Abstract
Rationale
Methylnaltrexone bromide (MTNX) is a peripherally acting mu-opioid receptor antagonist, prescribed for the treatment of opioid-induced constipation in patients with advanced illness who are receiving palliative care. Studies have used this drug to determine if other opioid-induced effects besides constipation are altered by MTNX in humans, and have suggested based on their results that these other effects are altered by peripheral opioid actions.
Objective
The primary objective of this report is to present results that provide indirect evidence that MTNX has centrally-mediated effects, albeit slight, and secondarily to describe the effects of MTNX on psychopharmacological effects of morphine.
Methods
In a crossover, randomized, placebo-controlled, double-blind study, 29 healthy volunteers received 0.45 mg/kg MTNX or saline subcutaneously, followed by saline intravenously. In three other conditions 0.143 mg/kg of morphine sulfate administered intravenously was preceded by subcutaneous administration of 0, 0.225, or 0.45 mg/kg MTNX. Before and after drug administration, subjective and physiological measures, including pupil diameter, were assessed.
Results
Two separate analyses confirmed that 0.45 mg/kg MTNX alone induced a slight degree of miosis, a centrally mediated opioid agonist effect. This dose had minimal subjective effects. MTNX at either or both the 0.225 and 0.45 mg/kg dose reduced some subjective effects of morphine without altering miosis.
Conclusions
We present indirect evidence that MTNX crosses the blood-brain barrier in humans. Therefore, whether the reductions in subjective effects of morphine by MTNX that were observed in past studies and in this study can be attributed to peripheral mechanisms is open to question.
Keywords: methynaltrexone, morphine, opioid, opioid antagonist, subjective effects, human
Introduction
In 2008, the Food and Drug Administration granted approval for the use of methylnaltrexone (MTNX) bromide (RELISTOR®, Salix Pharmaceuticals, Inc, Raleigh, NC), a peripherally acting mu-opioid receptor antagonist, for the treatment of opioid-induced constipation in patients with advanced illness who are receiving palliative care, and whose response to laxative therapy has not been sufficient. MTNX is a quaternary derivative of the pure opioid antagonist, naltrexone (Brown and Goldberg 1985). The addition of the methyl group at the amine in its ring forms a compound with greater polarity and lower lipid solubility, and is described in the literature as a compound that either does not cross the blood-brain barrier (BBB) (e.g., Murphy et al. 1997; Holzer 2009; Kast et al. 2009; Weinstock and Chang 2011; Gatti and Sabato 2012; Bader et al. 2013; Zand et al. 2014) or has limited , reduced, or restricted ability to cross the BBB (e.g., Brown and Goldberg 1985; Trujillo et al. 1989; Yuan et al. 1996; Rosow et al. 2007; Chamberlain et al 2009; Slatkin et al. 2009; Garnock-Jones and McKeage 2010). Constipation occurs in part because opiate receptors in the gut, when activated by exogenous opioids, slow down gastrointestinal motility - this effect is antagonized by MTNX via peripheral mu-opiate receptor blockade (Russell et al. 1982; Yuan et al. 1996, 2000, 2002; Yuan and Foss 1999). Studies have established that the centrally-mediated effect of mu opioid agonist, analgesia, still occurs in the presence of MTNX (Yuan et al. 1996; Portenoy et al. 2008; Thomas et al. 2008).
Several studies have examined whether other effects of mu opioid agonists are mediated at least in part by peripheral mechanisms. In 2007 Rosow et al. showed that remifentanil-induced urinary retention was reversed by intravenous methylnaltrexone in healthy volunteers. As a negative control, pupil diameter of the volunteers were assessed - mu opioid agonists constrict pupil size (miosis), and is a central effect occurring at the autonomic segment of the oculormotor nerve (Lee and Wang 1975; Murray et al. 1983; Lotsch et al. 2002), and therefore MTNX, a putative peripheral mu opioid antagonist, should not alter this effect. Indeed, the degree of miosis was the same with a MTNX challenge as with a saline challenge. The researchers attributed the reversal of urinary retention at least in part to actions occurring at peripheral opioid receptors.
Two earlier studies conducted during the development of MTNX examined whether some subjective effects of opioids might be mediated at peripheral mu-opiate receptors. In the first study (Yuan et al. 1998), ten healthy volunteers ingested oral MTNX at doses of 0, 0.64, or 19.2 mg/kg, and then 20 min later were given an intravenous injection of 0.05 mg/kg morphine. Subjects, 10 min before the morphine injection and 3 min afterwards, completed a subjective effects form that had been modeled after the Single Dose Questionnaire (Fraser et al. 1961) - the items were representative of common effects experienced from opioids. Morphine alone significantly increased ratings of "nauseous," "skin itch," "flushing," "stimulated," and "coasting" relative to baseline. MTNX 19.2 mg/kg compared to MTNX 0 mg/kg (placebo) significantly reduced the first four ratings 3 min after morphine dosing. Thus three prototypic subjective effects of mu opiate agonists, i.e., nausea, pruritis, and flushing, were reduced by MTNX. The magnitude of reductions was substantial. For example, average "nauseous" ratings were 1.7 and 0.2 (on a scale of 0–4) after morphine and morphine/MTNX, respectively. In a second study (Yuan et al. 2002), MTNX or placebo was administered subcutaneously 15 min prior to a placebo injection or a morphine injection (0.05 mg/kg, iv) to two groups of subjects (six per group), and gastrointestinal motility was measured as well as subjective effects. The MTNX dose was 0.15 mg/kg in one group and 0.3 mg/kg in the other group. The investigators in this study did not examine subjective effects individually (a limitation of the study) but grouped them together to yield a single measure, due to the small sample sizes. Both doses of MTNX decreased morphine-induced subjective ratings. These results again indicated that a peripheral opiate antagonist reduced some subjective effects of opioids, although which specific ones were not ascertained.
The Yuan et al. studies (1998, 2002) raise the possibility that some subjective effects of morphine, and perhaps other opioids, are mediated at peripheral opiate receptors. The results of the Yuan et al. (1998, 2002) studies were intriguing to us, but had limitations some of which Yuan et al. themselves raised. The studies used a small number of subjects, did not include a placebo control condition, tested a low dose of morphine (i.e., some prototypic effects of morphine were not increased by the low dose), assessed subjective effects only twice after drug administration, and used only one subjective effects questionnaire that did not include abuse liability-related effects (e.g., euphoria, drug liking). Further, the studies lacked a negative control measure such as miosis to demonstrate that the reduction in effects they observed were indeed peripherally mediated. However it should be noted that in an earlier study Yuan et al (1996) did use a negative control condition, analgesia, and demonstrated that MTNX prevented morphine-induced delay in oral-cecal transit time without affecting the central effect of morphine, analgesia. We designed our study with the intent to systematically replicate the Yuan et al. (1998, 2002) studies to address the limitations above, so as to more completely characterize the effects of MTNX on psychopharmacological effects of opioids. We hypothesized that MTNX would attenuate those subjective effects that were attenuated in the Yuan et al. (1998) study, but because we tested a larger dose of morphine and included several subjective effects questionnaires, we also hypothesized that other subjective effects of morphine would be altered by MTNX.
