Abstract
Chronic kidney disease and its worsening are recurring conditions in chronic heart failure (CHF) which are independently associated with poor patient outcome. The heart and kidney share many pathophysiological mechanisms which can determine dysfunction in each organ. Cardiorenal syndrome is the condition in which these two organs negatively affect each other, therefore an accurate evaluation of renal function in the clinical setting of CHF is essential. This review aims to revise the parameters currently used to evaluate renal dysfunction in CHF with particular reference to the usefulness and the limitations of biomarkers in evaluating glomerular dysfunction and tubular damage. Moreover, it is reported the possible utility of renal arterial resistance index (a parameter associated with abnormalities in renal vascular bed) for a better assesment of kidney disfunction.
Keywords: Heart failure, Biomarkers, Doppler, Renal resistance index, Chronic kidney disease
Core tip: In the clinical setting of chronic heart failure the evaluation of renal dysfunction is essential. This review revises the currently available markers of renal function in chronic heart failure for a better characterization of renal function. Moreover, it is discussed the potential utility of a Doppler derived parameter, the renal resistance index, which is associated with abnormalities in the kidney vascularization.
INTRODUCTION
Subjects with chronic heart failure (CHF) often present chronic kidney disease (CKD) as well as worsening of renal function (WRF), which are both responsible for a poor outcome[1-3]. Over the past few years interest in this link between the kidney and heart has increased as these organs share many pathophysiological mechanisms, and are therefore able to negatively affect each other. This reciprocal influence has recently been defined as cardiorenal syndrome[4].
In this clinical setting the pathophysiological background underlying renal impairment is, in part, different when acute and chronic heart failure are considered. In acute decompensated heart failure (ADHF) acute kidney injury (AKI) can often occur[5]. Both low cardiac output and venous congestion are the principal determinants of WRF. When cardiac output decreases, renal perfusion and, consequently, glomerular filtration rate are reduced. On the other hand, venous congestion is the cause of a rise in efferent arterioles and end glomerular capillary pressure thus inducing a decrease in net filtration pressure, an increase in interstitial pressure with damage to tubules[6].
In CHF patients, abnormalities in cardiac function lead to a gradual WRF rather than AKI and a steady decrease in renal perfusion due to low cardiac output which is associated with micro and macrovascular disease[5]. However, also the presence of increased central venous pressure in CHF can favour the occurrence of WRF[7,8]. Finally, neuro-hormonal activation further enhances pathophysiological mechanisms leading to WRF[1].
On the basis of these considerations it is clear that in ADHF and CHF patients the evaluation of kidney function is extremely relevant. The aim of this review is to revise the parameters currently used to evaluate renal function in the CHF clinical setting and discuss the usefulness and limitations of biomarkers in evaluating glomerular dysfunction and tubular damage. Moreover, it is reported the possible utility of renal arterial resistance index (a parameter associated with abnormalities in renal vascular bed) for a better assesment of kidney Dysfunction.
ESTIMATION OF GLOMERULAR FUNCTION
Glomerular filtration rate and serum creatinine
Currently glomerular filtration rate (GFR) is the most used index to assess kidney function. It measures kidney filtration capacity and all the guidelines on heart failure recommend its routine use in CHF patients[9,10]. GFR can be estimated using renal clearance of an exogenous substance (inulina or I-iothalamate), however, even though this approach is more accurate, it is limited as it is expensive and time consuming. Consequently, GFR estimation is generally performed using an endogenous marker, i.e., serum creatinine[11]. Creatinine is produced in the muscles from creatine phosphate and it is removed by kidneys through glomerular filtration, but also by proximal tubular secretion.
The traditional method of measuring the clearance of creatinine requires a 24 h urine sample, which can be difficult for patients to perform and is often not done correctly. Therefore GFR is generally estimated by using serum creatinine levels[9]. For this purpose, Cockroft-Gault (CG), simplified Modification of Diet in renal disease (MDRD) and Chronic Kidney Disease Epidemiology Collaboration (CKD EPI) are the most widespread equations. These equations include creatinine serum levels, age, gender, race and, in CG, weight. However, they present an imperfect performance: GFR is underestimated by MDRD and CKD EPI and overestimated by CG, particularly at lower creatine serum levels[11].
Smilde et al[12] were the first to validate these creatinine based equations in a large cohort of CHF patients. In particular, they showed that GFR assessed by MDRD formula is underestimated in patients with normal and near normal values and overestimated in patients with worsening renal function. MDRD has a good prognostic significance, but is lower than that of the real GFR.
More recently the CKD EPI formula has been introduced. It is currently the equation most used to evaluate GFR in CKD as it has been found to be more accurate than MDRD in patients with a preserved renal function[13,14]. Moreover, the metanalysis by Matsushita et al[15] showed that CKD EPI formula allowed a better risk stratification for mortality and end stage renal disease in a general population and in patients with cardiovascular diseases.
Valente et al[16] first evaluated the role of this equation in the setting of CHF, showing that CKD EPI classifies KDOQI stages more accurately than the MDRD equation, especially in patients with a higher GFR. Furthermore, in the metanalysis of McAlister et al[17] the EPI formula was more accurate in estimating the risk of patients with CHF. According to these studies the CKD EPI formula is the preferred method to estimate GFR in CHF patients, particularly in those with preserved or moderately impaired renal function.
