Summary
This study evaluated contrast-enhanced magnetic resonance angiography (CE-MRA) and three-dimensional time-of-flight magnetic resonance angiography (3D-TOF-MRA) through comparisons with digital subtraction angiography (DSA) for the follow-up of intracranial aneurysms treated with detachable coils.
Sixty-seven patients with 79 aneurysms underwent 3D-TOF-MRA, CE-MRA, and catheter angiography one year after coiling. Two independent observers classified recanalization status on images as neck or body remnant or no recanalization. For 3D-TOF-MRA and CE-MRA, the intermodality agreement, interobserver agreement, and correlation with angiography were assessed.
Sixty-seven patients with 79 coiled aneurysms agreed to participate in the study. Three aneurysms could not be detected on 3D-TOF-MRA, so they were excluded from this study. Interobserver agreement was very good for 3D-TOF-MRA and CE-MRA (kappa (κ): 0.87, 0.94, respectively). Correlation of TOF-MRA with angiography was good (κ: 0.76). Correlation of CE-MRA with angiography was excellent (κ: 0.91). The sensitivity and specificity of TOF-MRA were 92% and 98%, respectively. The sensitivity and specificity of CE-MRA were 96% and 98%, respectively.
After selective embolization of intracranial aneurysms, CE-MRA is useful and comparable to DSA in the assessment of aneurysmal recanalization. Agreement with the gold standard is stronger with CE-MRA than with 3D-TOF-MRA.
Keywords: TOF angiography, CE angiography, coiled aneurysm follow-up
Introduction
Endovascular treatment has now become a serious option for the treatment of intracranial aneurysms 1-3. One of the criticisms of endovascular aneurysm coiling compared with surgical clipping is the recurrence rate 4,5, which has been evaluated using several imaging techniques.
Digital subtraction angiography (DSA) has traditionally been the technique of choice for the follow-up of treated aneurysms. However, DSA is invasive and carries with it a measurable degree of morbidity 6,7. Other issues to consider with DSA are patient discomfort, cost of the procedure, and radiation exposure. Magnetic resonance angiography (MRA) may provide a noninvasive alternative with less discomfort and morbidity for patients. MRA has been performed by several groups to monitor coiled aneurysms 8,9. MRA can also be performed without contrast enhancement using three dimensional time-of-flight MRA (3D-TOF-MRA) or contrast-enhanced MRA (CE-MRA). Spin dephasing and saturation are two limitations of 3D TOF MRA. Progress in MRA has been reported with ultrafast CE-MRA (reduction of acquisition time and k-space elliptic central acquisition), which was mainly developed for carotid angiography, and it seems a promising technique for intracranial aneurysm detection 10. The aim of this study is to evaluate CE-MRA and 3D TOF MRA by comparison with DSA for the follow-up of intracranial aneurysms treated with detachable coils.
Materials and Methods
Patients
Our prospective study was approved by the local ethics committee and was conducted between May 2008 and November 2012. Written informed consent was obtained from all participants. The study included 67 patients (33 women, 34 men; mean age 59 ± 13.8, with a range of 33-78 years) with a total of 79 coiled aneurysms. Control 3D-TOF and CE-MRA as well as DSA were performed in all participants one year after the procedure. All patients were requested to undergo TOF-MRA and CE-MRA at 1.5 Tesla on the same day as their standard follow-up angiography. All patients included in the study had undergone endovascular coiling of intracranial aneurysms. Patients under the age of 18, suffering from claustrophobia (n:2) or fitted with a pacemaker (n:1), and those with additional aneurysms treated with neurosurgical clips were excluded from the study. Complications of catheter angiography and CE-MRA were recorded.