In our study, we included a condition which had not been employed by Yuan et al. (1998, 2002) or by Rosow et al. (2007) – a MTNX-alone condition. In an in vitro study using membranes prepared from Chinese hamster ovary cells, MTNX, as did morphine, stimulated [35S]GTPγS binding – MTNX had less than 1/10th the affinity to that of morphine, consistent with partial agonism (Beattie et al. 2007). We thought it unlikely in an in vivo study that MTNX would exhibit any activity by itself because of its classification as a peripheral opioid antagonist, and because of studies showing that two central effects of opioids, miosis (Rosow et al. 2007) and analgesia (Yuan et al. 1996), were not altered by MTNX. Much to our surprise we found that MTNX by itself did induce an agonist effect, miosis. As stated earlier, miosis is a central effect of mu opiate agonists, mediated by activation of the autonomic segment of the oculormotor nerve (Lee and Wang 1975; Murray et al. 1983; Lotsch et al. 2002). The fact that MTNX induced miosis indicated that it was crossing the BBB, something we had not anticipated based on the extant literature on this drug. We did find that MTNX reduced some subjective effects of morphine, as was found in the Yuan et al. (1998, 2002) studies, but whether these actions could be attributed to MTNX blocking morphine effects in the periphery, as opposed to it blocking morphine effects centrally (i.e., in the same manner as naloxone or naltrexone) could not be ascertained in our study. Thus the purpose of this report is to primarily focus on the effects of MTNX by itself, including its subjective and physiological effects, secondarily to enumerate the effects of MTNX on morphine effects, and then to discuss the ramifications of our findings.
Materials and methods
Subjects
The local Institutional Review Board approved the study. To be eligible for the study, subjects had to be between the ages of 21–39, have a BMI between 18 and 27, report consuming at least three alcoholic drinks per month or report some but not daily use of marijuana, be verbally fluent in English, and obtained a high school diploma or equivalent. Subjects were excluded if they had any medical problems or a history of Axis-I psychiatric disorders [American Psychiatric Association, 2000]. After providing written consent for pre-study screening procedures, volunteers underwent a semi-structured psychiatric interview, medical examination, and an orientation session in the laboratory. Those who fulfilled all our criteria were then asked if they wished to participate in the study and if they responded in the affirmative, written informed consent for the study proper was obtained.
In the study consent form, subjects were told the drug or drugs to be administered in the study were FDA approved and could be taken from one or more of 7 classes: sedative/tranquilizer, sedative blocker, stimulant, opiate, opiate blocker, antihistamine, and saline placebo. Upon completion of the study, a debriefing session was held and payment for participation in the study was remitted.
We enrolled 39 volunteers into the study (i.e., they participated in at least one experimental session), and of these, 29 had evaluable data (15 males and 14 females). The demographic data from the 29 subjects with evaluable data are shown in Table 1. For the sake of brevity we cannot list each of the reasons the other 10 volunteers did not complete the study, but it is important to point out that only two of them withdrew citing unpleasant effects of nausea and vomiting as their reasons for dropping out.
Table 1.
Demographics and substance use characteristics of study participants. Data are presented as N, mean±SD, or percent of participants.
| Male/female (N) | 15/14 |
| Age (years) | 26.6±4.6 |
| BMI (kg/m2) | 22.9±2.6 |
| Race | |
| White | 21 (72%) |
| Black | 3 (10%) |
| Asian | 4 (14%) |
| American Indian/Alaskan Native | 1 (3%) |
| Ethnicity | |
| Hispanic or Latino | 4 (14%) |
| Not Hispanic or Latino | 25 (86%) |
| Current drug use (past 30 days) | |
| Alcohol (drinks/week) | 4.0±3.7 |
| Caffeine (beverages/week) | 6.4±4.6 |
| Cigarettes (N) | 7 (24%) |
| Marijuana (N) | 9 (31%) |
| Lifetime Drug Use [N (%) ever used] | |
| Marijuana | 27 (93%) |
| Stimulant | 13 (45%) |
| Tranquilizer | 5 (17%) |
| Hallucinogen | 15 (52%) |
| Opioid | 7 (24%) |
| Inhalant | 5 (17%) |
Experimental design and drugs
A double-blind, placebo-controlled, crossover trial was used. Order of the five drug conditions in the study was determined by a Latin Square design. Methylnaltrexone bromide (RELISTOR®, Salix Pharmaceuticals, Inc., Raleigh, NC) (12 mg/0.6 mL single-use vial or pre-filled syringe) and morphine sulfate (10 mg/1 mL vial) were obtained from the Hospital Pharmacy. The five conditions were: 0 mg/kg MTNX/0 mg/kg morphine (abbreviated henceforth as 0MTNX/0MS); 0.45 mg/kg MTNX/0 mg/kg morphine (.45MTNX/0MS); 0 mg/kg MTNX/0.143 mg/kg morphine (0MTNX/.14MS); 0.225 mg/kg MTNX/0.143 mg/kg morphine (.225MTNX/.14MS); and 0.45 mg/kg MTNX/0.143 mg/kg morphine (.45MTNX/.14MS). Syringes were prepared by the medical center’s Investigational Drug Services.
Across sessions, doses of 0, 0.225, and 0.45 mg/kg MTNX were given subcutaneously in the upper arm in a volume of 2 cc over a 30-sec interval. The 0.45 mg/kg dose was chosen as the highest dose to be tested because it antagonized morphine-induced slowing of gastrointestinal motility (a peripherally-mediated effect) without affecting morphine-induced analgesia (a centrally-mediated effect) in healthy volunteers without adverse effects (Yuan et al., 1996). The lower dose of 0.225 mg/kg MTNX is close to the dose that a patient weighing 70 kg is recommended to take, 12 mg (approximately 0.17 mg/kg) (RELISTOR® package insert, August 2013). Morphine (0.143 mg/kg) or saline was given intravenously via an angiocatheter in a volume of 5 cc over a 30-sec period. Fifteen minutes separated the subcutaneous injection of MTNX or saline from the subsequent intravenous injection of morphine or saline. This interval was based on the MTNX pretreatment time used by Yuan et al. (2002) and the relatively short time to peak plasma concentration of MTNX (0.3 h, Yuan et al., 2002; 0.5 h, RELISTOR® package insert, August 2013).
Experimental sessions
The study consisted of five sessions (spaced at least one week apart) that took place in a departmental laboratory from 0800–1415 h. Subjects were instructed to not eat food or drink non-clear liquids for 4 h, drink clear liquids for 2 h, or use any drugs (excluding normal amounts of caffeine and nicotine) 24 h prior to sessions. Breath alcohol, urine toxicology, and pregnancy (for females) tests were given before sessions – all were negative.
Volunteers were in a semi-recumbent position in a hospital bed for all sessions. Prior to baseline testing, the anesthetist inserted an angiocatheter into a vein in the non-dominant hand or forearm of the subject. At baseline, vital signs of the subject were assessed by the technician, the subject completed several subjective effects forms and psychomotor tests, and then a picture of the subject’s right eye was taken. The anesthetist injected either MTNX or saline subcutaneously in the subject’s non-dominant upper arm. Ten min later, tests were administered, and the anesthetist was called into the room for the next injection to be given 15 min after the subcutaneous injection. Immediately prior to the intravenous injection, as well as the preceding subcutaneous injection, subjects were told by the technician that the “the injection you are about to receive may or may not contain a drug.” For 300 min after the intravenous injection, mood, psychomotor performance, and physiological measures were assessed at fixed time intervals. After the session ended and subjects were deemed fit to go home by the anesthetist, subjects were instructed not to engage in certain activities for the next 12 h, given questionnaires to complete at home 24 h after the session, and transported home via a livery service. If subjects felt nauseated or were vomiting during or at the end of the session this was discussed with the anesthetist, and the subject was given the option of receiving ondansetron (Zofran®, oral or iv).
Dependent measures
Table 2 shows when the different dependent measures were assessed. Subjective effects during the session were measured by four forms: a computerized, short form of the Addiction Research Center Inventory (ARCI) (Haertzen, 1966; Martin et al., 1971), a locally developed 32-item visual analog scale (VAS) including items sensitive to subjective effects of opioids (Fraser et al., 1961; Preston et al., 1989), and a Drug Effect/Drug Liking/Take Again (DEL/TA) questionnaire. Psychomotor performance was assessed with the Digit Symbol Substitution Test (DSST) (Wechsler, 1958). Six physiological measures were assessed: respiration rate, blood pressure, and exophoria (as measured by the Maddox Wing Test [Hannington-Kiff, 1970]), heart rate, arterial oxygen saturation, and pupil diameter. Pupil diameter was assessed one minute after the room was darkened with a commercial pupillometer (Neuroptics, San Clemente, CA).
