell function. They synthesize, secrete and assemble numerous extracellular matrix proteins in response to all these signals and this ECM also signals cells. They establish 3D gradients of nutrients, metabolites and cell signals as well as barriers to the transport of molecules. In short, the repertoire of cell functions in a multi-cellular spheroid is not constrained; cells are freed to assemble an in vivo-like 3D environment.
In contrast, 2D plastic substrates constrain the repertoire of cell functions. Cells adhere more strongly to the substrate than they do to each other. Cells spread to form a very thin and highly adherent monolayer whose shape is a poor replicate of cell shape in vivo. Cell-to-cell interactions and cell-to-cell signaling are minimized because attachment to the substrate dominates the 2D environment. Cells are exerting forces against and moving on an unyielding and unnaturally stiff substrate that is, from a biology perspective, unresponsive. This is true of cells on typical flat planar substrates or synthetic polymeric substrates that are in the form of fibers or foams. Likewise, the same is true, to some extent, for scaffolds or gels of natural proteins such as collagen or laminin. ECM bio-molecules do provide more biological information than synthetic polymers and cells will respond to these cues, but the information is not as dynamic, nor as biologically complex, as the exchange of signals that occurs when cells are in direct contact. Moreover, the bio-molecules in these scaffolds are often in random organizations, whereas the ECM produced by cells in contact with other cells is influenced by the mechanical and biochemical signals emanating from surrounding cells. Thus, multi-cellular spheroids are superior replicas of the in vivo environment because they maximize the cell-to-cell interactions that elicit the greatest repertoire of cell functions. Non-living substrates, (synthetic and natural) that interfere with cell-to-cell interactions can constrain cells, thereby limiting the repertoire of cell functions.
The new methods of forming spheroids and microtissues are expanding their use and driving new applications. By addressing the limitations and constraints to the biology of cells cultured on plastic, microtissues are beginning to emerge as more realistic and more predictive platforms for in vitro toxicity testing and drug discovery. The use of mixed spheroids with two or more different cell types is an especially exciting development because it takes another step closer to the in vivo environment. The robust biology of microtissues will also impact the fields of cell transplantation and tissue engineering by providing a stable and functional unit suitable for transplantation or use as building blocks for the biofabrication of larger tissue constructs.
With regards to applications in cell transplantation, the technology for making large numbers of well defined uniform spheroids is suitably advanced and near ready for the clinic. In many respects, spheroids are analogous to pancreatic islets which have been clinically used for many years. Needed are refinements to the methods currently in development to uniformly differentiate cells into desired cell types such as the beta cells of the pancreas. With regards to applications in the tissue engineering of large organs, the technology of spheroids and complex microtissues is limited by the same grand challenge that currently stymies all tissue engineering approaches. Due to the limitations of diffusion, thick tissues with high cell densities demand a functional vascular network to keep the cells alive. Research on many fronts is trying to address this grand challenge and in the future, spheroids and microtissues may be one part of the solution.
Article Highlights.
3D spheroids produced via self-assembly, in a scaffold-free environment, mimic the function and architecture of in vivo tissues making them important in vitro models.
New and more convenient methods to produce spheroids have expanded their use in biomedical research.
In addition to producing spheroids, non-adhesive micromolds can direct the self-assembly of microtissues with complex shapes, creating new opportunities.
New biochemical assays and microscopy techniques have been developed to image and analyze 3D spheroids.
Spheroids are commonly used in cancer biology to understand cancer-stromal cell interactions and mechanisms of cancer metastasis.
Mathematical models are being used to understand how cells aggregate and self assemble as well as how drugs diffuse into spheroids.
Due to their high biological content, spheroids are being used in high-throughput and high-content formats for toxicology testing and drug discovery.
Spheroids with tissue specific functionalities have been produced for cardiac, cartilage, bone, hepatic and pancreatic tissues.
Advances have been made in vascularizing spheroids, but this remains a major challenge to the field of tissue engineering.
Spheroids and microtissues with complex shapes are being used as building blocks for fabricating large tissue constructs.
Table 1.