Endovascular Treatment
Endovascular embolization of the aneurysms was performed by using detachable coils on a DSA system (Toshiba Infinix VC-i, Toshiba Medical Systems Corporation, Tokyo, Japan). All patients were treated under general anesthesia. Platinum coils were used, including detachable coils (Axium and Microvention, Inc). Only coiling was performed in 43 procedures; the balloon remodeling technique was performed in 19 procedures, and stent-assisted coiling was performed in 17 procedures. For stent-assisted coiling procedures, a flow diverter Pipeline embolization device (n:3), Enterprise stent (n:3) or Solitaire stent (n:11) were used. All the stents used were compatible with MRI. In cases of stent-assisted coiling, we gave the patient 100 mg of aspirin and 75 mg of Plavix (clopidogrel) per day for seven days before the procedure. In addition, we used VerifyNow (Accumetrics, San Diego, CA, USA) to calculate P2Y12 reaction units and percentage platelet inhibition immediately before the endovascular procedure. Clopidogrel resistance or low response was defined as percentage platelet inhibition ≤ 20%. After the stent-assisted coiling procedure, we maintained an intravenous heparin drip for up to 72 hours. The target activated partial thromboplastin time was 1.5-2.5 times the mean of the normal range (target activated partial thromboplastin time 65-90 seconds). The patients were given 100 mg aspirin (acetylsalicylic acid) for life and 75 mg Plavix per day for ≥ eight to nine weeks after the procedure.
Imaging Procedure
Angiographic evaluation
Follow-up angiography was performed on a flat-panel angiographic unit. Six to 8 mL of nonionic contrast material (iodixanol, Visipaque 320 mg I/mL; Amersham Health, Cork, Ireland) was injected into either the internal carotid or vertebral artery with a power injector at 4-6 mL/s. Three views were acquired of each patient, including the working projection of the endovascular treatment.
Contrast-enhanced MR angiography
All MRI examinations were performed using a 1.5-T MRI scanner (MagnetomAvanto, Siemens Healthcare) with an 18-channel body coil and high performance gradients (maximum gradient, 45 mT/m; maximum slew rate, 200 T/m/s). All examinations were performed with a standard head coil. A CE-MRA fast imaging with steady-state precession (FISP) sequence with a rectilinear k-space sampling (flip angle, 30°; field of view (FOV), 230 mm; matrix, 259×384; TR, 3.44; TE, 1.39; slice thickness, 1.0 mm) was acquired in the coronal plane with an acquisition time of 40 seconds. A bolus of 0.2 mmol/kg of gadolinium chelate (gadoterate meglumine; Dotarem, Guerbet, France) was injected at a rate of 2 mL/s using an MR-compatible power injector. Source images were then reconstructed using a maximum intensity projection (MIP) algorithm. Multiple projections using a large FOV were obtained every 15° over 180 in lateral and antero-posterior views, providing 12 views in total.
TOF MR angiography
TOF images were acquired in the transverse plane with the following parameters: TR/ TE, 25/7 ms; flip angle, 25 degrees; matrix, 211×256; FOV, 200×170 mm; slice thickness, 0.7 mm. Source images were then reconstructed using an MIP algorithm. Multiple projections using a large FOV were obtained every 15 degrees over 180 in lateral and antero-posterior views, providing 12 views in total.
Image evaluation
Aneurysm occlusion status on a 12-month follow-up angiogram was assessed by an experienced interventional neuroradiologist blinded to parallel MRA results. The occlusion statuses of the coiled aneurysms on TOF-MRA and CE-MRA were classified by a three-tier scale as complete occlusion, neck remnant, or body remnant. All TOF-MRA and CE-MRA images were evaluated independently and in random order by two experienced interventional neuroradiologists (LA and ES with 20 and 15 years of experience, respectively) unaware of the parallel angiography results. DSA images were fully and coherently evaluated by independent authors (YI and OO) unaware of MRI results. Source images, 3D MIP, and 3D volume-rendered reconstructions were available on a Vitrea workstation (Vitrea version 3.0.1; Vital Images, Plymouth, MN, USA). Occlusion status of the coiled aneurysms was classified in the same manner as for angiography.