Table 2.
Time line of events (min)*
| BL | −15 | −5 | 0 | 5 | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methylnaltrexone (MTNX) (or saline) | scI | ||||||||||||||||||
| Morphine (MS) (or saline) | ivI | ||||||||||||||||||
| ARCI | X | X | X | X | X | X | X | ||||||||||||
| VAS | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| DEL/TA | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| DSST | X | X | X | X | X | X | X | ||||||||||||
| HR, SaO2 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| RR, BP, MW | X | X | X | X | X | X | X | ||||||||||||
| Miosis | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
Abbreviations: BL, baseline; MTNX, methylnaltrexone; scI, subcutaneous injection; MS, morphine sulfate; ivI, intravenous injection; ARCI, Addiction Research Center Inventory; X, data collection time point; VAS, Visual Analog Scale; DEL/TA, Drug Effect/Liking/Take Again; DSST, Digit Symbol Substitution Test; HR, heart rate; SaO2, arterial oxygen saturation; RR, respiration rate; BP, blood pressure; MW, Maddox Wing
min is based on the intravenous injection at Time Point 0: −15 and −5 min are time points before the injection, and 5, 15, 30, 45, 60, 75, 90, 105, 120, 150, 180, 210, 240, 270, and 300 min are time points of data collection after the intravenous injection. To calculate time since the subcutaneous injection, add 15 min to each time point starting at −5 min. The x-axis of Figure 2 is based on the latter calculation.
Statistical analyses
The following two sets of analyses were conducted examining the effects of MTNX alone. First, a repeated-measures analysis of variance (rmANOVA; Stata Version 13, StataCorp., College Station, TX) examined change scores from baseline in all five conditions before 0.14 mg/kg MOR administration (i.e., value collected 10 min after the subcutaneous injection minus the baseline value) for all VAS measures, “feel drug effect” and drug liking from the DEL/TA, pupil diameter, heart rate, and oxygen saturation. This analysis allowed us to assess the effects of 0.45 mg/kg MTNX alone (2 replications, .45MTNX/0MS and .45MTNX/.14MS) and 0.225 mg/kg MTNX compared to placebo (two replications, 0MTNX/0MS and 0MTNX/.14MS). When significance was achieved for the drug condition main effect (Greenhouse-Geisser adjusted p<0.05), pairwise comparisons were performed with Tukey adjustment for multiple comparisons. The second set of analyses were time course analyses and assessed changes from baseline across the entire session, using rmANOVA, in the .45MTNX/0MS (i.e., 0.45 mg/kg MTNX alone) versus 0MTNX/0MS (i.e., 0 mg/kg MTNX, or saline-placebo) conditions for the above measures and also the DSST, blood pressure, and respiration rate measures.
Secondary analysis examined the effects of MTNX pretreatment on MS effects. For outcomes that were measured at multiple time points during each experimental session, peak (highest value obtained) or trough (lowest value obtained) values were used. In the peak and trough analyses, only values collected following the second injection (i.e., the iv injection) were included, and values were determined for each subject independent of time point. When significance was achieved for the drug condition main effect (Greenhouse-Geisser adjusted p<0.05), pairwise comparisons were performed with Tukey adjustment for multiple comparisons. Area-under-the-curve (AUC), using the trapezoidal rule, was calculated for selected measures (VAS, DEL/TA, pupil diameter, heart rate and arterial oxygen saturation) that were collected more than once an hour within the session. Using rmANOVA, AUC including time points 5–300 min post iv injection (AUC5–300) were examined.
Results
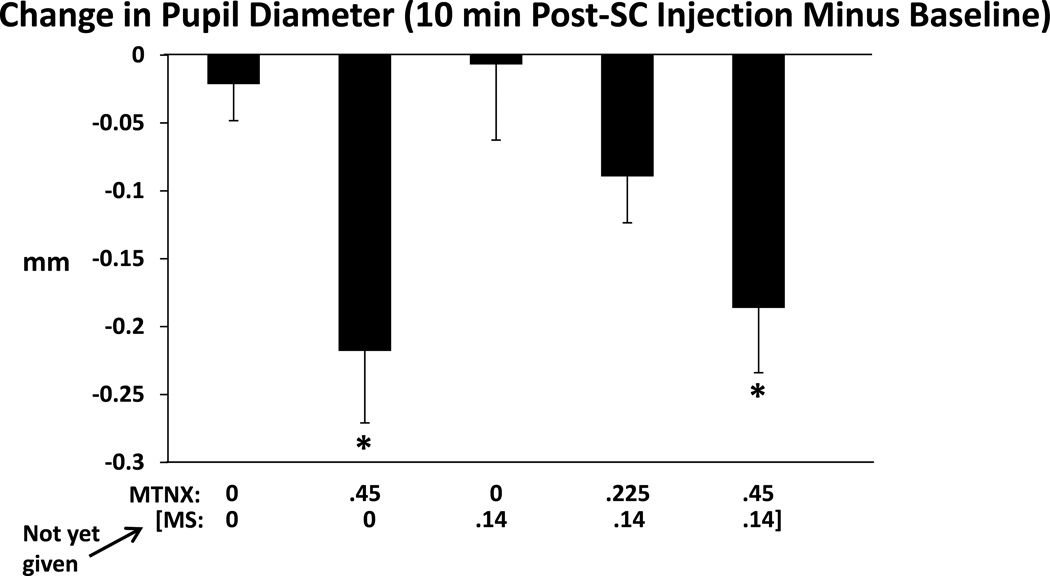
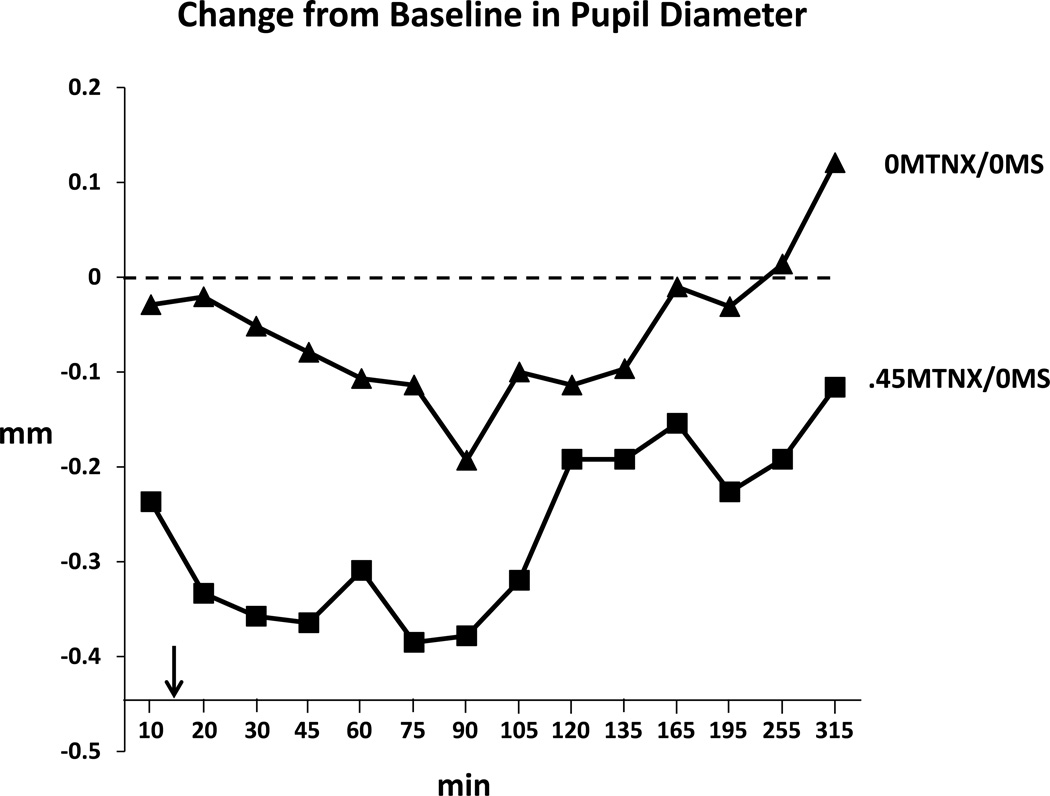
MTNX induced miosis - Figures 1 and 2 summarize these results. Figure 1 shows that change scores of pupil diameter (10 min after subcutaneous injection minus baseline) differed significantly across conditions (F(3.11,84.82, Greenhouse-Geisser adjusted)=5.03, p=0.0026). There were smaller pupil sizes in those conditions in which 0.45 mg/kg MTNX were given compared to the two conditions in which 0 mg MTNX was given. Change scores in the .225MTNX/.14MS condition did not differ significantly from the 0MTNX/0MS condition (or the 0MTNX/.14MS condition). Figure 2 shows time course of the pupillary effects across the course of the session in the 0MTNX/0MS and the.45MTNX/0MS conditions. There was a significant Condition effect, F(1,27, Greenhouse-Geisser adjusted)=15.4, p=0.0005, but not a Condition X Time interaction, F(5.61,150.92, Greenhouse-Geisser adjusted)=1.0, p=0.42. However, pairwise testing found the differences between the two conditions to be statistically significant at all time points except at 120, 135, and 165 minutes. After Bonferroni adjustment, differences were significant at the 20, 30, 45, and 75-minute time points. The slight miosis was therefore not a transient effect (e.g., limited only to the time point 10 minutes after the subcutaneous injection). Of note, early on in the session, pupil diameters decreased in the 0MTNX/0MS condition compared to baseline. The session started at 0830 h and this sort of diurnal variation in pupil diameter has been documented in other studies (Fraser and Isbell, 1961; Korey et al., 1979; Zilm, 1980).
