Abstract
A lysed preparation of isolated insulin secretory granules efficiently cleaved murine proopiomelanocortin (mPOMC) at physiologically important Lys-Arg processing sites. This processing was mostly attributed to an activity that co-eluted with the proinsulin processing type-II endopeptidase from anion exchange chromatography (Lys-Arg-directed; Davidson, H. W., Rhodes, C. J., and Hutton, J. C. (1988) Nature 333, 93–96). The principal peptide hormone products generated by the insulin secretory granule lysate were identified by specific radioimmunoassay and NH2-terminal microsequencing analysis of high performance liquid chromatography-separated products as α-melanocyte-stimulating hormone, corticotropin-like intermediate, γ-lipotropin, β-endorphin-(1–31), 18-kDa NH2-terminal fragment and, to a lesser extent, adrenocorticotrophin and β-lipotropin. This processing had an acidic pH optimum (pH 5–5.5) and was Ca2+-dependent (K0.5 activation = 5–80 µm). With increasing Ca2+ concentrations there was an increase in the extent to which mPOMC was processed. The in vitro processing of mPOMC by the insulin secretory granule endopeptidase activity reported here is in excellent agreement with the in vivo processing of this prohormone by a combination of PC2 and PC3, candidates of prohormone endpeptidase, in gene transfer studies with cells that express the regulated secretory pathway
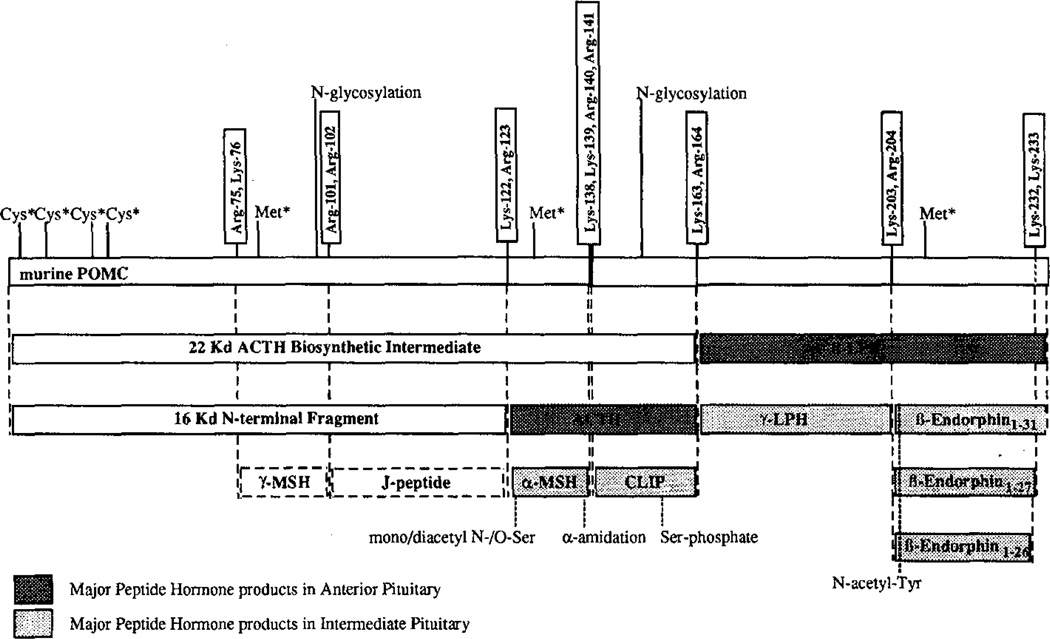
Many secreted proteins are synthesized as larger inactive precursors and processed post-translationally by limited proteolysis on the COOH-terminal side of a paired basic amino acid sequence (usually Lys-Arg or Arg-Arg) (1–3). This proteolytic step is shared in the maturation of precursor proteins in both the constitutive (e.g. albumin (4, 5) and the insulin receptor (6)) and regulated secretory pathways (e.g. insulin (7) and proopiomelanocortin (POMC)1 (8)). A number of precursor proteins that are expressed in multiple tissues are processed in a tissue-specific manner. For example, POMC is synthesized in the anterior and neurointermediate lobes of the pituitary as well as in certain areas of the brain and in other tissues (8) (Fig. 1). In the anterior lobe of the pituitary ACTH, β-LPH and a 16-kDa amino-terminal fragment are the major products of the proteolytic processing. In the neurointermediate lobe of the pituitary the ACTH and β-LPH domains of POMC are processed more extensively to α-MSH, CLIP, γ-LPH, N-acetyl-β-endorphin-(1–27), and N-acetyl-β-endorphin-(1–26) (8).
Figure 1. Schematic representation of mouse POMC processing in the pituitary.
The potential tetra/dibasic processing sites on the mPOMC precursor are indicated and the major peptide hormone products derived from differential mPOMC processing in the anterior verses intermediate pituitary are shown. The position of [35S]cysteine and [35S]methionine residues on the radiolabeled mPOMC substrates, the positions of other post-translational modifications of POMC, and processing products are indicated.
Studies by gene transfer identified two heterologous cell types which mimic the processing of POMC in the pituitary. Primary cultures of bovine adrenal medullary chromaffin (BAM) cells exhibited the same limited processing of murine POMC as anterior lobe corticotrophs producing primarily ACTH, β-LPH, and, to a lesser extent, γ-LPH and β-endorphin-(1–31) (9). In contrast, insulin secreting RIN m5F cells efficiently cleaved at all Lys-Arg sites examined, yielding a set of products reminiscent of neurointermediate lobe melanotrophs including α-MSH, CLIP, γ-LPH, and β-endorphin-(1–31) (10, 11) (no cleavage at Lys232-Lys233 of mPOMC was seen in RIN m5F cells (10, 11)).
The mechanism and regulation of specific proprotein proteolytic cleavage is poorly understood. A key to understanding the early events in prohormone maturation is the identification of the prohormone processing endopeptidases themselves. Several candidate endopeptidases have been proposed (see Refs. 12 and 13 for review), but to date the only unequivocally demonstrated proprotein-specific processing endopeptidase is the Kex2-protease in yeast. KexZp is a Ca2+-dependent serine endopeptidase which catalyses the maturation of the α-mating factor precursor by proteolytic cleavage on the COOH-terminal side of Lys-Arg residues (14). Certain lines of evidence suggest that mammalian proprotein processing endopeptidases might be functional (and hence structural) homologues of Kex2p. Co-expression of Kex2p and mPOMC in a number of mammalian cell types that are unable to process POMC (e.g. BSC-40 cells) results in the efficient processing of the prohormone to authentic pituitary peptides including γ-LPH and β-endorphin-(1–31) (15). Similarly, Kex2p can process proalbumin to albumin in vitro (16), and proinsulin is processed to insulin when expressed in yeast (17).
Although a complementation approach used to identify Kex2p is not possible in mammalian cells, two separate approaches have led to the identification of several endoproteases that share enzymatic and/or structural similarity to the yeast enzyme. First, a number of mammalian Ca2+-dependent endopeptidase activities with properties similar to Kex2p have been shown to correctly cleave precursor proteins at pairs of basic amino acids. Proinsulin processing is catalyzed by two distinct Ca2+-dependent endoproteolytic activities of acidic pH optima (18). One (type-I) specifically cleaves at the Arg31-Arg32 site, the proinsulin B/C-chain junction. The other (type-II) preferentially cleaves at the Lys64-Arg65 site, the proinsulin A/C-chain junction. Another related activity is a Ca2+-dependent proalbumin converting activity from liver Golgi membranes (19).
Second, polymerase chain reaction amplification of endocrine cell DNA yielded two neuroendocrine-specific cDNA sequences, PC2 (20) and PC3 (21) (also named PC1 (22,23)), which encode proteins related to Kex2p and have been reported. Co-expression of PC3 and mPOMC results in the production of ACTH and β-LPH, whereas co-expression of PC2 and POMC results in the expression of β-endorphin-(1–31) and a processing intermediate composed of ACTH and γ-LPH sequences. In contrast, co-expression of mPOMC together with PC2 and PC3 resulted in the production of ACTH, γ-LPH, and β-endorphin-(1–31) in cells containing only a constitutive secretory pathway, whereas expression of the same cDNAs in cells containing a regulated secretory pathway resulted in further cleavage of the ACTH domain to α-MSH and CLIP (11, 24).
In this study, we have addressed the possibility of whether the proinsulin processing type-I and -II activities are capable of differentially processing murine POMC in vitro. We show that only a type-II associated activity can efficiently cleave intact mPOMC. Analysis of mPOMC peptides excised by this endoproteolysis showed correct cleavage of the POMC substrate to a set of peptides produced by mPOMC-transfected insulinoma cells (10, 11).
MATERIALS AND METHODS
Vaccinia Virus
VV strain WR was used in these studies. Viral recombinants directing the expression of native murine POMC (mPOMC) were constructed and virus stocks maintained as described (25).
Nonradiolabeled POMC Substrate Production
150-mm plates of BSC-40 cells were infected with VV:mPOMC multiplicity of infection = 2) and after 30 min, the innoculum was replaced with serum- and phenol red-free MCDB 202 medium containing 25 µg/ml gentimycin. After 24 h, the medium was adjusted to 0.2% (v/v) trifluoroacetic acid, centrifuged at 18,000 rpm (SW 28 rotor) for 20 min, 4 °C, to remove particulate matter, and the cleared media applied directly to a C4 reversed phase HPLC column (Vydac No. 214TP54) at a flow rate of 1 ml/min. Retained material was eluted with a two-step linear gradient (from 12.8 to 20% ACN over 2 min, immediately followed by 20 to 39.5% ACN over 75 min) in 0.1% trifluoroacetic acid with a flow rate of 1 ml/min. One-ml fractions were collected and assayed for mPOMC by RIA (9–11, 25). Silver-stained polyacrylamide gels indicated mPOMC was at least 90% pure in peak fractions. Using this method, approximately 40 µg of mPOMC can be prepared from 1 × 108 cells. The correct NH2-terminal amino acid sequence of this mPOMC substrate has been determined by microsequence analysis (l0), and the production of β-endorphin-(1–31) in this and other studies (10) shows the correct mPOMC COOH terminus.
