Summary
Dural arteriovenous fistulas (DAVF) associated with our series of patients with vein of Galen malformations (VOGM) are analyzed and discussed.
We retrospectively analyzed 87 consecutive cases of VOGM treated between May 2002 and December 2011 and identified 26 patients with DAVF. We gathered information from the clinical case records, angiographic images, MRI on presentation and during follow-up. The findings were analyzed to aid discussion.
Among 87 patients treated by multi-stage endovascular embolization, age range from newborn to 19 years, 26(30%) had DAVF. In seven patients (8%), DAVF were found on initial angiogram and were all into the VOGM. Nineteen (21%) DAVF found on follow-up angiograms were all into the VOGM and distant locations. Sprouting and non-sprouting angiogenesis resulted in the formation of a network of vessels around partially thrombosed VOGM, recruiting blood from the surrounding dura mater resulting in a secondary network on the dura mater supplied by the blood vessels of dura mater in the region or from its natural collaterals. Embolization targeting DAVFs was done in 13 (52%) with complete cure in eight (32%) and recurrence in five (20%). Among 12 non-embolized patients (48%), eight (32%) had spontaneous regression with continued treatment of VOGM. In others, the DAVF either remained stable or progressed.
DAVF associated with VOGM represent the dural response to angiogenic stimuli. They are observed to regress spontaneously or mature while continuing to treat the primary feeders of VOGM. It is important to include the external carotid system during angiograms. Persistent DAVF with residual VOGM that do not have access though the pial vessels are used as a conduit to treat the dural shunt and to achieve obliteration of residual VOGM at later stages of treatment.
Keywords: vein of Galen, dural arteriovenous fistula, vein of Galen malformation
Introduction
Vein of Galen malformations (VOGM) are rare developmental vascular disorders of the brain. These high-flow vascular malformations are supplied by the choroidal vessels, thalamo-perforator vessels and rarely by the dural vessels. Endovascular embolization is the single most effective treatment, and it is done in multiple sessions. During the course of treatment the VOGM undergoes significant hemodynamic and structural modifications and alterations such as venous remodeling, reactive angiogenesis and development of dural arteriovenous fistula (DAVF). Although DAVF seen in association with VOGM either in the first angiogram or in follow-up angiogram during staged treatments, or following endovascular embolization, have been documented in the literature 1, it has not been described and discussed in detail. We have reviewed our series of 26 cases with DAVF among the 87 consecutive patients with VOGM. We have analyzed and discussed the incidence, morphology, anatomic basis, pathophysiologic mechanisms, hemodynamic nature, management options, and the outcomes of treatment.
Materials and Methods
We retrospectively analyzed 87 consecutive cases of VOGM treated at our center from May 2002 to December 2011. We only included choroid and mural type fistulous malformations. In all we had 87 cases that included both those still under treatment and those where the treatment had been completed. Among this cohort, we identified 26 patients with DAVF. We noticed DAVF in the initial angiogram before intervention in some cases and subsequent to endovascular intervention on a follow-up angiogram done after three months or more in most cases. We gathered information from the clinical case records, imaging by MRI on presentation and during follow-up, angiographic images and records during each of the procedures and during follow-up. We recorded the patient demographics, their clinical presentation, the timing of the procedure, the MRI characteristics, angio-architecture, management details, follow-up angiographic changes, their management and the outcome. The findings were analyzed to aid discussion on DAVF associated with VOGM.
Results
In total, we treated 87 patients with VOGM between May 2002 and December 2011 by multi-stage endovascular embolization. Their ages ranged from newborn to 19 years. We identified 26 patients (30%) with 34 DAVF among 87 patients treated for VOGM. In seven patients (8%) the DAVF was identified in the initial angiogram and they were all into the vein of Galen (Table 1) (Figure 1). This cohort of patients was relatively older (Mean age: 58 months) at initial angiogram compared to the patients who developed DAVF after endovascular embolization (Mean age: 11 months). In 19 patients (21%) the DAVF was identified in the follow-up angiograms following previous endovascular embolization. The DAVF identified following embolization were not confined to the vein of Galen itself, but had a tendency to develop at other locations including the torcula, superior sagittal sinus and transverse sigmoid sinus. (Figure 4) (Tables 1 and 2) In six of these patients, the DAVF developed in two to four isolated locations (Table 2). An interesting observation is that the patients with mural type VOGM had an increased tendency to develop DAVF at a distant location following treatment (Table 2). Following total obliteration of VOGM, DAVF was identified in two patients at the first follow-up angiogram done at six months and none in subsequent follow-up examinations.
