Summary
This study describes the peri-procedural and late complications and angiographic follow-up results of 32 patients with 34 complex aneurysms treated with flow diverter Silk stents in a single centre. In this retrospective study, 40 Silk stents (SS) were implanted in 34 complex intracranial aneurysms in 32 patients.
In our series, 20 (58.8%) carotid-ophthalmic internal carotid artery (ICA), six (17.6%) cavernous ICA, two (5.9%) supraclinoid ICA, two (5.9%) petrosal ICA (the same patient- bilateral) and four (11.8%) posterior circulation aneurysms were treated. One of the posterior circulation lesions was a fenestrated-type aneurysm. Twenty wide-necked, saccular; eight neck remnant; four fusiform and two blister-like aneurysms were included in our series. SS were successfully implanted in all patients (100%). Misdeployment occurred in 17.6% of patients. In two of these patients adequate stent openness was achieved via Hyperglide balloon dilatation. Coil embolization in addition to SS placement was utilized in four aneurysms. One patient (3%) experienced transient morbidity due to a thromboembolic event and there was one mortality (3%) due to remote intraparenchymal haemorrhage. Complete occlusion of 27/33 (81.8 %) and 29/33 (87.9 %) aneurysms was achieved six and 12 months after the procedure, respectively. In-stent intimal hyperplasia was detected in 6.1 % patients. Flow-diverter Silk stent implantation is an effective method of treating complex aneurysms with acceptable mortality and morbidity rates. Complete occlusion is achieved in most of the complex aneurysms.
Keywords: cerebral aneurysms, flow diverter, Silk stent, endovascular treatment
Introduction
The endovascular approach is an increasingly utilized method for intracranial aneurysm treatment. Occluding the aneurysmal sac with coils is a simple and repeatable technique with relatively low complication rates. Superiority of standard endovascular coil embolization is proven but still has some limitations 1. Embolization of wide-necked, blister-shaped and fusiform aneurysms is difficult with standard coiling techniques 2. Additional endovascular techniques like stenting or balloon remodelling are usually needed when treating these complex aneurysms. Recently, adjuvant stenting both to support the saccular aneurysm sac and decrease recurrence rates has become an accepted method 3,4. These stents support coils within the aneurysm sac and divert the blood flow in wide-necked aneurysms 5. Unfortunately, coil reconstruction of complex aneurysms even with self-expandable stents is accompanied with difficulties 6.
Stent technology shows an accelerated progress. New generation flow diverter (FD) stents cause flow changes around the aneurysmal sac ultimately resulting in occlusion 6. This study describes aneurysm characteristics and early and median period therapy results of 32 patients with 34 complex aneurysms treated with Silk flow diverter stents (SS) in a single centre.
Materials and Methods
Therapy results of 32 patients with 34 complex aneurysms treated with SS in a single centre between January 2009 and February 2013 were retrospectively evaluated. The study was approved by the ethics committee.
Patient Population
A total of 34 aneurysms in 32 patients with age range of 34-79 years (mean age: 55) were included in the study. Twenty-three (71.8 %) patients were female and nine (28.2%) were male. The neurointerventional radiologist and vascular neurosurgeon decided on patients and lesions appropriate for SS treatment by consensus. Initial complaints of patients included: headache (n=15), visual loss (n=1), diplopia in addition to headache (n=1), ptosis and visual loss (n=1). Six patients who were formerly treated with coil embolization had neck remnants and were asymptomatic. Three patients had subarachnoid haemorrhage (SAH) and one patient had cerebral infarct. Six patients had a history of SAH. Aneurysms were detected incidentally in two patients.
Aneurysm Characteristics
A total of 40 SSs were deployed to treat 34 aneurysms. Three aneurysms were ruptured. In our series, 20 (58.8%) carotid-ophthalmic internal carotid artery (ICA), six (17.6%) cavernous ICA, two (5.9%) supraclinoid ICA, two (5.9%) petrosal ICA (the same patient- bilateral) and four (11.8%) posterior circulation aneurysms were treated. One of the posterior circulation lesions was a fenestrated-type aneurysm. Twenty wide-necked, saccular; eight neck remnant; four fusiform and two blister-like aneurysms were included in our series. Mean aneurysm size was 11.8 mm (range: 4 mm-26 mm).
