Summary
The liquid embolic agents currently used for embolization of cerebral arteriovenous malformations are Onyx and NBCA. Glubran 2, a cyanoacrylate-based synthetic glue, has recently been applied for embolization of cerebral arteriovenous malformations (AVMs). We report the clinical results of selected cerebral AVMs treated with Glubran 2 targeting for curative embolization.
Between October 2011 and March 2013, 31 patients with cerebral AVMs were selected for curative embolization with Glubran 2. There were 19 men and 12 women with a mean age of 32 years (range 4–65 years). Initial clinical presentation included hemorrhage in 28 and seizures in three patients. AVM location was frontal in eight patients, parietal in four, occipital in eight temporal in six, cerebellar in two and cerebellar vermis in three patients. Follow-up was performed clinically and with angiography examination at three to six months. Clinical outcomes were evaluated based on the modified Rankin Scale (mRS).
A mean of 2.5 (range, 1–12) feeding pedicles were embolized per patient. Complete angiographic obliteration of AVM was achieved in 27 patients. A hemorrhagic complication was observed in one patient, an ischemic complication in one patient and technical complications in four patients. There was no procedure-related disabling neurological deficit or death at discharge. Additional gamma knife radiosurgery was performed in five patients, including one patient with recurrent AVM. All of the patients had favorable clinical outcomes at three to six month follow-up (mRS≤2).
The curative embolization technique with Glubran 2 for selected cerebral AVMs achieved a high initial complete obliteration rate with an acceptable complication frequency.
Keywords: arteriovenous malformation; cerebral; curative embolization; embolization, Glubran 2
Introduction
Treatment modalities for cerebral arteriovenous malformations (AVMs) include embolization, surgery and radiosurgery, either alone or in combination. With the improvements in materials and technique, especially with the advent of Onyx (ev3, Irvine, CA, USA), endovascular embolization is no longer restricted to partial AVM nidus obliteration of large AVMs to facilitate subsequent surgery or radiosurgery. In many centers, Onyx has replaced the use of acrylic glue for the obliteration of cerebral AVMs. Moreover, the curative rate for Onyx embolization of cerebral arteriovenous malformations aiming at complete obliteration ranged from 51% to 100%, with a morbidity of 0-8% and mortality of 0-3% 1-3.
In our department, Onyx has been applied for embolization of cerebral arteriovenous malformations since 2003. At the same time, we also used acrylic glue for embolization of cerebral AVMs. In recent years, with a better understanding of Onyx and acrylic glue, we chose Glubran 2, a cyanoacrylate-based synthetic glue, as the first-line liquid embolic agent for a subgroup of selected patients with cerebral AVMs. In this study, we report our results of cerebral AVMs treated with Glubran 2 targeting for curative embolization.
Materials and Methods
Patient Selection
Patients were carefully evaluated before making the decision for curative embolization with Glubran 2. Among the patients with cerebral arteriovenous malformations admitted to our department, curative embolization with Glubran 2 was considered only for low Spetzler-Martin grade patients with feeding arteries accessible for microcatheters 3. In practice, we limited the Spetzler-Martin grade to I-II and a considerable proportion of the subgroup patients were small- and medium-sized superficially located cerebral AVMs. In consideration of hemorrhage being the most common and life-threatening clinical presentation, curative embolization was considered as the first-line treatment for ruptured AVMs. By contrast, for unruptured low Spetzler-Martin grade cerebral AVMs, the initiation of treatment should be well considered while weighing potential benefits and treatment-related risk. For patients not suitable for curative embolization, such as large AVMs or eloquence region-evolved AVMs, partially targeted embolization was offered to eliminate the risk factors, such as AVM-associated aneurysms or arteriovenous fistula, and to reduce the volume of the AVMs to facilitate subsequent radiosurgery.