Table 1.
The locations of dural arteriovenous fistula associated with vein of Galen malformation.
| Location |
DAVF identified in Initial angiogram |
DAVF identified following endovascular embolization |
Total |
| VOGM | 7 | 18 | 25 |
| Torcula | 5 | 5 | |
| Superior sagittal sinus | 2 | 2 | |
| Transverse sigmoid sinus | 2 | 2 | |
| Total | 7 | 27 | 34 |
| DAVF, dural arteriovenous fistula; VOGM, vein of Galen malformation. | |||
Table 2.
Identified cases with dural arteriovenous fistula in vein of Galen malformation. In Cases 1-7, the fistula was identified in the initial angiogram and Cases 2 and 3 developed more extensive dural fistulization after embolization. In cases 8-26 the DAVF was identified after embolization. The location, feeders, embolization details, result and outcome are shown.
| No | Type |
Age at first treatment |
Location | Feeders |
DAVF treated |
Recurrence |
DAVF outcome |
FU angiogram |
| 1 | CH | 4 days | VOGM | PICA-Tentorial branch |
– | – | No Rx for VOGM | None |
| 2 | CH | 7 months | VOGM | PMA, Rt. & Lt. MTA, Lt. MMA |
+ | – | Embolized– Cured |
6 months |
| 3 | CH | 11 years | VOGM | Rt. & LT. MTA, Rt. & Lt. Occ, Rt. &Lt. MMA |
+ | + | Embolized– Partial Rx |
None after las embolization |
| 4 | CH | 12 months | VOGM | Rt. & Lt. PCA, Lt. SCA |
+ | – | Embolized–Cured | 9 months |
| 5 | CH | 9 months | VOGM | Rt. SCA | – | Spontaneous resolution |
3 months | |
| 6 | CH | 19 years | VOGM | Lt. SCA, PMA, Rt. MTA |
– | – | No Rx | None |
| 7 | CH | 16 months | VOGM | Rt. & Lt. MTA | + | – | Spontaneous resolution |
None after resolution. |
| 8 | CH | 7 Days | VOGM, Torcula , SSS |
Lt. MMA, Lt PMA, Lt. PCA |
+ | – | Cured | 3 yrs |
| 9 | CH | 14 days | VOGM | Rt. & Lt.MMA | + | + | Partial Rx | None after last embolization |
| 10 | CH | 3 days | VOGM | Rt. MMA | – | – | No Rx | – |
| 11 | CH | 14 days | VOGM | Rt. & Lt. MMA, Rt. & Lt. Occ, Lt. SCA, Lt. MTA |
– | – | Spontaneous resolution. |
5 months |
| 12 | CH | 7 months | Torcula | Rt. & Lt. MMA, Lt. Occ, PMA |
+ | – | Partial Rx | None after last embolization |
| 13 | CH | 7 days | VOGM | Rt. MMA | + | + | Cured | 15 months |
| 14 | CH | 14 days | VOGM | Rt. SCA | – | – | Spontaneous resolution |
8 months |
| 15 | CH | 2 days | VOGM | Rt. Occ, Lt. MTA |
– | – | Spontaneous resolution |
19 months |
| 16 | CH | 6 months | VOGM | Rt. MMA, Rt. Occ, Rt. & Lt. MTA, |
– | – | Spontaneous resolution |
13 months |
| 17 | CH | 14 months | VOGM | Rt. MMA, Rt. SCA, Rt. PCA |
+ | – | Partial Rx | None after last embolization |
| 18 | MU | 17 months | VOGM, Torcula |
Rt. MMA, Rt. & Lt. SCA, Rt.& Lt. Occ. |