Endovascular Procedure
All patients except the three with SAH underwent preprocedural premedication with 300 mg/day acetyl salicylic acid and 75 mg/day clopidogrel lasting for a minimum of three days. Three patients with SAH underwent nasogastric tubing placement before the procedure and 450-600 mg clopidogrel loading approximately one hour before stent placement. All endovascular procedures were carried out with the biplane digital subtraction angiography (DSA) unit (Axiom Artis; Siemens, Erlangen, Germany) under general anaesthesia. Systemic heparinization was initiated with 5000 IU bolus heparin injection at the beginning of the procedure and continued with 1000-1500 IU/hour infusion. Anticoagulation was evaluated with activated clotting time (ACT) measurements. Baseline ACT before heparin injection was measured and it was aimed to double this value during the procedure. Carotid artery was reached via a guiding catheter following femoral access. Fargo catheter (Balt, Montmorency, France) was delivered to the petrocavernous segment of the ICA or V2-V4 segment of the posterior circulation via the long introducer. A delivery catheter (Vasco 21 or 25; Balt, Montmoroncy, France) was placed at the distal segment of the parent artery (minimum 10-20 mm beyond the aneurysm neck) via a microguidewire (Terumo 0.016 inch, double angled, Terumo Medical Corporation). The stent was pushed out of the microcatheter and was unsheathed as the delivery catheter was pulled backwards. In cases when stent deployment was difficult (usually when the parent artery demonstrated a sharp angle), easy and secure stent deployment from the delivery catheter was assured with close placement of a Fargo catheter near the aneurysmal segment. Following stent deployment, the Vasco microcatheter was moved distally through the stent to ensure adequate device apposition against the parent artery wall was achieved. In cases with inadequate stent opening, adequate opening was provided by expanding the Silk stent by means of balloon (Hyperglide) angioplasty. Following stent placement DSA was performed to make sure no thrombus formation was detectable within the stent and distal vessels. Thrombolytic therapy was initiated when detected.
Post-Procedural Aspects and Follow-up
Systemic heparinization was continued for 24h with ACT control in patients who developed stent thrombosis, distal embolization or parent artery stenosis during the intervention. In the same group low molecular weight heparin (LMWH) (Clexane, Aventis, Pharma) was continued until day 5. Systemic heparinization was discontinued at 12 hours as a standard and LMWH was until day 3 in the remaining patients. The patients were prescribed lifelong aspirin (100-300 mg/day) and a minimum three months of oral clopidogrel (75mg/day). Control DSA of each patient was performed and evaluated before cessation of clopidogrel. First control DSAs were performed within three to seven months after the procedure.
Angiographic Grading
Angiographic grading during the procedure and follow-up was performed according to the visual scoring system below 4.
Immediate angiographic grading: Grade 0: No change in aneurysmal filling, Grade 1: Mild slow-flow in aneurysm; Grade 2: Significant slow-flow in aneurysm; Grade 3: Complete occlusion of the aneurysm.
Follow-up of angiographic grading: Grade 1: Complete occlusion of the aneurysmal sac; Grade 2: Incomplete occlusion of the aneurysmal sac; Grade 3: Saccular filling of the aneurysmal sac.
Results
Procedural Results
Thirty-three procedures were performed for 34 aneurysms in 32 patients. Stents were successfully deployed in all procedures. Thirty aneurysms were embolized solely with SS. Four wide-necked saccular aneurysms underwent partial coil embolization before SS placement. Six single aneurysms were treated with multiple stents (telescopic). Stent placement was difficult in six patients either because of tortuosity of anatomical structures (n=5) or technical problems with the stent (n=1). These aneurysms were located at carotid-ophthalmic (n=3), basilar (n=1) and cavernous ICA (n=2) segments. Dilatation with a Hyperglide balloon within the stent was performed in two patients (two aneurysms).
