Abstract
Recent advances in molecular targeted therapies, including targeting human epidermal growth factor receptor 2 (HER2), had a major forward step in the therapy for gastric cancer patients. Application of HER2-targeted therapies, in particular trastuzumab in combination with chemotherapy in metastatic HER2-positive gastric cancers, resulted in improvements in response rates, time to progression and overall survival. Nevertheless, as with breast cancer, many patients with gastric cancer develop resistance to trastuzumab. Several promising therapies are currently being developed in combination with chemotherapy to increase the efficacy and overcome the cancer-resistance. Here we review the current overview of clinical application of agents targeting HER2 in gastric cancer. We also discuss the ongoing trials supporting the use of HER2-targeted agents combined with cytotoxic agents or other monoclonal antibodies.
Keywords: Human epidermal growth factor receptor 2, Gastric cancer, Targeting therapy, Trastuzumab
Core tip: This review summarizes the development of diagnostic and therapeutic approach for the patients with human epidermal growth factor receptor 2 (HER2)-overexpressed/amplified gastric cancer. The biology of HER2-dependent signalling is also described. The ToGA trial highlighted the importance of accurate HER2 testing to guide treatment choice of gastric cancer. Future strategies beyond the ToGA trial to address EFGR family, including HER2 pathway are discussed according to current ongoing clinical trials, as well as experimental studies.
INTRODUCTION
Gastric cancer is the fourth most common malignant disease and the second leading cause of cancer-related death worldwide[1]. Despite the current improvements in survival of patients with gastric cancer, it is often diagnosed at an advanced stage and its prognosis is still unsatisfactory due to the high frequency of metastasis[2,3].
Recent studies have shown that several combination chemotherapies have been shown to significantly increase survival for patients with gastric cancer[4]. SPIRITS trial (S1 plus cisplatin vs S1 in RCT in the treatment of stomach cancer) showed that combined therapy of S1 with cisplatin significantly prolonged survival as a first-line treatment for advanced gastric cancer. Overall survival (OS) of patients treated with S1 plus cisplatin was 13.0 mo compared 11.0 mo with S1 alone[5]. Additionally, other cytotoxic agents, including docetaxel and irinotecan also prolonged survival[6,7]. Notably, capecitabine and oxaliplatin showed to be non-inferior to fluorouracil and cisplatin[8,9]. However, even with these treatments, most patients with advanced disease have a median overall survival in the range of 6-11 mo[2].
To date, with greater knowledge of the molecular basis of tumor initiation, several kinds of targeted agents have led to a better prognosis for solid tumors. One of the most important targets in human malignancy is the epidermal growth factor receptor (EGFR) family[10]. The human epidermal growth factor receptor-2 (HER2) is a receptor of tyrosine kinase and a member of the EGFR family[11]. HER2 is expressed in a significant proportion of gastric cancer[12]. Trastuzumab, a recombinant humanized monoclonal antibody that targets the extracellular domain IV of HER2, has recently been noticeably altered the treatment of gastric cancer. Trastuzumab has demonstrated a survival advantage in patients with HER2-overexpressed gastric cancer[13].
In this article, we will outline the issues concerning novel biologic agents for advanced gastric cancer, focusing on anti-HER2 therapies, such as trastuzumab, and other novel agents. We will also discuss the current clinical evidence and ongoing trials supporting the use of HER2-targeted agents combined with cytotoxic agents or other monoclonal antibodies.
MOLECULAR FEATURES OF HER2
HER2, a proto-oncogene encoded by ERBB2 on chro-mosome 17, is a cell membrane surface-bound receptor tyrosine kinase and belongs to EGFR family, including EGFR/HER1, HER2/neu, HER3, and HER4[11]. Each receptor has an extracellular domain, lipophilic transmembrane domain, and intracellular kinase domain (Figure 1). Although HER1, 3, 4 are activated by ligand binding, the specific ligand to HER2 have not been identified yet[14]. Nevertheless, aberrant HER2 activity and activation of the HER2 receptor leads to receptor dimerization (e.g., HER2/HER3), and subsequently activate downstream signals, including members of the Ras/Raf/mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase/protein kinase-B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathways (Figure 1)[15,16]. Overexpression of HER2 has been found to aggressively promote these signals which are responsible for regulating a variety of tumor biology, such as cancer cell growth, differentiation, invasion and survival[17,18]. Dimerization of HER2 and HER3 is known to be the most active HER signaling dimer. With regard to gastric cancer, HER2 and HER3 are significant predictors of poor survival in multivariate study. HER3 may become another candidate for molecular-targeted therapy in gastric cancer, especially for the diffuse histological type[19,20].
Figure 1.