Radiolabeled POMC Substrate Production
100-mm plates of BSC-40 cells (8 × l06 cells) were infected with VV:mPOMC (multiplicity of infection = 1). After 30 min, the innoculum was removed and replaced with minimal essential medium containing 10% fetal bovine serum for 4 h. The media was then replaced with methionine-free minimal essential medium (GIBCO Selectamine kit) containing 1% dialyzed serum for 1 h and replaced with the same medium containing 50 µg/ml leupeptin, 40 µg/ml soybean trypsin inhibitor, 100 µg/ml bacitracin, 10 µg/ml aprotinin, 50 µg/ml gentimycin, and 1 mCi of [35S]methionine and [35S]cysteine (Du Pont-New England Nuclear). After an additional 6 h, the labeling media was supplemented with 20 µl of minimal essential medium containing 10% nondialyzed fetal calf serum. After 24 h (final), the medium was removed, adjusted to 0.2% trifluoroacetic acid, and applied to a reversed phase cartridge (Baker).The cartridge was then washed with 4 ml of 12.8% ACN in 0.1% trifluoroacetic acid and then mPOMC was eluted with 4 ml of 80% ACN in 0.1% trifluoroacetic acid. The ACN was removed by evaporation, the sample was adjusted to 12.8% ACN in 0.1% trifluoroacetic acid, and then applied to a C4 reversed phase HPLC column (Vydac No. 214TP54). Murine POMC was eluted from the column with an ACN gradient as described above. Using this procedure, 3 × 106 cpm are incorporated into mPOMC (4 × 103 cpm/ng).
Tissue
Insulinoma tissue (26, 27) propagated in NEDH (New England Deaconess Hospital strain) rats was used as a source of purified β-granules and for the pancreatic β-cell subcellular fractions. Purified β-granules were prepared as previously described (28,29). The marker enzyme analysis and electron microscopy (27) showed this preparation to be essentially free from other subcellular organelles. It was either used immediately or stored in liquid nitrogen. Where indicated a soluble extract of an insulin secretory granule lysate was obtained using Triton X-114 as previously described (30).
Ion Exchange Chromatography
Separation of type-I and -II proinsulin processing endopeptidases was achieved by anion exchange chromatography of a β-granule lysate on DEAE-cellulose as previously described (18, 29, 31, 32).
POMC Processing Assay
Unless otherwise stated, POMC processing activity was assessed by incubating aliquots of a purified β-granule fraction (25–50 µg of protein) or DE-52 column fractions containing the enzyme activity for 1–6 h at 30 °C in 25 µ1 (final volume) of 0.1% (w/v) Brij-35, 20 mm sodium acetate, 5 mm CaCl2, 10 µm pepstatin A, 0.1 mm TPCK, 10 µm E-64, 0.1 mm phenylmethylsulfonyl fluoride (pH 5.5) containing 5–10 × 103 cpm of [35S]methionine or [35S]cysteine-radiolabeled mPOMC or 50 µg of nonradiolabeled mPOMC. The incubation was stopped by heating at 95 °C for 5 min. POMC processing was analyzed by either Tricine/SDS-PAGE (33) or HPLC (9–11, 25).
Electrophoretic Analysis
The assay incubation media was mixed with an equal volume of 100 mm Tris-HCI (pH 6.8), 4% (w/v) SDS, 24% (v/v) glycerol, 20 mm dithiothreitol, and 0.02% bromphenol blue and heated at 95 °C for 5 min, then applied to Tricine/SDS-PAGE as previously described (30).
HPLC Analysis of 35S-Radiolabeled mPOMC Processing Products
The incubation assay mixture was added to an equal volume of 20% (v/v) ACN, 0.2% (v/v) trifluoroacetic acid and applied to a C4 (Vydac No. 214TP54) reversed phase column at a flow rate of 1 ml/min. After 2.0 min at 12.8% (v/v) ACN in 0.1% trifluoroacetic acid, products from mPOMC processing were resolved over a two-step gradient (12.8 to 20.0% ACN over 3.0 min, followed by 20.0% to 33.6% ACN over a further 90 min). The 35S containing fractions (1 ml) were lyophilized, resuspended in 100 mm Tris-HCI (pH 6.8), 4% (w/v) SDS, 24% (v/v) glycerol, 20 mm dithiothreitol, and 0.02% bromphenol blue, and run on Tricine/SDS-PAGE. In other experiments mPOMC processing products were immunoprecipitated with antibodies to specific regions of the mPOMC molecule. In this case, HPLC isolated 35S radiolabeled products were collected, lyophilized, then resuspended in 95 µl of 50 mm sodium phosphate buffer (pH 7.6), 1 mm EDTA, 1% (v/v) Triton X-100,0.05% (w/v) bovine serum albumin, 10 µm phenylmethylsulfonyl fluoride, 0.1 mg/ml aprotinin, 0.1% (w/v) sodium azide. Five µl of undiluted antisera to either ACTH or β-endorphin (both a gift from Dr. Peng Loh, NIH, Bethesda, MD), or γ-MSH or POMC1–41 NH2-terminal (both from Dr. P. Lowry, Department of Biochemistry, University of Reading, United Kingdom) were added and incubated for 18 h at 4 °C. The antibody-antigen complex was isolated by adding 50 µl of Protein A-Sepharose (Sigma) suspension in phosphate-buffered saline (150 mg/ml; pH 7.4) and incubated for 2 h at 25 °C with rotary mixing. This was then centrifuged (Beckman Microcentrifuge) at 10,000 × g for 5 min, the supernatant was removed, and the pellet washed 5 times in the phosphate buffer. Material bound to the Protein A-Sepharose pellet was then eluted from the with 2 × 100 µl of 0.1 m HCl. This eluant and the first supernatant were then mixed with 10 ml of OptiPhase “Hisafe”’ II (LKB, Loughborough, United Kingdom) and counted in a Beckman-LS1801 β-scintillation counter.
HPLC for Microsequence Analysis
Separation of POMC processing products was by a reversed phase Vydac C4 column as described previously, but a different gradient profile was applied of: 0–6 min, 16.0% ACN; 7–10 min, 16.0–20.8% ACN 11–55 min, 20.8–32.0% ACN; 56–70 min 32.0–64.0% ACN). Fractions containing the products were collected, lyophilized, and sequenced directly with an Applied Biosystems 730A sequenator (Foster City, CA).
Ion Exchange HPLC of β-Endorphin and γ-LPH Peptides
Fractions from the C4 column containing β-endorphin and γ-LPH immunoreactivity were further resolved by cation exchange HPLC. Column fractions were diluted with 5 volumes of 10 mm HPLC grade NH4OAc (Baker) (pH 4.5) in 25% ACN and injected onto a Poly-Aspartic Acid WCX column (The Nest Group No. 1859-102). β-Endorphin samples were resolved with a two-step linear NH4OAc (pH 4.5) gradient (from 10 to 140 mm in 20 min, immediately followed by 140–300 mm in 10 min) in 25% ACN. γ-LPH samples were resolved with a linear NH4OAc pH 4.5 gradient (increasing from 10 to 500 mm in 30 min) in 25% ACN. Both gradients maintained a flow rate of 0.5 ml/min. One-min fractions were aliquoted into RIA tubes and lyophilized. To reduce the amount of residual acetate, 200 µl of 3% NH4OH (final concentration in water) was added to each tube and samples were again lyophilized before RIA.
Analysis of ACTH Immunoreactive Peptides
Two approaches were taken for this analysis.
Peptide Gel Analysis
Aliquots of C4 column fractions containing immunoreactive peptides were transferred to 1.5-ml Eppendorf tubes, lyophilized, resuspended in SDS sample buffer, and resolved by Tricine/SDS-PAGE (33). After electrophoresis, lanes containing molecular weight standards (cleavage fragments of horse myoglobin, Sigma MW-SDS-17 and Bio-Rad low Mr kit No. 1610304) were stained with Coomassie Blue. Lanes containing samples were cut into 2.5-mm thick slices which were incubated for 3.5 h at 55 °C in 0.5 ml/slice RIA buffer with occasional mixing. Aliquots of the eluants were assayed by RIA as described (9–11, 25).
Iodination and Sequence Analysis of ACTH Peptides
Tyrosine residues of ACTH immunoreactive peptides in HPLC C4 column fractions were iodinated with Na125I by the chloramine-T method as previously described (34). ACTH immunoreactive peptides were immunoprecipitated with an antiserum to ACTH (Henrietta) and the ACTH/γ-LPH processing intermediate peptide was immunoprecipitated using antiserum to γ-LPH (Molly). The antigen-antibody complex was then isolated using Protein A-Sepharose. An aliquot of the immunoprecipitated sample was resolved by Tricine/SDS-PAGE (33), to confirm size purity of the radiolabeled peptide. If no nonspecific background was detected, the remainder of the antigen-antibody-Protein A complexes were disrupted in 5 m acetic acid and the Protein A-Sepharose removed by centrifugation. The supernatant was transferred to an acid-washed glass tube, dried, and sequenced directly. If nonspecific background bands were detected, the remainder of the sample was resolved on Tricine/SDS-PAGE and electrotransferred onto Immobilon™ membranes (Millipore). 125I was detected by autoradiography. The section of Immobilon containing the peptide was cut out and placed directly in an acid-washed glass tube containing 0.5 µg of ACTH (Peninsula) as a carrier. NH2-terminal sequencing was performed as follows (all reagents were from Pierce Chemical Co. and were of sequencing grade quality). To the dried samples, 80 µl of H2O and 80 µl of 5% phenylisothiocyanante in pyridine were added, followed by a 45-min incubation at 45 °C under N2. Samples were then dried and incubated for 15 min incubation in 80 µ1 of anhydrous trifluoroacetic acid. Samples were dried again, resuspended in 200 µl of H2O, andex tracted 3 times with 300 µ1 of ethyl acetate. The organic phases were pooled and counted directly in a γ-counter. The aqueous phase was dried prior to starting the next cycle.