Statistical Analysis
κ statistics were used to assess interobserver agreement for TOF-MRA and CE-MRA and intermodality agreement between TOF-MRA and CE-MRA and to correlate the data of both MRA techniques with angiographic findings. The interpretation of κ was as follows: moderate for 0.4 < κ ≤ 0.60, good for 0.6 < κ ≤ 0.80, and excellent for κ > 0.80. Test characteristics of TOF-MRA and CE-MRA with corresponding 95% confidence intervals (CI) were calculated for the three-tier occlusion scale.
Results
MR angiography and DSA were successfully performed in all 67 patients (79 aneurysms). There were no complications, either from angiography or MRA. On 3D-TOF-MRA, three aneurysms could not be assessed due to coil artifacts and were excluded; the remaining aneurysms were evaluated. An illustrative case shows a large anterior communicating artery (ACoA) aneurysm before and after endovascular treatment (Figure 1). DSA revealed instances of adequate occlusion [neck remnant in 11 (14.4%) and no remnant in 59 (77.6%)] and incomplete occlusion [body remnant in six (7.8%)] of the 76 aneurysms. CE-MRA revealed complete occlusion in 61 aneurysms, neck remnants in ten aneurysms and body remnants in five aneurysms (Tables 1 and 2). The detailed cross-table for observers is displayed in Table 3. None of the cases experienced image loss associated with stent-assisted coiling. In three cases, TOF-MRA missed neck remnants (false negative) and in one case a competently coiled aneurysm was misdiagnosed as a neck remnant (false positive) (Figure 2). The aneurysms were located in the anterior circulation and in the posterior circulation. The locations of the aneurysms are presented in Table 4. The aneurysms ranged from three to 20 mm.
Table 1.
Correlation between TOF-MRA and DSA. Correlation for the three-tier classification was good (κ: 0.76; CI: 0.60-0.92) (p<0.05).
| Recanalization |
TOF Observer 1 |
TOF Observer 2 |
DSA |
| No | 62 | 65 | 59 |
| Neck | 9 | 5 | 11 |
| Body | 5 | 6 | 6 |
Table 2.
Correlation between CE-MRA and DSA. Correlation for the three-tier classification was excellent (κ: 0.91; CI: 0.82-1.00) (p<0.05).
| Recanalization |
CE Observer 1 |
CE Observer 2 |
DSA |
| No | 61 | 61 | 59 |
| Neck | 10 | 10 | 11 |
| Body | 5 | 5 | 6 |
Table 3.
A detailed cross table from observers for 3D TOF and CE MRA. Intermodality agreement for observer 1 and observer 2 (κ: 0.90; 95% CI, 0.80-1.00 and, κ: 0.84; 95% CI, 0.70-0.98, respectively) were excellent.
| CE MRA 1 | TOF 1 | |||
| No | Neck | Body | ||
| No | 60 | 1 | 0 | 61 (80.3%) |
| Neck | 2 | 8 | 0 | 10 (13.2%) |
| Body | 0 | 0 | 5 | 5 (6.6%) |
| Total | 62 (81.6%) |
9 (11.8%) |
5 (6.6%) |
76 |
| CE MRA 2 | TOF 2 | |||
| No | Neck | Body | ||
| No | 61 | 0 | 0 | 61 (80.3%) |
| Neck | 4 | 5 | 1 | 10 (13.2%) |
| Body | 0 | 0 | 5 | 5 (6.6%) |
| Total | 65 (85.5%) |
5 (6.6%) |
6 (7.9%) |
76 |
Table 4.