Advantages of the techniques for self-assembly of microtissues
| Pellet Culture | Spinner Culture | Hanging Drop | Liquid Overlay | Rotating Wall Vessel | External Force | Cell Sheets | Microfluidics | Micro-molded Hydrogels | |
|---|---|---|---|---|---|---|---|---|---|
| Good Size Control | + | + | + | + | + | ||||
| Low Shear Stresses | + | + | + | + | + | + | |||
| High Yield | + | + | + | + | |||||
| Good Control of Heterotypic Cell to Cell Ratio | + | + | + | + | + | ||||
| Good control of the microenvironment over time | + | + | + | + | + | ||||
| Minimal Labor | + | + | + | + | |||||
| Easy to Handle Micro-tissues | + | + | + | + | + | ||||
| Possibility of Complex Shapes | + | ||||||||
| Observe by Microscopy | + | + | + | + | + | ||||
| Cell Type Independent | + | + | + | + | + |
Acknowledgements
This work was funded, in part, by National Institutes of Health (NIH) grant R01EB008664-01A1. Conflict of interest disclosure: J.R.M has an equity interest in MicroTissues, Inc. This relationship has been reviewed and managed by Brown University in accordance with its conflict of interest policies.
References
- 1.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;810:839–45. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;145:526–32. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol. 2003;2532:309–23. doi: 10.1016/s0012-1606(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 4.Foty RA, Pfleger CM, Forgacs G, et al. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;1225:1611–20. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 5*.Moscona A, Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat. 1952;863:287–301. [One of the original papers showing the self-assembly and self-sorting of cells.] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda J, Nakazawa K. Orderly arrangement of hepatocyte spheroids on a microfabricated chip. Tissue Eng. 2005;117-8:1254–62. doi: 10.1089/ten.2005.11.1254. [DOI] [PubMed] [Google Scholar]
- 7*.Kelm JM, Fussenegger M. Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol. 2004;224:195–202. doi: 10.1016/j.tibtech.2004.02.002. [Scale up of the hanging drop method.] [DOI] [PubMed] [Google Scholar]
- 8*.Kunz-Schughart LA, Schroeder JA, Wondrak M, et al. Potential of fibroblasts to regulate the formation of three-dimensional vessel-like structures from endothelial cells in vitro. Am J Physiol Cell Physiol. 2006;2905:C1385–98. doi: 10.1152/ajpcell.00248.2005. [Demonstration of the endothelialization of spheroids and the role of spheroid size.] [DOI] [PubMed] [Google Scholar]
- 9.Desroches BR, Zhang P, Choi B, King ME, Maldonado AE, Li W, Rago A, Liu GX, Nath N, Hartmann KM, Yang B, Koren G, Morgan JR, Mende U. Functional Scaffold-Free 3D Cardiac Microtissues: a Novel Model for the Investigation of Heart Cells. Am. J of Physiol. 2012 doi: 10.1152/ajpheart.00743.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;73:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;3311:1469–90. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- 12.Li J, He F, Pei M. Creation of an in vitro microenvironment to enhance human fetal synovium-derived stem cell chondrogenesis. Cell Tissue Res. 2011;3453:357–65. doi: 10.1007/s00441-011-1212-8. [DOI] [PubMed] [Google Scholar]
- 13.Rouwkema J, de Boer J, Van Blitterswijk CA. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;129:2685–93. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 14.Qihao Z, Xigu C, Guanghui C, et al. Spheroid formation and differentiation into hepatocyte-like cells of rat mesenchymal stem cell induced by co-culture with liver cells. DNA Cell Biol. 2007;267:497–503. doi: 10.1089/dna.2006.0562. [DOI] [PubMed] [Google Scholar]
- 15.Jahn K, Richards RG, Archer CW, et al. Pellet culture model for human primary osteoblasts. Eur Cell Mater. 2010;20:149–61. doi: 10.22203/ecm.v020a13. [DOI] [PubMed] [Google Scholar]
- 16.Giovannini S, Diaz-Romero J, Aigner T, et al. Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cell Mater. 2010;20:245–59. doi: 10.22203/ecm.v020a20. [DOI] [PubMed] [Google Scholar]
- 17.Schrobback K, Klein TJ, Crawford R, et al. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1193-7. [DOI] [PubMed] [Google Scholar]
- 18.Markway BD, Tan GK, Brooke G, et al. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 2010;191:29–42. doi: 10.3727/096368909X478560. [DOI] [PubMed] [Google Scholar]