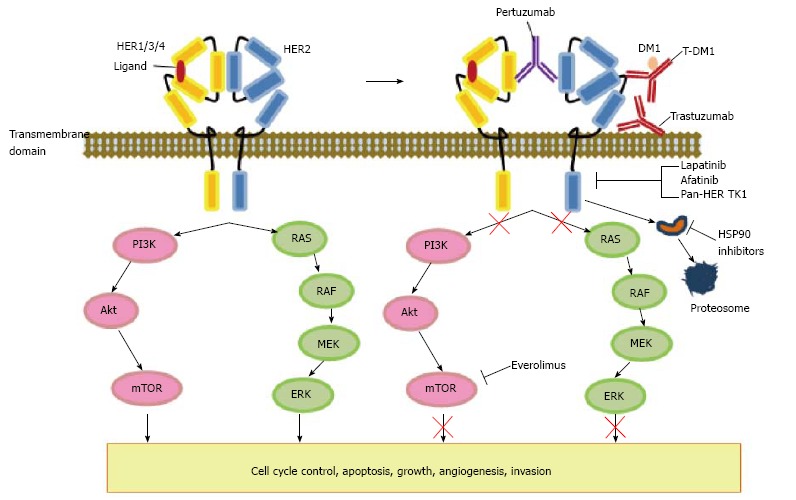
Human epidermal growth factor receptor 2 signaling pathway and interaction with other pathways. This demonstrates schematic representation of the HER-2 family of receptor and their interaction with downstream signalling, along the pathway which are responsible for a variety of biological processes involving cell cycle control, apoptosis, cellular growth, invasion, and angiogenesis. Examples of classes of drugs and corresponding compounds targeting the HER-2 network are also presented. HER1-4 are transmembrane proteins with associated tyrosine kinases. Heterodimerization result in tyrosine kinase activation with the subsequent signaling cascade, and subsequently activates downstream signals, including members of MAPK and PI3K/Akt/mTOR pathways. Trastuzumab and t-DM1 targtets to the extracellular domain IV of HER2. Anti-cancer activity of pertuzumab is interference with HER-receptor dimerization. Lapatinib, afatinib, and tyrosine kinase inhibitors (TKIs) compete for the binding of ATP in the intracellular domain of the receptors. HSP90 suppresses the NH2-terminal ATP binding site which leads to the degradation of client proteins by the ubiquitin proteasome pathway. HER: Human epidermal growth factor receptor; MAPK: Mitogen-activated protein kinase; MEK: Mitogen-activated protein kinase kinase; ERK: Extracellular signal-regulated kinase; PI3K: Phosphatidylinositol 3-kinase; mTOR: Mammalian target of rapamycin.
HER2 EXPRESSION AND GASTRIC CANCER
The reported HER2 amplification in patients with gastric cancer ranges from 6% to 23%[13,21,22]. The rates of HER2 amplification or overexpression in gastric cancers are different depending on the primary location of the tumor, which is more frequent in cancers located in the gastroesophageal junction compared with those from elsewhere in the stomach[13,23]. Histological evaluation revealed HER2 overexpression was predominantly seen in the intestinal-type than in diffuse-type cancers (32% vs 6%)[22].
HER2 amplification is associated with clinicop-athological features, such as age, male gender, tumor size, serosal invasion and lymph node metastasis[24,25]. HER2 expression is a biomarker for the prediction of trastuzumab response[26]. However, the prognostic significance of HER2 overexpression in gastric cancer remains controversial. A number of retrospective studies have demonstrated that HER2 positivity is a prognostic factor associated with increased risk of invasion, metastasis, and worse survival[19,27-29]. HER2 status has been reported as the second poorest prognostic variable following nodal status[30,31]. On the other hand, other studies found no association between HER2 and prognosis in both early and advanced stage cancers[13,32-38].
Several studies have investigated how differences in expression of HER2 between of primary gastric tumor and metastatic lesions. The majority of these reports has described that HER2 expression of primary and secondary sites revealed a high concordance rate, except two studies[35,39]. These data suggest that the evaluation of HER2 expression in the primary cancer is a reliable basis for determing treatment with anti-HER2 agents in patients with metastatic gastric cancer.
HER2 expression is usually determined by immunohis-tochemistry (IHC) or by the detection of HER2 gene amplification by fluorescence in situ hybridization (FISH). The evaluation of HER2 immunostained samples in gastric cancer is carried out as outlined by Hofmann et al[40] and optimized by Rüschoff et al[41]. HER2 testing is distinct from breast cancer immunohistochemistry testing. The major difference in scoring HER2 IHC staining between gastric cancer and breast cancer is that an incomplete basolateral or lateral staining alone is considered as a positive result, which lead to the frequent incidence of tumor heterogeneity[40]. This heterogeneity may represent the HER2 testing inaccuracy, resulting in the controversy of significance of HER2-expression in gastric cancer. Thus, further studies have been proposed to improve the quality of HER2 testing to make certain that patients receive the best possible therapy for their HER2-positive disease.
A recent study presented the Collaborative Enzyme Enhanced Reactive (CEER) immunoassay may be a useful technique to investigate the HER2 expression[42]. CEER-based assays showed higher sensitivity and specificity as compared to IHC-based assays. Evaluation with this high sensitivity of HER3 resulted in -20% of the IHC/FISH HER2 negative gastric cancers still expressed total HER2 although. Another study presented that the use of a quantitative variable that could be objectively measured, such as the HER2 gene copy number or the HER2 amplification ratio, which seems preferable to a subjective classification in accordance with IHC scores that are not regularly consistent[43]. These current development of technology may be useful for elucidating the expression of HER2 more precisely and improving prediction of clinical outcome in gastric cancer patients treated with trastuzumab.
TREATMENT FOR HER2 POSITIVE GASTRIC CANCER
Trastuzumab
Trastuzumab is the first molecular targeted agent approved as standard treatment in gastric cancer[13,44]. This agent induces antibody-dependent cellular cytotoxicity, inhibits HER2-mediated signaling and prevents cleavage of the extracellular domain of HER2 (Figure 1). Trastuzumab for Gastric Cancer (ToGA) study was an open-label phase III, randomized controlled trial undertaken in 122 centers among 24 countries[13]. ToGA trial showed that an addition of trastuzumab to conventional cytotoxic chemotherapy demonstrated a clinical benefit compared to chemotherapy alone in terms of tumor response, which suggested that combined chemotherapy with trastuzumab can be cited as a novel accepted option for patients with HER2-positive advanced gastric cancer. Recently, a role of trastuzumab as a second-line chemotherapy got attention because of the usefulness of trastuzumab as a first-line chemotherapy. In a recent retrospective analysis, gastric cancer patients with HER2 overexpression applied trastuzumab in addition to their first-line chemotherapy with or without trastuzumab maintenance therapy. The median PFS was 14.6 mo and OS was 16.4 mo with an acceptable benefit[45] (Table 1).