Creatinine serum levels and GFR are also used to estimate WRF. In CHF, WRF has generally been evaluated by assessing changes in creatinine[18]. In particular, an increase of > 0.3 mg/dL and/or > 25% between two time points[3] have been shown to be related to a worse outcome, hospitalizations for heart failure and higher mortality[19-23]. However, a rise in serum creatinine could be not associated with a significant reduction in GFR. A patient presenting higher baseline values of serum creatinine will show a less marked change in GFR than a patient with lower baseline values. Also the use of changes in GFR in order to assess WRF present some limitations due to the slight fluctuations in GFR which are common. For this reason current guidelines suggest that changes of 25% or more in estimated GFR should be considered as being associated with a change in GFR category[24].
Although creatinine serum levels and estimated GFR represent the corner stone in the evaluation of renal function and its worsening, several factors influencing the creatinine value and limiting its derived measures should also be considered. Creatinine values show a significant between-person and within-person variability, they are influenced by age, diet, gender and body mass. Moreover, when kidney filtration capacity decreases, GFR can be overestimated because of creatinine tubular active secretion[25]. Finally, in CHF patients there are several factors that can affect the validity of equations used to estimate GFR, such as the hemodynamic component of renal dysfunction, drugs interfering with renal function and the loss of muscle mass that is frequent in the end stages of the disease[12].
It is also worth noting that the kidneys use only part of their filtering capacity. A normal GFR could subtend an impairment in this increasing filtration capacity, in other words it could be observed in kidneys with a reduced renal reserve[26].
In order to avoid the limitations of creatinine, new biomarkers estimating glomerular function, such as Cystatin C, have been introduced.
Cystatin C
This is a protein of cysteine proteinase inhibitor family (13 kdal) which is secreted by all cells with nucleus. It passes freely through the glomerular basement membrane and then it is reabsorbed, but not secreted in the tubules. As a consequence, it is an ideal marker of kidney filtration capacity. Moreover age, gender, food and body mass affect less its serum levels[27]. The only clinical conditions that can influence it are inflammatory status, the use of corticosteroids and thyroid diseases[28,29]. Several studies have shown that, in patients with moderate renal impairment, Cystatin C serum levels estimate GFR better than the equations based on creatinine serum levels[30,31].
In fact the KDIGO guidelines[32] for CKD management recommend assessing Cystatin C in patients with eGFRcr of 45-60 mL/min per 1.73 m2 , on condition that they don’t present other manifestations of CKD, such as albumin-creatinine ratio > 30 mg/g.
Formulas based on Cystatin C values have also been presented in order to obtain an estimate of GFR thus facilitating the clinical use of this marker. Moreover, equations based on combined creatinine-cystatin serum levels give a more accurate estimate of GFR than those based on each single level[33,34].
Cystatin C has also been demonstrated more accurate than creatinine in stratifying the risk of mortality and cardiovascular events in old subjects[35] and people with coronary artery disease[36]. High levels are also predictors of heart failure onset[37]. It is likely that the high Cystatin C levels reflect a mild or moderate decrease in renal function that is not detected by creatinine[33].
There are limited data on the role of Cystatin C in CHF[38-40]. Arimoto et al[38] showed the usefulness of Cystatin C in prognostic stratification of mild or moderate CHF. Moreover, Cystatin C has been found to better estimate the increased risk of cardiovascular death in elderly affected by CHF[39] as well as in patients with preserved renal function[40].
Although Cystatin C could be an interesting marker for an early assessment of renal function and for risk stratification in CHF, at present it is not widely used to estimate GFR. This is due to the widespread and consolidated use of creatinine, as well as to the fact that the Cystatin C measurement is more expensive, even if other markers, such as NT proBNP or troponin, have a similar cost[32].
Microalbuminuria
Albumin is the protein with the highest concentration in serum but has a low concentration in urine because of its large size, the selectivity of the glomerular filtration barrier and tubular reabsorption[11]. An increased albumin urinary excretion is a recognized early marker of kidney damage and its quantification is used for monitoring patients with CKD as well as for estimating the risk of kidney disease progression[24], as shown in Table 1. The urinary excretion of albumin is currently evaluated by calculating the albumin-to-creatinine ratio (UACR) in urine samples. An UACR < 30 mg/g is defined as normoalbuminuria, a UACR between 30 and 299 mg/g as microalbuminuria, and a UACR > 300 mg/g as macroalbuminuria[41].
Table 1.
