Abstract
Introduction. Neoadjuvant chemoradiotherapy and total mesorectal excision are considered the standard treatment for locally advanced rectal cancer. Various studies have reported pathological downstaging and a complete pathological response rate of 15%–27% following neoadjuvant chemoradiotherapy which has translated into improved survival. We endeavour to determine the clinical outcome of patients attaining a complete pathological tumor response following neoadjuvant chemoradiotherapy in the Indian setting where most of our patient population is younger and presents with aggressive tumor biology. Materials and Methods. Clinicopathological and treatment details were recorded for 64 patients achieving pathological complete response from 2010 to 2013. Disease-free survival (DFS), overall survival (OS), and locoregional and systemic recurrence rates were evaluated for these patients. Results. After a median follow-up of 30.5 months (range 11–59 months), the 3-year overall survival (OS) was 94.6% and the 3-year disease-free survival (DFS) was 88.5%. The locoregional and systemic recurrence rates were 4.7% and 3.1%, respectively. Conclusion. In the Indian subcontinent, despite younger patients with aggressive tumor biology, outcome in complete responders is good.
1. Introduction
Total mesorectal excision coupled with neoadjuvant chemoradiotherapy is currently considered the standard treatment for patients with locally advanced rectal cancers (LARC). This multimodality treatment has resulted in improved local control rates, albeit showing no long-term survival benefits [1–5]. Various studies across literature have reported pathological downstaging and a complete pathological response rate (ypCR) of 15%–27% following neoadjuvant chemoradiotherapy (NACTRT) prior to radical surgery [6]. This has translated into not only a superior and improved survival but also decreased locoregional and systemic recurrence.
Through our study we endeavour to determine the clinical outcome of patients attaining a complete pathological tumor response following neoadjuvant chemoradiotherapy and radical rectal surgery in the Indian setting where most of our patient population is younger and presents with aggressive tumor histology.
2. Materials and Methods
Four hundred and thirty patients of LARC (cT3, cT4, N+) underwent surgical resection following NACTRT from 2010 to 2013 at the Tata Memorial Hospital. Of the 430 patients, sixty-four patients achieved complete pathological tumor response. Information on demographic data, stage at presentation, and treatment administered were recorded from a prospectively maintained database for these 64 patients. A colonoscopy/sigmoidoscopy was performed on all patients and a tissue diagnosis with biopsy was obtained prior to commencement of treatment. Staging investigations were comprised of a contrast enhanced CT scan of the thorax and a MRI of the pelvis or CT scan of the pelvis prior to receiving neoadjuvant chemoradiotherapy. After completion of NACTRT and prior to surgery all patients were imaged with a pelvic MRI. Patients were staged according to the UICC TNM 2010 classification for rectal cancer [7]. Patients received long course radiation between 40 Gy and 50 Gy in 25#–28# for 5 weeks with twice daily Capacetabine 825 mg/m2 concurrently with radiation, as part of NACTRT protocol. All patients were discussed in a multidisciplinary tumor board where treatment decisions were made. Disease-free survival (DFS), overall survival (OS), and locoregional and systemic recurrence rates were evaluated for these 64 patients. DFS was determined from the date of starting treatment, that is, neoadjuvant chemoradiotherapy, to the date of first locoregional or systemic relapse. Overall survival (OS) was calculated from the date of starting treatment, that is, neoadjuvant chemoradiotherapy, to the date of death or last follow-up. Kaplan-Meier curves were calculated to determine survival and log-rank test was used to compare survival outcomes between different subgroups of patients.