Table 1.
Clinical trials of HER2-targeted therapy in gastric cancer (after ToGA study)
| Trial | Study design | No. of patients | HER2 status | Regimen duration of treatment | Response rate | Prognosis |
| LOGiC | Phase III/1st Randomized Double Blind | 545 | HER2 amplification | Lapatinib + XELOX XELOX No description of duration | non-significant prolongation | |
| TyTAN | Phase III/2nd Parallel group Randomized | 1923 | HER2 amplification | Lapatinib +Paclitaxel Paclitaxel 24 mo | 27% vs 9% | OS: 11.0 mo vs 8.9 mo PFS: 5.4 mo vs 4.4 mo |
| HERBIS-1 | Phase II/1st Non-Randomized | 56 | IHC 3+ IHC 2+ FISH + | S-1/cisplatin + trastuzumab No description of duration | 68% | OS: 16.0 mo PFS: 7.8 mo |
| PF299804 | Phase II/2nd Non-Randomized | 28 (estimated) | IHC 3+ IHC 2+ FISH + | PF299804 Cycles of 28 d | 7.40% | Ongoing |
| HIROISE (NCT01450646) | Phase III/1st Randomized | 400 (estimated) | Trastuzumab + cisplatin + capecitabine 33 wk | Ongoing | ||
| NCT01472029 | Phase II/1st Non-Randomized | 53 | 5-FU, leucovorin, docetaxel, oxaliplatin (FLOT), trastuzumab No description of duration | Ongoing | ||
| NCT01130337 | Phase II/preoperative Non-Randomized | 36 | Trastuzumab + XELOX 12 mo | Ongoing | ||
| NCT01522768 | Phase II/advanced Non-Randomized | 40 | IHC 3+ IHC 2+ FISH + | Afatinib + trastuzumab 24 mo | Ongoing | |
| NCT01402401 | Phase II/2nd Non-Randomized | 21 | IHC 3+ IHC 2+ FISH + | AUY922 + trastuzumab Every 6 wk | Ongoing | |
| NCT01641939 | Phase II/III/1st Randomized | 412 (estimated) | IHC 3+ IHC 2+ FISH + | Trastuzumab emtansine Taxane 12 wk | Ongoing |
HER2: Human epidermal growth factor receptor; OS: Overall survival; PFS: Progression-free survival; IHC: Immunohistochemistry; FISH: Fluorescence in situ hybridization.
On the other hand, the efficacy of HER2-targeted agents have shown to be limited and unsatisfactory than that would be expected[13,46]. These insufficiencies may be overcome by the development of combined therapy with other cytotoxic agents, and strategies against primary or acquired resistance in the patients with gastric cancer. The HERBIS-1 study is the muticenter phase II trial undertaken at 22 hospitals in Japan[47]. Patients with HER2-positive advanced gastric cancer received S1 on day 1-14, cisplatin, on 1 d, and trastuzumab on day 1 of a 21-d cycle. The RR based on RECIST was 68% (95%CI: 54%-80%; 80%CI: 58%-76%) and the disease control rate was 94% (95%CI: 84%-99%). Median OS, PFS, and TTF were estimated as 16.0, 7.8, and 5.7 mo, respectively. Trastuzumab combined with SP would be a potential new strategy for patients with HER-2 positive advanced gastric cancer. Another study presented that the level of synergistic effect on combination therapy with trastuzumab and anti-cancer drug was different depending on the expression level of HER-2. The HER-2 expression may be applied to standard for drug selection in combination of trastuzumab with known cytotoxic agents in gastric cancer[48].
Pertuzumab
Pertuzumab, one of the HER2-targeted monoclonal antibody, is distinct and complementary to trastuzumab. Pertuzumab binds the extracellular domain II of the HER2 receptor and disrupts HER2 dimerization (Figure 1)[49]. Similar to trastuzumab, pertuzumab activates antibody-dependent cellular cytotoxicity, with equivalent efficacy, leading to cancer cell death[50]. The combination of trastuzumab plus pertuzumab was reported to be synergistic in breast cancer. This combination reduced HER2/EGFR and HER2/HER3 heterodimer formation, leading to in induction of apoptosis in vitro[49]. Thus the therapeutic achievement of pertuzumab in metastatic HER2 positive breast cancer provides hope for HER2 positive gastric cancer by using a similar approach. With regard to gastric cancer, a phase IIa study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2 positive advanced gastric cancer was carried out[51]. Patients were divided into two arms: pertuzumab 840 mg for cycle 1 and trastuzumab 420 mg q3w for cycle 2-6 vs pertuzumab 840 mg q3w for six cycles. Partial responses were achieved by 86% and 55% of patients, respectively.
