Abstract
WNT signaling was discovered in tumor models and has been recognized as a regulator of cancer development and progression for over 3 decades. Recent work has highlighted a critical role for WNT signaling in the metabolic homeostasis of mammals, where its misregulation has been heavily implicated in diabetes. While the majority of WNT metabolism research has focused on nontransformed tissues, the role of WNT in cancer metabolism remains underinvestigated. Cancer is also a metabolic disease where oncogenic signaling pathways regulate energy production and macromolecular synthesis to fuel rapidly proliferating tumors. This review highlights the emerging evidence for WNT signaling in the reprogramming of cancer cell metabolism and examines the role of these signaling pathways as mediators of tumor bioenergetics.
INTRODUCTION
Following the initial discovery of WNT1 in a murine breast cancer model in the early 1980s (1), 19 WNT genes have been identified in mammals. All of these genes encode secreted glycoproteins that signal through an array of receptors and coreceptors to elicit control over cell proliferation, stem cell self-renewal, and cellular differentiation in a variety of tissues. WNT ligands can signal via a number of pathways, which can be broadly subdivided into two categories based on whether or not they signal through an intracellular transcriptional coactivator called β-catenin. The WNT/β-catenin pathway is commonly referred to as the canonical WNT pathway, whereas the noncanonical pathway is an umbrella term for β-catenin-independent WNT signaling. In recent years, the WNT/β-catenin signaling pathway has been found to be involved in the development of diabetes mellitus and obesity. A number of genome-wide association studies and genetically engineered animal models have identified components of the WNT/β-catenin pathway in susceptibility to obesity and diabetes, in addition to the WNT ligands themselves (2–6). The majority of the genes that are associated with susceptibility to type 2 diabetes regulate β-cell function. Loss of β-catenin in the adult murine pancreas leads to glucose intolerance and protection from high-fat-diet-induced obesity and insulin resistance (7). Furthermore, WNT signaling is also strongly implicated in the control of adipose tissue to systemically regulate glucose homeostasis and adipogenesis during obesity (8–11). These findings highlight that the WNT/β-catenin signaling pathway regulates whole-body metabolism in mammals by altering the behavior of multiple cell types and tissues involved in growth, insulin secretion, and energy expenditure.
Although hormones and other extracellular signaling components facilitate metabolic communication between tissues and organs, at the cellular level, the expression of specific enzyme isoforms and regulatory molecules allows for localized, tissue-specific regulation of metabolism to support specialized cellular functions. Recently, evidence has emerged that highlights a role for WNT-mediated regulation of cellular metabolism as well, including the reprogramming of tumor cell bioenergetics (12–15). This review explores the evidence that components of the WNT signaling pathways regulate cellular metabolism with a specific focus on cancer cells. Furthermore, I explore whether this regulation is controlled either through direct interplay of these components with the cellular metabolism machinery or through cross talk with other oncogenic pathways that are already well-established regulators of tumor cell metabolism. Although our current understanding of the molecular mechanisms involved in this regulation are still incomplete, this work highlights a whole area of WNT signaling that up until now has been poorly investigated, with an aim to emphasize some of the most interesting opportunities for future research efforts.
WNT SIGNALING PATHWAYS
Canonical WNT signaling.