+ | + | Partial Rx | None after last embolization |
| 19 | CH | 18 months | VOGM | Lt. SCA, Rt. PCA |
– | – | No Rx | – |
| 20 | CH | 7 months | VOGM | Lt. Occ. | – | – | Spontaneous resolution |
23 months |
| 21 | CH | 14 days | VOGM, ISS | Rt. & Lt. MMA, Rt. & Lt. MTA |
+ | – | Partial Rx | None after last embolization |
| 22 | CH | 10 months | VOGM | Rt. & Lt. MTA | – | – | Spontaneous resolution |
5 months |
| 23 | CH | 3 months | VOGM | Rt. & Lt. MMA, Rt. & Lt. SCA,Lt. PCA |
+ | – | Cured | 3 months |
| 24 | MU | 5 months | VOGM, Torcula |
Rt. & Lt. Occ, PMA |
– | – | No Rx | – |
| 25 | MU | 5 months | VOGM, Torcula, Rt. & Lt. TS |
Rt. & Lt. MMA, Rt. & Lt. Occ, PMA, APA |
+ | + | Cured | 26 months |
| 26 | CH | 7 months | VOGM, SSS |
Rt. MMA | + | – | Cured | None after last embolization |
|
VOGM = vein of Galen malformation; CH, choroidal type; MU, mural type; SSS, superior sagittal sinus; ISS, inferior sagittal sinus; Rt., right; Lt., left; MMA, middle meningeal artery; SCA, superior cerebellar artery; Occ, occipital artery; PCA, posterior cerebral artery; MTA, marginal tentorial artery; APA, ascending pharyngeal artery; PMA, posterior meningeal artery; FU, follow–up. | ||||||||
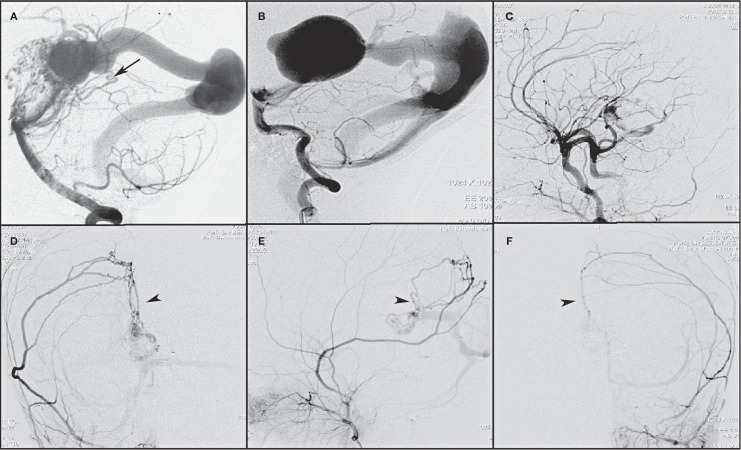
Figure 1.
Dural fistula identified in the initial left vertebral angiogram (A) in this patient is the marginal tentorial artery (arrow) from superior cerebellar artery. The fistulas that develop following endovascular embolization of vein of Galen malformation are shown in B-F. B) Pre-embolization left vertebral artery angiogram shows no fistulas. C) Right common carotid angiogram following first stage embolization reveals no dural fistulas. Follow-up angiograms of right external carotid artery (D,E) and left external carotid artery (F) show dural fistulas to the vein of Galen malformation with secondary arterial network along the falx cerebri (arrowhead) with feeders from both the middle meningeal arteries.
The dural fistulas that developed following endovascular embolization formed a network of vessels on the dura before draining into the venous channel. The feeders to the DAVF were from the blood vessels that normally supply the dura in the region and from the natural collaterals to the dura in the region of the network. In addition to the dural network of vessels, we identified in the subarachnoid space, around the wall of the partially thrombosed vein of Galen an extensive network of vessels like the weave around the “gourd rattle” that may be complete or incomplete (Figure 2).
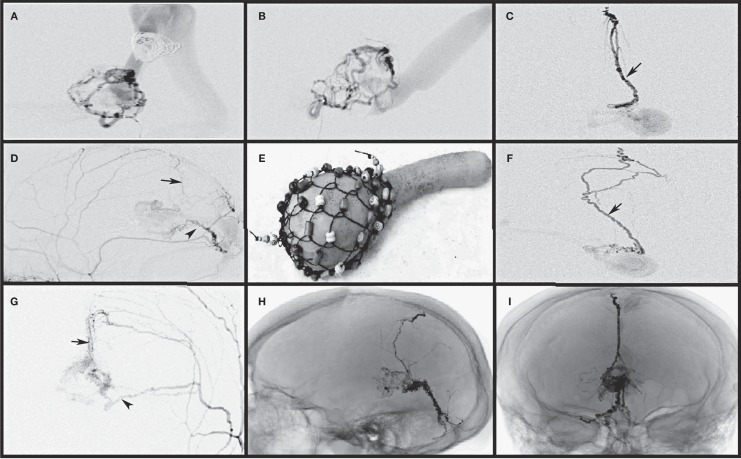
Figure 2.