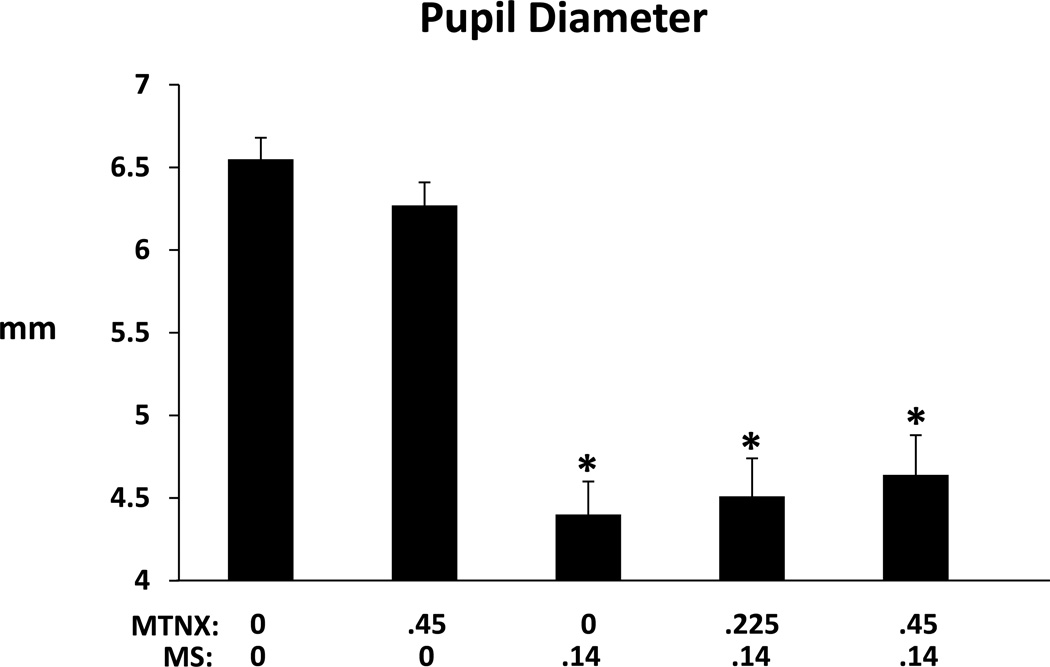
Figure 1.
Change in pupil diameter (mm), determined by subtracting the baseline value from the value collected 10 min after the subcutaneous injection, for the five drug conditions. Larger negative scores indicate a greater degree of miosis. Brackets represent SE. Asterisks indicate a significant difference from both the 0MTNX/0MS condition and the 0MTNX/.14MS condition. Figure 2.
Figure 2.
Time course of pupil diameter (mm), expressed as a change relative to the baseline value, from 10 to 315 minutes after the subcutaneous injection of 0MTNX/0MS (triangles) and .45MTNX/0MS (squares). Arrow indicates when the intravenous injection of saline was given. Each time point is the mean across the 29 subjects.
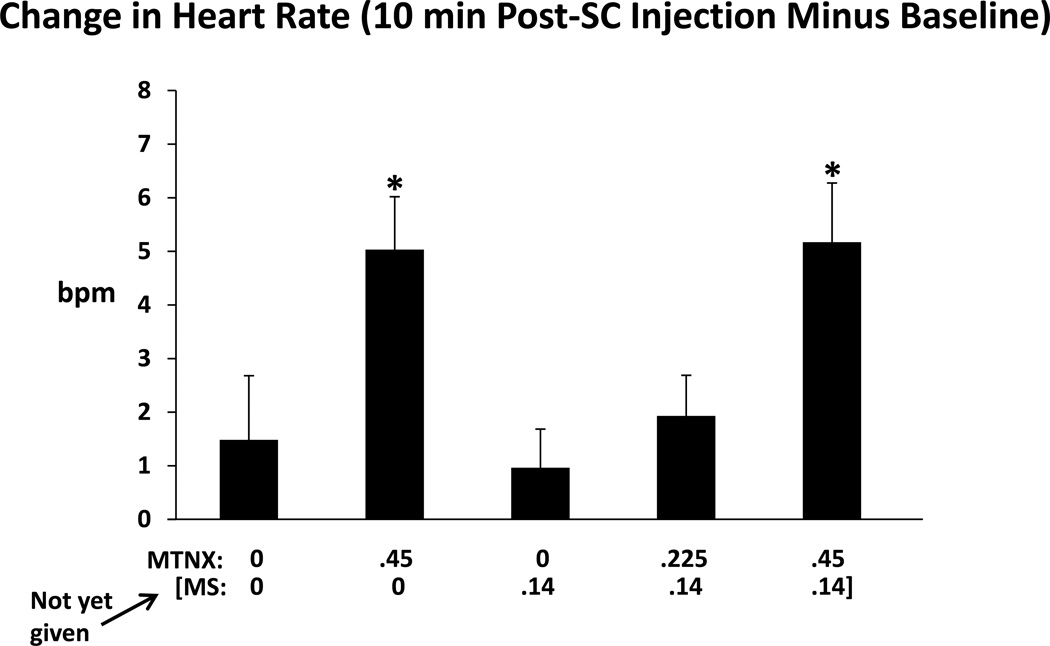
Another physiological parameter affected by MTNX was heart rate. As shown in Figure 3, change scores of heart rate (10 min after subcutaneous injection minus baseline) differed significantly across conditions (F(3.44,96.43, Greenhouse-Geisser adjusted)=4.58, p=0.0032). There were larger increases in heart rate when comparing the.45MTNX/0MS and.45MTNX/.14MS conditions to the 0MTNX/.14MS condition (MS had not yet been administered). A time course analysis comparing heart rate between the 0MTNX/0MS and the .45MTNX/0MS conditions revealed a significant Condition X Time interaction, F(7.46,208.82, Greenhouse-Geisser adjusted)=3.56, p<0.001. Pairwise testing found the differences between the two conditions to be statistically significant at seven time points but the differences were not consistent – at three of the seven time points, 10, 20, and 30 min post-injection (sc), heart rate was higher in the .45MTNX/0MS condition than in the 0MTNX/0MS condition, but at the other four time points, 120, 165, 255, and 315 min post injection (sc), heart rate was higher in the 0MTNX/0MS condition. After Bonferroni adjustment, differences were significant only at the 315-min time point.