Immediate Angiographic Results
During endovascular treatment; no aneurysm (0 %) demonstrated complete occlusion (Grade 3), nine aneurysms (26.4 %) showed significant slow flow (Grade 2), 21 aneurysms showed slow flow (61.7 %) (Grade 1) and four aneurysms (11.7 %) showed no significant change in aneurysmal filling (Grade 0).
Procedural and Peri-Procedural Complications
The total rate of procedural and peri-procedural complications was 15.1%. Thromboembolic complications occurred in four patients (12.1%) and in one patient resulted in a clinically adverse outcome. In patients developing thrombus formation during the procedure successful resorption of the thrombus was accomplished with 4-10 mg tirofiban application.
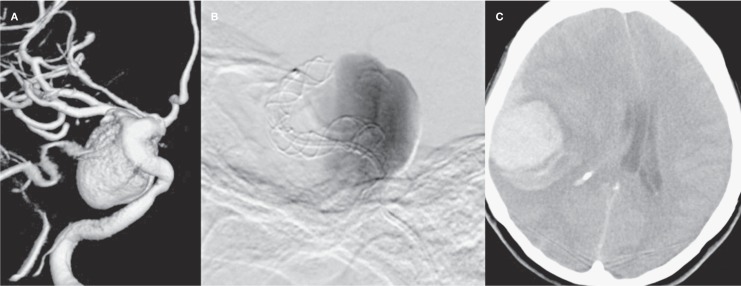
A haemorrhagic complication was observed in one patient (3%) who had a 25 mm, wide-necked, saccular aneurysm located at the cavernous segment of the ICA. A stent was applied successfully without any complications during the procedure. As the patient's preprocedural visual complaints continued, anti-oedema therapy with corticosteroids was initiated. The patient became unconscious 40 hours after the procedure. CT demonstrated intraparenchymal haemorrhage with a diameter of 5 cm within the right temporoparietal lobe. The patient died before being evaluated with angiography. Pre- and peri-procedural angiography and cranial CT images are demonstrated in Figure 1.
Figure 1.
A) 3D angiography image demonstrating the giant complex aneurysm at right cavernosal segment of ICA of the patient lost due to remote intraparenchymal bleeding within post-procedural 40h. B) DSA image showing wide neck saccular aneurysm with slow flow (Grade 2) during the procedure.C) Native cranial CT axial image demonstrating parenchymal haemorrhage.
Delayed Complications
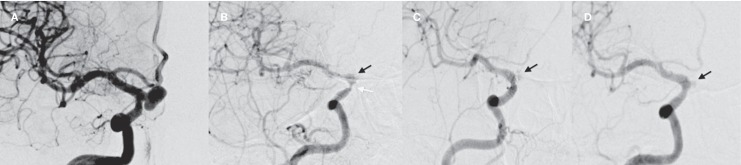
The delayed complication rate was 6.1%. Intimal hyperplasia (IH) was detected in three stents applied to three aneurysms in two patients. Both patients were asymptomatic. In the first patient, angiographic control six months after the procedure demonstrated significant IH within the stent. Dual antiplatelet therapy was continued and control angiographies at 12 and 18 months after the procedure showed regression of the intimal hyperplasia. Unfortunately, the aneurysm showed Grade 2 (incomplete) occlusion and total occlusion could not be achieved (Figure 2). The patient is still under clinical and radiological follow-up.
Figure 2.
A) Pre-procedural DSA image of the patient with intimal hyperplasia at 6-month follow-up shows a carotid-ophthalmic, wide-necked aneurysm. B) Significant luminal stenosis (white arrow) due to intimal hyperplasia and incomplete occlusion of the aneurysm (black arrow) was detected during angiographic control 6 months after the procedure. DSA images of ICA obtained 12 (C) and 18 months (D) after the procedure.
Imaging studies showed bilateral petrosal segment fusiform dissecting aneurysms in another patient. Two separate SS for both aneurysms were implanted in one session. Bilateral IH within the stents was depicted. Clopidogrel therapy was continued and control DSA images obtained 12 months after the procedure showed regression of the right-sided IH. The left-sided aneurysm was incompletely occluded and IH persisted. The patient is still under close clinical and angiographic follow-up.