Endovascular partial embolization of vein of Galen malformation results in the new formation of a fine arterial network of vessels due to angiogenic stimuli on the wall of the partially thrombosed vein of Galen sac. This fine network of vessels appears like a weave (I) around the gourd rattle, a traditional African musical instrument. This network may be complete or partial and receives blood supply from both the pial and dural vessels. A,B) The pial vessel microcatheterization and selective angiogram show the fine network and partially thrombosed vein of Galen. Dural vessel selective microcatheterization (C,D) and non-selective angiograms (G,H) show the middle meningeal artery feeders along the falx (arrow) and tentorium (arrowhead) supplying the fine network of vessels. E,F) Onyx cast following treatment in one of the patients reveals the falx and tentorial network along with the fine network on the wall of the vein of Galen.
Among the 26 patients with DAVF, one patient was not treated for VOGM owing to the severe pre-existing brain damage. In all other patients, endovascular embolization of VOGMs was performed in one or more sessions. With the progress in staged treatment of VOGM, DAVF developed or underwent dynamic changes in morphology by either regression or progression. Embolization targeted at DAVF was done in 13 patients (13/25, 52%) in one or more embolization sessions using NBCA or Onyx. Complete cure was achieved in eight patients (32%). Five patients (20%) had a recurrence of DAVF and only partial cure was achieved. No embolization so far has been done targeting the DAVF in 12 patients (48%). Among them, eight patients (32%) had spontaneous regression and complete obliteration of DAVF with continued treatment of VOGM (Tables 2 and 3). In the others, the DAVF either remained stable or progressed by recruitment of more supply from the dural vessels and subsequently increasing the shunt.
Table 3.
Management and results of DAVF associated with VOGM.
| Total number of VOGM | 87 |
| Total VOGM with DAVF | 26 (30%) |
| VOGM treated in cases with DAVF | 25 |
| Number of DAVF embolized | 13 (52%) |
| Complete Embolization | 8 (32%) |
| Partial Embolization | 5 (20%) |
| Number of DAVF Not Embolized | 12 (48%) |
| Spontaneous resolution | 8 (32%) |
| DAVF remaining and not treated so far | 4 (16%) |
Discussion
Dural arteriovenous fistulas (DAVF) associated high-flow vascular malformations of the brain have been described in the literature 2-4 but their association with vein of Galen malformation (VOGM) has been poorly documented 1. Arteriovenous malformations (AVM) of the brain can have spontaneous dural feeders ranging from 15% to as high as 50% in various reports 2-4. Development of dural feeders following embolization of brain AVM has been documented 4,5 but the exact incidence is not known as most high-flow vascular malformations are treated by more than one modality 6,3. Development of DAVF in the venous sinuses has been well-documented after sinus thrombosis, surgery, trauma and infection of the adjacent paranasal sinus 7-9. The exact mechanism of development of such DAVF is unclear, and the theories are controversial with the most accepted being the pathological recanalization of a thrombosed sinus.
The vein of Galen is a short transition venous structure between the pial veins to the dural sinus, located in the quadrigeminal cistern at the junction of supra and infratentorial compartments in the posterior incisural space. It drains posteriorly into the straight sinus or falcine sinus in VOGM located within the sleeves of the dura at the falcotentorial junction. The vein of Galen is surrounded by dura mater consisting of the broad tentorial margin posteriorly and laterally, the posterior edge of the falx cerebri posterosuperiorly and falx cerebelli posteroinferiorly. In VOGM, the enlarged vein occupies the entire cistern stretching the neural tissue anteriorly and the dura all around the space. Its anatomic location is significant in development of DAVF.
The dura mater is a thick, fibrous and inelastic membrane with a complex vascular network, with blood supply far in excess 10 of the expected metabolic needs of a membrane furnishing only mechanical support and it readily yields to the angiogenic stimuli. The arterial and venous networks are not present in the pia and arachnoid 11.