Other Procedures
Protein was determined by the method of Bradford (35) using bovine serum albumin as a standard. Free Ca2+ concentration was calculated from reference stability constants as previously described (36).
RESULTS
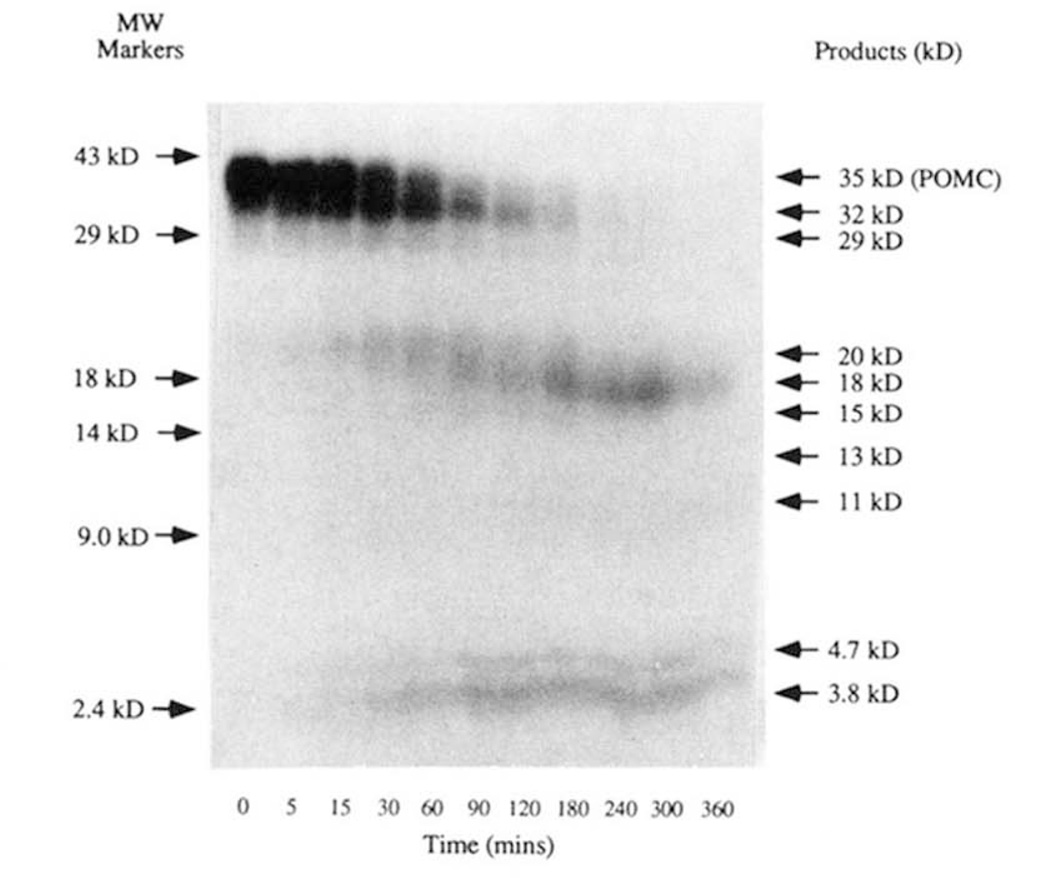
To compare the specificity of the insulin secretory granule type-I and -II endoproteolytic activities with previously characterized in vivo processing of mPOMC by insulinoma and BAM cells (9, 10, 25), as well as transfected PC2 and PC3 (11, 24), an in vitro POMC processing assay was developed. The [35S]methionine-labeled POMC used as substrate was produced in BSC-40 cells infected with VV:mPOMC, a recombinant vaccinia virus which directs the expression of mouse POMC. This monkey kidney cell line was previously shown to synthesize only intact prohormone (i.e. is maturation deficient for endocrine peptide precursors), facilitating precursor purification by allowing isolation from the culture media of metabolically labeled cells (see “Materials and Methods”). Incubation in vitro of the radiolabeled prohormone with endopeptidase activities present in insulin secretory granule lysates resulted in a time-dependent conversion of the prohormone to smaller peptides (Fig. 2). Some of the [35S]methionine radiolabeled processing products generated were identified by their apparent Mr on Tricine/SDS-PAGE (Fig. 2). A subset of these products (e.g. 32, 20, 15 kDa) were intermediates in that they appeared as the mPOMC substrate disappeared, then disappeared themselves with increasing time of incubation. The processing of mPOMC under optimal in vitro processing conditions (pH 5.5, 5 mm Ca2+, 30 °C, 25 µg of protein insulin secretory granule lysate) was completed within 4 h giving a stable pattern of final products (18, 11, 4.7, and 3.8 kDa) present for up to 18 h. The 29-kDa band observed appeared to be an minor unrelated contaminating peptide in the mPOMC substrate preparation and was not processed by the insulin secretory granule endopeptidases (Figs. 2 and 3).
Figure 2. Time course of [35S]methionine-labeled mPOMC processing by an insulin secretory granule lysate.
mPOMC processing by an insulin secretory granule lysate was assessed as described (see “Materials and Methods”) with analysis by Tricine/SDS-PAGE. A typical fluorograph of such an analysis at the indicated time points is shown and the apparent Mr of the products indicated.
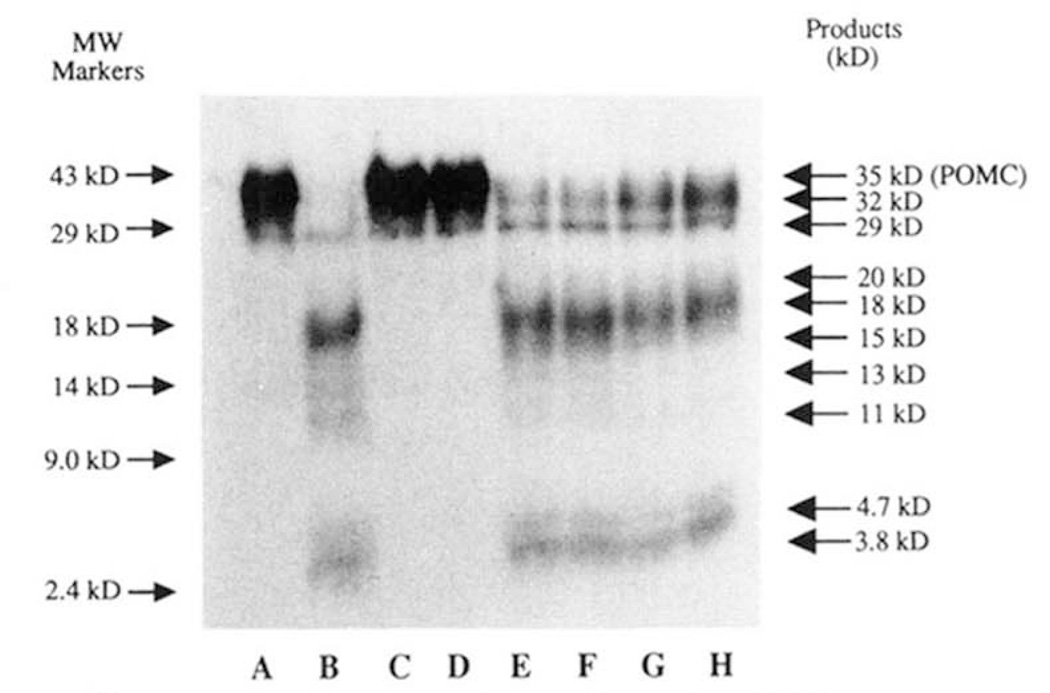
Figure 3. [35S]Methionine-labeled mPOMC processing by type-I and -II insulin secretory granule endopeptidases.
The type-I and -II endopeptidases were separated as previously described (18), and mPOMC processing was assessed over 3 h at 30 °C as described (see “Materials and Methods”) with analysis by Tricine/SDS-PAGE. A typical fluorograph of such an analysis is shown and the apparent Mr of the products indicated lane A, [35S]methionine-labeled mPOMC only; lane B, [35S]methionine-labeled mPOMC + insulin secretory granule lysate; lanes C and D (duplicate lanes), [35S] methionine-labeled mPOMC + type-I activity; lanes E and F (duplicate lanes), [35S]methionine-labeled mPOMC + type-II activity; lanes G and H (duplicate lanes), [35S]methionine-labeled mPOMC + type-I and -II activities recombined after anion exchange.
The paired basic site-specific proinsulin processing type-I (Arg-Arg-directed) and type-II (Lys-Arg-directed) activities can be separated by anion exchange chromatography (18,31). The processing of [35S]methionine mPOMC was only associated with endopeptidase activity that co-eluted with the proinsulin processing type-II activity (Fig. 3, lanes E and F), the type-I activity did not cleave the mPOMC substrate under the conditions used for up to 18-h incubations (Fig. 3, lanes C and D). Consistent with these observations, the combination of type-I and- II (Fig. 3, lanes G and H), gave a mPOMC processing pattern similar to type-II activity alone (Fig. 3, lanes E and F).