- 19.Castaneda F, Kinne RK. Short exposure to millimolar concentrations of ethanol induces apoptotic cell death in multicellular HepG2 spheroids. J Cancer Res Clin Oncol. 2000;1266:305–10. doi: 10.1007/s004320050348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;2781:255–63. doi: 10.1016/j.ydbio.2004.11.012. [Experimental evidence supporting the hypothesis that self-sorting is driven by differences in cell adhesion.] [DOI] [PubMed] [Google Scholar]
- 21.Nyberg SL, Hardin J, Amiot B, et al. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transpl. 2005;118:901–10. doi: 10.1002/lt.20446. [DOI] [PubMed] [Google Scholar]
- 22.Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;164:735–49. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- 23.Han Y, Liu XM, Liu H, et al. Cultivation of recombinant Chinese hamster ovary cells grown as suspended aggregates in stirred vessels. J Biosci Bioeng. 2006;1025:430–5. doi: 10.1263/jbb.102.430. [DOI] [PubMed] [Google Scholar]
- 24.Kelm JM, Timmins NE, Brown CJ, et al. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;832:173–80. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 25.Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol Med. 2007;140:141–51. doi: 10.1007/978-1-59745-443-8_8. [DOI] [PubMed] [Google Scholar]
- 26.Tung YC, Hsiao AY, Allen SG, et al. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;1363:473–8. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enmon RM, Jr., O'Connor KC, Lacks DJ, et al. Dynamics of spheroid self-assembly in liquid-overlay culture of DU 145 human prostate cancer cells. Biotechnol Bioeng. 2001;726:579–91. [PubMed] [Google Scholar]
- 28.Metzger W, Sossong D, Bachle A, et al. The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy. 2011;138:1000–12. doi: 10.3109/14653249.2011.583233. [DOI] [PubMed] [Google Scholar]
- 29.Landry J, Bernier D, Ouellet C, et al. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;1013:914–23. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingram M, Techy GB, Saroufeem R, et al. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell Dev Biol Anim. 1997;336:459–66. doi: 10.1007/s11626-997-0064-8. [DOI] [PubMed] [Google Scholar]
- 31.Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells. 2007;259:2224–34. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 32.Manley P, Lelkes PI. A novel real-time system to monitor cell aggregation and trajectories in rotating wall vessel bioreactors. J Biotechnol. 2006;1253:416–24. doi: 10.1016/j.jbiotec.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Sebastian A, Buckle AM, Markx GH. Tissue engineering with electric fields: immobilization of mammalian cells in multilayer aggregates using dielectrophoresis. Biotechnol Bioeng. 2007;983:694–700. doi: 10.1002/bit.21416. [DOI] [PubMed] [Google Scholar]
- 34.Ino K, Okochi M, Honda H. Application of magnetic force-based cell patterning for controlling cell-cell interactions in angiogenesis. Biotechnol Bioeng. 2009;1023:882–90. doi: 10.1002/bit.22104. [DOI] [PubMed] [Google Scholar]
- 35.Okochi M, Takano S, Isaji Y, et al. Three-dimensional cell culture array using magnetic force-based cell patterning for analysis of invasive capacity of BALB/3T3/v-src. Lab Chip. 2009;923:3378–84. doi: 10.1039/b909304d. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Kuznetsova LA, Edwards GO, et al. Functional three-dimensional HepG2 aggregate cultures generated from an ultrasound trap: comparison with HepG2 spheroids. J Cell Biochem. 2007;1025:1180–9. doi: 10.1002/jcb.21345. [DOI] [PubMed] [Google Scholar]
- 37.Park KH, Na K, Kim SW, et al. Phenotype of hepatocyte spheroids behavior within thermo-sensitive poly(NiPAAm-co-PEG-g-GRGDS) hydrogel as a cell delivery vehicle. Biotechnol Lett. 2005;2715:1081–6. doi: 10.1007/s10529-005-8453-0. [DOI] [PubMed] [Google Scholar]
- 38.Harimoto M, Yamato M, Hirose M, et al. Novel approach for achieving double-layered cell sheets co-culture: overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes. J Biomed Mater Res. 2002;623:464–70. doi: 10.1002/jbm.10228. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu T, Yamato M, Isoi Y, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;903:e40. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 40.Sekine H, Shimizu T, Dobashi I, et al. Cardiac Cell Sheet Transplantation Improves Damaged Heart Function via Superior Cell Survival in Comparison with Dissociated Cell Injection. Tissue Eng Part A. 2011;1723-24:2973–80. doi: 10.1089/ten.tea.2010.0659. [DOI] [PubMed] [Google Scholar]