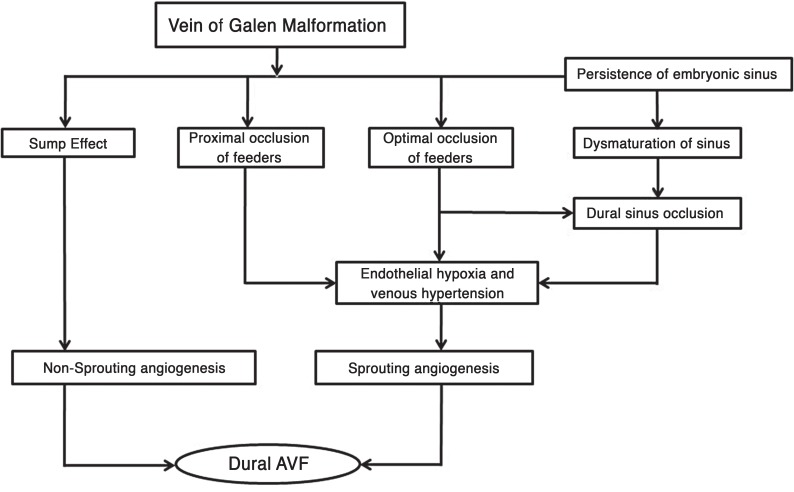
The pathophysiological mechanism involved in the development of DAVF in VOGM is associated with non-sprouting or sprouting angiogenesis (Figure 5).
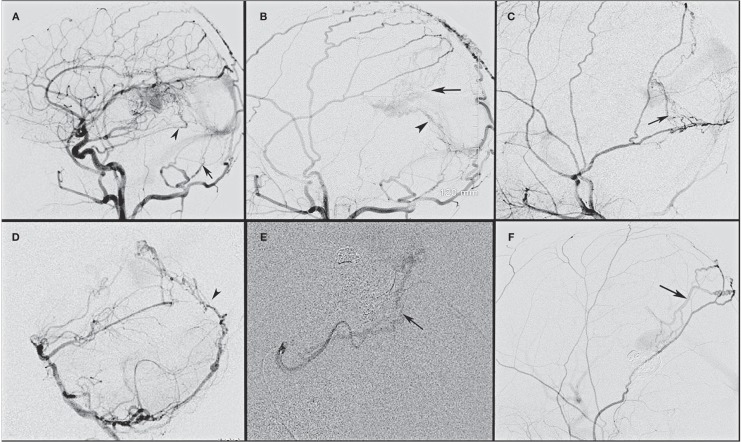
Figure 3.
Dural fistulas that develop following embolization of vein of Galen malformation develop from the natural collaterals that normally supply the dura mater of the falx and tentorium. A) The common carotid artery angiogram shows the basal tentorial artery from the meningohypophyseal trunk (arrowhead) supplying the dural fistula along with tentorial arteries recruiting supply from the transmastoid branch of the occipital artery (arrow). B) The external carotid artery from the same patient shows an additional supply from the middle meningeal artery along the falx (arrow) and the prominent tentorial network of vessels ending in a fine network of vessels around the partially thrombosed vein of Galen. C) An external carotid angiogram shows the supply from the petrosquamous branch of middle meningeal artery along the tentorium (arrow). D) A vertebral artery angiogram shows the supply from the posterior meningeal artery along the falx cerebelli and tentorium (arrowhead). E) A superselective superior cerebellar artery angiogram shows the supply from the marginal tentorial artery (arrow). F) An external carotid artery angiogram shows the parietal branch of the middle meningeal artery supplying the fistula by traversing along the falx cerebri (arrow).
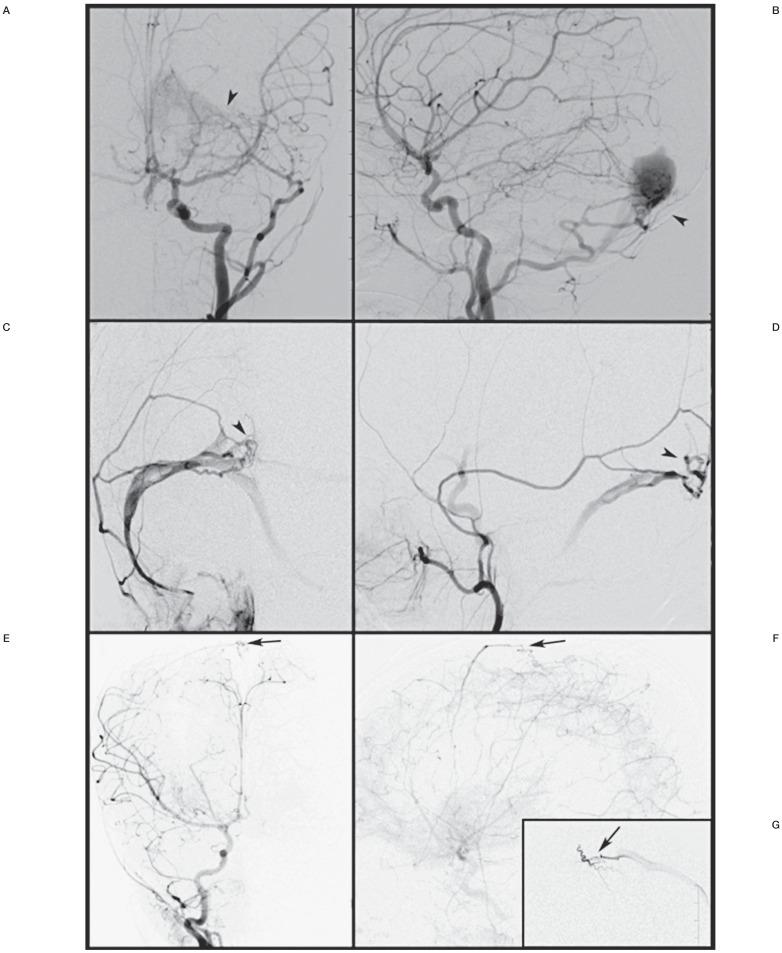
Figure 4.