Patients
Between October 2011 and March 2013, 31 patients with cerebral arteriovenous malformations were selected for curative embolization with Glubran 2. These patients' clinical records, image data, embolization procedures and follow-up data were collected and analyzed. Twenty-four patients were initially treated and seven had been partially treated with Onyx previously. There were 19 men and 12 women with a mean age of 32.6 years (range 4-65 years). The clinical presentations at admission were intracranial hemorrhage in 17 patients, intraventricular hemorrhage in six, subarachnoid hemorrhage in five, and seizures in three patients. AVM location was frontal in eight, parietal in four, occipital in eight, temporal in six, cerebellar in two and cerebellar vermis in three patients. AVM-associated aneurysms were identified in ten patients, including intranidal aneurysms in four and feeding artery aneurysms in six patients. Furthermore, three patients harbored multiple associated feeding artery aneurysms. AVM size and angioarchitecture were evaluated based on MR imaging and angiography. According to the Spetzler-Martin grading scale 4, 24 AVMs were grade I and seven were grade II (Table 1).
Table 1.
Baseline characteristics in 31 patients with cerebral arteriovenous malformations (AVM) treated with Glubran 2.
| Characteristics | No. (%) |
|
Demography Mean age in years Male Female Initial AVM presentation Hemorrhage Seizures Morphology Concurrent aneurysm Intranidal aneurysm Feeding artery aneurysm Spetzler-Martin grade I II |
32.6 19 (61.3%) 12 (38.7%) 28 (90.3%) 3 (9.7%) 3 (9.7%) 7 (22.6% 24 (77.4%) 7 (22.6%) |
Table 2.
Treatment and follow-up outcomes in 31 patients with cerebral arteriovenous malformations (AVM) treated with Glubran 2.
| Outcomes | No. (%) |
|
Treatment outcomes Total occlusion Near-total occlusion (>90%) Gamma knife radiosurgery Treatment-related complications Technical Hemorrhagic Ischemic Follow-up outcomes Recurrence of AVM Clinical outcomes (mRS) mRS≤2 mRS≥3 |
27 (87.1%) 4 (12.9%) 5 (16.1%) 4 (12.9%) 1 (3.2%) 1 (3.2%) 1 (3.2%) 31 (100%) 0 |
| mRS, modified Rankin Scale. | |
Embolization Technique with Glubran 2
All embolizations were performed aiming at complete obliteration of the arteriovenous malformations. For patients with associated aneurysms, complete occlusion of the aneurysms was also our target. All procedures were carried out under general anesthesia without systemic heparinization. Through a trans-femoral route, a 6F guiding catheter, continuously flushed with heparinized saline (1,500 IU/L), was positioned in the internal carotid or vertebral artery supplying the AVM. Generally, the microcatheter (Marathon, ev3) was introduced into the feeding pedicle as near as possible to the nidus of the AVM, while for patients harboring associated feeding artery aneurysms, the microcatheter tip was placed near the proximal part of the aneurysm for embolization of the aneurysm and the AVM nidus at the same time.
Superselective angiography via the microcatheter with a 1mL syringe was performed to evaluate any normal branches, AVM architecture, flow volume, and to illustrate the anatomy of the proximal part of the draining vein. Then the microcatheter was flushed with 5% glucose solution, and 20% Glubran 2 was injected under subtracted fluoroscopy. Once reflux occurred, the injection was terminated and the microcatheter was withdrawn. Control angiography was performed to evaluate the AVM nidus. If there was another feeding pedicle, the above procedure was repeated with a new microcatheter.
Total occlusion was defined as complete angiographic obliteration of the AVM nidus, and for patients with associated aneurysms, total occlusion also meant complete obliteration of the aneurysms (Figure 1). Near-total occlusion meant more than 90% reduction of the AVM volume. The following parameters were recorded: the number of embolization sessions, the number of feeding pedicles embolized, complete obliteration rate, and procedure-related complications.