Risk of chronic renal disease progression according to K/DOQI clinical practice guidelines[25]
| Glomerular filtration rate categories |
Microalbuminuria |
||||
| A1 normal to mildly increased < 30 mg/g | A2 moderately increased 30-299 mg/g | A3 severely increased > 300 mg/g | |||
| 1 | Normal or high | > 90 mL/min | Low risk | Moderately increased risk | High risk |
| 2 | Mildly decreased | 60-89 mL/min | |||
| 3a | Mildly to moderately decreased | 45-59 mL/min | Moderately increased risk | High risk | Very high risk |
| 3b | Moderately to severely decreased | 30-44 mL/min | High risk | Very high risk | Very high risk |
| 4 | Severely decreased | 15-29 mL/min | Very high risk | Very high risk | Very high risk |
| 5 | Kidney failure | < 15 or dialysis mL/min | Very high risk | Very high risk | Very high risk |
Microalbuminuria has been shown to be associated not only with CKD progression, but also with an increased risk of cardiovascular death in the general population as well as in subjects with diabetes and hypertension[42].
The greater prevalence of microalbuminuria in CHF subjects than in the general population was demonstrated for the first time by Van der Wall[43]. In CHF albuminuria and reduced GFR can coexist, but sometimes only one of the conditions was found because they are caused by different pathophysiological mechanisms. Albuminuria can be the consequence of damage to the glomerular basement membrane secondary to endothelial dysfunction and inflammatory cytokine activation[44]. Moreover, it can be caused by hyperfiltration due to a reduction in the number of nephrons. This condition could occur in the event of a post glomerular ischemia which leads to hypoxia, oxidative stress and tubulointerstitial injury[45]. Other mechanisms involved in the genesis of microalbuminuria could be renal congestion[46,47] and reduced tubular reabsorption of albumin as a consequence of tubular dysfunction[48].
The large substudies from CHARM and GISSI HF trials[49,50] have confirmed the high prevalence of micro and macroalbuminuria in CHF patients (microalbuminuria was more common than macroalbuminuria) and its association with a worse outcome. HF patients with higher albumin urinary levels showed a greater mortality and an increased risk of HF hospitalizations. This prognostic value was independent from serum creatinine levels, eGFR and the comorbidities that worsen renal filtration function. Another important finding of these studies was that mortality risk increased with the increase in albumin excretion, even in the normal range (< 30 mg/g) suggesting that this parameter is a continuous measure of risk.
All of these studies showed that albumin urinary excretion is not affected by treatment with angiotensin receptor blockade, contrary to that observed in patients with diabetes, hypertension and renal disease. This may be due to the different pathophysiological mechanisms underlying albuminuria in CHF[51].
MARKERS OF TUBULAR DAMAGE
Different markers reflecting tubular damage have been studied in order to obtain a more accurate evaluation of renal function.
The Neutrophil gelatinase-associated lipocalin (NGAL) is a lipocalin protein (25 kdal), which is produced by the kidney, but also by other organs (the trachea, lung, stomach and colon). After being filtered through the glomerulus, NGAL is reabsorbed in the proximal tubule. When the proximal tubule is damaged, filtered NGAL can not be totally reabsorbed and its urinary levels increase[52]. Furthermore, during tubular damage NGAL mRNA is transcribed and overexpressed in loop of Henle and collecting duct and its plasma and urinary levels rise considerably[53,54]. NGAL plasma levels can also be increased by systemic diseases such as inflammation and cancer, but urinary levels are less influenced by these conditions[54].
The N-acetyl beta glucosaminidase (NAG) is a protein produced in the proximal tubular cells which is excreted into the urine when a tubular injury occurs[55]. Its urinary levels are increased in acute and chronic kidney disease[56] but also in diabetes and hypertension.
The Kidney injury molecule (KIM1) is a transmembrane glycoprotein and its expression in proximal tubule cells increases significantly after hypoxic or nephrotoxic tubular injury[57]. Its urinary levels are high also in patients with polycystic kidney[58] and renal cancer[59].
NAG, NGAL and KIM 1 have been widely studied in the setting of AKI (Acute Kidney Injury),where they have had a diagnostic and prognostic role, and show an increase 24 h prior to creatinine[60-63]. Recently, the utility of these markers in cardiorenal syndrome[25] has been evaluated by a number of studies, mainly involving patients with ADHF[64-67].
On the other hand, there are few studies which examine these markers in patients with CHF[68]. Damman et al[69] have shown a significant increase in urinary NGAL levels in these patients when compared to healthy controls, suggesting that renal dysfunction in CHF is characterised not only by a decrease in GRF and an increase in urinary albumin excretion but also by tubular damage. The renal perfusion decrease that occurs in CHF produces a GRF reduction and determines hypoxia leading to tubular damage.
Another study by Damman et al[70] showed that NGAL urinary levels were not correlated with prognosis, while urinary NAG and KIM1 were associated to a worse outcome (death, heart failure, hospitalisations and heart transplantation) and have an additional prognostic value compared to GRF. According to the authors, one explanation for this difference could be that NAG and KIM1 are produced in two different parts of the nephron and damage to the proximal part of the tubule may be more significant for the prognosis.
Finally, in a substudy of the GISSI HF trial[71] it has been confirmed that markers of tubular damage were strongly associated with HF hospitalizations and all cause mortality. In particular, NAG was the only marker remaining significantly correlated with a worse prognosis at multivariate analysis. This association was independent from estimated GFR and urinary protein excretion.