Angiographic Follow-up
Angiographic follow-up of 33 aneurysms in 31 patients was performed. No follow-up was possible in one patient who died. The first control DSA was performed within seven months after the procedure. Clinical and radiological follow-up durations of the patients varied between three and 42 months with a mean interval of 17 months; 64.5 % of patients were followed up for 12 months or more. DSA images obtained six months after the procedure showed 81.8% complete occlusion (Grade 1), 12.1% incomplete occlusion (Grade 2) and 6.1% saccular filling (Grade 3). Patients with aneurysms showing complete occlusion (27 aneurysms) underwent only clinical follow-up and further control DSA was not performed. Seven patients with aneurysms showing incomplete occlusion underwent routine DSA follow-up. Six of these patients underwent DSA 12 months after the procedure. Three patients with Grade 2 and Grade 3 filling patterns at the follow-up DSA images obtained six months after the procedure showed no significant change. These patients are under follow-up. One patient with an aneurysm showing a Grade 3 filling pattern and two other patients with aneurysms showing a Grade 2 filling pattern during the first control DSA, demonstrated Grade 1 (complete occlusion) on DSA performed 12 months after the procedure. One patient has not yet reached the 12-month control period and is under clinical follow-up.
DSAs of two patients with rarely seen blister-shaped aneurysms demonstrated complete occlusion. Both patients were referred to our institute with clinical findings of SAH. Neither of these patients developed peri- or post-procedural complications. Peri- and post-procedural complications, procedural events and immediate and control angiography findings of the patients are summarised in Table 1.
Table 1.
Peri- and post-procedural complications, adjunctive therapies and immediate and control angiography findings of the patients included in the study.
|
Parent artery |
Branch | Stent | Distal | Deployment | Angiographic results | |||||
| Stenosis | Occlusion | Thrombosis | Embolism | Difficulty | Immediate* | ≤6 months** | >7months** | |||
| Procedure | 2 | None | 3 | 1 | 6 | Grade 0; 4 | Grade 1; 27 | Grade 1; 29 | ||
| Grade 1; 21 | Grade 2; 4 | Grade 2; 2 | ||||||||
| (n) | (Hyperglide) | Agrestat | Grade 2; 9 | Grade 3; 2 | Grade 3; 1 | |||||
| 4 -10 mg | Grade 3; None | |||||||||
|
* Immediate angiography grade: Grade 0: Unchanged; Grade 1: Slow flow; Grade 2: Significant slow flow; Grade 3: Complete occlusion ** Control angiography grade: Grade 1: Complete occlusion; Grade 2: Incomplete occlusion; Grade 3: Saccular filling | ||||||||||
Discussion
Endovascular therapy has been accepted as one of the first line treatment choices for ruptured or unruptured aneurysms for years 1,7. The treatment of complex aneurysms significantly improved after the development of new endovascular techniques in addition to standard methods and stents enabling endovascular reconstruction of aneurysms 8-10. Recently, FD stents promoting occlusion of the aneurysmal sac as a result of the ability of endovascular reconstruction have been developed 11-16. FD stents stand out as a specific instrument in the treatment of complex aneurysms not easily treated with a conventional endovascular approach 17. SS permit resheathing and repositioning but they have lower radial force 6. There are also some specific problems with SS implantation 4. Reporting these problems and user experiences would help more successful and safe implementation of this new technique in the future.