- 41.Collett J, Crawford A, Hatton PV, et al. Thermally responsive polymeric hydrogel brushes: synthesis, physical properties and use for the culture of chondrocytes. J R Soc Interface. 2007;412:117–26. doi: 10.1098/rsif.2006.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams C, Xie AW, Yamato M, et al. Stacking of aligned cell sheets for layer-by-layer control of complex tissue structure. Biomaterials. 2011;3224:5625–32. doi: 10.1016/j.biomaterials.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 43.Okuyama T, Yamazoe H, Mochizuki N, et al. Preparation of arrays of cell spheroids and spheroid-monolayer cocultures within a microfluidic device. J Biosci Bioeng. 2010;1105:572–6. doi: 10.1016/j.jbiosc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Huang CP, Lu J, Seon H, et al. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip. 2009;912:1740–8. doi: 10.1039/b818401a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsiao AY, Torisawa YS, Tung YC, et al. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials. 2009;3016:3020–7. doi: 10.1016/j.biomaterials.2009.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin HJ, Cho YH, Gu JM, et al. A multicellular spheroid formation and extraction chip using removable cell trapping barriers. Lab Chip. 2011;111:115–9. doi: 10.1039/c0lc00134a. [DOI] [PubMed] [Google Scholar]
- 47.Agastin S, Giang UB, Geng Y, et al. Continuously perfused microbubble array for 3D tumor spheroid model. Biomicrofluidics. 2011;52:24110. doi: 10.1063/1.3596530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toh YC, Zhang C, Zhang J, et al. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip. 2007;73:302–9. doi: 10.1039/b614872g. [DOI] [PubMed] [Google Scholar]
- 49*.Dean DM, Napolitano AP, Youssef J, et al. Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J. 2007;2114:4005–12. doi: 10.1096/fj.07-8710com. [The first demonstration that self-assembly is not limited to the spheroid geometry.] [DOI] [PubMed] [Google Scholar]
- 50.Napolitano AP, Chai P, Dean DM, et al. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007;138:2087–94. doi: 10.1089/ten.2006.0190. [DOI] [PubMed] [Google Scholar]
- 51.Bao B, Jiang J, Yanase T, et al. Connexon-mediated cell adhesion drives microtissue self-assembly. FASEB J. 2011;251:255–64. doi: 10.1096/fj.10-155291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bao B, Lai CP, Naus CC, et al. Pannexin1 drives multicellular aggregate compaction via a signaling cascade that remodels the actin cytoskeleton. J Biol Chem. 2012 doi: 10.1074/jbc.M111.306522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krotz SP, Robins JC, Ferruccio TM, et al. In vitro maturation of oocytes via the pre fabricated self-assembled artificial human ovary. J Assist Reprod Genet. 2010;2712:743–50. doi: 10.1007/s10815-010-9468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Youssef J, Nurse AK, Freund LB, et al. Quantification of the forces driving self-assembly of three-dimensional microtissues. Proc Natl Acad Sci U S A. 2011;10817:6993–8. doi: 10.1073/pnas.1102559108. [The first quantitative measurement of the self-assembly process in terms of work performed by the assembling cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Achilli TM, McCalla S, Tripathi A, et al. Quantification of the Kinetics and Extent of Self-Sorting in Three Dimensional Spheroids. Tissue Eng Part C Methods. 2011 doi: 10.1089/ten.tec.2011.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zajac M, Jones GL, Glazier JA. Simulating convergent extension by way of anisotropic differential adhesion. J Theor Biol. 2003;2222:247–59. doi: 10.1016/s0022-5193(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 57.Poplawski NJ, Swat M, Gens JS, et al. Adhesion between cells, diffusion of growth factors, and elasticity of the AER produce the paddle shape of the chick limb. Physica A. 2007;373:521–32. doi: 10.1016/j.physa.2006.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen N, Glazier JA, Izaguirre JA, et al. A parallel implementation of the Cellular Potts Model for simulation of cell-based morphogenesis. Comput Phys Commun. 2007;17611-12:670–81. doi: 10.1016/j.cpc.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graner F, Glazier JA. Simulation of biological cell sorting using a two-dimensional extended Potts model. Phys Rev Lett. 1992;6913:2013–6. doi: 10.1103/PhysRevLett.69.2013. [DOI] [PubMed] [Google Scholar]