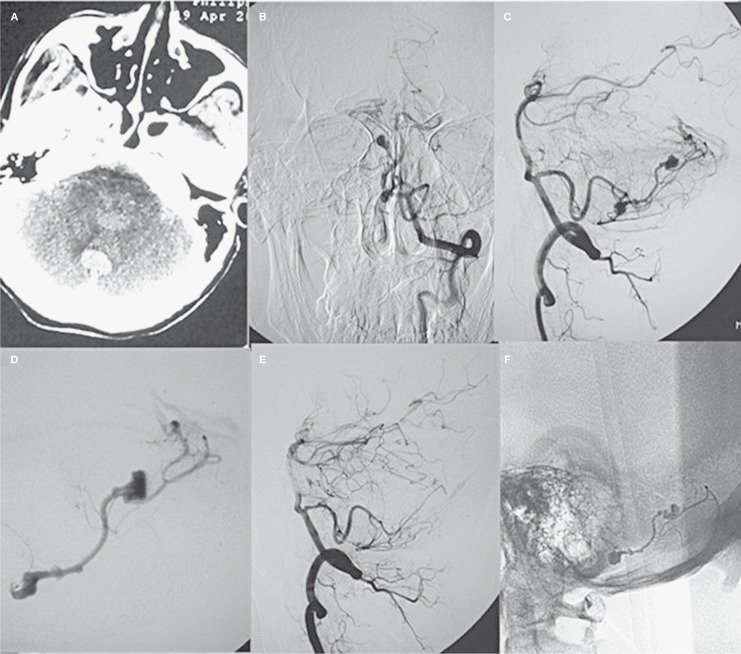
Figure 1.
A middle-aged patient presented with sudden headache. A) CT scan revealed fourth ventricular hemorrhage. B,C) Frontal and lateral view of digital subtraction angiography showed AVM and associated multiple feeding artery aneurysms. D) Super-selective angiography demonstrated associated aneurysms and the AVM. E) Lateral view of the left vertebral artery showed complete obliteration of the AVM and associated aneurysms after embolization. F) Cast of Glubran 2 after the procedure.
Follow-up Protocol
After complete obliteration of arteriovenous malformations, patient follow-up was performed clinically and with angiography examination at three to six months. Clinical outcomes were evaluated according to the modified Rankin Scale (mRS).
Results
Embolization Results
We performed 34 embolization sessions in these 31 patients with three patients treated for two sessions. A mean of 2.5 (range, 1-12) feeding pedicles were embolized per patient.
One feeding pedicle was embolized in 11 patients, two feeding pedicles in 13 patients, three feeding pedicles in three patients, five feeding pedicles in two patients, ten feeding pedicles in one patient, and 12 feeding pedicles in one patient. Total occlusion of the arteriovenous malformation with Glubran 2 was achieved in 27 patients (Figure 2). For the ten patients with coexisting aneurysms, the associated aneurysms were also completely occluded with Glubran 2.
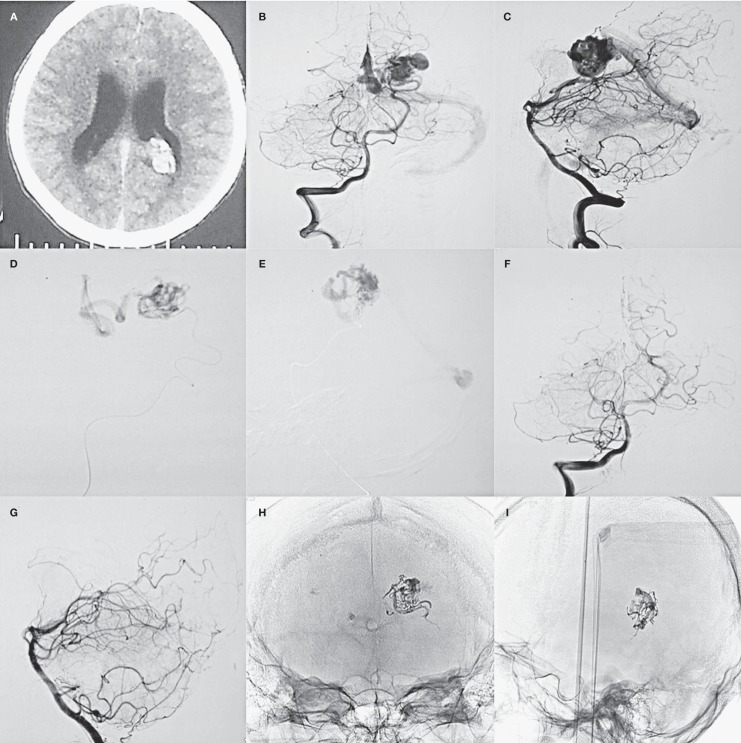
Figure 2.