Lapatinib
Lapatinib is a small tyrosine kinase inhibitor of EGFR and HER2 that interferes activation by binding to the intracellular ATP binding site of these kinases (Figure 1). This agent inhibits HER2 and EGFR dependent activation of PI3K and Ras pathways, leading to downregulation of receptor tyrosine kinase phosphorylation in tumor cells (Figure 1). The synergistic activity was shown by lapatinib in combination with trastuzumab for HER2 positive breast cancer that presented through two lines of trastuzumab related treatments[52]. Regarding to gastric cancer, modest activity was demonstrated with single-agent lapatinib in the Southwest Oncology Group (SWOG) 0413 trial[53]. Subsequently, Tykerb with Taxol in Asian ErbB2 gastric cancer (TyTAN), an open-label randomized phase III study comparing paclitaxel with paclitaxel plus lapatinib in patients with HER2 FISH-positive IHC3 + gastric cancer as a second-line therapy was performed. Although the median OS was prolonged by two months with lapatinib, this trial failed to achieve any OS and PFS benefit because statistically significance was not obtained[46]. Moreover, the Lapatinib Optimization study in HER2 positive Gastric Cancer (LOGiC) trial is a phase III trial of capecitabine and oxaliplatin with or without lapatinib in first-line advanced HER2 FISH amplified gastric and GEJ cancers suggested not any signs of activity for lapatinib[54]. These negative results of TyTAN and LOGiC trials indicate that there is a presence of drug resistance or an alternative pathways from HER2-targeted therapy. On the other hand, lapatinib may be useful for treating the trastuzumab-resistant HER2-positive gastric cancer. A previous study described that lapatinib showed the antitumor efficacy for trastuzumab-resistant cell lines which was due to both G1 cell-cycle arrest and apoptosis induction[55].
Trastuzumab-emtansine
Antibody-drug conjugates have been said to transfer cytotoxic drugs directly to tumor cells. Trastuzumab emtansine (TDM-1) is an antibody-drug conjugate of trastuzumab and, a potent microtubule inhibitor, DM1(derivative of maytansine). In preclinical gastric cancer models, TDM-1 has shown more aggressive tumor activity than trastuzumab[56]. A multicenter adaptive phase II/III of TDM-1 is currently underway with HER2 positive advanced gastric cancer after progression following first line treatment (NIH study trial registration number NCT01641939; ClinicalTrials. gov).
STRATEGIES TO OVERCOME TRASTUZUMAB RESISTANCE IN GASTRIC CANCER
Although HER2 targeting therapy has been advanced, most patients with gastric cancer still develop acquired resistance to trastuzumab[54]. To achieve better benefits for HER2-targeted therapy in patients with HER2-positive gastric cancer, there is an urgent need to clarify the mechanisms underlying the cancer-resistance. Some papers described that intra-tumoral heterogeneity of gastric cancer may play a role in the resistance[57,58]. In this section, we will discuss selected novel agents including those based on proposed mechanisms of resistance to HER2-targeted therapy (Figure 1).
Afatinib
Afatinib (Gilotrif, Boehringer Ingelheim), an irreversible inhibitor of EGFR, HER2, and HER4, has been shown to be effective in the elimination of cancer cells with HER2 gene mutations[59]. This orally-bioavailable compound binds to its targets and has potential against receptors with acquired mutations that are resistant to first-generation inhibitors. The usefulness of this agent is currently examined in phase III study in EGFR-positive non small cell lung cancer, breast cancer, and head and neck squamous cell carcinoma[59]. Phase II study in metastatic HER2 positive trastuzumab refractory esophagogastric cancer is underway (NIH study trial registration number NCT01522768; ClinicalTrials.gov).
mTOR inhibitors
One of the most important mechanisms underlying trastuzumab resistance is dysregulation of HER2 downstream signal substrate, including the PI3K/Akt/mTOR pathway. It is well known that PIK3CA mutations and phosphate and tensin homolog (PTEN) inactivation result in constitutive activation of the downstream signals. HER2 overexpression is said to be significantly associated with p-Akt expression, suggesting that PIK3CA mutation and PTEN inactivation may affect the effectiveness of HER2-targeted therapy[60]. Inhibition of the mTOR/S6K signal by mTOR inhibitor, everolimus, enhanced fluorouracil-induced apoptosis in gastric cancer cells with HER2 amplification. Thus, it is plausible that concomitant therapy between HER2-targeted agents and mTOR inhibitor may provide substantial benefit in patients with HER2-positive gastric cancer.
HSP90 inhibitors
HSP90 is an ATP-dependent, conserved molecular chaperone that is involved in the structural folding and stability of proteins. Suppression at the NH2-terminal ATP binding site results in the degradation of client proteins by the ubiquitin proteasome pathway, which lead to the degradation of HER2 (Figure 1)[61]. Furthermore, p95-HER2, which is amino-terminal truncated form of HER2 and also a major factor of trastuzumab resistance due to the lacks of the trastuzumab binding site, shown to be degraded by HSP90 inhibitors. Several HSP90 inhibitors are undergoing early clinical evaluation. AUY922 is part of the isoxazole HSP90-inhibitor family. HSP90 inhibition by AUY922 leads to decrease the ErbB2 protein level and downstream signaling via PI3K in HER2-positive gastric cancer[62]. A recent study described that a significant synergy exists between AUY922 and trastuzumab in HER2-amplified gastric cancer. The combination of AUY922 and trastuzumab is also synergistic in HER2-amplified trastuzumb-progressed gastric cancer, which may be due to reinforce the rationale behind dual mechanisms of blockade in HER2-amplified diseases[63]. Clinical trials on the basis of this study are currently underway in gastric cancers (NIH study trial registration number NCT01402401; ClinicalTrials.gov). Thus, the dual inhibition of HSP90 and HER2 may enhance the attenuate effects on downstream signaling, especially in trastuzumab-resistant patients.