- 60.Brodland GW. The differential interfacial tension hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J Biomech Eng. 2002;1242:188–97. doi: 10.1115/1.1449491. [DOI] [PubMed] [Google Scholar]
- 61.Klopper AV, Krens G, Grill SW, et al. Finite-size corrections to scaling behavior in sorted cell aggregates. Eur Phys J E Soft Matter. 2010;332:99–103. doi: 10.1140/epje/i2010-10642-y. [DOI] [PubMed] [Google Scholar]
- 62.Jiang Y, Pjesivac-Grbovic J, Cantrell C, et al. A multiscale model for avascular tumor growth. Biophys J. 2005;896:3884–94. doi: 10.1529/biophysj.105.060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ribba B, Saut O, Colin T, et al. A multiscale mathematical model of avascular tumor growth to investigate the therapeutic benefit of anti-invasive agents. J Theor Biol. 2006;2434:532–41. doi: 10.1016/j.jtbi.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 64.Ramis-Conde I, Drasdo D, Anderson AR, et al. Modeling the influence of the E-cadherin-beta-catenin pathway in cancer cell invasion: a multiscale approach. Biophys J. 2008;951:155–65. doi: 10.1529/biophysj.107.114678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quaranta V, Rejniak KA, Gerlee P, et al. Invasion emerges from cancer cell adaptation to competitive microenvironments: quantitative predictions from multiscale mathematical models. Semin Cancer Biol. 2008;185:338–48. doi: 10.1016/j.semcancer.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein AM, Demuth T, Mobley D, et al. A mathematical model of glioblastoma tumor spheroid invasion in a three-dimensional in vitro experiment. Biophys J. 2007;921:356–65. doi: 10.1529/biophysj.106.093468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mueller-Klieser W. Multicellular spheroids. A review on cellular aggregates in cancer research. J Cancer Res Clin Oncol. 1987;1132:101–22. doi: 10.1007/BF00391431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elliott NT, Yuan F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J Pharm Sci. 2011;1001:59–74. doi: 10.1002/jps.22257. [DOI] [PubMed] [Google Scholar]
- 69.Frieboes HB, Edgerton ME, Fruehauf JP, et al. Prediction of drug response in breast cancer using integrative experimental/computational modeling. Cancer Res. 2009;6910:4484–92. doi: 10.1158/0008-5472.CAN-08-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sinek JP, Sanga S, Zheng X, et al. Predicting drug pharmacokinetics and effect in vascularized tumors using computer simulation. J Math Biol. 2009;584-5:485–510. doi: 10.1007/s00285-008-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kunwar PS, Siekhaus DE, Lehmann R. In vivo migration: a germ cell perspective. Annu Rev Cell Dev Biol. 2006;22:237–65. doi: 10.1146/annurev.cellbio.22.010305.103337. [DOI] [PubMed] [Google Scholar]
- 72.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;1304:601–10. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 73*.Dean DM, Morgan JR. Cytoskeletal-mediated tension modulates the directed self-assembly of microtissues. Tissue Eng Part A. 2008;1412:1989–97. doi: 10.1089/ten.tea.2007.0320. [The demonstration that in addition to surface adhesion forces, cytoskeletal forces also contribute to self-assembly and self-sorting.] [DOI] [PubMed] [Google Scholar]
- 74.Krieg M, Arboleda-Estudillo Y, Puech PH, et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;104:429–36. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 75.Tzanakakis ES, Hansen LK, Hu WS. The role of actin filaments and microtubules in hepatocyte spheroid self-assembly. Cell Motil Cytoskeleton. 2001;483:175–89. doi: 10.1002/1097-0169(200103)48:3<175::AID-CM1007>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura M, Shinji T, Ujike K, et al. Cytoskeletal inhibitors, anti-adhesion molecule antibodies, and lectins inhibit hepatocyte spheroid formation. Acta Med Okayama. 2002;561:43–50. doi: 10.18926/AMO/31727. [DOI] [PubMed] [Google Scholar]