The major problem with SS implantation is the difficulty of deployment 18. It is important to effectively manipulate the delivery catheter to open and position the stent at the vessel wall and this is the critical step when experience of the interventionist has a direct effect on the result. This manoeuvre is particularly important in vessels with acute angles and curves like the ICA siphon. Adequate stent openness is obtained only when the stent is slowly deployed from the microcatheter and it is also important to make sure that the stent is securely placed at the vessel wall via pushing the microcatheter distally through the stent. These steps require the utmost care as inadequate stent openness due to low radial force can cause vessel occlusion. Careful length and diameter selection is also obligatory as erroneous selection can cause stent migration 19. All these details highlight the fact that SS placement is more difficult than conventional techniques and results are significantly correlated with the experience of the interventionist. In our opinion, the heterogeneity of the results in the literature is attributable to aneurysm location, patient-related factors and the experience of the interventionist. In our series, misdeployment was experienced in six patients (17.6 %). These patients had carotid-ophthalmic (n=3), cavernosal ICA (n=2) and basilar artery (n=1) aneurysms. Two of these patients underwent balloon dilatation (Hyperglide). Five of the six aneurysms (except one fenestrated basilar artery aneurysm) were located at curved vessel segments as reported in the literature.
Angiographic grading systems after stent placement show variability within studies in the literature. In our study we performed immediate angiographic grading based on Velioglu et al. 4. This grading is a scoring system based on flow haemodynamics. However during follow-up, we decided that a modified scoring system based on aneurysm occlusion similar to that of Maimon et al. 6 but not reported in the literature before would describe our results better.
In the literature two meta-analysis studies on FD stents drew our attention. The study by Arrese et al. 20 analysed a total of 1018 aneurysms in 897 patients. The total occlusion rate was reported as 76.2%. Occlusion rates of SS and pipeline stents were separately reported as 68% and 88% respectively with significant differences. When SS studies included in the meta-analysis are examined, studies with lower occlusion rates are the results of the first six months after the procedure. We interpret the lower occlusion rates as a result of this short follow-up period. In the second meta-analysis, 1654 aneurysms in 1451 patients were included 21. This study reported complete occlusion rates at six months as 76%. In our series, the complete occlusion rate at six months was 81.8%, compatible with the literature. The complete occlusion rate at 12 months was 87.9%. In some cases, incomplete occlusion at six-month follow-up turned to complete occlusion during angiographic follow-up. Occlusion rates in FD stents can change during long-term follow-up.
In our study, morbidity and mortality rates were 3% and 3% respectively, which is within acceptable limits when compared with rates reported in the literature.
Thromboembolic events and parental artery occlusion risk accompany endovascular stent implementation. Therefore, appropriate anticoagulation and antiplatelet therapy is warranted 17. Anticoagulation and antiplatelet therapies vary in the literature with significant variations regarding systemic heparinization periods. In some studies, heparinization was discontinued at the end of the procedure 22 while in others it was continued for 24 hours (4) or 48 hours (5). In some studies, LMWH was used until day 3 23. Katsaridis et al. reported utilization of LMWH after stent implementation in wide-neck aneurysms as safe and effective. In our routine protocol, systemic heparinization was discontinued 12 hours after the procedure and LMWH was continued until day 3. However, in patients who developed thromboembolic events, systemic heparinization was continued for 24 hours under ACT control and LMWH was used until day 5.
It is suggested that rapid flow decrease within the aneurysmal sac triggers massive intra-aneurysmal platelet aggregation and lytic enzyme discharge from the platelets following FD stent application and that can cause aneurysmal wall rupture 25. Increased turbulent flow at the neck region due to altered haemodynamic mechanisms or peripheral fresh persistent thrombus can also cause aneurysmal rupture 22. We did not experience early or late aneurysmal rupture in our series. Non-aneurysm-related remote intraparenchymal haemorrhage (IPH) after FD stent implantation is documented in the literature. In the meta-analysis series of Brinjikji et al. 21 and Arrese et al. 20 intraparenchymal haemorrhage rates were reported as 3% and 1.7% respectively. In our series this rate was 3%. The mechanism of IPH is not clearly understood. Haemodynamic changes, aneurysm dimensions and antiplatelet therapy are thought to be responsible, however Brinjikji et al. 21 reported that no significant relationship exists between IPH and aneurysm dimensions or antiplatelet therapy.