Aneurysm location.
| Anterior Circulation | Posterior Circulation | ||
| ACoA | 27 | PCA | 4 |
| ICA | 23 | VA | 1 |
| ACA | 2 | ||
| MCA | 18 | ||
| PCoA | 4 | ||
| Total | 74 | Total | 5 |
| ACoA: anterior communicating artery; ICA: internal cerebral artery; ACA: anterior cerebral artery; MCA: middle cerebral artery; PCoA: posterior communicating artery; PCA: posterior cerebral artery; VA: vertebral artery. | |||
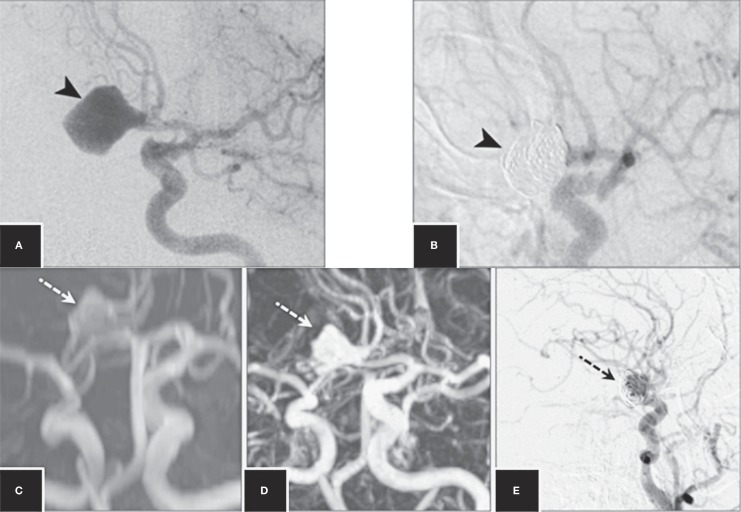
Figure 1.
DSA image shows an ACoA aneurysm (A) and coiled aneurysm (B) after endovascular treatment (arrowheads). Follow-up 3D-TOF-MRA (C), CE-MRA (D) and DSA (E) images at 12 months show a body remnant (dashed arrows).
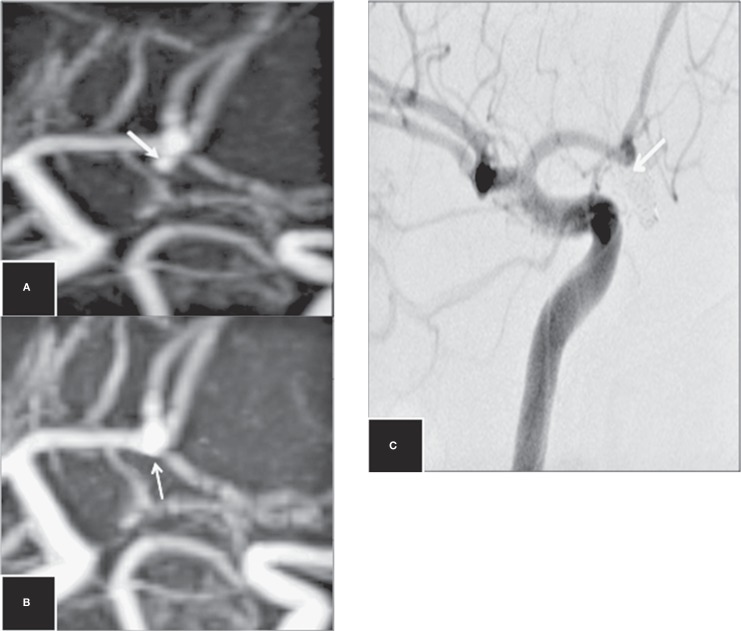
Figure 2.
Disagreement between 3D-TOF-MRA and DSA on the occlusion of an ACoA aneurysm. Follow-up TOF-MRA (A) and CE-MRA (B) images at 12 months show a small neck remnant (false positive neck remnant). Follow-up DSA image (C) at 12 months shows complete occlusion (arrows).