Pan-HER TK1
Recent studies have suggested that HER3 plays a pivotal role in tumor resistance to molecular agents targeting HER2[64], and is causing the maintenance of HER2-amplified cell growth. Therefore, it is plausible that a pan-HER TK1, which targets all HER family members, may have more potent activity in HER expressed malignancies. PF00299804 (Decomitib) is an orally bioavailable, second-generation, irreversible pan-HER TKI currently under clinical development[65]. The combination of PF00299804 with chemotherapeutic agents or molecular-targeted agents including trastuzumab produced synergistic effects. These data suggested that PF00299804 may augment the antitumor efficacy of chemotherapy and/or molecular-targeted agents.
Met inhibitors
c-Met is a cell surface receptor for hepatocyte growth factor (HGF), which regulates a variety of cellular processes involved in cell growth, invasion and angio-genesis[66]. A functional crosstalk between Met and HER family members has been shown to acquire an invasive and progressive phenotype, suggesting that Met can be the dimerization partner or crosstalk with HER2[67]. Furthermore, the Met signaling has been implicated as a mediator of resistance to therapies targeting members of the HER family in several solid tumors[68]. These data indicate that HER2 targeted agents combined with Met inhibitor may be useful to facilitate the efficacy and overcome the resistance of HER2 therapy in patients with HER2-positive gastric cancer. Similarly, HGF activation of MET receptors has been described to rescue cells from lapatinib-induced growth inhibition by restimulating the downstream pathways[69]. This effect was abrogated by inhibiting MET with PHA-665752 (a highly specific MET inhibitor), suggesting that lapatinib-induced growth inhibition may be abrogated through the activation of MET. Thus, the dual application of lapatinib and MET inhibitor may be a favorite model to overcome the lapatinib-resistant gastric cancer.
FUTURE PERSPECTIVES
This review has discussed some of a large number of data showing the clinical benefits of trastuzumab for treating HER2-positive gastric cancer. Combination therapy between trastuzumab and conventional chemotherapy is now cited as a standard first-line treatment for HER2 overexpressing gastric cancer in patient with advanced stage. This treatment remains to be under investigation for more potent utilization. Abundant clinical trials are planned or currently underway to evaluate the role of anti-HER2 agents in metastatic disease in combination with cytotoxic chemotherapy as well as with targeted therapy. As previously described, some studies with anti-HER2 combination treatments indicate that the use of more than one HER2-targeted therapy was superior to one of these agents alone in HER2 positive gastric cancer[63,69-71]. With the expansion of utilizing anti-HER2 agents, identifying the right combination of these various novel agents will be urgent to benefit the clinical outcomes in patients with advanced gastric cancer.
There still are several subjects to be discussed and settled for the advancement of HER2-targeted therapy in gastric cancer.
Dose increases of trastuzumab
To date, the clinical relevance of trastuzumab’s kinetic variable is not defined. It has been considered that higher dosing may be required in patients with gastric cancer (Roche, Inc. Herceptin package insert. Available at: http://www.medsafe.govt.nz/profs/datasheet/h). Pharmacokinetics data showed in the ToGA study described that the trastuzumab clearance is 0.378 L/d based on the current standard dosing, 70% higher than the rates in trastuzumab-treated patients with breast cancer. The HELOISE study: a study of trastuzumab in combination with chemotherapy in patients with HER2-positive metastatic gastric or gastro-esophageal cancer is currently under investigation (NIH study trial registration number NCT01450696; ClinicalTrials.gov.). In this phase III study, patients are randomized to either standard dosing or higher dosing arm (trastuzumab given at 8 mg/kg loading dose followed by 10 mg/kg every 3 wk) in combination with cisplatin based chemotherapy.
Possible candidates as a new biomarker
To date, there are no predictive biomarkers of response to trastuzumab. Thus, identifying a biomarker is one of the most importance in the development of an effective targeted agent. The NeoSphere trial, examining 16 different biomarkers, including HER2 expression, PI3KCA mutation, p95HER2, and insulin-like growth factor receptor expression in patients with breast cancer, resulted in failure of predicting response to pertuzumab[72]. In contrast, the presence of HER2 phosphorylation and low HER3 mRNA expression were associated with improved efficacy in patients with ovarian cancer treated with pertuzumab[73]. In some clinical trials, PIK3CA mutation or PTEN loss has been evaluated as a possible predictive biomarker. Therefor, to clarify the association between HER2 expression and PI3K/Akt pathway alterations is necessary to develop a new therapeutic strategy.
With regard to gastric cancer, several experimental studies showed the possibility of several factors which may be useful for predicting the efficacy of trastuzumab alone or combined chemotherapy. Trastuzumab resistant cells were noted to express the decreased amount of p27KIP1 levels and increased CDK2 activity[74]. As a result, p27Kip1 level may be utilized as a marker of trastuzumab response and potential therapeutic target in trastuzumab-resistant gastric cancer. The serum level of HER2-extracellular domain (ECD) has been said to evaluate the HER2 status of patients with gastric cancer. Moreover, the serum HER2-ECD test could enable monitoring of the dynamic changes in HER2 status over the clinical course of the disease. It can be used as an easily accessible real-time biomarker for longitudinal assessments of disease status[75].