Markers of tubular function could be also related to WRF. In another substudy of the GISSI HF trial[47] patients with WRF had higher NGAL, NAG and KIM1 levels. These outpatients with WFR had a worse outcome. A possible explanation could be that the increase in these markers is caused by renal hypoxia, leading to a progressive deterioration of renal function that makes the kidney more vulnerable. At multivariate analysis KIM1 was the strongest independent predictor of WRF among all associated variables (eGFR and albuminuria included).
Beside NAG, NGAL and KIM 1, also other markers of tubular damage have been proposed.
IL 18 is a cytokine secreted by proximal tubular cells. When AKI occurs, it has been shown to increase more quickly than serum creatinine levels. It also increases in inflammatory status, therefore it is not highly specific[72,73]. Mallat et al[74] showed that, in ADHF, IL 18 plasma levels increased and were higher in patients who died during follow-up.
The fatty acid-binding proteins (FABPs) are proteins able to bind free fatty acids. Among the several tissue-specific isoforms, urinary levels of liver FABP (FABP-1) and heart FABP (FABP-3) can reflect AKI, because excreted when an ischemic tubular injury occurs[25]. Moreover, serum FABP levels have been associated to worse outcome in CHF patients[75].
RENAL CIRCULATION AND RENAL RESISTANCE INDEX
Markers of GFR and tubular damage offer information about the status of nephrons. However, kidney circulation also plays a key role in renal function. Consequently, the evaluation of renal circulation could provide useful parameters to add to the information obtained from the above mentioned biomarkers.
Renal circulation
The kidney is a highly perfused organ, receiving 22% of cardiac output at rest[76]. After entering the hilum, the renal arteries divide into the interlobar, arcuate and interlobular arteries. Afferent arterioles originate from interlobular arteries, leading to the glomerular capillaries, and then to efferent arterioles. Efferent arterioles are followed by a second capillary network, i.e., peritubular capillaries, and finally by the venous system. The regulation of renal blood flow (RBF) is not dependent on oxygen demand, but on reflex and neurohormonal mechanisms underlying the control of afferent and efferent arteriole tone. These mechanisms are not discussed in this review, however it is worth noting that arteriole tone changes are the main determinants of arterial renal resistance. The arteriolar tone is determined by reflex mechanisms, such as myogenic effect and tubular-glomerular feedback, and neurohormonal mechanisms. Of the neurohormonal systems, sympathetic and renin-angiotensin-aldosterone are those which most influence efferent arteriolar tone, determining vasoconstriction and increased water and sodium reabsorption. Endothelin, nitric oxide, prostaglandins, bradykinin and natriuretic peptides are also involved in the regulation of arteriolar tone.
The self-regulation of RBF allows renal perfusion to be kept constant through a wide range of arterial pressure (70-180 mmHg). On the other hand, changes in venous renal pressure could affect RBF more than those of arterial pressure. In 1931 Winton[77] evaluated the reduction of RBF by changing arterial and venous pressures in isolated kidneys. The results demonstrated that the changes were greater when renal venous pressure had been increased. More recent studies have confirmed the association between central venous pressure and renal function worsening in acute, as well as in chronic heart failure[77-79]. The mechanisms by which central venous pressure could affect RBF are different. In particular, it can increase both intra-abdominal pressure and renal venous pressure. This leads not only to an increase in capillary pressure and a reduction in artero-venous gradient but also to greater interstitial pressures and, consequently, greater arterial renal resistances.
Functional abnormalities of RBF and of renal resistances due to neurohormonal and hemodynamic changes could also lead to structural changes. Chade[76] demonstrated that the functional increase in renal vascular resistances could lead to ischemia, endothelial dysfunction, cytokine production and finally to fibrosis. This cascade of events causes a renal vascular rarefaction which could further induce CKD worsening.
On the basis of this pathophysiological background we can conclude that an increase in arterial renal resistances could represent an altered neuro-hormonal status, an increased central venous pressure, but it could also reflect the presence of parenchymal abnormalities and vascular rarefaction which favour CKD progression.
Arterial renal resistance index
Arterial renal resistance index (RRI) is a non-invasive measurement of renal arterial resistance which is easily evaluated by Doppler technique (Figure 1). It is calculated using Pourcelot’s formula[80] since the peak systolic velocity and the end-diastolic velocity are obtained. Doppler evaluation of renal arteries is generally performed at the level of one or more interlobar arteries. In healthy adult subjects the RRI mean value is around 0.60 with no significant differences between the two kidneys[81]. An RRI greater than 0.70 is generally considered abnormal[82].
Figure 1.
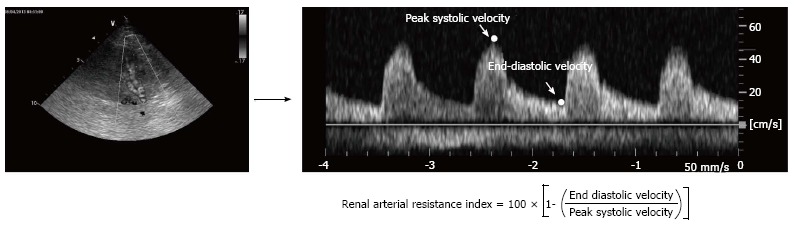
Calculation of Renal arterial Resistance Index. Renal arterial Doppler was performed using a 4 MHz probe, with the patient in the sitting position and using a posterior approach to visualise the kidney. The course of the right or left kidney segmental arteries is visualised by color Doppler flow and, at the middle tract level of the best one visualised, pulsed Doppler is performed. Peak systolic velocity and end diastolic velocity are used to calculate the renal arterial resistance index according to Pourcelot’s formula.