Figure 3.
Change in heart rate (bpm), determined by subtracting the baseline value from the value collected 10 min after the subcutaneous injection, for the five drug conditions. Brackets represent SE. Asterisks indicate a significant difference from the 0MTNX/.14MS condition (MS had not yet been administered).
MTNX had minimal subjective effects. Ten minutes after its injection at both the 0.225 and 0.45 mg/kg dose, VAS ratings and “feel drug effect” and drug liking ratings from the DEL/TA expressed as change scores from baseline did not differ significantly from the 0MTNX/0MS or 0MTNX/.14 MS conditions. Time course analyses comparing 0MTNX/0MS and .45MTNX/0MS revealed significant Condition effects for only “heavy, sluggish feeling” (F(1,28)=8.41, p=0.0072) and “feel bad” (F(1,28)=4.81, p=0.037) ratings. The actual differences between the conditions were small, keeping in mind effects were measured on a 100-mm scale, and were not concordant with each other. That is, mean (±SE) change scores (averaged over all time points) in the .45MTNX/0MS condition were higher (6.5±0.8) for “heavy, sluggish feeling” than in the 0MTNX/0MS (−0.3±0.4) condition, but lower for “feel bad” (−2.0±0.3) than in the 0MTNX/0MS condition (0.1±0.2).
MTNX had no impact on arterial oxygen saturation in the two sets of analyses. Systolic and diastolic blood pressures, and performance on the DSST and Maddox Wing Test did not differ across conditions in the time course analyses (neither a Condition nor a Condition X time interaction).
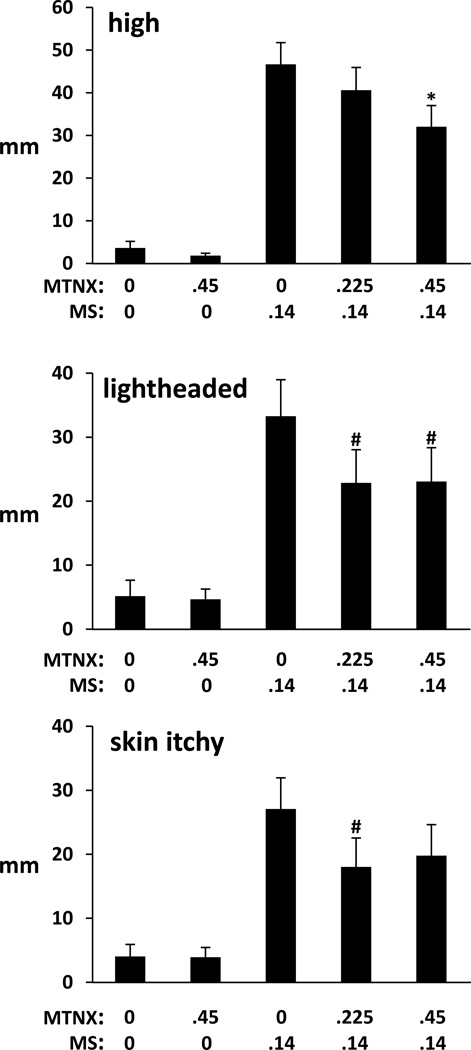
We will briefly present the results of the effects of pretreatment of MTNX on MS effects. There were several measures where MTNX pretreatment altered the magnitude of the effects of 0.14 mg/kg MS. For “drug high,” peak ratings were significantly greater in the 0MTNX/.14MS, .225MTNX/.14MS, and .45MTNX/.14MS conditions compared to both the 0MTNX/0MS and .45MTNX/0MS conditions, but the effects of .14 MS were decreased by MTNX in a dose-related manner. The difference between .45MTNX/.14MS and 0MTNX/.14MS reached statistical significance (p=0.033) (Figure 4 top frame). For “lightheaded,” the lower ratings in the .225MTNX/.14MS and .45MTNX/.14MS conditions compared to the 0MTNX/.14MS condition approached significance (p=0.051 and 0.057, respectively) (Figure 4, middle frame). For “skin itchy” (Figure 4, bottom frame) and “dry mouth,” the lower ratings in the .225MTNX/.14MS condition compared to the 0MTNX/.14MS condition approached significance (p=0.06 and 0.082, respectively). On the global rating of ‘feel drug effect,” a measure of drug strength as measured in the DEL/TA (scale of 1–5 where 1=I feel no effect from the drug(s) at all and 5=I feel a very strong effect), there was an indication that MTNX pretreatment reduced the effects of MS. Although all three MS conditions increased this effect relative to the 0MTNX/0MS and .45MTNX/0MS conditions (peak ratings of 4.0±0.1, 3.7±0.1, and 3.7±0.1 in the 0MTNX/0.14MS, .225MTNX/.14 MS, .45MTNX/.14 MS conditions and 1.9±0.1 and 2.2±0.2 in the 0MTNX/0MS and .45MTNX/0MS conditions, respectively), the lower ratings in the .225MTNX/.14 MS and .45MTNX/.14MS conditions compared to the 0MTNX/.14MS condition approached significance (p=0.088 for both comparisons).
Figure 4.
Peak visual analog scale ratings of “high (drug ‘high’) (top frame),” “lightheaded” (middle frame), and “skin itchy” (bottom frame) in the five drug conditions: 0MTNX/0MS; .45MTNX/0MS; 0MTNX/.14MS; .225MTNX/.14MS; and .45MTNX/.14MS. Labels at the far ends of the 100-mm visual analog scales were “not at all” (0) and “extremely” (100). Brackets represent SE. Asterisks indicate a significant difference from the 0MTNX/.14MS condition; the pound sign indicates a trend towards a significance difference (p<0.10) from the 0MTNX/.14 MS condition. For each of the subjective effects, ratings from the three conditions in which morphine was administered differed significantly from the 0MTNX/0MS and.45MTNX/0MS conditions.
Morphine produced numerous alterations in subjective effects that were not altered by either dose of MTNX. These effects included increases in peak PCAG and LSD scores of the ARCI, increases in peak VAS ratings of coasting (spaced out), dizzy, difficulty concentrating, dry mouth, having pleasant bodily sensations, having unpleasant bodily sensations, heavy or sluggish feeling, nauseated, and sleepy, and both increases (peak) and decreases (trough) in DEL/TA ratings of liking and/or “take again”. Trough heart rate values were lower in the 0MTNX/.14MS condition than in the .45MTNX/0MS and 0MTNX/0MS conditions, but there were no significant differences between the three conditions in which morphine was administered. On the Maddox Wing Test, peak exophoria was significantly greater in the three conditions in which morphine was administered compared to the two conditions in which it was not administered (.45MTNX/0MS and 0MTNX/0MS), but neither dose of MTNX altered this effect of morphine. Blood pressure, respiration rate, and arterial oxygen saturation were not altered by morphine.
Figure 5 shows mean trough pupil diameter values in the five conditions. Only measures collected after the intravenous injection of either saline or MTNX were included in this analysis. Pupil diameters were smaller in the three conditions in which morphine was administered compared to the 0 MTNX/0 condition. There were no significant differences between the .45MTNX/0MS and 0MTNX/0MS conditions, and no differences between the three conditions in which 0.14 mg/kg MS was administered (confirmed in the AUC analysis). The figure does show a trend for pupil diameter to be bigger (less miosis) as the dose of MTNX increased. Finally, we conducted a time course analysis comparing pupil diameters in the 0MTNX/.14MS and .45MTNX/.14 MS conditions, using difference scores from baseline, and including only values collected following the iv injection. There was neither a significant main effect of condition (p=0.47) nor a significant condition by time interaction (p=0.59).