- 77.Youssef J, Chen P, Shenoy VB, et al. Mechanotransduction is enhanced by the synergistic action of heterotypic cell interactions and TGF-beta1. FASEB J. 2012 doi: 10.1096/fj.11-199414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78*.Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988;2404849:177–84. doi: 10.1126/science.2451290. [Review by a pioneer in the field whose work demonstrated the importance of spheroids in cancer and how the spheroid environment more closely mimics tumor biology.] [DOI] [PubMed] [Google Scholar]
- 79.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;155:405–12. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 80.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;65:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 81.Kaur P, Ward B, Saha B, et al. Human breast cancer histoid: an in vitro 3-dimensional co-culture model that mimics breast cancer tissue. J Histochem Cytochem. 2011;5912:1087–100. doi: 10.1369/0022155411423680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Upreti M, Jamshidi-Parsian A, Koonce NA, et al. Tumor-Endothelial Cell Three-dimensional Spheroids: New Aspects to Enhance Radiation and Drug Therapeutics. Transl Oncol. 2011;46:365–76. doi: 10.1593/tlo.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;59:675–88. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 84.Graff CP, Wittrup KD. Theoretical analysis of antibody targeting of tumor spheroids: importance of dosage for penetration, and affinity for retention. Cancer Res. 2003;636:1288–96. [PubMed] [Google Scholar]
- 85.Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;6012:1421–34. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong HL, Shen Z, Lu Z, et al. Paclitaxel tumor-priming enhances siRNA delivery and transfection in 3-dimensional tumor cultures. Mol Pharm. 2011;83:833–40. doi: 10.1021/mp1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharkey RM, Goldenberg DM. Use of antibodies and immunoconjugates for the therapy of more accessible cancers. Adv Drug Deliv Rev. 2008;6012:1407–20. doi: 10.1016/j.addr.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;68:583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 89.Huang L, Ao Q, Zhang Q, et al. Hypoxia induced paclitaxel resistance in human ovarian cancers via hypoxia-inducible factor 1alpha. J Cancer Res Clin Oncol. 2010;1363:447–56. doi: 10.1007/s00432-009-0675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kerr DJ, Wheldon TE, Kerr AM, et al. In vitro chemosensitivity testing using the multicellular tumor spheroid model. Cancer Drug Deliv. 1987;42:63–74. doi: 10.1089/cdd.1987.4.63. [DOI] [PubMed] [Google Scholar]
- 91.Tannock IF, Lee CM, Tunggal JK, et al. Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res. 2002;83:878–84. [PubMed] [Google Scholar]
- 92.Layer PG, Robitzki A, Rothermel A, et al. Of layers and spheres: the reaggregate approach in tissue engineering. Trends Neurosci. 2002;253:131–4. doi: 10.1016/s0166-2236(00)02036-1. [DOI] [PubMed] [Google Scholar]
- 93.Kelm JM, Ehler E, Nielsen LK, et al. Design of artificial myocardial microtissues. Tissue Eng. 2004;101-2:201–14. doi: 10.1089/107632704322791853. [DOI] [PubMed] [Google Scholar]
- 94.Mahmoudifar N, Doran PM. Chondrogenesis and cartilage tissue engineering: the longer road to technology development. Trends Biotechnol. 2011 doi: 10.1016/j.tibtech.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 95.Revell CM, Reynolds CE, Athanasiou KA. Effects of initial cell seeding in self assembly of articular cartilage. Ann Biomed Eng. 2008;369:1441–8. doi: 10.1007/s10439-008-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96*.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;124:969–79. doi: 10.1089/ten.2006.12.969. [An important paper demonstrating the application of self-assembly in the tissue engineering of cartilage.] [DOI] [PubMed] [Google Scholar]
- 97.Abu-Absi SF, Friend JR, Hansen LK, et al. Structural polarity and functional bile canaliculi in rat hepatocyte spheroids. Exp Cell Res. 2002;2741:56–67. doi: 10.1006/excr.2001.5467. [DOI] [PubMed] [Google Scholar]
- 98.Riccalton-Banks L, Liew C, Bhandari R, et al. Long-term culture of functional liver tissue: three-dimensional coculture of primary hepatocytes and stellate cells. Tissue Eng. 2003;93:401–10. doi: 10.1089/107632703322066589. [DOI] [PubMed] [Google Scholar]