FD stents can induce IH and intraluminal stenosis within the parent artery like other aneurysm modelling stents. Lylyk et al. (Pipeline FD stent series) reported stent stenosis regression rates of 18% and 10% three months and six months after the procedure respectively 12. Saatci et al. reported an IH rate of 4.2% in their Pipeline FD stent series. All these patients were asymptomatic except one (0.5%) 25. Berge et al. also reported 7.8% transient in-stent stenosis for Silk FD stents and that the patients were asymptomatic 18. In our series, 9.1% (3/33) in-stent IH was detected six months after therapy. Following controls (12 and 18 months) demonstrated regression of two and the rate was 3% (1/33). All our patients were asymptomatic. On IH detection, clopidogrel treatment was continued as described in the literature and regression was observed 25. According to our experience, clopidogrel treatment inhibiting IH progression and maintaining intraluminal in-stent stenosis regression should be continued in this group of patients.
Our study has some limitations. The patient population is small and results are not compared with a control group, conventional techniques or another FD stent.
In conclusion, SS is a good treatment choice with acceptable mortality and morbidity rates in complicated aneurysms unlikely to be treated with other techniques. Unfortunately, all complications, intracranial haemodynamic alterations caused by the system and long-term results are unknown. As further new data are added to the literature and experience and knowledge from various institutions and interventionalists accumulate, we believe therapy and patient management will improve.
References
- 1.Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro M, Babb J, Becske T, et al. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: a literature review. Am J Neuroradiol. 2008;29(9):1777–1781. doi: 10.3174/ajnr.A1216. doi: 10.3174/ajnr.A1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanzino G, Wakhloo AK, Fessler RD, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91(4):538–546. doi: 10.3171/jns.1999.91.4.0538. doi: 10.3171/jns.1999.91.4.0538. [DOI] [PubMed] [Google Scholar]
- 4.Velioglu M, Kizilkilic O, Selcuk H, et al. Early and midterm results of complex cerebral aneurysms treated with Silk stent. Neuroradiology. 2012;54(12):1355–1365. doi: 10.1007/s00234-012-1051-7. doi: 10.1007/s00234-012-1051-7. [DOI] [PubMed] [Google Scholar]
- 5.Leonardi M, Cirillo L, Toni F, et al. Treatment of intracranial aneurysms using flow-diverting silk stents (BALT): a single centre experience. Interv Neuroradiol. 2011;17(3):306–315. doi: 10.1177/159101991101700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maimon S, Gonen L, Nossek E, et al. Treatment of intra-cranial aneurysms with the SILK flow diverter: 2 years' experience with 28 patients at a single center. Acta Neurochir (Wien) 2012;154(6):979–987. doi: 10.1007/s00701-012-1316-2. doi: 10.1007/s00701-012-1316-2. [DOI] [PubMed] [Google Scholar]
- 7.Pierot L, Spelle L, Vitry F, et al. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke. 2008;39(9):2497–2504. doi: 10.1161/STROKEAHA.107.512756. doi: 10.1161/STROKEAHA.107.512756. [DOI] [PubMed] [Google Scholar]
- 8.Pierot L, Spelle L, Leclerc X, et al. Endovascular treatment of unruptured intracranial aneurysms: comparison of safety of remodeling technique and standard treatment with coils. Radiology. 2009;251(3):846–855. doi: 10.1148/radiol.2513081056. doi: 10.1148/radiol.2513081056. [DOI] [PubMed] [Google Scholar]
- 9.Piotin M, Blanc R, Spelle L, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010;41(1):110–115. doi: 10.1161/STROKEAHA.109.558114. doi: 10.1161/STROKEAHA.109.558114. [DOI] [PubMed] [Google Scholar]