RRI has been found to be associated with renal parenchymal abnormalities. Platt e coll[83] first demo-nstrated the relationship between an increased RRI and parenchymal and vascular abnormalities assessed by renal biopsies. Later RRI has been found to be strongly associated with arteriolosclerosis, glomerulosclerosis, interstitial renal fibrosis and tubulo-interstitial lesions[84-88].
Our study group evaluated the independent predictors of RRI values in a large group of CHF outpatients with reduced ejection fraction. In a multivariate logistic regression model including univariate predictors, only age, systolic pulmonary pressure, central venous pressure, GFR, diabetes, logarithm of NT-proBNP, pulse arterial pressure and NYHA class remained significantly correlated to RRI. These results help to confirm the pathophysiological background responsible for the modification of RRI in CHF. In particular, RRI seems to carry information about renal artery abnormalities and/or alterations of arterial stiffness, as suggested by the correlation we found with the related parameters such as age, diabetes and pulse pressure. Moreover, the relationship between RRI and central venous pressure highlights the role of intra abdominal pressure and renal venous pressure in determining an increase in renal arterial resistances[89].
In a group of patients with CHF and preserved left ventricular ejection fraction, Ennezat et al[90] first demonstrated the significance of RRI in predicting prognosis. In our series of outpatients affected by CHF (mainly due to a reduced left ventricular ejection fraction), we have also demonstrated the prognostic significance of RRI[89]. The combined end-point that we considered was death, urgent heart transplantation or hospitalization due to heart failure worsening. RRI was associated with events in an univariate Cox regression model (HR = 1.14; 95%CI: 1.09-1.19; P < 0.001; C-index: 0.77), but also in a multivariate model (HR = 1.08; 95%CI: 1.02-1.13; P = 0.004; C-index: 0.86) correcting for the independent predictors, i.e. LVEF, GFR and logNT-proBNP. Moreover, with the addition of RRI, the reclassification model showed an important rise according to both category-free net reclassification improvement NRI (47%; 95%CI: 13%-80%; P = 0.006) and integrated discrimination improvement IDI (0.034; 95%CI: 0.006-0.061; P = 0.016). Likewise, in our series, RRI has been also found to be independently associated with an increased risk of death (at multivariate analysis: HR = 1.06; 95%CI: 1.01-1.12; P = 0.023; C-index = 0.783)[91].
The pathophysiological factors influencing RRI offer an explanation to the incremental prognostic value of this parameter when added to a model already including GFR. In addition to GFR, RRI could provide further information about renal function by reflecting not only glomerular function but also the other factors which influence the progression of kidney disease. Therefore, RRI could complete the information carried by GFR and allow a better characterization of renal dysfunction in CHF.
This is also supported by the fact that in our series RRI was able not only to predict heart failure progression but also worsening of renal function. At multivariate analysis a RRI > 70 was independently associated with an increase in creatinine > 0.3 mg/dL[92], regardless of baseline GFR values.
CONCLUSION
The evaluation of renal dysfunction is a cornerstone for CHF patients’ management. GFR estimation is, at present, considered the best parameter to assess overall kidney function and it is recommended for routinely use. However, serum creatinine, traditionally used to estimate glomerular filtration rate, has several limitations. As a consequence the use of other parameters to evaluate hyperfiltration, tubular function and hemodynamic status could be useful in order to better define renal function. To this purpose RRI, an echo-Doppler derived parameter, which reflects abnormalities of the renal vascular bed, seems to be very promising as it carries an independent and incremental information to detect patients with worse prognosis and increased risk of WRF.