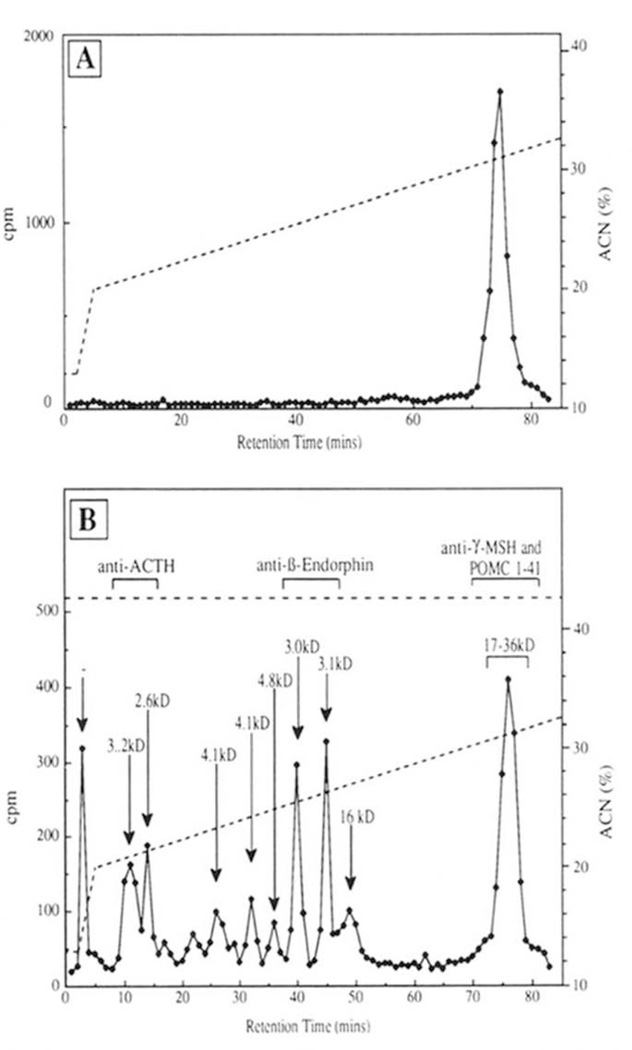
To identify the [35S]methionine mPOMC peptides excised by the insulin secretory granule endopeptidase activity, the incubated samples were resolved by reversed phase HPLC (Fig. 4). Processing products were detected by scintillation counting of the collected fractions (Fig. 4, panel B). The material present in the peaks of radioactivity were collected and then further separated by Tricine/SDS-PAGE. [35S]Methionine-labeled mPOMC eluted as a single peak between 73 and 78 min on this HPLC protocol (Fig. 4, panel A). After a 3-h incubation with an insulin secretory granule lysate, the predominant peptide that eluted in this peak was the 18-kDa product, as well as lesser amounts of the 20- and 32-kDa processing intermediates and only traces of intact mPOMC (35 kDa). Several of the early eluting peaks had estimated molecular masses between 3 and 5 kDa (Fig. 4, panel B), and most likely corresponding to the 5- and 4-kDa previously seen on Tricine/SDS-PAGE (Figs. 2 and 3). Two peaks eluting between 10 and 15 min were immunoprecipitable with an ACTH cross-reacting antisera, whereas two peaks at 40 and 45 min (3 kDa) were immunoprecipitable by anti-β-endorphin antisera (Fig. 4, panel B). The [35S]methionine peptides eluting in the single peak between 73 and 78 min were immunoprecipitated (<85%) by both anti-POMC1–41 and anti-γ-MSH antisera (Fig. 4, panel B). These results suggest that the predominant 18-kDa peptide in this peak is the glycosylated NH2-terminal fragment resulting from cleavage of POMC at the J peptide/ACTH junction (Lys122-Arg123) (37). Furthermore, the lack of any lower Mr peptides containing γ-MSH immunoreactivity suggests that little or no [35S]methionine γ-MSH was generated from the mPOMC processing by an insulin secretory granule lysate.
Figure 4. HPLC analysis of [35S]methionine-labeled mPOMC processing by an insulin secretory granule lysate.
mPOMC processing by an insulin secretory granule lysate was assessed as described (see “Materials and Methods”) with analysis by Vydac reversed phase C4 HPLC. The ACN gradient applied is indicated. Panel A, [35S]methionine-labeled mPOMC only. Panel B represents [35S]methionine-labeled mPOMC + insulin secretory granule lysate (3 h at 30 °C) where peaks of [35S]methionine mPOMC processing products are generated. The apparent Mr of these products (determined by Tricine/SDS-PAGE (see “Materials and Methods”)) and immunoprecipitation with antibodies specific to mPOMC regions (ACTH, β-endorphin, γ-MSH, and POMC1–41; see “Materials and Methods”) is indicated.
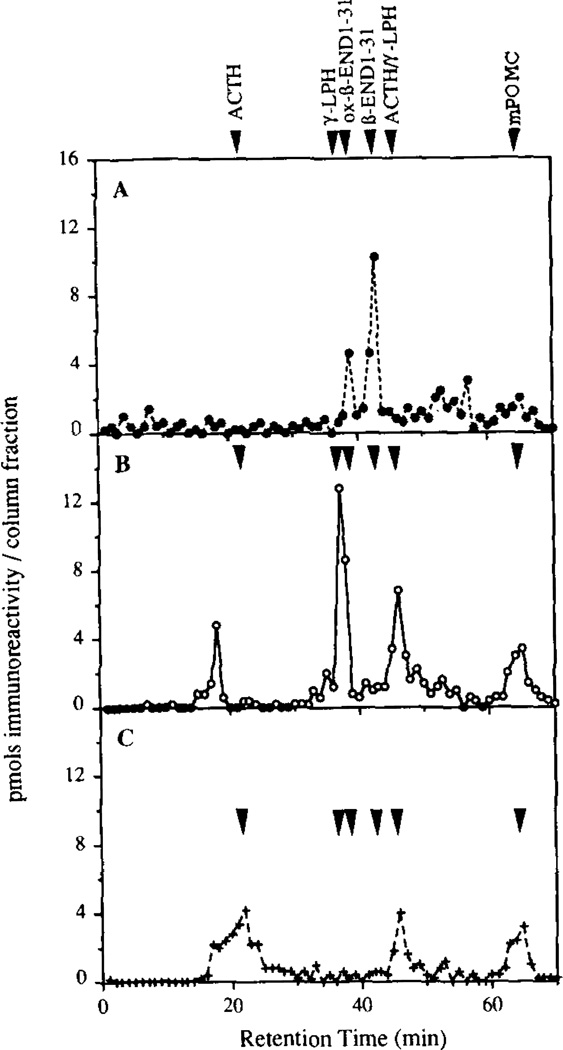
To unequivocally identify mPOMC cleavage products by direct NH2-terminal sequencing, 10 µg of nonradiolabeled mPOMC was incubated (30 °C for 3.5 h) with an aqueous phase Triton X-114 extract of insulin secretory granules. The digested sample was resolved by reversed phase HPLC and5-µ1 aliquots of the 1-ml resultant fractions were assayed with domain-specific RIAs as previously described (9–11, 25). It was calculated that from the 85 pmol of mPOMC applied to the C4 column that 66 pmol were detected in the assay (a recovery of 77%). RIA analysis showed two major peaks of β-endorphin immunoreactivity (39 and 43 min) which co-eluted with oxidized and native β-endorphin-(1–31), respectively (Fig. 5, panel A). The major peak of γ-LPH immunoreactivity at 37 min co-eluted with authentic γ-LPH (Fig. 5, panel B), whereas the minor peak at 18 min co-eluted with the NH2-terminal truncated form produced in Rin m5F cells (10), presumably by cleavage at the Leu10-Glu11 bond in γ-LPH. Using an antibody directed at the mid-portion of ACTH (no cross-reactivity with α-MSH or CLIP), a broad heterologous peak of ACTH immunoreactivity was detected between 17 and 24 min (Fig. 5, panel C). This material, resulting from cleavage of POMC synthesized in VV:mPOMC-infected BSC-40 cells, eluted several minutes earlier than ACTH synthesized in either AtT20 cells or VV:mPOMC-infected BAM cells (9), but co-eluted with ACTH derived from PC3 cleavage in transfected BSC-40 cells (11).
Figure 5. Radioimmunoassay analysis of mPOMC processing products generated by insulin secretory granule endopeptidase activity.
mPOMC processing by a Triton X-114 aqueous phase-extracted insulin secretory granule lysate over 3.5 h at 30 °C was assessed as described (see “Materials and Methods”). The mPOMC products were resolved by Vydac reversed phase C4 HPLC, 5-µl aliquots of the 1-ml fractions were assayed by specific RIA to regions of mPOMC and the total amount of product per fraction calculated. The elution position of various POMC processing product standards is indicated. RIA analysis was for β-endorphin (panel A, ●), γ-LPH (panel B, ○), and ACTH (panel C, +).