3. Results
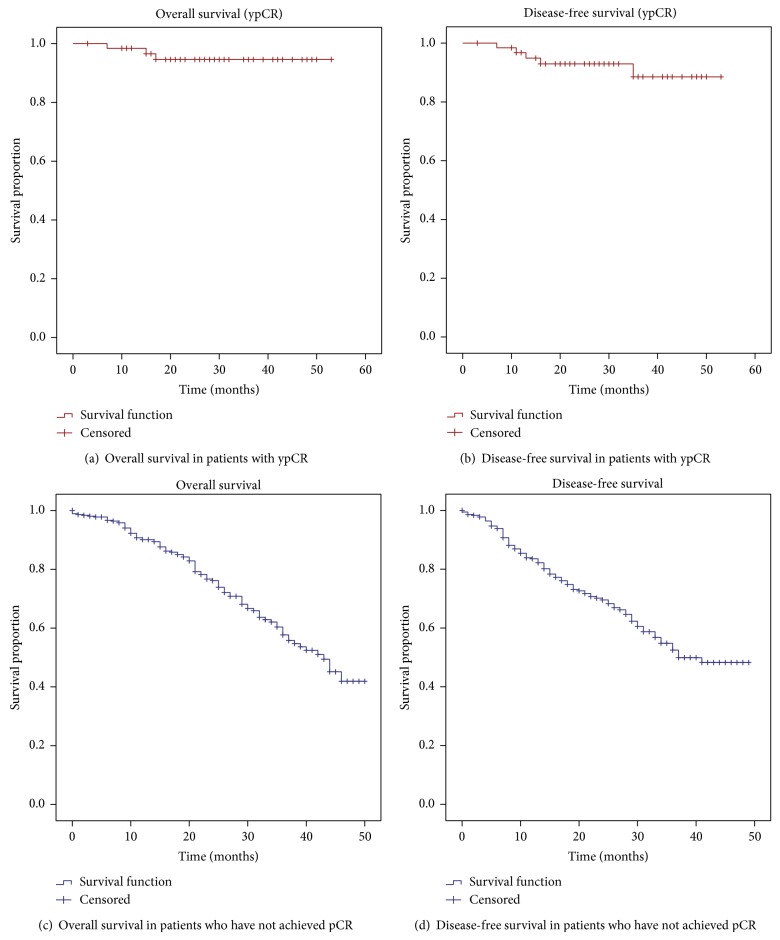
Clinicopathological characteristics of this cohort are summarized in Table 1. In our study, 65.6% of our patients were male. The median age of presentation was 47 years (range 18–77 years). The median pretreatment carcinoembryonic (CEA) tumor marker level was 3.2 (range 1.1–127 ng/mL). 95.7% received long course radiation as part of NACTRT. The median interval between completion of NACTRT and surgery was 56 days (range 12–206 days). Abdominoperineal resection (APR) was the most common surgical procedure performed. Adjuvant chemotherapy was administered in 60.9% patients. 14.8% patients had a complete pathological tumor response rate (ypCR) with a TRG score of 1/5 (the Mandard scoring system was used) [8]. The commonest histology was classical adenocarcinoma. Signet cell carcinoma and mucinous carcinoma were seen in 14.06% and 7.8% of cases, respectively. The median nodes dissected at surgery were 8 (range 1–21). After a median follow-up of 30.5 months (range 11–59 months), the 3-year overall survival (OS) was 94.6% and the 3-year disease-free survival (DFS) was 88.5% (Figures 1(a) and 1(b)). In patients without complete pathological response, the 3 year OD was 57% and DFS was 52% (Figures 1(c) and 1(d)). The locoregional recurrence rate and systemic recurrence rate were 4.7% and 3.1%, respectively.
Table 1.
Demographic and clinicopathological characteristics.
| Characteristics | n = 64 (%) |
|---|---|
| Gender | |
| Male | 42 (65.6%) |
| Female | 22 (34.4%) |
| Age (yr) | |
| Range | 18–77 |
| Median | 47 |
| Stage at presentationa | |
| cT3N0 | 39 (60.9%) |
| cT4N0 | 2 (3.1%) |
| cT3N+x | 23 (35.9%) |
| Distance from anal verge (AV)b | |
| AV-2 cms | 25 (39.1%) |
| 2–5 cms | 15 (23.4%) |
| 5–10 cms | 20 (31.3%) |
| >10 cms | 04 (6.3%) |
| Pretreatment CEA (ng/mL)c | |
| Range | 1.1–127 |
| Median | 3.2 |
| Surgery | |
| Abdominoperineal resection (APR)d | 29 (45.3%) |
| Low anterior resection | 10 (15.6%) |
| Anterior resection | 22 (34.3%) |
| Intersphincteric resection | 03 (4.6%) |
| Histology | |
| Classical adenocarcinoma | 50 (78.1%) |
| Mucinous adenocarcinoma | 5 (7.8%) |
| Signet cell adenocarcinoma | 9 (14.06%) |
| Extracellular mucin pool | |
| Yes | 24 (37.5%) |
| No | 40 (62.5%) |
| Total nodes dissected | |
| Range | 1–21 |
| Median | 08 |
| Radiotherapy type | |
| Long course | 67 (95.7%) |
| Short course | 03 (4.3%) |
| Radiotherapy dose | |
| 50 GY | 48 (75%) |
| 40–45 GY | 14 (22%) |
| 25 GY | 02 (3.1%) |
| Laparoscopy | |
| Yes | 11 (17.2%) |
| No | 53 (82.8%) |
| Interval of NACTRT to surgery (days) | |
| Range | 12–206 |
| Median | 56.5 |
| Postoperative chemotherapy | |
| Yes | 39 (60.9%) |
| No | 25 (39.1%) |
aClinicoradiological staging.
bAV: anal verge.
cCEA: carcinoembryonic antigen.
dAPR indicates abdominoperineal resection.
xN+: positive nodal status.