Footnotes
P- Reviewer: Artunc F, Schoenhagen P S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 30, 2014
First decision: September 16, 2014
Article in press: December 23, 2014
References
- 1.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 2.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 3.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH) Eur J Heart Fail. 2009;11:847–854. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 4.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 5.Ronco C, Ronco F. Cardio-renal syndromes: a systematic approach for consensus definition and classification. Heart Fail Rev. 2012;17:151–160. doi: 10.1007/s10741-010-9224-0. [DOI] [PubMed] [Google Scholar]
- 6.Ronco C, Di Lullo L. Cardiorenal syndrome. Heart Fail Clin. 2014;10:251–280. doi: 10.1016/j.hfc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–878. doi: 10.1016/j.ejheart.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Iacoviello M, Puzzovivo A, Monitillo F, Saulle D, Lattarulo MS, Guida P, Forleo C, Gesualdo L, Favale S. Independent role of high central venous pressure in predicting worsening of renal function in chronic heart failure outpatients. Int J Cardiol. 2013;162:261–263. doi: 10.1016/j.ijcard.2012.06.088. [DOI] [PubMed] [Google Scholar]
- 9.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson MA, Waikar SS. Established and emerging markers of kidney function. Clin Chem. 2012;58:680–689. doi: 10.1373/clinchem.2011.167494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smilde TD, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation. 2006;114:1572–1580. doi: 10.1161/CIRCULATIONAHA.105.610642. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56:486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valente MA, Hillege HL, Navis G, Voors AA, Dunselman PH, van Veldhuisen DJ, Damman K. The Chronic Kidney Disease Epidemiology Collaboration equation outperforms the Modification of Diet in Renal Disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur J Heart Fail. 2014;16:86–94. doi: 10.1093/eurjhf/hft128. [DOI] [PubMed] [Google Scholar]
- 17.McAlister FA, Ezekowitz J, Tarantini L, Squire I, Komajda M, Bayes-Genis A, Gotsman I, Whalley G, Earle N, Poppe KK, et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ Heart Fail. 2012;5:309–314. doi: 10.1161/CIRCHEARTFAILURE.111.966242. [DOI] [PubMed] [Google Scholar]
- 18.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Smith GL, Shlipak MG, Havranek EP, Masoudi FA, McClellan WM, Foody JM, Rathore SS, Krumholz HM. Race and renal impairment in heart failure: mortality in blacks versus whites. Circulation. 2005;111:1270–1277. doi: 10.1161/01.CIR.0000158131.78881.D5. [DOI] [PubMed] [Google Scholar]
- 20.Akhter MW, Aronson D, Bitar F, Khan S, Singh H, Singh RP, Burger AJ, Elkayam U. Effect of elevated admission serum creatinine and its worsening on outcome in hospitalized patients with decompensated heart failure. Am J Cardiol. 2004;94:957–960. doi: 10.1016/j.amjcard.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 21.Khan NA, Ma I, Thompson CR, Humphries K, Salem DN, Sarnak MJ, Levin A. Kidney function and mortality among patients with left ventricular systolic dysfunction. J Am Soc Nephrol. 2006;17:244–253. doi: 10.1681/ASN.2005030270. [DOI] [PubMed] [Google Scholar]
- 22.Krumholz HM, Chen YT, Vaccarino V, Wang Y, Radford MJ, Bradford WD, Horwitz RI. Correlates and impact on outcomes of worsening renal function in patients & gt; or =65 years of age with heart failure. Am J Cardiol. 2000;85:1110–1113. doi: 10.1016/s0002-9149(00)00705-0. [DOI] [PubMed] [Google Scholar]
- 23.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, et al. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–195. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 25.Damman K, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL. Current and novel renal biomarkers in heart failure. Heart Fail Rev. 2012;17:241–250. doi: 10.1007/s10741-011-9254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosch JP. Renal reserve: a functional view of glomerular filtration rate. Semin Nephrol. 1995;15:381–385. [PubMed] [Google Scholar]
- 27.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 28.Singh D, Whooley MA, Ix JH, Ali S, Shlipak MG. Association of cystatin C and estimated GFR with inflammatory biomarkers: the Heart and Soul Study. Nephrol Dial Transplant. 2007;22:1087–1092. doi: 10.1093/ndt/gfl744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944–1947. doi: 10.1046/j.1523-1755.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 30.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 31.Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18:2024–2031. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- 32.Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis. 2013;62:595–603. doi: 10.1053/j.ajkd.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lassus J, Harjola VP. Cystatin C: a step forward in assessing kidney function and cardiovascular risk. Heart Fail Rev. 2012;17:251–261. doi: 10.1007/s10741-011-9242-6. [DOI] [PubMed] [Google Scholar]
- 34.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 36.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 38.Arimoto T, Takeishi Y, Niizeki T, Takabatake N, Okuyama H, Fukui A, Tachibana H, Nozaki N, Hirono O, Tsunoda Y, et al. Cystatin C, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail. 2005;11:595–601. doi: 10.1016/j.cardfail.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Alehagen U, Dahlström U, Lindahl TL. Cystatin C and NT-proBNP, a powerful combination of biomarkers for predicting cardiovascular mortality in elderly patients with heart failure: results from a 10-year study in primary care. Eur J Heart Fail. 2009;11:354–360. doi: 10.1093/eurjhf/hfp024. [DOI] [PubMed] [Google Scholar]