Mechanism of resistance
Resistance to trastuzumab is currently emerging in HER2 positive gastric cancers. PI3K pathway may confer resistance to tratuzumab in preclinical studies. Enhanced signaling from HER family receptors, including overexpression of HER3 and formation of high levels of HER2-HER3 heterodimers, and insulin-like growth factor-1 receptor (IGF-1R) are also correlated with PI3K signaling activity and resistance of anti-HER2 agents[76]. Recently, several studies have presented the mechanisms of resistance in patients with gastric cancer. Cancer stem cells may be postulated mediators of the chemoresistance. The c-Met inhibitor may be a promising target molecule for irinotecan-based chemotherapy of gastric cancer[77]. Heregulin 1 can cause resistance to lapatinib in gastric cancers in vitro through HER3 and Akt activation[78]. Interestingly, epithelial-mesenchymal transition plays an important role in acquiring resistance to HER2-directed treatment in HER2 positive gastric cancer[79].
CONCLUSION
HER2 signaling lead to receptor dimerization such as HER2/HER3 and positively mediates its downstream signals which lead to a variety of tumor biology. Dimerization of HER2 and HER3 is known to be the most active HER signaling dimer and HER3 may be a subsequent target of therapy in gastric cancer as well as HER2, especially for the diffuse histological type. Current development of technology for identifying HER2 expression is convincing and may result in the improved clinical outcome in trastuzumab applied patients with gastric cancer. There are several molecular HER2-targeted agents applied practically and showing clinical benefits compared to chemotherapy alone in gastric cancer. However, the efficacy of these agents has shown to be modest and unsatisfactory than that would be expected which may be due to the acquisition of resistance or an unmatched combination to known cytotoxic agents. To overcome the trastuzumab resistance, several examinations have elucidated the mechanisms of resistance in gastric cancer. Clinical and experimental studies using several other molecules, which shows synergistic activity by concomitant use with trastuzumab or has potential against receptors with acquired resistance, are under investigation. There still are several subjects to be discussed for the advancement of HER2-targeted therapy in gastric cancer, such as determining the suitable dose of trastuzumab, identifying a predictive biomarker. In conclusion, HER2-targeted therapy is now acceptable for management of patients with gastric cancer and recent studies including resistance-control will provide superior strategies for treating HER2-positive gastric cancer patients. In the meanwhile, considering the low expression (6%-23%) of HER2 and modest effect of ToGA trial (only 2.7 mo prolonged survival) in gastric cancer, the development of a novel molecular targeted therapy which has a more potent activity for gastric cancer might be desirable.
Footnotes
P- Reviewer: Kapischke M, Lee JI, Omboni S, Pavlidis TE, Tang Y S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Supported by KAKENHI (Grant-in-Aid for Scientific Research), No. 23390329; and the National Cancer Center Research and Development Fund (23-A-9).
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 6, 2014
First decision: September 16, 2014
Article in press: December 23, 2014
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;(3):CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27. doi: 10.1007/s10120-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 7.Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, Wenczl M, Goker E, Cisar L, Wang K, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 9.Okines AF, Norman AR, McCloud P, Kang YK, Cunningham D. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. 2009;20:1529–1534. doi: 10.1093/annonc/mdp047. [DOI] [PubMed] [Google Scholar]
- 10.Fornaro L, Lucchesi M, Caparello C, Vasile E, Caponi S, Ginocchi L, Masi G, Falcone A. Anti-HER agents in gastric cancer: from bench to bedside. Nat Rev Gastroenterol Hepatol. 2011;8:369–383. doi: 10.1038/nrgastro.2011.81. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 12.Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146:264–275. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 14.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in devel-opment and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97:453–457. doi: 10.1038/sj.bjc.6603910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol. 2011;8:492–503. doi: 10.1038/nrclinonc.2011.45. [DOI] [PubMed] [Google Scholar]
- 17.Li SG, Li L. Targeted therapy in HER2-positive breast cancer. Biomed Rep. 2013;1:499–505. doi: 10.3892/br.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komoto M, Nakata B, Amano R, Yamada N, Yashiro M, Ohira M, Wakasa K, Hirakawa K. HER2 overexpression correlates with survival after curative resection of pancreatic cancer. Cancer Sci. 2009;100:1243–1247. doi: 10.1111/j.1349-7006.2009.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, da Costa WL, Soares FA. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29:3030–3036. doi: 10.1200/JCO.2010.33.6313. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XL, Yang YS, Xu DP, Qu JH, Guo MZ, Gong Y, Huang J. Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J Surg. 2009;33:2112–2118. doi: 10.1007/s00268-009-0142-z. [DOI] [PubMed] [Google Scholar]
- 21.Sheng WQ, Huang D, Ying JM, Lu N, Wu HM, Liu YH, Liu JP, Bu H, Zhou XY, Du X. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol. 2013;24:2360–2364. doi: 10.1093/annonc/mdt232. [DOI] [PubMed] [Google Scholar]