A dural fistula developed over the torcula following embolization of vein of Galen malformation. A,B) Left common carotid artery angiogram show the enlarged transmastoid branch of the occipital artery supplying the dural fistula around the torcula (arrowhead). C,D) Left external carotid artery angiogram shows the fistula around the torcula (arrowhead) supplied by the middle meningeal artery branch. E,F) Right common carotid artery angiogram during follow-up shows the newly developed dural fistula along the superior sagittal sinus (arrow). G) Superselective injection of the right middle meningeal artery revealing the dural fistula (arrow).
Figure 5.
Schematic representation of mechanisms involved in sprouting and non-sprouting angiogenesis resulting in development of dural arteriovenous fistula in vein of Galen malformation.
Non-sprouting angiogenesis is the persistence and maturation of existing dura to pia arterial connections that occurs due to a possible sump effect. The sump effect is created due to the pressure difference between the high pressure and low velocity in the dural vessel and low pressure and high velocity in the distal part of the feeding arteries and draining galenic venous sac. The longer the sump effect is in place, the greater the chances of developing a fistula. The mean age of patients where the DAVF was seen on the first angiogram was five times higher than the group that developed DAVF following embolization. As we treat the VOGM by embolization, the flow decreases resulting in reduced velocity and more turbulent flow. These changes can cause a reduction and ultimately elimination of the sump effect 12 resulting in spontaneous resolution of the primary DAVF as we observed in two cases. The non-sprouting angiogenesis can co-exist with a sprouting angiogenesis.
Sprouting angiogenesis is a complex process involving the interplay of various chemical mediators. Clinical experience and experimental studies involving rats have shown that venous or intra-sinus thrombosis and hypertension are important factors related to the pathogenesis of DAVF 8,13-15. Endovascular embolization results in hemodynamic changes in the veins and sinus that create a slow and turbulent flow. The changes are not uniform with some parts affected more than others as decided by the altered flow pattern, septations and segmentation in the venous sinus channels. The resulting thrombosis leads to hypoxia of the endothelium and secondarily localized hypertension. In addition, the persistence of the embryonic sinus in VOGM leads to desaturation of the dural venous sinus at the skull base. Following embolization the rapid reduction in the flow of blood can cause further narrowing or occlusion of these already immature venous sinuses. This results in a further pressure rise in the venous channels leading to more turbulent flow resulting in thrombosis and localized hypertension. The pathophysiological mechanism following the initiating event is subclinical resulting in the production of vascular endothelial growth factor and other angiogenic factors 16-19. The angiogenic factors produce local angiogenesis either by sprouting or splitting of pre-existing vascular structure 20. Our interval between embolization of the vein of Galen malformation and identification of the new DAVF ranged from one month to 12 years, but this does not mean that the fistula did not occur earlier.
The post embolization pressure changes and thrombus formation peak at the dilated VOGM followed by the straight sinus and torcula as does the development of DAVF: most were into the vein of Galen followed by the torcula, transverse sigmoid sinus and less frequently the superior sagittal sinus (Figure 4).
Angiogenesis is evidenced by the formation of a fine network of vessels in the subarachnoid space or on the wall of the partially thrombosed vein of Galen. It looks like the weaving of the “gourd rattle” and may be complete or incomplete (Figure 2). This network of vessels is well-interconnected neo-angiogenic vessels and may also comprise the remnant of the distal artery-to-artery anastamosis of feeders found in VOGM 1. This vascular network receives blood supply from the brain parenchymal vessels that for convenience in this paper we call pial vessels and dural vessels draining into the partially thrombosed VOGM sac. The point of drainage of this network into the vein is variable and may be well away from the pial or dural feeder to the network.