Figure 5.
Trough pupil diameter (expressed in mm) in the five drug conditions: 0MTNX/0MS; .45MTNX/0MS; 0MTNX /.14MS; 0.225MTNX/.14MS; and .45MTNX/.14MS. Brackets represent SE. Asterisks indicate a significant difference from the 0MTNX/0MS condition.
Discussion
In designing this study, its purpose was to determine if any effects of morphine, primarily its subjective effects, were altered by MTNX – if effects were reduced we believed this would provide evidence that the effects were peripherally mediated for two reasons: MTNX is a quaternary antagonist that has been frequently described in original research articles and in review articles as a compound that does not cross the BBB (e.g., Murphy et al. 1997; Holzer 2009; Kast et al. 2009; Weinstock and Chang 2011; Gatti and Sabato 2012; Bader et al. 2013; Zand et al. 2014), and centrally-mediated effects of opioids, miosis and analgesia, were shown in at least two studies to not be altered by MTNX (Yuan et al. 1996; Rosow et al. 2007). However in the present study it was shown in two separate analyses that 0.45 mg/kg MTNX induced miosis (Figures 1 and 2). Also, in the first analysis (Figure 1), the effect was replicated – 0.45 mg/kg MTNX was included in two conditions of the study and miosis was induced in each of them. We believe this is the first time that the effects of MTNX by itself on a centrally mediated effect of mu opioid agonists has been systematically tested. We provide indirect evidence that MTNX crosses the BBB. Therefore although we showed that MTNX reduced some of the subjective effects of morphine as did Yuan et al. (1998, 2002), whether those effects involved peripheral mechanisms is certainly open to question. It is possible that central effects of MTNX were altering those effects of morphine.
The fact that MTNX alone induced miosis was surprising to us for two reasons: first, miosis is a prototypic effect of mu opioid agonists (Reisine and Pasternak 1996), not antagonists, and second, MTNX has been classified as having effects in the periphery but opioid-induced miosis is a central effect. We will address each of these issues separately.
After we found that MTNX induced miosis, we conducted a comprehensive literature search on mu opioid agonists and miosis and found two studies, with one of them referencing another study, indicating that naloxone induced miosis. In 1980, Zilm examined the effects of 1.2 mg naloxone administered intravenously in non-dependent volunteers and found slight miosis, and two other effects which could be considered agonist in nature, a decrease in systolic blood pressure and increased rate of core temperature drop. In 1990, Loimer and colleagues tested 0.4 mg naloxone, intravenously administered, on pupil size in ten healthy volunteers. Naloxone induced miosis in a time-dependent manner – the decreases relative to baseline were not significant at 3, 6, or 15 min post injection but were significant at 20 and 30 min after injection. In a saline control condition, pupil diameter did not differ at any time point post-injection relative to baseline. Naloxone-induced miosis was not marked, i.e., an approximate 10% decrease relative to baseline at 30 min. The finding of slight miosis in the Zilm (1980) and Loimer et al. (1990) studies is important to point out, for we too did not see marked miosis. The greatest decrease in pupil diameter compared to baseline in the time course analysis (Fig 2) was 5.5% (approximately 75 min after MTNX injection). In contrast, the greatest decrease in pupil diameter when morphine was administered with saline pretreatment (compared to baseline) was a 28.2% decrease at 60 min after its injection. Loimer et al. (1990) also made the observation that in an earlier study by Jasinski and colleagues (1967), a brief mention was made in the introduction of their research manuscript that parenteral administration of 5 mg naloxone produced a “slight miosis” in two non-dependent volunteer prisoners, although the total number of subjects in the dose run-up study was not reported. Jasinski et al. (1967) did not observe any miosis at a dose range of 6–25 mg/70 kg. Kumor et al. (1988) examined the effects of very large doses of naloxone administered intramuscularly (150 and 300 mg) in non-dependent opioid abusers, and also did not detect changes in pupil size. As well, miosis was not found in a study in healthy volunteers in which a cumulative dose of 0.057 mg/kg naloxone (4 mg in a 70-kg individual) was administered (Evans et al. 1974). In sum, although not uniformly consistent, there are studies in the literature showing slight miosis, a prototypic mu agonist effect, induced by naloxone that has strong binding capacity at the mu-opiate receptor but putatively limited efficacy (Reisine and Pasternak, 1996). It is interesting to note that the studies in which naloxone induced miosis used relatively low doses of the drug, in contrast to the studies in which naloxone did not induce miosis that used high doses of the drug. In our study we found slight miosis with a mu opiate antagonist at a dose higher than the prescribed dose but not orders of magnitude higher such as those used in the Jasinski et al. (1967), Evans et al (1974), and Kumor et al. (1988) studies. Thus, whether or not a mu antagonist induces miosis may be dependent on dose, and merits further investigation.
The second issue, and the one that casts doubt on the supposition that the effects of MTNX on some of morphine’s subjective effects were due to MTNX’s peripheral actions, is that we provide indirect evidence that MTNX crosses the BBB, since miosis is a centrally-mediated effect of mu opioid agonists. Our results would have to challenge the notion that MTNX’s effects are limited to the periphery, or one would have to take the approach that miosis produced by systemic administration of opioids is a peripherally mediated effect. The latter assertion is something we have not been able to find in the extant scientific literature. We provide indirect evidence that MTNX crosses the BBB, but more research is needed to directly determine if this is the case, and if so, what the underlying biological substrates are that mediate the effect. A more definitive study is needed at this point to determine if indeed MTNX, and at what doses (e.g., therapeutic versus supra-therapeutic), crosses the BBB. Such a study might involve [11C]carfentanil positron emission tomography (PET) imaging to determine µ-opioid receptor binding potential in the brain after administration of different doses of MTNX (Ray et al., 2011).
It is important to point out that if indeed MTNX was exerting a centrally-mediated opioid agonist effect, it was small in magnitude. As mentioned above, the degree of miosis was far less than that of 0.14 mg/kg MS, approximately 5-fold less in magnitude. Consistent with this slight effect on miosis the primary dependent measures in this study, subjective effects, were altered minimally by 0.45 mg/kg MTNX alone. Ten minutes after its injection when miosis was detected, no subjective effects that we measured were altered by MTNX. In the time course analysis, only two subjective effects measures were altered by MTNX, and the effects were small in magnitude and opposite in direction to each other when one would think they should be in the same direction (i.e., “heavy sluggish feeling” and “feel bad”). We should mention that Yuan et al. (2005) in a pharmacokinetic study examined repeated doses of intravenous MTNX (12 consecutive infusions of 0.3 mg/kg every 6 h) in healthy volunteers. Subjective effects were assessed periodically as a secondary measure, using the same instrument used in the Yuan et al. (1998) study. No subjective effects measures were increased or decreased relative to baseline. In light of this discussion that effects of MTNX by itself were slight, another prototypic effect of mu opioid agonists, particularly at high doses, respiratory depression as measured by arterial oxygen saturation, was not evident after MTNX administration. However, in our study heart rate was transiently increased by 0.45 mg/kg MTNX, which is interesting but perplexing because increase in heart rate is not a prototypic effect of either mu opioid agonists or antagonists (Willer et al. 1979; Rubin et al. 1981; Reisine and Pasternak 1996).