Interobserver agreement was very good for TOF-MRA (κ: 0.87; 95% confidence interval [CI], 0.74-0.99) and CE-MRA (κ: 0.94; 95% CI: 0.86-1.00). Correlation of TOF-MRA with angiography was good (κ: 0.76; CI: 0.60-0.92) (Table 1). Correlation of CE-MRA with angiography was excellent (κ: 0.91; CI:0.82-1.00) (Table 2). The sensitivity and specificity of TOF-MRA for both observers 1 and 2 were 92% (95% CI, 83%-96%) and 98% (95% CI, 90%-99%), respectively. The sensitivity and specificity of CE-MRA for observers 1 and 2 were 96% (95% CI, 88%-99%) and 98% (95% CI, 90%-99%), respectively. Intermodality agreement for observers 1 and 2 (κ: 0.84; 95% CI, 0.70-0.98 and κ: 0.90; 95% CI, 0.80-1.00, respectively) were excellent (Table 3).
Discussion
Long-term follow-up is required for embolized aneurysms to determine the stability of treatment and identify a possible recanalization, which may require retreatment due to the risk of bleeding. Considering the drawbacks inherent in DSA, MRI has been suggested as a non-invasive method of monitoring embolized cerebral aneurysms 11,12 .
Several studies have compared various MRA techniques to DSA with the ultimate goal of obviating repeated DSA in these patients 13-15. Earlier studies suggested non-enhanced 3D TOF MRA as the method of choice for monitoring coiled aneurysms. Although some of these reports showed encouraging results for 3D TOF MRA 16, others did not because neck remnants and even recurrent aneurysms were repeatedly overlooked using this technique. Furthermore, a recent study of TOF MRA showed that the needs for retreatment were underestimated compared with DSA 17. TOF-MRA is insensitive to a slow or complex flow and can interfere with the visualization of a residual neck or aneurysm. This signal loss can be attributed to intravoxel dephasing and saturation effects. On the other hand, TOF MRA is more sensitive to susceptibility artifacts because of coil packing. In our study, three cases were excluded because of these artifacts. In addition, our study showed three false negatives and one false positive with 3D TOF-MRA. DSA and CE-MRA images were consistent with each other in these cases (Figure 2). This was explained by reduced flow within the aneurysm leading to a hypointensity misinterpreted as a complete occlusion and the presence of residual hemorrhage or artifacts leading to a hyperintensity misinterpreted as residual flow 18,19. In a similar study among 26 treated aneurysms, Derdeyn et al. detected residual flow in coils with 3D TOF MRA, two false negatives and two false positives 20.
CE-MRA may have potential advantages compared with the 3D TOF technique because the flow within an embolized aneurysm is complex. CE-MRA is less sensitive to flow turbulences and saturation effects than TOF sequences because of the high signal intensity within the arterial lumen caused by T1-shortening effects. Contrast enhancement allows for the imaging of low-flow signals, which theoretically allows for greater conspicuity of a residual aneurysm. Moreover, CE-MRA has demonstrated a relative insensitivity to coil-related artifacts that may potentially degrade image quality and hinder visualization of a residual neck.
Several authors have evaluated CE-MRA versus TOF-MRA for coiled aneurysms, and most have reported the superiority of CE-MRA 8,15. We found similar results, especially that neck remnant aneurysms are more visible with CE-MRA. In our four cases, a residual neck appeared in DSA and CE-MRA, but not in TOF-MRA. Although this result is not statistically significant, it should be evaluated in a larger series.
Most interestingly, several studies revealed that CE-MRA is more compatible than DSA for patients in whom aneurysm body remnants extend or invaginate into the coil mass. This can be explained by the radiodensity of the coil mass itself. In these instances, the coils surrounding the aneurysmal remnant create an impenetrable radiodense helmet around the remnant. This makes it difficult to identify the remnant on routine DSA (anteroposterior, lateral, and rotational views) due to lack of photon penetration through the coil mass 20. Therefore, because the helmet phenomenon may result in a false positive result, such cases should be examined more carefully. MRA enjoys a distinct advantage over DSA for the visualization of this type of remnant because it is not affected by the helmet phenomenon and there are only minimal susceptibility artifacts arising from platinum coils 13,17.