A young patient presented with sudden headache. A) CT scan showed intraventricular hemorrhage. B-E) Frontal and lateral view of digital subtraction angiography and super-selective angiography via the microcatheter demonstrated the AVM fed by left posterior choroidal artery. F,G) Frontal and lateral view of digital subtraction angiography after Glubran 2 injection showed that the AVM was completely obliterated. H,I) Cast of Glubran 2 after the procedure.
Near-total occlusion was achieved in the remaining four patients (12.9%). Gamma knife radiosurgery was performed on the four patients with a remnant of the AVM within one month after embolization.
Treatment-Related Complications
Procedure-related complications included technical, hemorrhagic, and ischemic complications. Perforation of the feeding pedicle by the guidewire occurred in four patients, and vessel perforation was treated immediately by injection of Glubran 2. All of these perforations occurred without clinical consequences. One hemorrhagic complication was caused by retraction of the microcatheter in a young patient with the AVM located in occipital lobe. The injured vessel was completely occluded with Glubran 2. Post-procedure cerebral CT scan showed intra-parenchymal and intra-ventricular hemorrhage.
The patient was treated by surgery and had a favorable recovery at discharge (mRS=2). One patient with a ruptured right parietal AVM exhibited weakness of the left lower limb just after embolization, but the deficit recovered completely in a few days. There were no permanent disabling neurological deficits or death at discharge in our patient group.
Follow-up Results
In our series, we looked at angiographic and clinical follow-up in 21 patients with completely obliterated AVMs at three to six months, and the results demonstrated a small remnant of the AVM in one patient, which was subsequently treated with gamma knife radiosurgery. Angiography confirmed lasting complete obliteration of the AVMs in the remaining 20 patients. Six of the patients with completely obliterated AVMs refused angiographic follow-up, and clinical interviews were conducted. We took clinical follow-up for the four patients with near-total occluded AVMs at six months after gamma knife radiosurgery. In our study group, all of the patients had favorable clinical outcomes at three to six month follow-up (mRS≤2).
Discussion
Of the 31 patients with Spetzler-Martin grade I-II AVMs targeted for curative embolization with Glubran 2, total occlusion was achieved in 27 patients (87.1%). There were six treatment-related complications. Neither permanent disabling neurological deficits nor death occurred in our patient group.
Glubran 2, a cyanoacrylate-based synthetic glue modified by the addition of a monomer, has been applied for treating spinal tumors, extracerebral tumors, cerebral and spinal arteriovenous malformations and dural fistulae 5,6. A histological experiment demonstrated that arterial occlusion caused by Glubran 2 was stable at 30 and 60 days 7,8. Like n-butyl-cyanoacrylate glue (NBCA), AVM embolization with Glubran 2 requires considerable experience since the material polymerizes very quickly and the microcatheter can become glued to the vessel under treatment. Therefore, multiple catheterizations may be needed in some cases. Although keeping the guidewire within the tip of the microcatheter during catheterization may avoid vessel perforation 6, for a targeted wedge position of the microcatheter tip, navigation with the guidewire may be necessary in some circumstances. This may achieve better penetration of Glubran 2, though the injection time is short. The majority of our patients were ruptured AVMs, so aggressive treatment to prevent recurrent hemorrhage was reasonable, considering the relatively higher rebleeding rate for AVM patients with hemorrhage presentation 9-11. Several factors such as the angioarchitecture of the AVM, blood flow rate, and operator experience could affect the final results. For success in curative embolization of AVMs with Glubran 2, we should take the following factors into consideration. First, the AVM nidus should be small or medium-sized, not located in eloquent region such as brain stem or basal ganglia. Second, the feeding pedicles could be accessible by microcatheters and the perforating arteries should not be used.