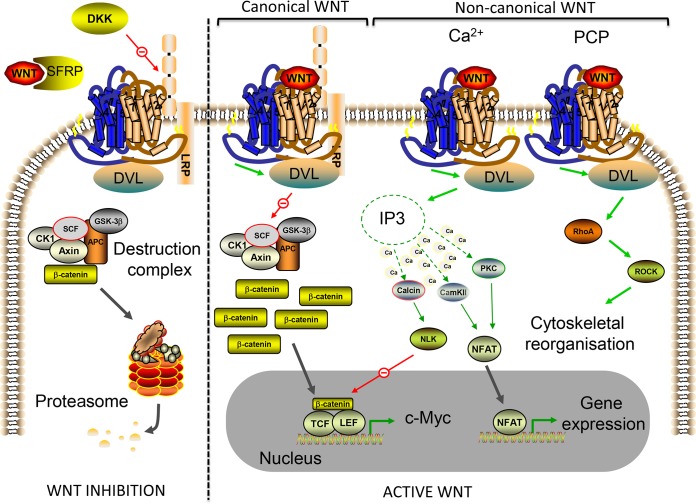
WNT signaling pathways regulate a myriad of biological functions in animals through embryonic development and in adult tissues (16). The canonical WNT pathway involves activation of the key effector molecule β-catenin. In this signaling pathway, β-catenin functions as part of a bipartite transcription factor that activates WNT target genes by interacting with other transcription factors, classically, those belonging to the T cell factor/lymphoid-enhanced binding factor 1 (TCF/LEF1) family (Fig. 1). The WNT/β-catenin pathway has been well defined by numerous studies showing that WNT receptor interaction results in the stabilization of a cytosolic pool of β-catenin by inactivating the anchoring axin-adenomatosis polyposis coli (APC) complex, which is essential for the function of a β-catenin destruction complex. In the absence of an extracellular WNT signal, β-catenin is anchored by the axin-APC complex, subsequently phosphorylated by casein kinase Iα (CKIα) and glycogen synthase kinase 3β (GSK3β), and then ubiquitinated by the SKP1–cullin1–F-box (SCF-β-TRCP) E3 ubiquitin ligase, leading to 26S proteasome-mediated degradation. Upon WNT binding to the frizzled (FZD) and LRP5/6 coreceptors, the axin-APC destruction complex is inactivated through recruitment of the intracellular signaling protein dishevelled (DVL), which prevents β-catenin degradation and allows nuclear translocation of the stabilized pool to activate gene transcription (Fig. 1) (17, 18). WNT signaling is antagonized by a number of endogenous mechanisms, two of which involve the secretion of WNT activity inhibitors, i.e., secreted frizzled receptor-related proteins (SFRPs) and dickkopf proteins (DKKs) that function as LRP and FZD decoys, respectively (19).
FIG 1.
Overview of the WNT signaling network. WNT ligands signal via a number of intracellular pathways in a highly tissue context-dependent manner. In the “off” state, WNT signaling is inhibited (left of the broken line). Extracellularly, this occurs through secreted frizzled receptor-related protein (SFRP) sequestering of WNT ligands to inhibit both canonical (β-catenin-dependent) and noncanonical (β-catenin-independent) WNT signaling. Dickkopf (DKK) is an extracellular antagonist of the LRP5/6 receptor, which is specific to the canonical WNT pathway. For the canonical pathway, in the absence of a WNT ligand, β-catenin levels in the cell are kept low (except at adherens junctions, where β-catenin exists in a cadherin-bound pool at the cell membrane) by a multiprotein destruction complex. The destruction complex binds and phosphorylates β-catenin, targeting it for polyubiquitination and proteasome-mediated degradation. In the presence of WNT ligands, the signaling network becomes activated (right of the broken line). In the canonical WNT pathway, WNT ligands bind to the FZD receptors (large seven-transmembrane domain receptors; blue/brown in schematic) and LRP5/6 coreceptors to activate DVL. DVL inactivates the destruction complex, which in reality occurs through recruitment of the scaffold protein axin to the cytoplasmic tail of LRP5/6, preventing β-catenin degradation. Following inactivation of the destruction complex, cytoplasmic levels of β-catenin rise and the protein is eventually translocated to the nucleus, where it associates with TCF/LEF transcription factors to control the expression of WNT target genes such as the c-Myc gene. Noncanonical WNT signaling consists of two main pathways, the WNT/Ca2+ and WNT/PCP pathways. Both of these pathways involve WNT ligand binding of FZD receptors (also involving coreceptors; not shown) and subsequent activation of DVL. In the WNT/Ca2+ pathway, intracellular Ca2+ levels increase following WNT-induced activation of phospholipase C, resulting in inositol 1,4,5-triphosphate-3 (IP3) generation and subsequent Ca2+ release from intracellular stores. Increased Ca2+ levels result in activation of Ca2+-dependent enzymes and subsequent NFAT-mediated gene activation and inhibition of TCF gene transcription through activated NLK. The WNT/PCP pathway involves WNT-mediated activation of the small GTPase RhoA, which activates Rho-associated kinase (ROCK) to remodel the cytoskeleton and regulate cell movement and tissue polarity. Red arrows represent inhibition, green arrows represent activation, gray arrows represent translocation, and broken green arrows represent second-messenger signaling. See the text for additional details.