We would like to briefly discuss that part of the study that did examine the effects of MTNX pretreatment on morphine effects. Although the majority of effects that we measured were not altered by either dose of MTNX, there were several subjective effects that were attenuated by MTNX, including ratings of “high,” “lightheaded,” and “skin itchy.” In concordance with our study, Yuan et al. (1998) also found that MTNX attenuated morphine-induced increases in ratings of “skin itch.” There are studies that do support the notion that opioid-induced pruritis is mediated at least in part by peripheral mechanisms (Levy et al. 1989; Bigliardi et al. 2007; Yamamoto et al. 2010). There was another prototypic effect of morphine (and other mu agonist opioids) that Yuan et al. (1998) observed that was reduced by MTNX, self-reported ratings of nausea. Rosow et al. (2007) in their study examining MTNX’s effects of remifentanil’s effects on urinary retention also found a reduction in incidence of self-reports of nausea, compared to remifentanil accompanied by a saline challenge. We found no evidence of such a reduction: morphine increased peak VAS ratings of “nauseated” relative to saline-placebo, but ratings in the 0MTNX/.14MS, .225MTNX/.14MS, and .45MTNX/.14MS did not differ significantly from each other (22.2±5.3, 28.0±5.7, and 24.1±5.5, respectively).
Finally, using three separate analyses (trough, AUC, time course), we found no evidence that MTNX antagonized the miotic effects of morphine. If we had not included the 0.45 mg/kg MTNX-alone condition in this study we would have concluded based on the lack of effect of MTNX on morphine-induced miosis that the reductions in some subjective effects of morphine in this study may have been at least in part mediated by peripheral opiate receptor blockade. However, because we provide indirect evidence that MTNX was exerting central effects at least when administered alone, whether those reductions in morphine effects were reduced by peripheral effects of MTNX, we feel, is open to question. In closing, the ramifications of the finding that MTNX induces miosis are discussed below.
It is possible that there is some explanation that we are not aware of for why MTNX induced miosis that is not opioid-related. We would find this to be more plausible if not for the finding that other investigators have found that naloxone, a mu opioid antagonist, also produces the seemingly paradoxical effect of miosis (Zilm 1980; Loimer et al. 1990). A statement was made in a previous report (Yuan et al. 2002) that in part influenced our decision to design and conduct this study: that MTNX can be used as a “probe” to differentiate putatively peripherally mediated or centrally mediated subjective symptoms (p. 122). Until a study is done that unequivocally demonstrates the inability of MTNX to cross the BBB in humans, we would suggest that the results of our study would argue against using MTNX as a probe to parse out peripheral from central effects of mu opioid agonists. In addition, many research reports or reviews that focus on MTNX characterize MTNX as a drug that does not cross the BBB (Kast et al. 2009; Weinstock and Chang 2011; Gatti and Sabato 2012; Bader et al. 2013; Zand et al. 2014). We feel that such an unequivocal statement is not warranted at this time, and would suggest that until proven otherwise that MTNX should be characterized as a peripheral opioid antagonist that “does not readily traverse or cross the BBB” (e.g., Brown and Goldberg 1985; Butelman et al. 2004) or “has restricted or limited ability to cross the BBB” (e.g., Foss et al. 1997; Garnock-Jones and McKeage 2010).
Acknowledgements
Research was supported in part by grant R21 DA031318 from the National Institute on Drug Abuse. We wish to thank Drs. Ravi Singh, William (Scott) Jones, Joseph Enayati, Michael Esposito, Hasan Chowdhury, Kelly Eaton, Amy Gruber, Annie Amin, and Keith Rodriguez for administering the drugs and monitoring the physiological status of the research volunteers, Karin Kirulis for screening potential subjects and conducting the structured interviews, Katarzyna Zoszak, Sean M. Apfelbaum, and Sandra Gutierrez for assistance in conducting the experimental sessions, and for the research volunteers who participated in the study. We also wish to thank an anonymous reviewer who offered critical and valuable comments on earlier iterations of this manuscript that resulted in a more coherent and parsimonious representation of the findings of the study.
Footnotes
Conflicts of interest The authors have no conflicts of interest to report.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. Text revision. [Google Scholar]
- Bader S, Dürk T, Becker G. Methylnaltrexone for the treatment of opioid-induced constipation. Expert Rev Gastroenterol Hepatol. 2013;7:13–26. doi: 10.1586/egh.12.63. [DOI] [PubMed] [Google Scholar]
- Beattie DT, Cheruvu M, Mai N, O'Keefe M, Johnson-Rabidoux S, Peterson C, Kaufman E, Vickery R. The in vitro pharmacology of the peripherally restricted opioid receptor antagonists, alvimopan, ADL 08-0011 and methylnaltrexone. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:205–220. doi: 10.1007/s00210-007-0146-x. [DOI] [PubMed] [Google Scholar]
- Bigliardi PL, Stammer H, Jost G, Rufli T, Buchner S, Bigliardi-Qi M. Treatment of pruritis with topically applied opiate receptor antagonist. J Am Acad Dermatol. 2007;56:979–988. doi: 10.1016/j.jaad.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Brown DR, Goldberg LI. The use of quaternary narcotic antagonists in opiate research. Neuropharmacology. 1985;24:181–191. doi: 10.1016/0028-3908(85)90072-3. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek MJ. Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. J Pharmacol Exp Ther. 2004;311:155–163. doi: 10.1124/jpet.104.068411. [DOI] [PubMed] [Google Scholar]
- Chamberlain BH1, Cross K, Winston JL, Thomas J, Wang W, Su C, Israel RJ. Methylnaltrexone treatment of opioid-induced constipation in patients with advanced illness. J Pain Symptom Manage. 2009;38:683–690. doi: 10.1016/j.jpainsymman.2009.02.234. [DOI] [PubMed] [Google Scholar]
- Evans JM, Hogg MI, Lunn JN, Rosen M. A comparative study of the narcotic against activity of naloxone and levallorphan. Anaesthesia. 1974;29:721–727. doi: 10.1111/j.1365-2044.1974.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Foss JF, O'Connor MF, Yuan CS, Murphy M, Moss J, Roizen MF. Safety and tolerance of methylnaltrexone in healthy humans: a randomized, placebo-controlled, intravenous, ascending-dose, pharmacokinetic study. J Clin Pharmacol. 1997;37:25–30. doi: 10.1177/009127009703700105. [DOI] [PubMed] [Google Scholar]
- Fraser HF, Isbell H. Human pharmacology and addictiveness of ethyl 1-(3-cyano-3,3-phenylpropyl)-4-phenyl-4-piperadine carboxylate hydrochloride. Bull Narcotics Jan-March. 1961:29–43. [Google Scholar]
- Fraser HF, van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (a) “attitude” of opiate addicts toward opiate-like drugs, (b) a short-term “direct” addiction test. J Pharmacol Exp Ther. 1961;133:371–387. [PubMed] [Google Scholar]
- Garnock-Jones KP, McKeage K. Methylnaltrexone. Drugs. 2010;70:919–928. doi: 10.2165/11204520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Gatti A, Sabato AF. Management of opioid-induced constipation in cancer patients: focus on methylnaltrexone. Clin Drug Investig. 2012;32:293–301. doi: 10.2165/11598000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Haertzen CA. Development of scales based on patterns of drug effects, using the Addiction Research Center Inventory (ARCI) Psychol Rep. 1966;18:163–194. doi: 10.2466/pr0.1966.18.1.163. [DOI] [PubMed] [Google Scholar]