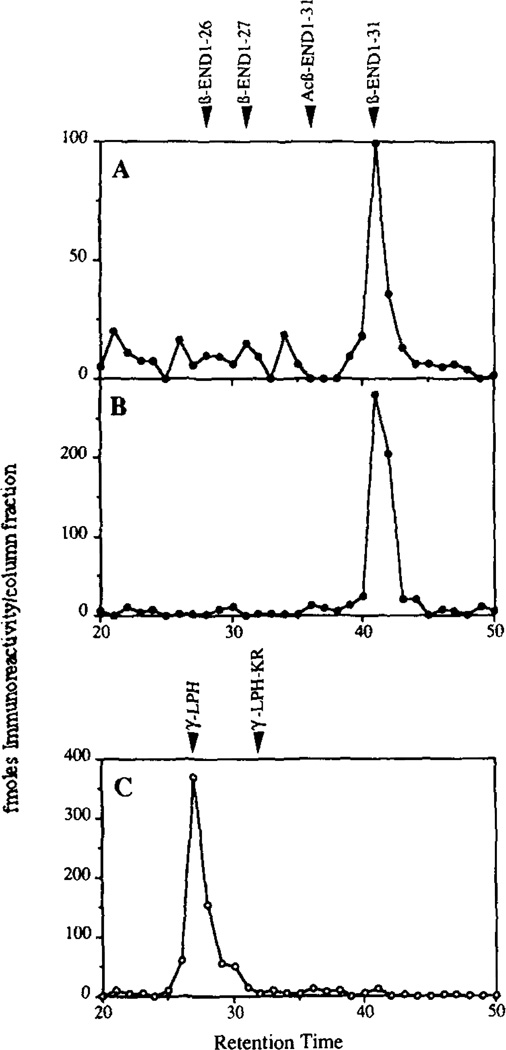
To confirm the identities of the β-endorphin immunoreactive peptides eluting at 39 and 43 min, material from each of these fractions was further resolved by cation exchange HPLC. Analysis of column fractions by RIA showed that both peptides only co-eluted with des-acetyl-β-endorphin-(1–31) (Fig. 6, panels A and B). The γ-LPH immunoreactive peptide eluting at 37 min similarly co-eluted with authentic carboxyl-shortened γ-LPH (Fig. 6, panel C). Thus, like the endogenous RIN m5F cell endoproteases (10) and transfected PC2 (11, 24), insulin secretory granule endopeptidase activity efficiently cleaves the Lys163-Arg164 site at the ACTH/β-LPH and Lys203-Arg204 γ-LPH/β-endorphin junctions but not the Lys232-Lys233 at the COOH terminus of β-endorphin (refer to Fig. 1).
Figure 6. Analysis of β-endorphin and γ-LPH products generated by an insulin secretory granule endopeptidase activity.
An aliquot of the column fractions eluting from the C4 reversed phase column (see Fig. 5) containing β-endorphin immunoreactivity (39 and 43 min) and γ-LPH immunoreactivity (37 min) were subjected to cation exchange HPLC analysis (“Materials and Methods”) and fractions were assayed by specific RIA to β-endorphin or γ-LPH. The elution position of various β-endorphin and γ-LPH standards are indicated. Recovery of imputimmunoreactivity in the peak fractions from cation exchange HPLC column was 48, 52, and 58% for panels A, B, and C, respectively. Panel A is analysis for the 39-min β-endorphin fraction, panel B for the 43-min β-endorphin fraction, and panel C for the γ-LPH fraction.
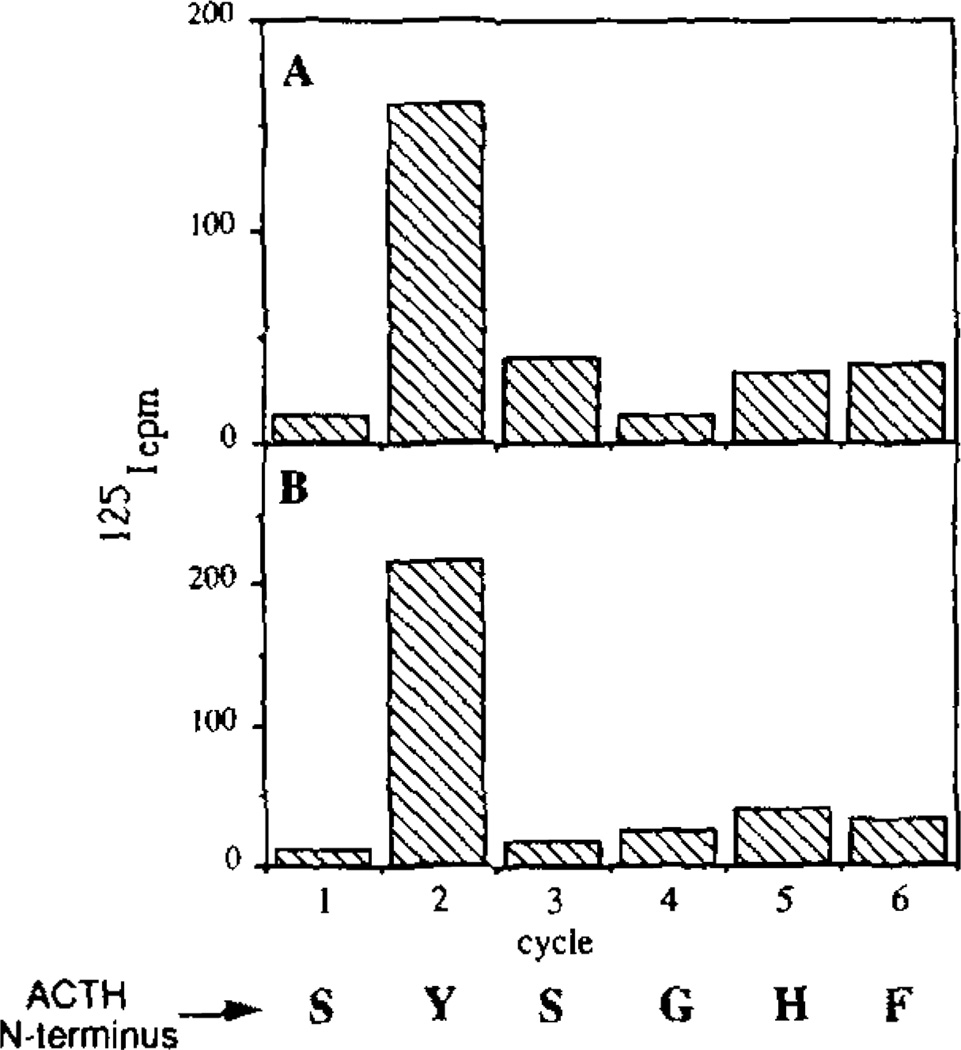
TO confirm the identities of the ACTH immunoreactive peptides, the material from reversed phase HPLC eluting at 46 min and pooled fractions between 17 and 24 min (pooled) were resolved by Tricine/SDS-PAGE and eluates of 2.5-mm slices were analyzed for ACTH and γ-LPH immunoreactivity. The peak at 46 min migrated as a single 20-kDa band with overlapping ACTH and γ-LPH immunoreactivity. The pooled 17–24-min fractions were resolved into two major forms; one with an apparent molecular mass of 16 kDa, and the second which co-migrated with 4.5-kDa synthetic ACTH1–39. A second aliquot from each of the 17–24- and 46-min reversed phase column fractions was then radioiodinated and the material immunoprecipitated with either an ACTH (fractions 17–24) or a γ-LPH (fraction 46) antisera. The immunoprecipitated material was then resolved by Tricine/SDS-PAGE, transferred to Immobilon membrane, and subjected to manual NH2-terminal sequence analysis (see “Materials and Methods”). For both the 16-kDa ACTH immunoreactive and the 20-kDa ACTH/γ-LPH immunoreactive peptides, 125I was released in the second cycle of Edman degradation (a calculated recovery of about 60%) indicating the position of tyrosine at residue 2 (Fig. 7). No release of 125I was detected from the 4.5-kDa ACTH peptide, suggesting either a different or blocked NH2 terminus. Thus, like PC2 and PC3 expressed in transfected BSC-40 cells (11, 24), insulin secretory granule endoproteolytic activity can correctly cleave at the Lys122-Arg123 site between the J-peptide/ACTH junction (Fig. 1). Furthermore, like PC2 expressed in transfected cells (11, 24), the insulin secretory granule lysate excised a 20-kDa ACTH/γ-LPH processing intermediate from mPOMC. The earlier elution of ACTH derived from BSC-40 cells on reversed phase HPLC columns compared to ACTH produced in either AtT20 cells or VV:mPOMC transfected BAM-40 cells (9) suggests that this domain is differentially modified in the BSC-40 cells (e.g. glycosylation or phosphorylation).
Figure 7. NH2-terminal sequence analysis of ACTH immunoreactive products generated by an insulin secretory granule endopeptidase activity.
mPOMC processing by a Triton X-114 aqueous phase-extracted insulin secretory granule lysate over 3.5 h at 30 °C was assessed as described (see “Materials and Methods”). The mPOMC products were resolved by Vydac reversed phase C4 HPLC and the fractions containing ACTH immunoreactivity were iodinated, followed by SDS-PAGE, transfer to Immobilon membranes, and then subjected to manual Edman degradation (“Materials and Methods”). A, sequence analysis of 16-kDa ACTH immunoreactive peptide and B, that for the 20-kDa ACTH immunoreactive peptide. The ACTH amino acid sequence is indicated.