- 40.Tang WH, Van Lente F, Shrestha K, Troughton RW, Francis GS, Tong W, Martin MG, Borowski AG, Jasper S, Starling RC, et al. Impact of myocardial function on cystatin C measurements in chronic systolic heart failure. J Card Fail. 2008;14:394–399. doi: 10.1016/j.cardfail.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, Itoh Y, Lieske JC, Seccombe DW, Jones G, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55:24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- 42.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 43.van de Wal RM, Asselbergs FW, Plokker HW, Smilde TD, Lok D, van Veldhuisen DJ, van Gilst WH, Voors AA. High prevalence of microalbuminuria in chronic heart failure patients. J Card Fail. 2005;11:602–606. doi: 10.1016/j.cardfail.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Niizeki T, Takeishi Y, Sasaki T, Kaneko K, Sugawara S, Watanabe T, Kubota I. Usefulness of albuminuria as a prognostic indicator in patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2013;111:1180–1186. doi: 10.1016/j.amjcard.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 45.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 46.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 47.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 48.Damman K, Masson S, Hillege HL, Voors AA, van Veldhuisen DJ, Rossignol P, Proietti G, Barbuzzi S, Nicolosi GL, Tavazzi L, et al. Tubular damage and worsening renal function in chronic heart failure. JACC Heart Fail. 2013;1:417–424. doi: 10.1016/j.jchf.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, Granger CB, Swedberg K, Pfeffer MA, Yusuf S, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374:543–550. doi: 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed] [Google Scholar]
- 50.Masson S, Latini R, Milani V, Moretti L, Rossi MG, Carbonieri E, Frisinghelli A, Minneci C, Valisi M, Maggioni AP, et al. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: data from the GISSI-Heart Failure trial. Circ Heart Fail. 2010;3:65–72. doi: 10.1161/CIRCHEARTFAILURE.109.881805. [DOI] [PubMed] [Google Scholar]
- 51.Damman K, Hillege HL, van Veldhuisen DJ. Albuminuria in heart failure: a CHARMing new risk factor? Lancet. 2009;374:506–508. doi: 10.1016/S0140-6736(09)61469-0. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 53.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt-Ott KM, Mori K, Kalandadze A, Li JY, Paragas N, Nicholas T, Devarajan P, Barasch J. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15:442–449. doi: 10.1097/01.mnh.0000232886.81142.58. [DOI] [PubMed] [Google Scholar]
- 55.Bazzi C, Petrini C, Rizza V, Arrigo G, Napodano P, Paparella M, D’Amico G. Urinary N-acetyl-beta-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol Dial Transplant. 2002;17:1890–1896. doi: 10.1093/ndt/17.11.1890. [DOI] [PubMed] [Google Scholar]
- 56.Wellwood JM, Ellis BG, Price RG, Hammond K, Thompson AE, Jones NF. Urinary N-acetyl- beta-D-glucosaminidase activities in patients with renal disease. Br Med J. 1975;3:408–411. doi: 10.1136/bmj.3.5980.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 58.Meijer E, Boertien WE, Nauta FL, Bakker SJ, van Oeveren W, Rook M, van der Jagt EJ, van Goor H, Peters DJ, Navis G, et al. Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: a cross-sectional analysis. Am J Kidney Dis. 2010;56:883–895. doi: 10.1053/j.ajkd.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 59.Han WK, Alinani A, Wu CL, Michaelson D, Loda M, McGovern FJ, Thadhani R, Bonventre JV. Human kidney injury molecule-1 is a tissue and urinary tumor marker of renal cell carcinoma. J Am Soc Nephrol. 2005;16:1126–1134. doi: 10.1681/ASN.2004070530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haase M, Bellomo R, Devarajan P, Ma Q, Bennett MR, Möckel M, Matalanis G, Dragun D, Haase-Fielitz A. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg. 2009;88:124–130. doi: 10.1016/j.athoracsur.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 61.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 62.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 63.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choudhary R, Gopal D, Kipper BA, De La Parra Landa A, Aramin H, Lee E, Shah S, Maisel AS. Cardiorenal biomarkers in acute heart failure. J Geriatr Cardiol. 2012;9:292–304. doi: 10.3724/SP.J.1263.2012.02291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alvelos M, Lourenço P, Dias C, Amorim M, Rema J, Leite AB, Guimarães JT, Almeida P, Bettencourt P. Prognostic value of neutrophil gelatinase-associated lipocalin in acute heart failure. Int J Cardiol. 2013;165:51–55. doi: 10.1016/j.ijcard.2011.07.080. [DOI] [PubMed] [Google Scholar]