Figure 1.
(a) and (b) Survival in patients with a complete pathological response (ypCR). (c) and (d) Survival in patients who have not achieved a complete pathological response.
4. Discussion
The combination of NACTRT and meticulous radical surgery has contributed to increased local control in patients with advanced rectal cancers. Despite this multimodality treatment, the reported 5-year survival in patients with locally advanced rectal cancer is 45%–75% with recurrences occurring in 5%–15% of patients [9]. In India, however, survival remains low, the reported 5-year survival being between 30 and 40% [10, 11]. There is, however, a cohort of patients who achieve complete pathological response following NACTRT and definitive surgery. Several studies across literature have reported a ypCR rate of 15%–27% and a favourable survival in these patients. In a retrospective cohort study of 725 patients, oncologic outcomes after preoperative chemoradiotherapy and radical resection for locally advanced rectal cancer correlated with pathological complete response. This study reported no local recurrences and a distal metastatic rate of 7% in patients achieving a complete pathological response [12].
In our study 14.8% of patients achieved a ypCR (64/430). Our study showed that this cohort of patients with pathological complete response enjoyed a good overall survival as well as disease-free survival. In a recent systematic review and meta-analysis evaluating the clinical outcome of complete pathological response following neoadjuvant chemotherapy in 3363 patients, the reported five-year overall and disease-free survival rates were 90.2% and 87.0%, respectively. A local recurrence rate of 0.7% with a distant failure rate of 8.7% was also reported in this review. This meta-analysis concluded that a complete pathological response following NACTRT was associated with excellent long-term survival, with low rates of local recurrence and distant failure [13]. Capirci et al. in their largest published experience of patients with a pathological complete response following neoadjuvant chemoradiotherapy and radical rectal surgery reported a local failure rate of 0.9% with distant failure occurring in 8.9% [14]. Maas and coworkers in a comparative analysis of pooled individual data of 3105 patients to determine the oncological outcomes for patients with and without a pathological complete response (ypCR) after neoadjuvant chemoradiotherapy showed a 5-year DFS rate of 83.3% in patients with a ypCR as compared with 65.6% for incomplete responders. Five-year local recurrence rates reported were 2.8% versus 9.7% and the rate of distant metastasis was 11.2% versus 25.2% [6]. The findings of our study are in concordance with those published in the literature. In our patients with ypCR the 3-year OS and DFS were 94.6% and 88.5%, respectively. We reported a locoregional and systemic recurrence rate of 4.7% and 3.1%, respectively. This holds true despite a high proportion of our tumors presenting with extracellular mucin (37.5%) and showing aggressive histology (signet cell 14.06%, mucinous 7.8%).
Despite achieving a complete response in the primary tumor, approximately 6.6%–17% of tumor specimens have residual disease in the mesorectal nodes (ypT0N+) [15–17]. Several studies have emphasized the prognostic significance of residual nodal disease [17–19]. Yeo and colleagues in their study of 333 LARC patients concluded that patients achieving a pathological complete response after preoperative NACTRT enjoyed favorable long-term outcomes. They also showed that the most significant independent prognostic factor for DFS and OS, even after total regression of the primary tumor by preoperative NACTRT, was residual nodal disease [15]. Evidence in the literature has shown that ypCR is associated with improved survival. One of the most studied strategies to improve ypCR is the optimum interval between NACTRT and surgery. Several studies have shown that a minimum duration of 6–8 weeks between NACTRT and surgery improves downstaging and increases the chances of a ypCR [20–23]. In our study, the median interval between NACTRT and surgery was 56 days (range 12–127 days).
In our study 60.9% of patients achieving a pathological complete response received postoperative adjuvant chemotherapy. There is however a lack of consensus about the benefit of adjuvant chemotherapy in patients achieving a ypCR. A few studies have shown an improved survival in patients with pathological downstaging and postoperative chemotherapy [15, 24, 25], while others have suggested that adjuvant chemotherapy be administered only in patients with residual nodal disease [26].