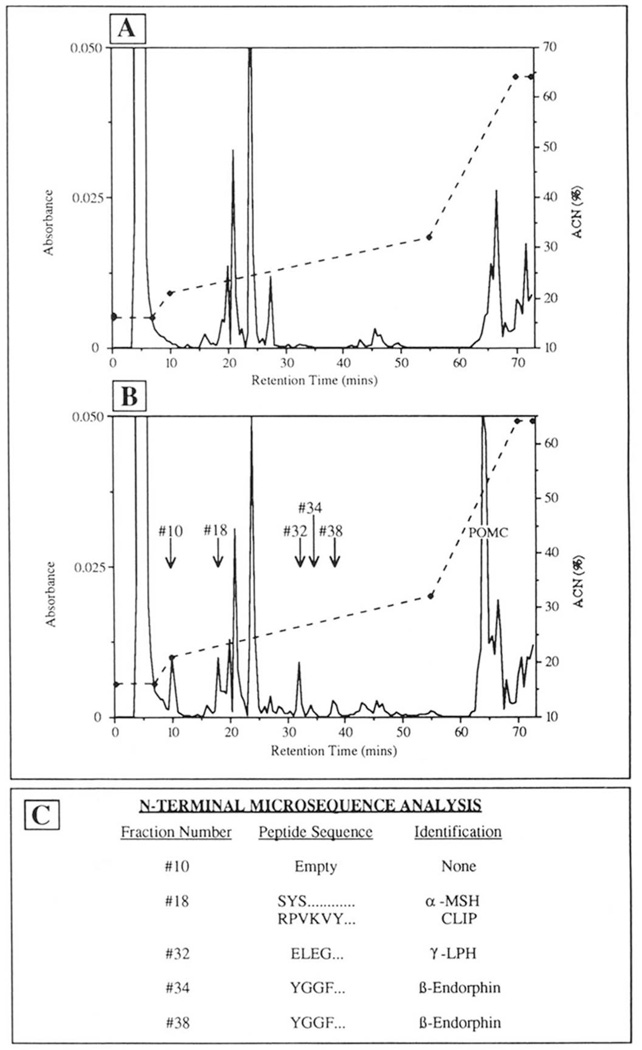
To further confirm the sites of mPOMC endoproteolysis by an insulin secretory granule lysate, NH2-terminal microsequence analysis was performed on mPOMC-derived peptide products that had been separated by reversed phase Vydac C4 HPLC with a modified ACN gradient (see “Materials and Methods”). 85 pmol of mPOMC substrate were incubated with an insulin secretory granule lysate for 3.5 h at 30 °C resulting in about 60% mPOMC processing (i e. around 50 pmol of mPOMC processing products generated). Fig. 8 (panel A) shows the HPLC profile of absorbance at 214 nm of a Triton X-114 aqueous phase extract of insulin secretory granules alone, and panel B a similar profile of the same extract incubated for 3.5 h with 10 µg of unlabeled mPOMC. On this ACN gradient system, peaks specific to POMC digest were found in fractions 10, 18, 32, 34, and 38 (Fig. 8, panel B). The fractions containing these peaks were subjected to NH2-terminal microsequence analysis (Fig. 8, panel C), at a detection level of 10–20 pmol per cycle. Fraction 18 contained two peptides that had NH2-terminal sequences corresponding to α-MSH and CLIP, respectively, indicating cleavage COOH terminally of Lys122-Arg123 at the J-peptide/ACTH junction and Lys139-Arg140 within the tetrabasic in the ACTH sequence (Fig. 1). Fraction 32 contained a peptide that had the NH2-terminal sequence of γ-LPH (or β-LPH), indicating a cleavage COOH terminally of mPOMC Lys163-Arg164 (Fig. 1).Fractions 34 and 38 contained peptides of NH2-terminal amino acid sequence of mPOMC β-endorphin, indicating cleavage COOH terminally of Lys203-Arg204 (Fig. 1).
Figure 8. Microsequencing of nonradiolabeled mPOMC processing products generated by an insulin secretory granule endopeptidase.
mPOMC processing by a Triton X-114 aqueous phase-extracted insulin secretory granule lysate extract was assessed as described (see “Materials and Methods”) with analysis by Vydac reversed phase C4 HPLC. The HPLC separated products from mPOMC processing were then subjected to microsequencing analysis (see “Materials and Methods”). Panel A, A214 nm profile for insulin secretory granules only; panel B, A214 nm profile for insulin secretory granules + mPOMC (10 µg) incubated for 3.5 h a t 30 °C, where additional peaks of mPOMC processing products are indicated that were subsequently subjected to NH2-terminal microsequence analysis; panel C, NH2-terminal microsequence analysis of mPOMC processing products indicated from panel B.
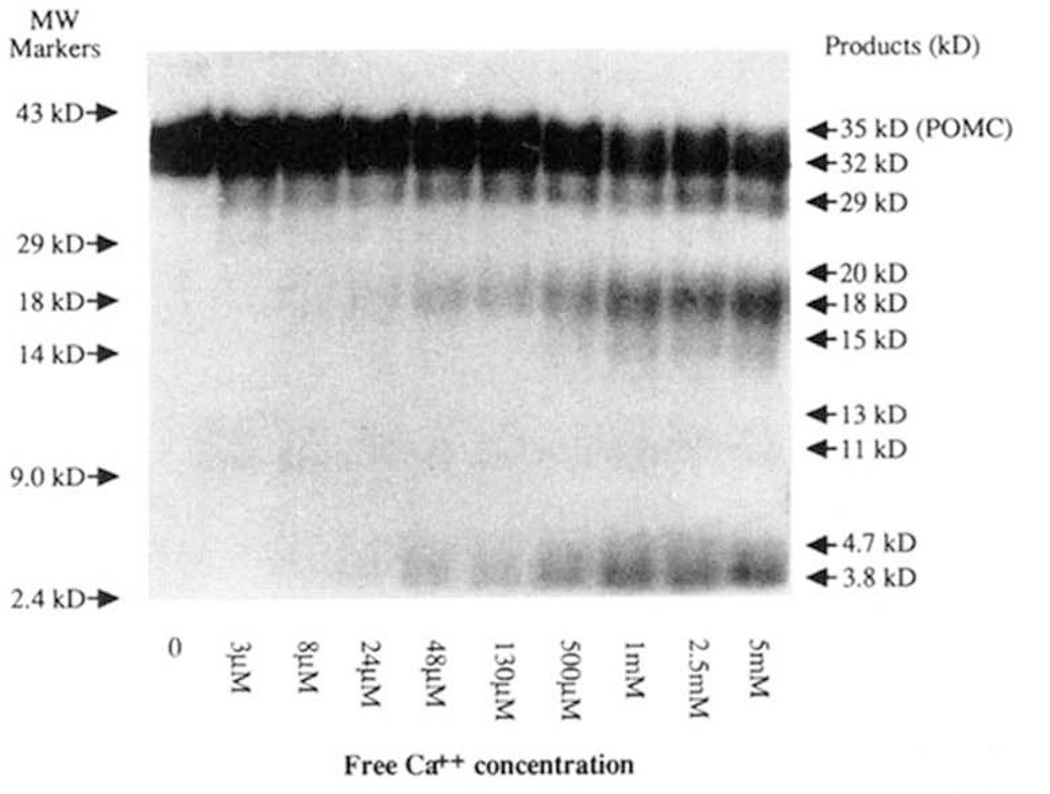
The processing of [35S]methionine POMC by the insulin secretory granule lysate was Cap2+-dependent (Fig. 9). In the absence of Ca2+, no conversion of substrate occurred. Maximum processing (at pH 5.5,30 °C) of mPOMC was seen above 1 mm free Ca2+, however, detectable processing was observed at 3 µm free Ca2+. The generation of individual mPOMC products (from Tricine/SDS-PAGE analysis) by the insulin secretory granule endopeptidase activity had different apparent Ca2+ dependence; the apparent K0.5 maximum Ca2+ concentrations for the major products observed by Tricine/SDS-PAGE analysis were calculated as 8.0 µm Ca2+ for disappearance of the 35-kDa mPOMC substrate; 5.5 µm Ca2+ for generation of the 32-kDa intermediate; 63 µm Ca2+ for 20 kDa; 80 µm Ca2+ for 18 kDa; 40 µm Ca2+ for 15 kDa; 18 µm Ca2+ for 4.7 kDa; 22 µm Ca2+ for 3.8 kDa. This micromolar Ca2+ requirement of mPOMC processing is reminiscent of the type-II endopeptidase activity for proinsulin processing (18), rather than the type-I (which has a millimolar Ca2+ requirement). The general effect of limiting the Ca2+ available for insulin secretory granule endopeptidase activity was to limit the rate at which the POMC precursor was cleaved (compare Figs. 2 and 9).
Figure 9. Ca2+ dependence of [35S]methionine-labeled mPOMC processing by an insulin secretory granule endopeptidase.
mPOMC processing by an insulin secretory granule lysate was assessed as described over 3 h at 30 °C at various Ca2+ concentrations (see “Materials and Methods”; the free [Ca2+] was calculated as previously described (36)) with analysis by Tricine/SDS-PAGE. A typical fluorograph is shown of the mPOMC processing products generated at various free [Ca2+].
The pH requirements for mPOMC processing by the insulin secretory granule lysate was also determined. To simplify the analysis [35S]cysteine-labeled mPOMC was used as a substrate. Cysteine is only found in the NH2-terminal of region of mPOMC (see Fig. l), and product analysis was reduced to monitoring the specific generation of [35S]cysteine-labeled 18-kDa NH2-terminal fragment (a final product of mPOMC processing (Fig. 2)) from the 35-kDa mPOMC precursor by Tricine/SDS-PAGE. The pH optimum for mPOMC processing by the insulin secretory granule endopeptidase was between 5 and 5.5, although significant processing of [35S] cysteine mPOMC was observed between 4.5 and 7.0.
Finally, an inhibitor profile of mPOMC processing by the granule lysate was determined and compared to the inhibitor profiles previously reported for the type-I and type-II endoproteases (31) and PC2 (38). Group specific inhibitors of serine, cysteinyl, or aspartyl proteinases (39) had no effect on mPOMC processing by the insulin secretory granule endopeptidase (Table I). The cysteinyl protease inhibitor p-chloromercuribenzoate inhibited this activity but thiol-alkylating reagents (iodoacetic acid, iodoacetamide, and N-ethylmaleimide) had no inhibitory effect. The metal ion chelators EDTA and CDTA, but not 1,10-phenanthroline, did inhibit mPOMC processing activity, however, inhibition by EDTA and CDTA was reversed by the addition of excess Ca2+ but not by other divalent cations (e.g. Ba2+ and Mg2+; Table I). The protease active site-directed inhibitors TLCK and TPCK had no of mPOMC processing activity. The equivalent dibasic tripeptide Ala-Lys-Arg-CH2Cl caused over 90% inhibition, whereas the monobasic tripeptide Ala-Nle-Arg-CH2Cl inhibited to a much lesser extent (Table I), consistent with the apparent dibasic substrate-specificity for mPOMC processing by insulin secretory granule endopeptidase activity (31). In general, the effects of proteinase inhibitors on mPOMC processing by a n insulin secretory granule lysate are indistinguishable from those previously observed for type-I and -II processing of proinsulin (31), and for PC2 activity (38).