Although the mechanistic understanding of WNT/β-catenin signaling has been the subject of intense investigation, we are still learning about the complex and often context-dependent effects of WNT signaling in disease progression, such as in cancer. WNT signaling and its numerous downstream effectors regulate a number of biological processes associated with carcinogenesis, including tumor formation, proliferation, cell death, senescence, cell differentiation, metastasis, and more recently (as reviewed here) also in tumor metabolism. Components of the WNT/β-catenin pathway are frequently mutated in tumors, highlighting the importance of this pathway in the oncogenic process. One of the best examples is the loss of functional APC expression in colorectal cancer (CRC) patients, where APC mutations mimic active WNT/β-catenin signaling and are associated with initiation of CRC (20). Other colorectal tumors and types of cancer (including lung cancer, hepatocarcinoma, medulloblastoma, ovarian cancer, and breast cancer) can also exhibit loss of functional axin or other mutations that stabilize β-catenin expression (21–26). These findings have prompted researchers to develop inhibitors of the WNT/β-catenin signaling pathway for therapeutic use to treat cancer, although the vast majority of these are still at the preclinical testing stage (27). The use of such therapeutics in cancer treatment is, however, confounded by the response of specific tumor types to WNT/β-catenin signaling. In some cancers, for example, melanoma and prostate cancer, patients with higher levels of active β-catenin signaling possess improved predicted outcomes (28, 29). This highlights the need for a better understanding of the context-dependent nature of WNT signaling in cancer.
Noncanonical WNT signaling.
Noncanonical WNT signaling is a collective term for a number of β-catenin-independent pathways that are composed of different types of ligands and receptors. Noncanonical WNT signals are transduced either through intracellular Ca2+ levels or by small GTPases that regulate cytoskeletal remodeling; these are referred to as the Ca2+ and planar cell polarity (PCP) pathways, respectively (Fig. 1). We have known for over a decade now that Wnt ligands are able to induce the release of intracellular Ca2+ to activate Ca2+-dependent enzymes such as phosphatase, calcineurin (Calcin), protein kinase C (PKC), and calmodulin-dependent kinase II (CamKII) (30–32) to mediate diverse effects in animal tissues. PKC and CamKII control dorsoventral patterning in embryos (32, 33) through modulation of cell adhesion, migration, and differentiation, which are regulated by the transcription factor nuclear factor of activated T cells (NFAT) (34, 35). Calcineurin, on the other hand, activates nemo-like kinase (NLK) to phosphorylate TCF transcription factors and antagonize canonical WNT signaling (36). Unlike the WNT/Ca2+ pathway, the WNT/PCP pathway is not directly involved in transcriptional regulation, but rather, FZD activation leads to increased Rac and Rho small GTPase activity that results in cytoskeletal reorganization and changes to cell polarity and migration (37, 38). Of the noncanonical pathways, it is the WNT/Ca2+ signaling pathway that has been most strongly associated with tumor formation and progression. The most common WNT ligand that has been found to activate WNT/Ca2+ signaling in cancer cells is WNT5A, and its expression is associated with both tumor-suppressive and pro-oncogenic roles, depending on the tumor type. For example, enhanced WNT5A expression correlates with a good patient prognosis in breast and colon cancers (39, 40) yet poor survival in melanoma and gastric cancer (41, 42), again highlighting the context-dependent nature of WNT signaling in carcinogenesis.