- Hannington-Kiff JG. Measurement of recovery from outpatient general anaesthesia with a simple ocular test. Br Med J. 1970;3:132–135. doi: 10.1136/bmj.3.5715.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept. 2009;155:11–17. doi: 10.1016/j.regpep.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR, Martin WR, Haertzen CA. The human pharmacology and abuse potential of N-allylnoroxymorphone (naloxone) J Pharmacol Exp Ther. 1967;157:420–426. [PubMed] [Google Scholar]
- Kast RE. Use of FDA approved methamphetamine to allow adjunctive use of methylnaltrexone to mediate core anti-growth factor signaling effects in glioblastoma. J Neurooncol. 2009;94:163–167. doi: 10.1007/s11060-009-9863-y. [DOI] [PubMed] [Google Scholar]
- Korey A, Zilm DH, Sellers EM. A comparison of the dependence liabilities of two antidiarrheal agents, nufenoxole and loperamide, in volunteer subjects (abstract) Clin Pharmacol Ther. 1979;25:232. [Google Scholar]
- Kumor KM, Haertzen CA, Jasinski DR, Johnson RE. The psychopharmacologic and prolactin response after large doses of naloxone in man. Pharmacol Biochem Behav. 1988;30:967–975. doi: 10.1016/0091-3057(88)90128-1. [DOI] [PubMed] [Google Scholar]
- Lee HK, Wang SC. Mechanism of morphine-induced miosis in the dog. J Pharmacol Exp Ther. 1975;192:415–431. [PubMed] [Google Scholar]
- Levy JH, Brister NW, Shearin A, Ziegler J, Hug CC, Adelson DM, Walker BF. Wheal and flare responses to opioids in humans. Anesthesiology. 1989;70:756–760. doi: 10.1097/00000542-198905000-00008. [DOI] [PubMed] [Google Scholar]
- Loimer N, Schmid R, Grünberger J, Linzmayer L. Naloxone induces miosis in normal subjects. Psychopharmacology (Berl) 1990;101(2):282–283. doi: 10.1007/BF02244141. 1990. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Skarke C, Grösch S, Darimont J, Schmidt H, Geisslinger G. The polymorphism A118G of the human mu-opioid receptor gene decreases the pupil constrictory effect of morphine-6-glucuronide but not that of morphine. Pharmacogenetics. 2002;12:3–9. doi: 10.1097/00008571-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Murphy DB, Sutton JA, Prescott LF, Murphy MB. Opioid-induced delay in gastric emptying: a peripheral mechanism in humans. Anesthesiology. 1997;87:765–770. doi: 10.1097/00000542-199710000-00008. [DOI] [PubMed] [Google Scholar]
- Murray RB, Adler MW, Korczyn AD. The pupillary effects of opioids. Life Sci. 1983;33:495–509. doi: 10.1016/0024-3205(83)90123-6. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Thomas J, Boatwright MLM, Tran D, Galasso FL, Stambler N, Von Gunten CF, Israel RJ. Subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with advanced illness: a double-blind, randomized, parallel group, dose-ranging study. J Pain Symptom Manage. 2008;35:458–468. doi: 10.1016/j.jpainsymman.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Bickel WK, Liebson IA. Drug discrimination in human postaddicts: Agonist-antagonist opioids. J Pharmacol Exp Ther. 1989;250:184–196. [PubMed] [Google Scholar]
- Ray R, Ruparel K, Newberg A, Wileyto EP, Loughead JW, Divgi C, Blendy JA, Logan J, Zubieta JK, Lerman C. Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc Natl Acad Sci U S A. 2011;108:9268–9273. doi: 10.1073/pnas.1018699108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T, Pasternak G. Opioid analgesics and antagonists. In: Hardman JG, Gilman AG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996. pp. 521–555. [Google Scholar]
- Rosow CE, Gomery P, Chen TY, Stefanovich P, Stambler N, Israel R. Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther. 2007;82:48–53. doi: 10.1038/sj.clpt.6100164. [DOI] [PubMed] [Google Scholar]
- Rubin P, Blaschke TF, Guilleminault C. Effect of naloxone, a specific opioid inhibitor, on blood pressure fall during sleep. Circulation. 1981;63:117–121. doi: 10.1161/01.cir.63.1.117. [DOI] [PubMed] [Google Scholar]
- Russell J, Bass P, Goldberg LI, Schuster CR, Merz H. Antagonism of gut, but not central effects of morphine with quaternary opiate antagonists. Eur J Pharmacol. 1982;78:255–261. doi: 10.1016/0014-2999(82)90026-7. [DOI] [PubMed] [Google Scholar]
- Slatkin N, Thomas J, Lipman AG, Wilson G, Boatwright ML, Wellman C, Zhukovsky DS, Stephenson R, Portenoy R, Stambler N, Israel R. Methylnaltrexone for treatment of opioid-induced constipation in advanced illness patients. J Support Oncol. 2009;7:39–46. [PubMed] [Google Scholar]
- Thomas J, Karver S, Cooney GA, Chamberlain BH, Watt CK, Slatkin NE, Stambler N, Kremer AB, Israel RJ. Methylnaltrexone for opioid-induced constipation in advanced illness. NEJM. 2008;358:2332–2343. doi: 10.1056/NEJMoa0707377. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Belluzzi JD, Stein L. Effects of opiate antagonists and their quaternary analogues on nucleus accumbens self-stimulation. Behav Brain Res. 1989;33:181–188. doi: 10.1016/s0166-4328(89)80049-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Measurement and Appraisal of Adult Intelligence. Baltimore, MD: Williams and Wilkins; 1958. [Google Scholar]
- Weinstock LB1, Chang AC. Methylnaltrexone for treatment of acute colonic pseudo-obstruction. J Clin Gastroenterol. 2011;45:883–884. doi: 10.1097/MCG.0b013e31821100ab. [DOI] [PubMed] [Google Scholar]
- Willer JC, Boreau F, Dauthier C, Bonora M. Study of naloxone in normal awake man: effects on heart rate and respiration. Neuropharmacology. 1979;18:469–472. doi: 10.1016/0028-3908(79)90072-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Sugimoto Y. Involvement of peripheral mu opioid receptors in scratching behavior in mice. Eur J Pharmacol. 2010;649:336–341. doi: 10.1016/j.ejphar.2010.07.039. [DOI] [PubMed] [Google Scholar]
- Yuan C-S, Foss JF, O'Connor M, Toledano A, Roizen MF, Moss J. Methylnaltrexone prevents morphine-induced delay in oral-cecal transit time without affecting analgesia: a double-blind, randomized placebo-controlled trial. Clin Pharmacol Ther. 1996;59:469–475. doi: 10.1016/S0009-9236(96)90117-4. [DOI] [PubMed] [Google Scholar]
- Yuan C-S, Foss JF, O'Connor M, Osinski J, Roizen MF, Moss J. Efficacy of orally administered methylnaltrexone in decreasing subjective effects after intravenous morphine. Drug Alcohol Depend. 1998;52:161–165. doi: 10.1016/s0376-8716(98)00087-8. [DOI] [PubMed] [Google Scholar]
- Yuan C-S, Foss JF. Gastric effects of methylnaltrexone on mu, kappa, and delta opioid agonists induced brainstem unitary responses. Neuropharmacology. 1999;38:425–432. doi: 10.1016/s0028-3908(98)00192-0. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Foss JF, O'Connor M, Osinski J, Karrison T, Moss J, Roizen MF. Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial. JAMA. 2000;283:367–372. doi: 10.1001/jama.283.3.367. [DOI] [PubMed] [Google Scholar]
- Yuan C-S, Wei G, Foss JF, O'Connor M, Karrison T, Osinski J. Effects of subcutaneous methylnaltrexone on morphine-induced peripherally mediated side effects: a double-blind randomized placebo-controlled trial. J Pharmacol Exp Ther. 2002;300:118–123. doi: 10.1124/jpet.300.1.118. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Doshan H, Charney MR, O'Connor M, Karrison T, Maleckar SA, Israel RJ, Moss J. Tolerability, gut effects, and pharmacokinetics of methylnaltrexone following repeated intravenous administration in humans. J Clin Pharmacol. 2005;45:538–546. doi: 10.1177/0091270004273491. [DOI] [PubMed] [Google Scholar]
- Zand F, Amini A, Asadi S, Farbood A. The effect of methylnaltrexone on the side effects of intrathecal morphine after orthopedic surgery under spinal anesthesia. Pain Pract. 2014 Feb 27; doi: 10.1111/papr.12185. [DOI] [PubMed] [Google Scholar]
- Zilm DH. Naloxone response in non-dependent man: effect on six physiological variables. Neuropharmacology. 1980;19:591–595. doi: 10.1016/0028-3908(80)90031-3. [DOI] [PubMed] [Google Scholar]