- 10.Lubicz B, Collignon L, Raphaeli G, et al. Solitaire stent for endovascular treatment of intracranial aneurysms: immediate and mid-term results in 15 patients with 17 aneurysms. J Neuroradiol. 2010;37(2):83–88. doi: 10.1016/j.neurad.2010.02.003. doi: 10.1016/j.neurad.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62(5):1115–1120. doi: 10.1227/01.neu.0000325873.44881.6e. doi: 10.1227/01.neu.0000325873.44881.6e. [DOI] [PubMed] [Google Scholar]
- 12.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64(4):632–642. doi: 10.1227/01.NEU.0000339109.98070.65. doi: 10.1227/01.NEU.0000339109.98070.65. [DOI] [PubMed] [Google Scholar]
- 13.Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. Am J Neuroradiol. 2010;31(6):1139–1147. doi: 10.3174/ajnr.A2023. doi: 10.3174/ajnr.A2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadasivan C, Cesar L, Seong J, et al. An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase-induced model. Stroke. 2009;40(3):952–958. doi: 10.1161/STROKEAHA.108.533760. doi: 10.1161/STROKEAHA.108.533760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ionita CN, Paciorek AM, Dohatcu A, et al. The asymmetric vascular stent: efficacy in a rabbit aneurysm model. Stroke. 2009;40(3):959–965. doi: 10.1161/STROKEAHA.108.524124. doi: 10.1161/STROKEAHA.108.524124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelboom G, Kadri K, Hassan F, et al. Infectious aneurysm of the cavernous carotid artery in a child treated with a new-generation of flow-diverting stent graft: case report. Neurosurgery. 2010;66(3):623–624. doi: 10.1227/01.NEU.0000365370.82554.08. doi: 10.1227/01.NEU.0000365370.82554.08. [DOI] [PubMed] [Google Scholar]
- 17.Tähtinen OI, Manninen HI, Vanninen RL, et al. The silk flow-diverting stent in the endovascular treatment of complex intracranial aneurysms: technical aspects and midterm results in 24 consecutive patients. Neurosurgery. 2012;70(3):617–623. doi: 10.1227/NEU.0b013e31823387d4. doi: 10.1227/NEU.0b013e31823387d4. [DOI] [PubMed] [Google Scholar]
- 18.Berge J, Biondi A, Machi P, et al. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. Am J Neuroradiol. 2012;33(6):1150–1155. doi: 10.3174/ajnr.A2907. doi: 10.3174/ajnr.A2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubicz B, Collignon L, Raphaeli G, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke. 2010;41(10):2247–2253. doi: 10.1161/STROKEAHA.110.589911. doi: 10.1161/STROKEAHA.110.589911. [DOI] [PubMed] [Google Scholar]
- 20.Arrese I, Sarabia R, Pintado R, et al. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery. 2013;73(2):193–199. doi: 10.1227/01.neu.0000430297.17961.f1. doi: 10.1227/01.neu.0000430297.17961.f1. [DOI] [PubMed] [Google Scholar]
- 21.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013;44(2):442–447. doi: 10.1161/STROKEAHA.112.678151. doi: 10.1161/STROKEAHA.112.678151. [DOI] [PubMed] [Google Scholar]
- 22.Briganti F, Napoli M, Tortora F, et al. Italian multicenter experience with flow-diverter devices for intracranial unruptured aneurysm treatment with periprocedural complications--a retrospective data analysis. Neuroradiology. 2012;54(10):1145–1152. doi: 10.1007/s00234-012-1047-3. doi: 10.1007/s00234-012-1047-3. [DOI] [PubMed] [Google Scholar]
- 23.Jiang WJ, Xu XT, Jin M, et al. Apollo stent for symptomatic atherosclerotic intracranial stenosis: study results. Am J Neuroradiol. 2007;28(5):830–834. [PMC free article] [PubMed] [Google Scholar]
- 24.Katsaridis V, Papagiannaki C, Violaris C. Embolization of acutely ruptured and unruptured wide-necked cerebral aneurysms using the neuroform2 stent without pretreatment with antiplatelets: a single center experience. Am J Neuroradiol. 2006;27(5):1123–1128. [PMC free article] [PubMed] [Google Scholar]
- 25.Saatci I, Yavuz K, Ozer C, et al. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. Am J Neuroradiol. 2012;33(8):1436–1446. doi: 10.3174/ajnr.A3246. doi: 10.3174/ajnr.A3246. [DOI] [PMC free article] [PubMed] [Google Scholar]