In a study related to CE-MRA for follow-up on aneurysms post stent-assisted coiling, CE-MRA showed false stenosis of the stented artery in six cases and exaggerated stenosis in two cases. In 18 cases, CE-MRA showed a short focal “pseudo-stenosis” (marker band effect) where the stent's marker bands were located. Despite metal artifacts such as these, the study showed that the presence of a metal stent in the parent artery at the aneurysmal neck does not impair the ability of CE-MRA to reveal remnants 21. Similarly, these artifacts did not prevent evaluation of any neck remnant in our study.
In a similar study, Sprengers et al. discovered that TOF-MRA and CE-MRA at 3T were equivalent in evaluating the occlusion status of intracranial aneurysms after coiling 17. This result is most likely related to the ability of TOF-MRA to produce better images at 3T because of the length of T1. Thus, background tissue is suppressed and better images are provided in the vascular structures.
The limitations of our study should be recognized. Firstly, the patient population was relatively small. Our results will need to be confirmed in a larger prospective study. Secondly, although the helmet phenomenon may be present in false positive cases, such cases should be examined more carefully.
Conclusions
Follow-up imaging with CE-MRA after selective embolization of intracranial aneurysms can be used to detect and categorize aneurysmal recanalization as either residual neck or aneurysmal sac.
Although TOF MRA is inferior to CE-MRA, it performed quite well and only missed small neck remnants.
References
- 1.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–1274. doi: 10.1016/s0140-6736(02)11314-6. doi: 10.1016/S0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 2.Vrsajkov V, Kolak R, Uram-Benka A, et al. Anesthesia, complications, and clinical outcome for ruptured intracranial aneurysms: a retrospective comparison between endovascular coiling and neurosurgical clipping. Turk J Med Sci. 2012;42(3):477–483. [Google Scholar]
- 3.Hirsch JA, Bendok BR, Paulsen RD, et al. Midterm clinical experience with a complex-shaped detachable platinum coil system for the treatment of cerebral aneurysms: Trufill DCS Orbit detachable coil system registry interim results. J Vasc Interv Radiol. 2007;18(12):1487–1494. doi: 10.1016/j.jvir.2007.07.020. doi: 10.1016/j.jvir.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 5.Shankar JJ, Lum C, Parikh N, et al. Long-term prospective follow-up of intracranial aneurysms treated with endovascular coiling using contrast-enhanced MR angiography. Am J Neuroradiol. 2010;31(7):1211–1215. doi: 10.3174/ajnr.A2064. doi: 10.3174/ajnr.A2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willinsky RA, Taylor SM, terBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227(2):522–528. doi: 10.1148/radiol.2272012071. doi: 10.1148/radiol.2272012071. [DOI] [PubMed] [Google Scholar]
- 7.Peker A, Ustüner E, Ozkavukcu E, et al. Performance analysis of 8-channel MDCT angiography in detection, localization, and sizing of intracranial aneurysms identified on DSA. Diagn Interv Radiol. 2009;15(2):81–85. [PubMed] [Google Scholar]
- 8.Leclerc X, Navez J, Gauvrit J, et al. Aneurysms of the anterior communicating artery treated with Guglielmi detachable coils: follow-up with contrast-enhanced MR angiography. Am J Neuroradiol. 2002;23(7):1121–1127. [PMC free article] [PubMed] [Google Scholar]