WNT SIGNALING CROSS TALK
The concept that the WNT signaling pathways, as presented above, function as autonomous signal transduction processes has been replaced in the field with an understanding that, rather, the pathways function as a network of integrated WNT signaling activities. A plethora of evidence now exists showing that WNT ligands and their downstream signaling components are involved in more than one of the pathways, that the pathways regulate many of the same biological processes, and that within tissues the pathways regulate one another, which, taken together, demonstrates that the WNT proteins activate a signaling network (43, 44). Here I explore how this WNT signaling network regulates tumor cell metabolism and, for clarity, provide examples based on the individual WNT signaling pathways, canonical and noncanonical.
WNT AND NORMAL CELL METABOLISM
Carbohydrate metabolism is central to energy generation in cells, and for well over a century, biochemists have worked on generating detailed maps of central carbon metabolism, highlighting the substrates, enzymes, cofactors, and metabolites involved in these biochemical reactions. There are several core metabolic pathways involved, including glycolysis, the pentose phosphate pathway (PPP), the tricarboxylic acid (TCA) cycle, and the electron transport chain (ETC). Glucose is a key substrate, where it can be converted to the nucleotide component ribose-5-phosphate and NADPH by the PPP or oxidized in glycolysis to convert it into pyruvate and generate ATP. In normal cells, the fate of pyruvate is determined by O2 availability; in normoxia, pyruvate is further degraded to form acetyl coenzyme A (acetyl-CoA), which can be used by the TCA cycle and ETC to generate more ATP through oxidative phosphorylation (OXPHOS). In hypoxia, however, pyruvate is converted to the waste product lactic acid by the enzyme lactate dehydrogenase (LDH) and removed from the cell. It is now understood that determining how the flow of metabolites through metabolic networks is regulated will be essential in determining where best to apply therapeutic interventions to treat metabolic disorders (45), and in recent years, there has been a surge in uncovering new mechanisms of metabolic regulation. One such regulator that has been discovered is the WNT signaling network.
WNT signaling is now known to effect cellular metabolism in normal cell types, which has been the subject of a previous excellent review (46). For example, in fibroblasts and myoblasts, active WNT3A/β-catenin signaling enhances mitochondrial biogenesis and O2 consumption (47). Furthermore, the WNT repressor SFRP-5 has been shown to suppress oxidative metabolism in adipocytes through inhibition of WNT3A activity (8). In contrast, a recent study showed that WNT3A signaling in a noncanonical manner (i.e., independent of axin, GSK3β, and β-catenin) increased glycolytic activity during osteoblast differentiation (48). These studies provide direct evidence of the context-dependent nature of WNT signaling in regulating glucose metabolism, at least in normal cells, and raises the question of whether WNT signaling can also control cancer cell metabolism, where well-characterized perturbed metabolic processes exist to facilitate tumor growth.