- 99.Lehnert L, Trost H, Schmiegel W, et al. Hollow-spheres: a new model for analyses of differentiation of pancreatic duct epithelial cells. Ann N Y Acad Sci. 1999;880:83–93. doi: 10.1111/j.1749-6632.1999.tb09512.x. [DOI] [PubMed] [Google Scholar]
- 100.Lehnert L, Lerch MM, Hirai Y, et al. Autocrine stimulation of human pancreatic duct-like development by soluble isoforms of epimorphin in vitro. J Cell Biol. 2001;1525:911–22. doi: 10.1083/jcb.152.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rothermel A, Biedermann T, Weigel W, et al. Artificial design of three-dimensional retina-like tissue from dissociated cells of the mammalian retina by rotation-mediated cell aggregation. Tissue Eng. 2005;1111-12:1749–56. doi: 10.1089/ten.2005.11.1749. [DOI] [PubMed] [Google Scholar]
- 102.Griffith CK, Miller C, Sainson RC, et al. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;111-2:257–66. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 103.Khademhosseini A, Langer R, Borenstein J, et al. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;1038:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104*.Kelm JM, Djonov V, Ittner LM, et al. Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng. 2006;128:2151–60. doi: 10.1089/ten.2006.12.2151. [An important paper demonstrating the use of endothelialized spheroids as building blocks.] [DOI] [PubMed] [Google Scholar]
- 105.Alajati A, Laib AM, Weber H, et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods. 2008;55:439–45. doi: 10.1038/nmeth.1198. [DOI] [PubMed] [Google Scholar]
- 106.Wenger A, Stahl A, Weber H, et al. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng. 2004;109-10:1536–47. doi: 10.1089/ten.2004.10.1536. [DOI] [PubMed] [Google Scholar]
- 107.Boland T, Mironov V, Gutowska A, et al. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat Rec A Discov Mol Cell Evol Biol. 2003;2722:497–502. doi: 10.1002/ar.a.10059. [DOI] [PubMed] [Google Scholar]
- 108.Jakab K, Neagu A, Mironov V, et al. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci U S A. 2004;1019:2864–9. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jakab K, Norotte C, Marga F, et al. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;22:022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mironov V, Boland T, Trusk T, et al. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;214:157–61. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 111.Mironov V, Visconti RP, Kasyanov V, et al. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;3012:2164–74. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith CM, Stone AL, Parkhill RL, et al. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 2004;109-10:1566–76. doi: 10.1089/ten.2004.10.1566. [DOI] [PubMed] [Google Scholar]
- 113.Wilson WC, Jr., Boland T. Cell and organ printing 1: protein and cell printers. Anat Rec A Discov Mol Cell Evol Biol. 2003;2722:491–6. doi: 10.1002/ar.a.10057. [DOI] [PubMed] [Google Scholar]
- 114.Rago AP, Dean DM, Morgan JR. Controlling cell position in complex heterotypic 3D microtissues by tissue fusion. Biotechnol Bioeng. 2009;1024:1231–41. doi: 10.1002/bit.22162. [DOI] [PubMed] [Google Scholar]
- 115.McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci U S A. 2006;10331:11461–6. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang J, Yamato M, Kohno C, et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;2633:6415–22. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 117.Ito A, Ino K, Hayashida M, et al. Novel methodology for fabrication of tissue-engineered tubular constructs using magnetite nanoparticles and magnetic force. Tissue Eng. 2005;119-10:1553–61. doi: 10.1089/ten.2005.11.1553. [DOI] [PubMed] [Google Scholar]
- 118*.Livoti CM, Morgan JR. Self-assembly and tissue fusion of toroid-shaped minimal building units. Tissue Eng Part A. 2010;166:2051–61. doi: 10.1089/ten.tea.2009.0607. [The demonstration that building blocks with new shapes, such as the toroid, open up new possibilities.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tejavibulya N, Youssef J, Bao B, et al. Directed self-assembly of large scaffold-free multi-cellular honeycomb structures. Biofabrication. 2011;33:034110. doi: 10.1088/1758-5082/3/3/034110. [DOI] [PMC free article] [PubMed] [Google Scholar]