In light of the available evidence in the literature, the clinical ramifications of such a pathological complete response could be manifold. There is no doubt that patients achieving a ypCR enjoy improved survival. Hence efforts need to be made to increase the rates of ypCR. This becomes imperative especially in context of the Indian population as majority of our patients are younger and present with an aggressive histology, that is, mucinous and signet cell carcinoma (approx. 7.8% and 14.06%, resp.) [Dr. A Saklani, Tata Memorial Hospital]. Efforts to improve ypCR rates with the addition of oxaliplatin or irinotecan [27, 28] or targeted agents [29, 30] have been disappointing. The integration of translational research and gene expression profiling may assist in identifying factors that may predict tumor response and guide in achieving a higher proportion of ypCR.
5. Conclusion
In the Indian scenario, despite younger age and higher proportion of mucinous and signet cell tumors, outcome in complete responders is good and is in concordance with world literature. Efforts need to be made to increase complete response rates in order to avail our patients with maximum benefits in terms of survival and local control.
Ethical Approval
The authors would like to state that in view of the retrospective nature of this study it was not deemed necessary to obtain approval from the institutional review board and the permission and approval of the Head of Department of Surgical Oncology were taken prior to commencement of this study. This study did not involve the direct use of tissue or blood samples or animal studies.
Conflict of Interests
The authors do not have any conflict of interests. The authors do not have any relationships or interests to disclose that could bias or influence this study.
References
- 1.Heald R. J., Ryall R. D. H. Recurrence and survival after total mesorectal excision for rectal cancer. The Lancet. 1986;1(8496):1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R., Becker H., German Rectal Cancer Study Group, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. The New England Journal of Medicine. 2004;351:1731–1740. doi: 10.1056/nejmoa040694. [DOI] [PubMed] [Google Scholar]
- 3.Sebag-Montefiore D., Stephens R. J., Steele R., et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. The Lancet. 2009;373(9666):811–820. doi: 10.1016/s0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pucciarelli S., Gagliardi G., Maretto I., et al. Long-term oncologic results and complications after preoperative chemoradiotherapy for rectal cancer: a single-institution experience after a median follow-up of 95 months. Annals of Surgical Oncology. 2009;16(4):893–899. doi: 10.1245/s10434-009-0335-6. [DOI] [PubMed] [Google Scholar]
- 5.Kusters M., Marijnen C. A. M., van de Velde C. J. H., et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. European Journal of Surgical Oncology. 2010;36(5):470–476. doi: 10.1016/j.ejso.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Maas M., Nelemans P. J., Valentini V., et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. The Lancet Oncology. 2010;11(9):835–844. doi: 10.1016/s1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 7.Edge S. B., Byrd D. R., Compton C. C., et al., editors. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 7th. New York, NY, USA: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 8.Mandard A. M., Dalibard F., Mandard J. C., et al. Pathological assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–2686. doi: 10.1002/1097-0142(19940601)73:1160;2680::aid-cncr282073110562;3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.de Wilt J. H. W., Vermaas M., Ferenschild F. T. J., Verhoef C. Management of locally advanced primary and recurrent rectal cancer. Clinics in Colon and Rectal Surgery. 2007;20(3):255–264. doi: 10.1055/s-2007-984870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pathy S., Lambert R., Sauvaget C., Sankaranarayanan R. The incidence and survival rates of colorectal cancer in india remain low compared with rising rates in east Asia. Diseases of the Colon and Rectum. 2012;55(8):900–906. doi: 10.1097/dcr.0b013e31825afic4e. [DOI] [PubMed] [Google Scholar]