The feeders to this neo-angiogenic network can follow three different patterns. In the first, the feeders are predominantly from the pial vessels, in the second, from pial and dural vessels, and in the third, predominantly from the dural vessels (Figure 1 B-F). In patterns two and three, the angiogenic stimuli from the partially thrombosed Galenic venous pouch located in the subarachnoid space and in near direct contact with the dura baring the thin arachnoid results in an angiogenic response from the dura. This results in the formation of a vascular network on the dura, frequently on the tentorium and falx cerebri. This network is the result of secondary recruitment of blood supplies to the shunt from the vessels that normally supply the tentorium, falx cerebri and cerebelli and their natural collaterals.
The association of DAVF in VOGM is not uncommon and so it is important to involve the external carotid system during the initial and each follow-up angiogram to detect these dural fistulas. Some are seen in the initial angiogram, but due to the very high-flow nature of the malformation some may be missed initially and become more prominent later. So it is not clear if they were all present initially and not seen or most of them had initiated after treatment. However embolization certainly aggravated their enlargement.
On identification of the DAVF in the initial angiogram and on follow-up angiograms, our strategy is to continue with the treatment of the primary vein of Galen feeders and to continue to observe the dural fistula for various reasons. As we embolize more and more primary pial feeders along with the angiogenic network, the DAVF undergoes spontaneous resolution (32%) on achieving complete or near complete cure of VOGM. This spontaneous resolution may be due to loss of endothelial angiogenic stimuli and due to the embolization of the subarachnoid network of vessels that are the final common pathway with feeders from the pial and dural feeders. In some patients the focus of neo-angiogenesis may shift to the dural feeders and this allows the dural feeders to mature, enabling effective embolization of the subarachnoid network through them at a later stage. In all cases of dural feeders embolized in our series, there were no large pial feeders to the residual malformation and the dural feeders were considered safe access to obliterate the dural shunt and to achieve cure of the residual VOGM.
The goal of embolization through the dural feeders was to achieve good penetration of the embolic material to the fine angiogenic network around the venous sac. The material used is based on individual choice and experience. We embolized localized simple dural fistulas with NBCA, and multi-segmental, extensive dural networks with Onyx. We used both NBCA and Onyx in two cases of extensive and complex DAVF without good penetration to the angiogenic network around the venous sac; both had recurrence and remain partially occluded. In our experience, it is difficult to achieve deep penetration to obliterate the angiogenic network, and we were forced in some cases to access multiple feeders before achieving adequate penetration. As long as the embolization is on the dural sleeves, it is safe. As embolization proceeds to the dural edge and beyond into the subarachnoid or the venous wall plexus, extreme caution is mandatory as this network has feeding vessels from pial vessels. We had neurophysiologic monitoring and were extremely cautious with regard to the penetration of onyx and did not have any complications while embolizing through the dural feeders. Among the 15 cured cases, 13 patients had follow-up angiograms in the period ranging from three months to three years to confirm complete occlusion, and the remaining two were treated recently and are yet to be followed.
Conclusion
DAVF associated with high-flow VOGM is not uncommon. They represent the dural response to the angiogenic stimuli. It is important to include the external carotid system during the initial and follow-up angiograms while treating the VOGM. These DAVF can be observed to regress spontaneously or mature while we continue to treat the primary feeders to the VOGM. The persisting DAVF with residual VOGM that do not have access though the pial vessels are used as conduits to treat the dural shunt and achieve obliteration of residual VOGM at later stages of treatment.