- 9.Cottier J, Bleuzen-Couthon A, Gallas S, et al. Intracranial aneurysms treated with Guglielmi detachable coils: is contrast material necessary in the follow-up with 3D time-of-flight MR angiography? Am J Neuroradiol. 2003;24(9):1797–1803. [PMC free article] [PubMed] [Google Scholar]
- 10.Pierot L, Delcourt C, Bouquigny F, et al. Follow up of intracranial aneurysms selectively treated with coils: prospective evaluation of contrast-enhanced MR angiography. Am J Neuroradiol. 2006;27(4):744–749. [PMC free article] [PubMed] [Google Scholar]
- 11.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke. 1999;30(2):317–320. doi: 10.1161/01.str.30.2.317. doi: 10.1161/01.STR.30.2.317. [DOI] [PubMed] [Google Scholar]
- 12.Agid R, Willinsky RA, Lee SK, et al. Characterization of aneurysm remnants after endovascular treatment: contrast-enhanced MR angiography versus catheter digital subtraction angiography. Am J Neuroradiol. 2008;29(8):1570–1574. doi: 10.3174/ajnr.A1124. doi: 10.3174/ajnr.A1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okahara M, Kiyosue H, Hori Y, et al. Three-dimensional time-of-flight MR angiography for evaluation of intracranial aneurysms after endosaccular packing with Guglielmi detachable coils: comparison with 3D digital subtraction angiography. Eur Radiol. 2004;14(7):1162–1168. doi: 10.1007/s00330-004-2277-5. doi: 10.1007/s00330-004-2277-5. [DOI] [PubMed] [Google Scholar]
- 14.Boulin A, Pierot L. Follow-up of intracranial aneurysms treated with detachable coils: comparison of gadolinium-enhanced 3D time-of-flight MR angiography and digital subtraction angiography. Radiology. 2001;219(1):108–113. doi: 10.1148/radiology.219.1.r01mr06108. doi: 10.1148/radiology.219.1.r01mr06108. [DOI] [PubMed] [Google Scholar]
- 15.Majoie CB, Sprengers ME, van Rooij WJ, et al. MR angiography at 3T versus digital subtraction angiography in the follow-up of intracranial aneurysms treated with detachable coils. Am J Neuroradiol. 2005;26(6):1349–1356. [PMC free article] [PubMed] [Google Scholar]
- 16.Costalat V, Lebars E, Sarry L, et al. In vitro evaluation of 2D-digital subtraction angiography versus 3D-time-of-flight in assessment of intracranial cerebral aneurysm filling after endovascular therapy. Am J Neuroradiol. 2006;27(1):177–184. [PMC free article] [PubMed] [Google Scholar]
- 17.Sprengers ME, Schaafsma JD, van Rooij WJ, et al. Evaluation of the occlusion status of coiled intracranial aneurysms with MR angiography at 3T: is contrast enhancement necessary? Am J Neuroradiol. 2009;30(9):1665–1671. doi: 10.3174/ajnr.A1678. doi: 10.3174/ajnr.A1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anzalone N, Righi C, Simionato F, et al. Three-dimensional time-of-flight MR angiography in the evaluation of intracranial aneurysms treated with Guglielmi detachable coils. Am J Neuroradiol. 2000;21(4):746–752. [PMC free article] [PubMed] [Google Scholar]
- 19.Gauvrit JY, Leclerc X, Caron S, et al. Intracranial aneurysms treated with Guglielmi detachable coils: imaging follow-up with contrast-enhanced MR angiography. Stroke. 2006;37(4):1033–1037. doi: 10.1161/01.STR.0000209236.06451.3b. doi: 10.1161/01.STR.0000209236.06451.3b. [DOI] [PubMed] [Google Scholar]
- 20.Derdeyn CP, Graves VB, Turski PA, et al. MR angiography of saccular aneurysms after treatment with Guglielmi detachable coils: preliminary experience. Am J Neuroradiol. 1997;18:279–286. [PMC free article] [PubMed] [Google Scholar]
- 21.Agid R, Schaaf M, Farb R. CE-MRA for follow-up of aneurysms post stent-assisted coiling. Interv Neuroradiol. 2012;18:275–283. doi: 10.1177/159101991201800305. [DOI] [PMC free article] [PubMed] [Google Scholar]