- 66.Dupont M, Shrestha K, Singh D, Awad A, Kovach C, Scarcipino M, Maroo AP, Tang WH. Lack of significant renal tubular injury despite acute kidney injury in acute decompensated heart failure. Eur J Heart Fail. 2012;14:597–604. doi: 10.1093/eurjhf/hfs039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collins SP, Hart KW, Lindsell CJ, Fermann GJ, Weintraub NL, Miller KF, Roll SN, Sperling MI, Sawyer DB, Storrow AB. Elevated urinary neutrophil gelatinase-associated lipocalcin after acute heart failure treatment is associated with worsening renal function and adverse events. Eur J Heart Fail. 2012;14:1020–1029. doi: 10.1093/eurjhf/hfs087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Testani JM, Tang WH. Biomarkers of acute kidney injury in chronic heart failure: what do the signals mean? JACC Heart Fail. 2013;1:425–426. doi: 10.1016/j.jchf.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Damman K, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail. 2008;10:997–1000. doi: 10.1016/j.ejheart.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD, Bonventre JV, Voors AA, Hillege HL. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96:1297–1302. doi: 10.1136/hrt.2010.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damman K, Masson S, Hillege HL, Maggioni AP, Voors AA, Opasich C, van Veldhuisen DJ, Montagna L, Cosmi F, Tognoni G, et al. Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J. 2011;32:2705–2712. doi: 10.1093/eurheartj/ehr190. [DOI] [PubMed] [Google Scholar]
- 72.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 73.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 74.Mallat Z, Heymes C, Corbaz A, Logeart D, Alouani S, Cohen-Solal A, Seidler T, Hasenfuss G, Chvatchko Y, Shah AM, et al. Evidence for altered interleukin 18 (IL)-18 pathway in human heart failure. FASEB J. 2004;18:1752–1754. doi: 10.1096/fj.04-2426fje. [DOI] [PubMed] [Google Scholar]
- 75.Niizeki T, Takeishi Y, Arimoto T, Nozaki N, Hirono O, Watanabe T, Nitobe J, Miyashita T, Miyamoto T, Koyama Y, et al. Persistently increased serum concentration of heart-type fatty acid-binding protein predicts adverse clinical outcomes in patients with chronic heart failure. Circ J. 2008;72:109–114. doi: 10.1253/circj.72.109. [DOI] [PubMed] [Google Scholar]
- 76.Chade AR. Renal vascular structure and rarefaction. Compr Physiol. 2013;3:817–831. doi: 10.1002/cphy.c120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol. 1931;72:49–61. doi: 10.1113/jphysiol.1931.sp002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jessup M, Costanzo MR. The cardiorenal syndrome: do we need a change of strategy or a change of tactics? J Am Coll Cardiol. 2009;53:597–599. doi: 10.1016/j.jacc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 80.Pourcelot L. Velocimetrie ultrasonore Doppler. Seminaire INSERM. Paris, France: Edition INSERM; 1974. pp. 213–240. [Google Scholar]
- 81.Parolini C, Noce A, Staffolani E, Giarrizzo GF, Costanzi S, Splendiani G. Renal resistive index and long-term outcome in chronic nephropathies. Radiology. 2009;252:888–896. doi: 10.1148/radiol.2523080351. [DOI] [PubMed] [Google Scholar]
- 82.Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol. 2003;180:885–892. doi: 10.2214/ajr.180.4.1800885. [DOI] [PubMed] [Google Scholar]
- 83.Platt JF, Ellis JH, Rubin JM, DiPietro MA, Sedman AB. Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. AJR Am J Roentgenol. 1990;154:1223–1227. doi: 10.2214/ajr.154.6.2110732. [DOI] [PubMed] [Google Scholar]
- 84.Ikee R, Kobayashi S, Hemmi N, Imakiire T, Kikuchi Y, Moriya H, Suzuki S, Miura S. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis. 2005;46:603–609. doi: 10.1053/j.ajkd.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 85.Hanamura K, Tojo A, Kinugasa S, Asaba K, Fujita T. The resistive index is a marker of renal function, pathology, prognosis, and responsiveness to steroid therapy in chronic kidney disease patients. Int J Nephrol. 2012;2012:139565. doi: 10.1155/2012/139565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sugiura T, Nakamori A, Wada A, Fukuhara Y. Evaluation of tubulointerstitial injury by Doppler ultrasonography in glomerular diseases. Clin Nephrol. 2004;61:119–126. doi: 10.5414/cnp61119. [DOI] [PubMed] [Google Scholar]
- 87.Sugiura T, Wada A. Resistive index predicts renal prognosis in chronic kidney disease. Nephrol Dial Transplant. 2009;24:2780–2785. doi: 10.1093/ndt/gfp121. [DOI] [PubMed] [Google Scholar]
- 88.Boddi M, Cecioni I, Poggesi L, Fiorentino F, Olianti K, Berardino S, La Cava G, Gensini G. Renal resistive index early detects chronic tubulointerstitial nephropathy in normo- and hypertensive patients. Am J Nephrol. 2006;26:16–21. doi: 10.1159/000090786. [DOI] [PubMed] [Google Scholar]
- 89.Ciccone MM, Iacoviello M, Gesualdo L, Puzzovivo A, Antoncecchi V, Doronzo A, Monitillo F, Citarelli G, Paradies V, Favale S. The renal arterial resistance index: a marker of renal function with an independent and incremental role in predicting heart failure progression. Eur J Heart Fail. 2014;16:210–216. doi: 10.1002/ejhf.34. [DOI] [PubMed] [Google Scholar]
- 90.Ennezat PV, Maréchaux S, Six-Carpentier M, Pinçon C, Sediri I, Delsart P, Gras M, Mounier-Véhier C, Gautier C, Montaigne D, Jude B, Asseman P, Le Jemtel TH. Renal resistance index and its prognostic significance in patients with heart failure with preserved ejection fraction. Nephrol Dial Transplant. 2011;26:3908–3913. doi: 10.1093/ndt/gfr116. [DOI] [PubMed] [Google Scholar]
- 91.Monitillo F, Iacoviello M. Citarelli G, Leone M. Independent and incremental role of renal resistance index in predicting mortality among heart failure outpatients. Eur J of Heart Fail. 2014;16:126. doi: 10.1002/ejhf.34. [DOI] [PubMed] [Google Scholar]
- 92.Citarelli G, Iacoviello M, Monitillo F, Leone M. A high renal arterial resistance index is associated to one year worsening of renal function in heart failure outpatients. Eur J of Heart Fail. 2014;16:126. doi: 10.1002/ejhf.34. [DOI] [PubMed] [Google Scholar]