- 22.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 23.Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37 Suppl 4:S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 24.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 25.Cho J, Jeong J, Sung J, Sung CO, Kim KM, Park CK, Choi MG, Sohn TS, Bae JM, Kim S. A large cohort of consecutive patients confirmed frequent HER2 positivity in gastric carcinomas with advanced stages. Ann Surg Oncol. 2013;20 Suppl 3:S477–S484. doi: 10.1245/s10434-012-2818-0. [DOI] [PubMed] [Google Scholar]
- 26.Moelans CB, van Diest PJ, Milne AN, Offerhaus GJ. Her-2/neu testing and therapy in gastroesophageal adenocarcinoma. Patholog Res Int. 2011;2011:674182. doi: 10.4061/2011/674182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu MZ, Li Q, Wang ZQ, Liu TS, Liu Q, Wei XL, Jin Y, Wang DS, Ren C, Bai L, et al. HER2-positive patients receiving trastuzumab treatment have a comparable prognosis with HER2-negative advanced gastric cancer patients: a prospective cohort observation. Int J Cancer. 2014;134:2468–2477. doi: 10.1002/ijc.28559. [DOI] [PubMed] [Google Scholar]
- 28.Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, Jang SJ, Park YS. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18:2833–2840. doi: 10.1245/s10434-011-1695-2. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen JT. Role of human epidermal growth factor receptor 2 in gastric cancer: biological and pharmacological aspects. World J Gastroenterol. 2014;20:4526–4535. doi: 10.3748/wjg.v20.i16.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T, Nakano H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Yoo CH, et al. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci. 2006;51:1371–1379. doi: 10.1007/s10620-005-9057-1. [DOI] [PubMed] [Google Scholar]
- 32.Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, Lopez-Sanchez RI, Price T, Gladkov O, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2014:Jul 20; Epub ahead of print. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, Katai H, Sano T, Imamura H, Sasako M. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992–6000. doi: 10.1158/1078-0432.CCR-12-1318. [DOI] [PubMed] [Google Scholar]
- 34.Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57–65. doi: 10.3233/CLO-2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, Altmannsberger HM, Robinson E, Tafe LJ, Tang LH, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol. 2012;23:2656–2662. doi: 10.1093/annonc/mds104. [DOI] [PubMed] [Google Scholar]
- 36.Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–1553. doi: 10.1200/JCO.2009.25.4706. [DOI] [PubMed] [Google Scholar]
- 37.Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JG, Piedbois P, Paillot B, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648–2657. doi: 10.1200/JCO.2000.18.14.2648. [DOI] [PubMed] [Google Scholar]
- 38.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 39.Bozzetti C, Negri FV, Lagrasta CA, Crafa P, Bassano C, Tamagnini I, Gardini G, Nizzoli R, Leonardi F, Gasparro D, et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer. 2011;104:1372–1376. doi: 10.1038/bjc.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 41.Rüschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, Chenard MP, Penault-Llorca F, Nagelmeier I, Schlake W, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299–307. doi: 10.1007/s00428-010-0952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J, Kim S, Kim P, Liu X, Lee T, Kim KM, Do IG, Park JO, Park SH, Jang J, et al. A novel proteomics-based clinical diagnostics technology identifies heterogeneity in activated signaling pathways in gastric cancers. PLoS One. 2013;8:e54644. doi: 10.1371/journal.pone.0054644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez-Martin C, Plaza JC, Pazo-Cid R, Salud A, Pons F, Fonseca P, Leon A, Alsina M, Visa L, Rivera F, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol. 2013;31:4445–4452. doi: 10.1200/JCO.2013.48.9070. [DOI] [PubMed] [Google Scholar]
- 44.Roukos DH. Targeting gastric cancer with trastuzumab: new clinical practice and innovative developments to overcome resistance. Ann Surg Oncol. 2010;17:14–17. doi: 10.1245/s10434-009-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palacio S, Loaiza-Bonilla A, Kittaneh M, Kyriakopoulos C, Ochoa RE, Escobar M, Arango B, Restrepo MH, Merchan JR, Rocha Lima CM, et al. Successful use of Trastuzumab with anthracycline-based chemotherapy followed by trastuzumab maintenance in patients with advanced HER2-positive gastric cancer. Anticancer Res. 2014;34:301–306. [PubMed] [Google Scholar]
- 46.Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32:2039–2049. doi: 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]
- 47.Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, Imamura H, Gamoh M, Sakai D, Shimokawa T, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1) Br J Cancer. 2014;110:1163–1168. doi: 10.1038/bjc.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui H, Cheng Y, Piao SZ, Xu YJ, Sun HH, Cui X, Li XZ, Zhang SN, Piao LZ, Jin YM, et al. Correlation between HER-2/neu(erbB-2) expression level and therapeutic effect of combination treatment with HERCEPTIN and chemotherapeutic agents in gastric cancer cell lines. Cancer Cell Int. 2014;14:10. doi: 10.1186/1475-2867-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 50.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 51.Kang YK, Rha SY, Tassone P, Barriuso J, Yu R, Szado T, Garg A, Bang YJ. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br J Cancer. 2014;111:660–666. doi: 10.1038/bjc.2014.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, Ellis B, Pennisi C, Horne E, Lackey K, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–6659. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 53.Iqbal S, Goldman B, Fenoglio-Preiser CM, Lenz HJ, Zhang W, Danenberg KD, Shibata SI, Blanke CD. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22:2610–2615. doi: 10.1093/annonc/mdr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimoyama S. Unraveling trastuzumab and lapatinib inefficiency in gastric cancer: Future steps (Review) Mol Clin Oncol. 2014;2:175–181. doi: 10.3892/mco.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oshima Y, Tanaka H, Murakami H, Ito Y, Furuya T, Kondo E, Kodera Y, Nakanishi H. Lapatinib sensitivities of two novel trastuzumab-resistant HER2 gene-amplified gastric cancer cell lines. Gastric Cancer. 2014;17:450–462. doi: 10.1007/s10120-013-0290-6. [DOI] [PubMed] [Google Scholar]