- 11.Yeole B. B., Sunny L., Swaminathan R., Sankaranarayanan R., Parkin D. M. Population-based survival from colorectal cancer in Mumbai, (Bombay) India. European Journal of Cancer. 2001;37(11):1402–1408. doi: 10.1016/s0959-8049(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 12.Park I. J., You Y. N., Agarwal A., et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. Journal of Clinical Oncology. 2012;30(15):1770–1776. doi: 10.1200/jco.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin S. T., Heneghan H. M., Winter D. C. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. British Journal of Surgery. 2012;99(7):918–928. doi: 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- 14.Capirci C., Valentini V., Cionini L., et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. International Journal of Radiation Oncology Biology Physics. 2008;72(1):99–107. doi: 10.1016/j.ijrobp.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Yeo S.-G., Kim D. Y., Kim T. H., et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: Long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01) Annals of Surgery. 2010;252(6):998–1004. doi: 10.1097/SLA.0b013e3181f3f1b1. [DOI] [PubMed] [Google Scholar]
- 16.Hughes R., Glynne-Jones R., Grainger J., et al. Can pathological complete response in the primary tumour following pre-operative pelvic chemoradiotherapy for T3-T4 rectal cancer predict for sterilisation of pelvic lymph nodes, a low risk of local recurrence and the appropriateness of local excision? International Journal of Colorectal Disease. 2006;21(1):11–17. doi: 10.1007/s00384-005-0749-y. [DOI] [PubMed] [Google Scholar]
- 17.Glynne-Jones R., Wallace M., Livingstone J. I. L., Meyrick-Thomas J. Complete clinical response after preoperative chemoradiation in rectal cancer: is a “wait and see” policy justified? Diseases of the Colon and Rectum. 2008;51(1):10–19. doi: 10.1007/s10350-007-9080-8. [DOI] [PubMed] [Google Scholar]
- 18.Valentini V., Coco C., Picciocchi A., et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. International Journal of Radiation Oncology, Biology, Physics. 2002;53(3):664–674. doi: 10.1016/s0360-3016(02)02764-5. [DOI] [PubMed] [Google Scholar]
- 19.Shivnani A. T., Small W., Jr., Stryker S. J., et al. Preoperative chemoradiation for rectal cancer: results of multimodality management and analysis of prognostic factors. American Journal of Surgery. 2007;193(3):389–394. doi: 10.1016/j.amjsurg.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Kalady M. F., De Campos-Lobato L. F., Stocchi L., et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Annals of Surgery. 2009;250(4):582–588. doi: 10.1097/SLA.0b013e3181b91e63. [DOI] [PubMed] [Google Scholar]
- 21.Tulchinsky H., Shmueli E., Figer A., Klausner J. M., Rabau M. An interval > 7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Annals of Surgical Oncology. 2008;15(10):2661–2667. doi: 10.1245/s10434-008-9892-3. [DOI] [PubMed] [Google Scholar]
- 22.de Campos-Lobato L. F., Geisler D. P., da Luz Moreira A., Stocchi L., Dietz D., Kalady M. F. Neoadjuvant therapy for rectal cancer: the impact of longer interval between chemoradiation and surgery. Journal of Gastrointestinal Surgery. 2011;15(3):444–450. doi: 10.1007/s11605-010-1197-8. [DOI] [PubMed] [Google Scholar]
- 23.Perez R. O., Habr-Gama A., São Julião G. P., et al. Optimal timing for assessment of tumor response to neoadjuvant chemoradiation in patients with rectal cancer: do all patients benefit from waiting longer than 6 weeks? International Journal of Radiation Oncology Biology Physics. 2012;84(5):1159–1165. doi: 10.1016/j.ijrobp.2012.01.096. [DOI] [PubMed] [Google Scholar]
- 24.Janjan N. A., Crane C., Feig B. W., et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. American Journal of Clinical Oncology. 2001;24(2):107–112. doi: 10.1097/00000421-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Collette L., Bosset J.-F., Den Dulk M., et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. Journal of Clinical Oncology. 2007;25(28):4379–4386. doi: 10.1200/jco.2007.11.9685. [DOI] [PubMed] [Google Scholar]
- 26.Fietkau R., Barten M., Klautke G., et al. Postoperative chemotherapy may not be necessary for patients with ypN0-category after neoadjuvant chemoradiotherapy of rectal cancer. Diseases of the Colon and Rectum. 2006;49(9):1284–1292. doi: 10.1007/s10350-006-0570-x. [DOI] [PubMed] [Google Scholar]
- 27.Allegra C. J., Yothers G., O'Connell M. J., et al. Neoadjuvant therapy for rectal cancer: Mature results from NSABP protocol R-04. Journal of Clinical Oncology. 2014;32(supplement 3, abstract 390) [Google Scholar]
- 28.Mohiuddin M., Paulus R., Mitchell E., et al. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. International Journal of Radiation Oncology Biology Physics. 2013;86(3):523–528. doi: 10.1016/j.ijrobp.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dewdney A., Cunningham D., Tabernero J., et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) Journal of Clinical Oncology. 2012;30(14):1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 30.Velenik V., Ocvirk J., Music M., et al. Neoadjuvant capecitabine, radiotherapy, and bevacizumab (CRAB) in locally advanced rectal cancer: results of an open-label phase II study. Radiation Oncology. 2011;6, article 105 doi: 10.1186/1748-717x-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]