References
- 1.Raybaud CA, Strother CM, Hald JK. Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology. 1989;31(2):109–128. doi: 10.1007/BF00698838. doi: 10.1007/BF00698838. [DOI] [PubMed] [Google Scholar]
- 2.Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969;93(5):1071–1078. doi: 10.1148/93.5.1071. [DOI] [PubMed] [Google Scholar]
- 3.Miyachi S, Negoro M, Handa T, et al. Contribution of meningeal arteries to cerebral arteriovenous malformations. Neuroradiology. 1993;35(3):205–209. doi: 10.1007/BF00588495. doi: 10.1007/BF00588495. [DOI] [PubMed] [Google Scholar]
- 4.Söderman M, Rodesh G, Lasjuanias P. Transdural blood supply to cerebral arteriovenous malformations adjacent to the dura mater. Am J Neuroradiol. 2002;23(8):1295–1300. [PMC free article] [PubMed] [Google Scholar]
- 5.Paramasivam S, Toma N, Niimi Y, et al. De novo development of dural arteriovenous fistula after endovascular embolization of pial arteriovenous fistula. J Neurointerv Surg. 2013;5(4):321–326. doi: 10.1136/neurintsurg-2012-010318. doi: 10.1136/neurintsurg-2012-010318. [DOI] [PubMed] [Google Scholar]
- 6.Willinsky R, Lasjaunias P, Terbrugge K, et al. Brain arteriovenous malformations: analysis of the angio-architecture in relationship to hemorrhage (based on 152 patients explored and/or treated at the hopital de Bicêtre between 1981 and 1986) J Neuroradiol. 1988;15(3):225–237. [PubMed] [Google Scholar]
- 7.Kinjo T, Mukawa J, Miyagi K, et al. [A case of successfully removed posttraumatic high flow dural arteriovenous fistula in the posterior fossa] No Shinkei Geka. 1991;19(6):577–581. [PubMed] [Google Scholar]
- 8.Sasaki T, Hoya K, Kinone K, et al. Postsurgical development of dural arteriovenous malformations after transpetrosal and transtentorial operations: case report. Neurosurgery. 1995;37(4):820–824. doi: 10.1227/00006123-199510000-00029. discussion 824-825. doi: 10.1227/00006123-199510000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Touho H, Ohnishi H, Komatsu T, et al. Dural arteriovenous fistula caused by sinus thrombosis--case report. Neurol Med Chir (Tokyo) 1994;34(8):543–546. doi: 10.2176/nmc.34.543. doi: 10.2176/nmc.34.543. [DOI] [PubMed] [Google Scholar]
- 10.Kerber CW, Newton TH. The macro and microvasculature of the dura mater. Neuroradiology. 1973;6(4):175–179. doi: 10.1007/BF00335317. doi: 10.1007/BF00335317. [DOI] [PubMed] [Google Scholar]
- 11.Rowbotham GF, Little E. New concepts on the aetiology and vascularization of meningiomata; the mechanism of migraine; the chemical processes of the cerebrospinal fluid; and the formation of collections of blood or fluid in the subdural space. Br J Surg. 1965;52:21–24. doi: 10.1002/bjs.1800520105. doi: 10.1002/bjs.1800520105. [DOI] [PubMed] [Google Scholar]
- 12.Debrun G, Viñuela F, Fox A, et al. Embolization of cerebral arteriovenous malformations with bucrylate. J Neurosurg. 1982;56(5):615–627. doi: 10.3171/jns.1982.56.5.0615. doi: 10.3171/jns.1982.56.5.0615. [DOI] [PubMed] [Google Scholar]
- 13.Herman JM, Spetzler RF, Bederson JB, et al. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83(3):539–545. doi: 10.3171/jns.1995.83.3.0539. doi: 10.3171/jns.1995.83.3.0539. [DOI] [PubMed] [Google Scholar]
- 14.Nishijima M, Takaku A, Endo S, et al. Etiological evaluation of dural arteriovenous malformations of the lateral and sigmoid sinuses based on histopathological examinations. J Neurosurg. 1992;76(4):600–606. doi: 10.3171/jns.1992.76.4.0600. doi: 10.3171/jns.1992.76.4.0600. [DOI] [PubMed] [Google Scholar]
- 15.Terada T, Higashida RT, Halbach VV, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80(5):884–889. doi: 10.3171/jns.1994.80.5.0884. doi: 10.3171/jns.1994.80.5.0884. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235(4787):442–447. doi: 10.1126/science.2432664. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 17.Wiesener MS, Turley H, Allen WE, et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92(7):2260–2268. [PubMed] [Google Scholar]
- 18.Persson AB, Buschmann IR. Vascular growth in health and disease. Front Mol Neurosci. 2011;4:14. doi: 10.3389/fnmol.2011.00014. doi: 10.3389/fnmol.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miano JM, Vlasic N, Tota RR, et al. Smooth muscle cell immediate-early gene and growth factor activation follows vascular injury. A putative in vivo mechanism for autocrine growth. Arterioscler Thromb. 1993;13(2):211–219. doi: 10.1161/01.atv.13.2.211. doi: 10.1161/01.ATV.13.2.211. [DOI] [PubMed] [Google Scholar]
- 20.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]