- 56.Barok M, Tanner M, Köninki K, Isola J. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett. 2011;306:171–179. doi: 10.1016/j.canlet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Barros-Silva JD, Leitão D, Afonso L, Vieira J, Dinis-Ribeiro M, Fragoso M, Bento MJ, Santos L, Ferreira P, Rêgo S, et al. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer. 2009;100:487–493. doi: 10.1038/sj.bjc.6604885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong SJ, Jin CJ, Rha SY, Chung HC. Growth inhibitory effects of trastuzumab and chemotherapeutic drugs in gastric cancer cell lines. Cancer Lett. 2004;214:215–224. doi: 10.1016/j.canlet.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 59.Dungo RT, Keating GM. Afatinib: first global approval. Drugs. 2013;73:1503–1515. doi: 10.1007/s40265-013-0111-6. [DOI] [PubMed] [Google Scholar]
- 60.Matsuoka T, Yashiro M. The Role of PI3K/Akt/mTOR Signaling in Gastric Carcinoma. Cancers (Basel) 2014;6:1441–1463. doi: 10.3390/cancers6031441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banerji U. Heat shock protein 90 as a drug target: some like it hot. Clin Cancer Res. 2009;15:9–14. doi: 10.1158/1078-0432.CCR-08-0132. [DOI] [PubMed] [Google Scholar]
- 62.Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, Patterson L, de Haven Brandon A, Gowan S, Boxall F, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850–2860. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- 63.Wainberg ZA, Anghel A, Rogers AM, Desai AJ, Kalous O, Conklin D, Ayala R, O’Brien NA, Quadt C, Akimov M, et al. Inhibition of HSP90 with AUY922 induces synergy in HER2-amplified trastuzumab-resistant breast and gastric cancer. Mol Cancer Ther. 2013;12:509–519. doi: 10.1158/1535-7163.MCT-12-0507. [DOI] [PubMed] [Google Scholar]
- 64.Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A, Elbi C, Lutterbach B. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 2008;68:2340–2348. doi: 10.1158/0008-5472.CAN-07-5229. [DOI] [PubMed] [Google Scholar]
- 65.Nam HJ, Ching KA, Kan J, Kim HP, Han SW, Im SA, Kim TY, Christensen JG, Oh DY, Bang YJ. Evaluation of the antitumor effects and mechanisms of PF00299804, a pan-HER inhibitor, alone or in combination with chemotherapy or targeted agents in gastric cancer. Mol Cancer Ther. 2012;11:439–451. doi: 10.1158/1535-7163.MCT-11-0494. [DOI] [PubMed] [Google Scholar]
- 66.Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol. 2011;3:S21–S35. doi: 10.1177/1758834011422557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19:542–551. doi: 10.1016/j.tcb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 69.Chen CT, Kim H, Liska D, Gao S, Christensen JG, Weiser MR. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol Cancer Ther. 2012;11:660–669. doi: 10.1158/1535-7163.MCT-11-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh R, Kim WJ, Kim PH, Hong HJ. Combined blockade of HER2 and VEGF exerts greater growth inhibition of HER2-overexpressing gastric cancer xenografts than individual blockade. Exp Mol Med. 2013;45:e52. doi: 10.1038/emm.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, Fujimoto-Ouchi K. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17:5060–5070. doi: 10.1158/1078-0432.CCR-10-2927. [DOI] [PubMed] [Google Scholar]
- 72.Sendur MA, Aksoy S, Altundag K. Pertuzumab in HER2-positive breast cancer. Curr Med Res Opin. 2012;28:1709–1716. doi: 10.1185/03007995.2012.728132. [DOI] [PubMed] [Google Scholar]
- 73.Nagumo Y, Faratian D, Mullen P, Harrison DJ, Hasmann M, Langdon SP. Modulation of HER3 is a marker of dynamic cell signaling in ovarian cancer: implications for pertuzumab sensitivity. Mol Cancer Res. 2009;7:1563–1571. doi: 10.1158/1541-7786.MCR-09-0101. [DOI] [PubMed] [Google Scholar]
- 74.Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ. P27(kip1) down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004;64:3981–3986. doi: 10.1158/0008-5472.CAN-03-3900. [DOI] [PubMed] [Google Scholar]
- 75.Oyama K, Fushida S, Tsukada T, Kinoshita J, Watanabe T, Shoji M, Nakanuma S, Okamoto K, Sakai S, Makino I, et al. Evaluation of serum HER2-ECD levels in patients with gastric cancer. J Gastroenterol. 2014:Feb 21; Epub ahead of print. doi: 10.1007/s00535-014-0941-3. [DOI] [PubMed] [Google Scholar]
- 76.Martin HL, Smith L, Tomlinson DC. Multidrug-resistant breast cancer: current perspectives. Breast Cancer (Dove Med Press) 2014;6:1–13. doi: 10.2147/BCTT.S37638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yashiro M, Nishii T, Hasegawa T, Matsuzaki T, Morisaki T, Fukuoka T, Hirakawa K. A c-Met inhibitor increases the chemosensitivity of cancer stem cells to the irinotecan in gastric carcinoma. Br J Cancer. 2013;109:2619–2628. doi: 10.1038/bjc.2013.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sato Y, Yashiro M, Takakura N. Heregulin induces resistance to lapatinib-mediated growth inhibition of HER2-amplified cancer cells. Cancer Sci. 2013;104:1618–1625. doi: 10.1111/cas.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim HP, Han SW, Song SH, Jeong EG, Lee MY, Hwang D, Im SA, Bang YJ, Kim TY. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene. 2014;33:3334–3341. doi: 10.1038/onc.2013.285. [DOI] [PubMed] [Google Scholar]