TABLE I. Effect of various proteinase inhibitors on mPOMC processing by an insulin secretory granule endopeptidase.
[35S]Methionine-labeled mPOMC processing was assessed over a 2-h period at 30 °C, with analysis by electrophoresis as previously described (see “Materials and Methods”). In control samples (with no added extra proteinase inhibitor in the assay medium), 76.4 ± 8.3% (n = 4) of mPOMC had been processed. The effects of proteinase inhibitors are expressed as a percentage of the appropriate parallel control value of the assay. Each value is a mean of at least 2 independent observations.
| Inhibitor | Control activity |
|---|---|
| mM | % |
| Phenylmethanesulfonyl fluoride (10) | 102.3 |
| Phenylmethanesulfonyl fluoride (1) | 97.4 |
| Diisopropyl phosphofluoridate (1) | 82.3 |
| Iodoacetic acid (1) | 111.4 |
| Iodoacetamide (1) | 96.7 |
| N-Ethvlmaleimide (1) | 106.1 |
| Pepstatin-A (1) | 107.7 |
| E-64 (1) | 102.8 |
| TLCK (1) | 96.9 |
| TPCK (1) | 103.5 |
| EDTA (10) | 6.2 |
| EDTA (10) + CaCl2 (15) | 88.3 |
| CDTA (10) | 3.1 |
| CDTA (10) + CaCl2 (15) | 79.4 |
| 1,10-Phenanthroline (10) | 89.3 |
| Orthovanadate (0.1) | 107.4 |
| Ala-Nle-Arg-CH2Cl (0.1) | 78.0 |
| Ala-Lys-Arg-CH2Cl (0.1) | 7.3 |
| Guanidinoethylmercaptosuccinic acid (0.1) | 101.6 |
| Leupeptin (1) | 86.7 |
| Antipain (1) | 12.4 |
| p-Chloromercuribenzoate (0.1) | 0 |
| HgCl2 (0.1) | 0 |
DISCUSSION
The majority of polypeptide hormones are synthesized as proprotein precursors and the bulk of this post-translational proteolysis occurs in the secretory granule compartment (40, 41), although the process may, in part at least, be initiated the trans-Golgi network (18, 42). Gene transfer studies have demonstrated that the ability of neuroendocrine cells to process prohormones is not restricted to the endeogenous precursor, suggesting a general mechanism shared by a wide variety of cells to synthesize peptide hormones (see Ref. 43 for review). For example, proinsulin cDNA transfected into AtT20 cells (a mouse pituitary cell line), which normally produce ACTH from the POMC precursor, results in correct processing of proinsulin to insulin (44). Similarly, expression of POMC in the insulin secreting cell line Rin m5F, results in the correct processing of the prohormone to γ-LPH and β-endorphin-(1–31) (25). However, the precise pattern of peptides produced can be cell type-specific, suggesting that select components of the processing machinery may vary between cell types. For example, expression of mPOMC in BAM cells resulted in only limited processing of POMC, mostly to ACTH and β-LPH with only partial conversion of β-LPH to γ-LPH and β-endorphin-(1–31) (9).
We report here that insulinoma candidate processing endoproteases, previously characterized for proinsulin processing (18), were capable of processing mPOMC in vitro to peptides previously reported in gene transfer studies (25). This activity was mostly attributed a Lys-Arg-directed endopeptidase activity that co-eluted with proinsulin processing type-II activity from anion exchange chromatography (18). This activity had a similar micromolar Ca2+ requirement, acidic pH optimum, and group-specific proteinase inhibitor profile to the type-II processing of proinsulin (18, 31). The most susceptible processing site for cleavage of mPOMC by insulin secretory granule endopeptidase activity was at Lys232-Arg233 which generated β-endorphin-(1–31) and a 32-kDa peptide (these were the earliest products yielded from mPOMC by the type-II-like Activity in vitro). The 32-kDa fragment was a conversion intermediate which was processed further at Lys122-Arg123 (the J-peptide/ACTH junction) to yield the NH2-terminal fragment 18-kDa peptide) and an ACTH/γ-LPH processing intermediate. More extensive processing of mPOMC and/or conversion intermediates by insulin secretory granule endopeptidase activity resulted in the production of ACTH and γ-LPH (via cleavage at Lys163-Arg164 and further cleavage of the ACTH domain (after mPOMC Lys139-Arg140) to yield α-MSH (the extent of acetylation and amidation was not determined) and CLIP. The cleavage COOH terminally of Lys139-Arg140 is characteristic of the cleavage pattern in the pituitary intermediate lobe and mPOMC-transfected insulinoma cells (25). Also in good agreement with the processing of POMC in insulinoma cells (25) is the lack of processing at Lys232-Lys233 at the COOH-terminal region of the β-endorphin domain. There was no appreciable processing of mPOMC by insulin secretory granule endopeptidase activities at the Arg75-Lys76 or Arg101-Arg102 POMC processing sites to generate γ-MSH (see Fig. 1). Processing at Arg101-Arg102 might have been anticipated by the Arg-Arg-directed type-I activity (18), but it was not observed. In general, the cleavage specificity of insulin secretory granule POMC processing is consistent with the preferred Lys-Arg-directed substrate specificity of the type-II endopeptidase activity previously reported for proinsulin processing (18, 31, 32). There was no detectable processing at POMC Lys232-Lys233 or Arg75-Lys76 or Arg101-Arg102. POMC processing was only observed at Lys-Arg processing sites on this precursor molecule, and this specificity was particularly emphasized in cleavage COOH terminally of the Lys-Arg sequence within the Lys138-Lys139-Arg140↓-Arg141 ACTH tetrabasic site (as indicated by the arrow), rather than COOH terminally of LYS138-Lys139 or Arg140-Arg141.
It has recently been shown that the proinsulin processing type-II endopeptidase activity and the PC2 Kex2-homologue are equivalent proteins (45). The order of preferred cleavage site recognition by the type-II-like activity for the POMC substrate is in good agreement with that previously reported for the processing of POMC by PC2 transfected cells (11,24). This could further indicate that type-II and PC2 are matching endopeptidase activities. The efficient production of β-endorphin1–31 and the ACTH/γ-LPH processing intermediate by type-II activity in vitro is identical to the cleavage specificity of transfected PC2 for POMC in vivo (11). In addition, the less efficient processing of the tetrabasic sequence (Lys138-Lys139-Arg140↓-Arg141 of the ACTH domain compared to Lys203-Arg204 (the γ-LPH/β-endorphin junction) also compares well with the processing site preference of PC2 in POMC-transfected cells (11, 24). One noted difference, however, is that PC3, and not PC2, was shown to cleave the ACTH/β-LPH junction (Lys163-Arg164), whereas in this study there was apparent cleavage at this site by the type-II activity in vitro. However, there is a minor component of a type-I-like activity (10–20%) capable of cleaving at proinsulin Arg31-Arg32 that is allied with the Lys-Arg-directed type-II activity (18, 31). It is unlikely that this associated Arg-Arg-directed activity can be attributable to the type-II itself since it has been partially separated from type-II activity, eluting slightly earlier on the NaCl gradient applied for anion exchange chromatography (46, 47). Furthermore, there is recent biochemical and immunological evidence that has suggested that type-I activity might be equivalent to PC3 (47, 48). In this study type-II activity was not entirely separated from its associated type-I-like activity. It therefore seems likely that the cleavage at the ACTH/β-LPH junction (Lys163-Arg164 observed might be due to a type-I/PC3 activity that associates with the type-II activity, rather than type-II/PC2 itself. However, type-I activity alone (eluting in the anion exchange column void (18)) did not process intact mPOMC. It should then be considered that the type-I/PC3 activity associated with type-II/PC2 could be acting secondary to a prior endopeptidic clip, preferentially cleaving a partially processed intermediate form of mPOMC rather than the intact precursor itself. Nevertheless, whether there are other minor contaminating endopeptidases present in the “type-II” preparation that are contributing to in vitro mPOMC processing cannot be ruled out until purified type-II/PC2 and type-I/PC3 enzyme activities are available.
The mechanism for differential processing of a proprotein such as mPOMC has not been fully resolved. A two-tiered mechanism has been suggested for tissue-specific processing of a proprotein (9), where regulation of factors which influence cleavage site accessibility, endopeptidase specificity, and/or rates of catalysis may act coordinately with the differential expression of a limited number of processing enzymes. Such factors may be intragranular Ca2+ or pH. Although the intragranular pH of insulin and chromaffin secretory granules are similar, the intragranular Ca2+ in insulin secretory granules is higher (by an order of magnitude) than that in chromaffin granules (49) (indeed the intragranlular Ca2+ concentration varies greatly in the secretory granules from different neuroendocrine cell types). In this in vitro study we have demonstrated that the insulin secretory granule endopeptidase activity for POMC processing has a requirement for the Ca2+ ion, similar to that for proinsulin processing (18). Higher concentrations of Ca2+ appeared to be required to generate some of the smaller molecular size POMC-derived products. This is most likely related to the fact that these products are generated by a series of Ca2+-dependent proteolytic steps which might be sequential in nature. Further analyses need to be performed with each of the POMC processing intermediates as substrates to resolve this issue. Nevertheless the results suggest that kinetic regulation of proprotein processing endopeptidases could be a determinant of the spectrum of peptide products generated and thus be a significant factor in the tissue-dependent differential processing of a prohormone precursor such as POMC.