For patients with associated feeding artery aneurysms, although the microcatheter tip was placed just at the proximal part of the associated aneurysm, the AVM nidus was also completely obliterated. And we thought that excellent penetration of Glubran 2 was achieved in these patients due to its high fluidity. Although we could not conclude that Glubran 2 was better than Onyx for these patients, Glubran 2 may be a better choice for its high fluidity, considering the distance between the associated aneurysm and the AVM nidus. While the adhesive feature makes it necessary to monitor the penetration of Glubran 2 closely during injection, venous penetration must be carefully recognized, especially for AVMs with a single draining vein 6. Once reflux occurs, rapid retraction may avoid retention of the microcatheter. Control angiography can be used to observe the situation of the AVM. In our series, blood vessel damage by retraction of the microcatheter was responsible for the hemorrhage complication. Although the AVM was completely obliterated, evacuation of hematoma and the AVM was conducted by surgery, and the patient recovered without neurological deficit at follow-up. A hemorrhagic complication may go undetected during embolization, therefore cerebral CT scan is recommended to detect such a complication promptly 3. At our department, CT scan was not a routine protocol for all patients after the procedure. At any time when a new neurological symptom or sign developed, the patient had a CT scan to rule out intracranial hemorrhage immediately. Inadvertent occlusion of normal arteries, either by reflux of glue along the microcatheter or by unrecognized reflux into remote arterial feeders, may cause an ischemic complication. Generally, this complication may not cause disastrous consequences. With the advent of Onyx and growing experience in embolization of cerebral arteriovenous malformations, the non-adhesive liquid embolic agent has been rapidly replacing the use of acrylic glue 1-3,12-14. Unlike NBCA or Glubran 2, Onyx injection requires more fluoroscopy and procedure times, and it is a controllable embolic agent 15. In our department, complete obliteration of AVMs with Onyx aiming to cure was achieved in about 20% of the whole patient group, most of whom had Spetzler-Martin grade I-II AVMs 16. Like several recently published studies, a higher cure rate was achieved for selected AVMs embolized with Onyx. Considering the relatively poor penetration of Onyx, for selected AVMs with a low blood flow rate, we recently employed Glubran 2 as the embolic agent targeting for curative embolization. Our initial results are encouraging.
The limitations of this study are those inherent in a retrospective study, and only 21 patients got follow-up angiograms after complete obliteration of the AVMs at three to six months. Yu et al. 17 reported that initial angiographic evidence of complete embolization of arteriovenous malformations with NBCA indicated permanent cure, but long-term angiographic follow-up is also necessary to confirm lasting complete obliteration after curative embolization with Glubran 2.
Conclusion
Curative Glubran 2 embolization for selected cerebral arteriovenous malformations achieved a relatively high rate of complete obliteration with an acceptable rate of complications.
Funding
This work was supported by the Beijing Talents Training Project, China (Category D) and Beijing Hygiene System High-level Hygienic Technical Personnel Training Program, China (Grant 2013-3-048).
References
- 1.Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50(7):589–597. doi: 10.1007/s00234-008-0382-x. doi: 10.1007/s00234-008-0382-x. [DOI] [PubMed] [Google Scholar]
- 2.Saatci I, Geyik S, Yavuz K, et al. Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg. 2011;115(1):78–88. doi: 10.3171/2011.2.JNS09830. doi: 10.3171/2011.2.JNS09830. [DOI] [PubMed] [Google Scholar]
- 3.van Rooij WJ, Jacobs S, Sluzewski M, et al. Curative embolization of brain arteriovenous malformations with onyx: patient selection, embolization technique, and results. Am J Neuroradiol. 2012;33(7):1299–1304. doi: 10.3174/ajnr.A2947. doi: 10.3174/ajnr.A2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speizler RF, Martin NA. A proposed grading system for arteriovenous malformations 1986. J Neurosurg. 2008;108(1):186–193. doi: 10.3171/JNS/2008/108/01/0186. doi: 10.3171/JNS/2008/108/01/0186. [DOI] [PubMed] [Google Scholar]
- 5.Raffi L, Simonetti L, Cenni P, et al. Use of Glubran 2 acrylic glue in interventional neuroradiology. Neuroradiology. 2007;49(10):829–836. doi: 10.1007/s00234-007-0238-9. doi: 10.1007/s00234-007-0238-9. [DOI] [PubMed] [Google Scholar]
- 6.Desal HA, Toulgoat F, Raoul S, et al. Brain arteriovenous malformations technical note of endovascular treatment with glubran(r) Interv Neuroradiol. 2005;11(Suppl 1):125–130. doi: 10.1177/15910199050110S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonardi M, Barbara C, Simonetti L, et al. Glubran 2: a new acrylic glue for neuroradiological endovascular use. Experimental study on animals. Interv Neuroradiol. 2002;8(3):245–250. doi: 10.1177/159101990200800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonardi M, Cenni P, Simonetti L, et al. Glubran 2((r)):a new acrylic glue for neuroradiological endovascular use: a complementary histological study. Interv Neuroradiol. 2003;9(3):249–254. doi: 10.1177/159101990300900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada S, Takagi Y, Nozaki K, et al. Risk factors for subsequent hemorrhage in patients with cerebral arteriovenous malformations. J Neurosurg. 2007;107(5):965–972. doi: 10.3171/JNS-07/11/0965. doi: 10.3171/JNS-07/11/0965. [DOI] [PubMed] [Google Scholar]
- 10.Hernesniemi JA, Dashti R, Juvela S, et al. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008;63(5):823–829. doi: 10.1227/01.NEU.0000330401.82582.5E. discussion 829-831. doi: 10.1227/01.NEU.0000330401.82582.5E. [DOI] [PubMed] [Google Scholar]
- 11.da Costa L, Wallace MC, Ter Brugge KG, et al. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke. 2009;40(1):100–105. doi: 10.1161/STROKEAHA.108.524678. doi: 10.1161/STROKEAHA.108.524678. [DOI] [PubMed] [Google Scholar]
- 12.van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. Am J Neuroradiol. 2007;28(1):172–177. discussion 178. [PMC free article] [PubMed] [Google Scholar]
- 13.Panagiotopoulos V, Gizewski E, Asgari S, et al. Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx) Am J Neuroradiol. 2009;30(1):99–106. doi: 10.3174/ajnr.A1314. doi: 10.3174/ajnr.A1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahlein DH, Mora P, Becske T, et al. Nidal embolization of brain arteriovenous malformations: rates of cure, partial embolization, and clinical outcome. J Neurosurg. 2012;117(1):65–77. doi: 10.3171/2012.3.JNS111405. doi: 10.3171/2012.3.JNS111405. [DOI] [PubMed] [Google Scholar]
- 15.Velat GJ, Reavey-Cantwell JF, Sistrom C, et al. Comparison of N-butyl cyanoacrylate and onyx for the embolization of intracranial arteriovenous malformations: analysis of fluoroscopy and procedure times. Neurosurgery. 2008;63(1) Suppl 1:ONS73–ONS78. doi: 10.1227/01.neu.0000335015.83616.12. discussion ONS78- ONS80. doi: 10.1227/01.neu.0000335015.83616.12. [DOI] [PubMed] [Google Scholar]
- 16.Lv X, Wu Z, Li Y, et al. Hemorrhage risk after partial endovascular NBCA and ONYX embolization for brain arteriovenous malformation. Neurol Res. 2012;34(6):552–556. doi: 10.1179/1743132812Y.0000000044. doi: 10.1179/1743132812Y.0000000044. [DOI] [PubMed] [Google Scholar]
- 17.Yu SC, Chan MS, Lam JM, et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. Am J Neuroradiol. 2004;25(7):1139–1143. [PMC free article] [PubMed] [Google Scholar]