Acknowledgments
We thank Drs. Peng Loh and Robert Lowry for providing antisera to specific regions of POMC.
Footnotes
This work was supported in part by National Institutes of Health Grants DK36836, DK37274, and BRGS07RR05673, the Juvenile Diabetes Foundation International, the American Diabetes Association (Oregon Chapter), the Wellcome Trust, the Medical Research Council of Great Britain, and the British Diabetic Association.
The abbreviations used are: POMC, proopiomelanocortin; ACTH, adrenocorticotropin; LPH, lipotropin; MSH, melanocyte-stimulating hormone; CLIP, corticotropin-like intermediate lobe peptide; TLCK, Nα-p-tosyl-l-lysine chloromethyl ketone; TPCK, tosylphenylalanyl chloromethyl ketone; E-64, trans-epoxysuccinyl-l-leucylamido-(4-guanidino)butane; Tricine, N-[2-hydroxy-l,l-bis(hydroxymethyl)-ethyl]glycine; PAGE, polyacrylamide gel electrophoresis; HPLC, high performance liquid chromatography; ACN, acetonitrile; RIA, radioimmunoassay; CDTA, trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid Ala-Nle-Arg-CH2Cl, l-alanyl-l-norleucyl-l-arginylchloromethane; Ala-Lys-Arg-CH2Cl, l-alanyl-l-lysyl-l-arginylchloromethane; BAM, bovine adrenal medullary; mPOMC, murine POMC; VV, vaccina virus.
References
- 1.Docherty KD, Steiner DF. Ann. Rev. Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- 2.Douglass J, Civelli O, Herbert E. Annu. Rev. Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- 3.Loh YP, Brownstein MJ, Gainer H. Annu. Rev. Neurosci. 1984;7:189–222. doi: 10.1146/annurev.ne.07.030184.001201. [DOI] [PubMed] [Google Scholar]
- 4.Edwards K, Fleischer B, Dryburgh H, Fleischer S, Schreiber G. Biochem. Biophys. Res. Commun. 1976;72:310–318. doi: 10.1016/0006-291x(76)90995-5. [DOI] [PubMed] [Google Scholar]
- 5.Ikehara Y, Oda K, Kato K. Biochem. Biophys. Res. Commun. 1976;72:319–326. doi: 10.1016/0006-291x(76)90996-7. [DOI] [PubMed] [Google Scholar]
- 6.Olson TS, Bamberger MJ, Lane MD. J. Biol. Chem. 1988;263:7342–7351. [PubMed] [Google Scholar]
- 7.Rhodes CJ, Halban PA. J. Cell Biol. 1987;105:145–153. doi: 10.1083/jcb.105.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith AI, Funder JW. Endocrine. Rev. 1988;9:159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 9.Thorne BA, Viveros OH, Thomas G. J. Biol. Chem. 1991;266:13607–13615. [PubMed] [Google Scholar]
- 10.Thorne BA, Caton LW, Thomas G. J. Biol. Chem. 1989;264:3545–3452. [PubMed] [Google Scholar]
- 11.Thomas L, Leduc R, Thorne BA, Smeekens SP, Steiner DF, Thomas G. Proc. Natl. Acad. Sci. U. S. A. 1991;88:5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutton JC. Curr. Opin. Cell. Biol. 1991;2:1131–1142. doi: 10.1016/0955-0674(90)90167-d. [DOI] [PubMed] [Google Scholar]
- 13.Lindberg I, Hutton JC. In: The Biosynthesis of Peptide Hormones and Neurotransmitters. Fricker LD, editor. Boca Raton, Florida: CRC Press; 1991. pp. 141–173. [Google Scholar]
- 14.Fuller RS, Sterne RE, Thorner J. Annu. Rev. Physiol. 1988;50:345–362. doi: 10.1146/annurev.ph.50.030188.002021. [DOI] [PubMed] [Google Scholar]
- 15.Thomas G, Thorne BA, Thomas L, Allen RG, Hruby DE, Fuller R, Thorner J. Science. 1988;241:226–230. doi: 10.1126/science.3291117. [DOI] [PubMed] [Google Scholar]
- 16.Bathurst IC, Brennan SO, Carrell RW, Cousens LS, Brake AJ, Barr PJ. Science. 1987;235:348–350. doi: 10.1126/science.3541206. [DOI] [PubMed] [Google Scholar]
- 17.Thim L, Hansen MT, Norris K, Hoegh I, Boel E, Forstrom J, Ammerer G, Fiil NP. Proc. Natl. Acad. Sci. U. S. A. 1986;83:6766–6770. doi: 10.1073/pnas.83.18.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson HW, Rhodes CJ, Hutton JC. Nature. 1988;333:93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- 19.Brennan SO, Peach RJ. FEBS. Lett. 1988;229:167–170. doi: 10.1016/0014-5793(88)80819-6. [DOI] [PubMed] [Google Scholar]
- 20.Smeekens SP, Steiner DF. J. Biol. Chem. 1990;265:2997–3000. [PubMed] [Google Scholar]
- 21.Smeekens SP, Avruch AS, LaMendola J, Chan SJ, Steiner DF. Proc. Natl. Acad. Sci. U. S. A. 1991;88:340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidah NG, Gaspar L, Mion P, Marcinkiewicz M, Mbikay M, Chrétien M. DNA. Cell Biol. 1990;9:415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- 23.Seidah NG, Marcinkiewicz M, Benjannet S, Gaspar L, Beaubien G, Mattei MG, Lazure C, Mbikay M, Chrétien M. Mol. Endocrinol. 1991;5:111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- 24.Benjannet S, Rondeau N, Day R, Chrétien M, Seidah NG. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorne BA, Thomas G. J. Biol. Chem. 1990;265:8436–8443. [PubMed] [Google Scholar]
- 26.Chick WL, Warren S, Chute RN, Like AA, Lauris V, Kitchen KC. Proc. Natl. Acad. Sci. U. S. A. 1977;74:628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutton JC, Penn EJ, Peshavaria M. Diabetologia. 1982;23:365–373. doi: 10.1007/BF00253746. [DOI] [PubMed] [Google Scholar]
- 28.Hutton JC. Diabetologia. 1989;32:271–281. doi: 10.1007/BF00265542. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes CJ, Brennan SO, Hutton JC. J. Biol. Chem. 1989;264:14240–14245. [PubMed] [Google Scholar]
- 30.Hutton JC, Bailyes EM, Rhodes CJ, Guest PC. In: Peptide Hormone Secretion. Hutton JC, Siddle K, editors. Oxford: IRL Press; 1990. pp. 309–336. [Google Scholar]
- 31.Rhodes CJ, Zumbrunn A, Bailyes EM, Shaw E, Hutton JC. Biochem. J. 1989;258:305–308. doi: 10.1042/bj2580305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Docherty KD, Rhodes CJ, Taylor NA, Shennan KIJ, Hutton JC. J. Biol. Chem. 1989;264:18335–18339. [PubMed] [Google Scholar]
- 33.Schagger H, von Jagow G. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 34.Edwards R. In: Peptide Hormone Secretion: A Practical Approach. Huton JC, Siddle K, editors. Oxford: IRL Press; 1990. pp. 71–95. [Google Scholar]
- 35.Bradford MM. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 36.Storer AC, Cornish-Bowden A. Biochem. J. 1976;159:1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhler M, Herbert E. J. Biol. Chem. 1983;258:257–261. [PubMed] [Google Scholar]
- 38.Shennan KIJ, Smeekens SP, Steiner DF, Docherty K. FEBS Lett. 1991;284:277–280. doi: 10.1016/0014-5793(91)80703-6. [DOI] [PubMed] [Google Scholar]
- 39.Bond JS, Butler PE. Annu. Rev. Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- 40.Orci L. Diabetologia. 1985;28:528–546. doi: 10.1007/BF00281987. [DOI] [PubMed] [Google Scholar]
- 41.Tooze J, Hollinshead M, Frank R, Burke B. J. Cell. Biol. 1987;105:155–162. doi: 10.1083/jcb.105.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnabel E, Mains RM, Farquhar MG. Mol. Endocrinol. 1989;3:1223–1235. doi: 10.1210/mend-3-8-1223. [DOI] [PubMed] [Google Scholar]
- 43.Thomas G, Thorne BA, Hruby DE. Annu. Rev. Physiol. 1988;50:323–332. doi: 10.1146/annurev.ph.50.030188.001543. [DOI] [PubMed] [Google Scholar]
- 44.Moore H-PH, Walker MD, Lee F, Keljy RB. Cell. 1983;35:531–538. doi: 10.1016/0092-8674(83)90187-3. [DOI] [PubMed] [Google Scholar]
- 45.Bennett DL, Bailyes EM, Nielson E, Guest PC, Rutherford NG, Arden SD, Hutton JC. J. Biol. Chem. 1992;267:15229–15263. [PubMed] [Google Scholar]
- 46.Rhodes CJ, Lincoln B, Shoelson SE. J. Biol. Chem. 1992;267:22719–22727. [PubMed] [Google Scholar]
- 47.Bailyes EM, Bennett DL, Hutton JC. Enzyme. 1991;45:301–313. doi: 10.1159/000468903. [DOI] [PubMed] [Google Scholar]
- 48.Bailyes EM, Shennan KIJ, Seal AJ, Smeekens SP, Steiner D, Hutton JC, Docherty K. Biochem. J. 1991;285:391–394. doi: 10.1042/bj2850391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Njus D, Kelley PM, Harnadek GJ. Biochim. Biophys. Acta. 1986;853:237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]