CANCER CELL METABOLISM
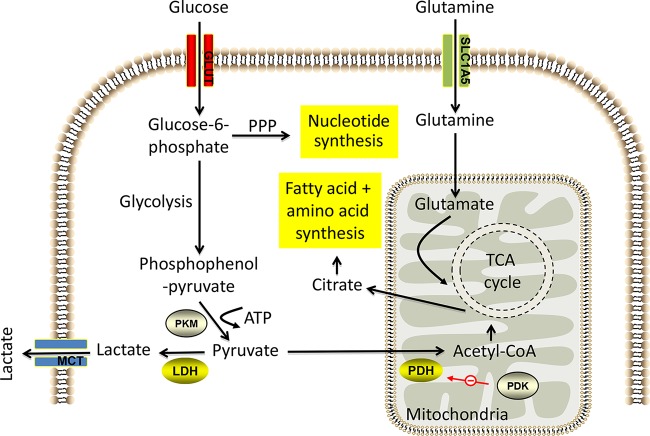
It has long been known that cellular metabolism in tumors is vastly different from that in normal tissues (Fig. 2). For example, in the first half of the last century, the German biochemist Otto Warburg observed that cancer cells exhibit aerobic glycolysis (elevated glycolysis even in normoxia) (49), which facilitates high levels of biosynthetic fluxes during rapid proliferation, resulting in increased glucose consumption and lactate secretion (50). This metabolic shift away from mitochondrion-dependent energy production toward anoxic breakdown of glucose involves the coordinated upregulation of glucose transporters and glycolysis enzymes by oncogenes, such as c-Myc and protein kinase B (Akt). Another crucial metabolic adaptation of cancer cells is glutaminolysis, where the anaplerotic flux of glutamine catabolism to α-ketoglutarate, as a carbon source for the TCA cycle, facilitates amino acid, nucleotide, and lipid synthesis (51). c-Myc has been shown to be a key regulator of glutaminolysis in tumors (52, 53). In addition, many tumors also exhibit a lipogenic phenotype by upregulating enzymes that facilitate de novo fatty acid synthesis required for biological membrane production (54). Intensive research in recent years has endeavored to determine how the metabolic activity of tumors is regulated, and as a result of this work, evidence has emerged that the WNT signaling pathways represent regulatory mediators of the perturbed metabolic activities of cancer cells.
FIG 2.
Summary of metabolic pathways in cancer cells. Highly proliferative cells upregulate key metabolic pathways to facilitate glucose catabolism and glutamine catabolism, which are interlinked to drive macromolecular synthesis and maintain energy balance. Cancer cells use aerobic glycolysis (left side) to produce ATP (two molecules for every molecule of glucose), which is promoted by increased expression of glycolytic enzymes such as pyruvate kinase isozymes M1/M2 (PKM) and LDH. Secretion of the accumulated lactate is achieved by the monocarboxylate transporter (MCT). Furthermore, oncogene-driven pyruvate dehydrogenase kinase (PDK) expression inhibits pyruvate dehydrogenase (PDH) to prevent TCA cycle flux and promote glycolysis. The PPP is parallel to glycolysis and generates NADPH and five-C sugars. Glutaminolysis (right side) is the metabolic process by which glutamine is deaminated to form glutamate and subsequently α-ketoglutarate to maintain the TCA cycle. Glutamine is taken up into the cell by amino acid transporters such as the SLC1A5 transporter. Glucose-derived citrate from the TCA cycle is exported to the cytosol, where it is further converted to acetyl-CoA for lipid synthesis or to oxaloacetate for amino acid synthesis. See the text for additional details.
CANONICAL WNT SIGNALING AND CANCER CELL METABOLISM
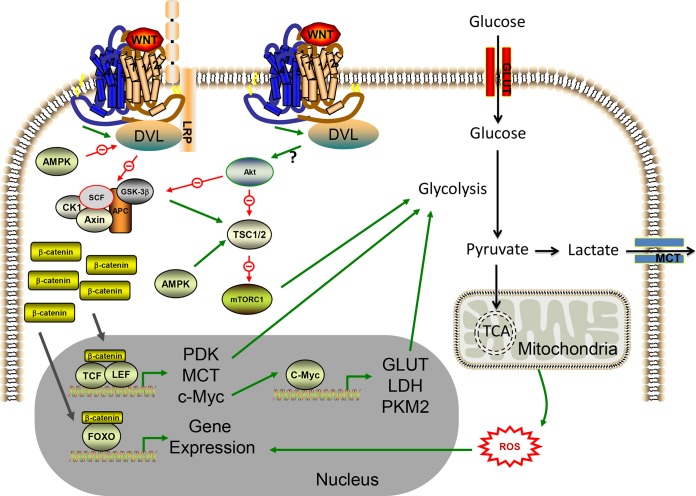
There is an ever-increasing body of evidence demonstrating that WNT/β-catenin signaling regulates cellular metabolism in tumors. Early observations drew speculative links, as it was demonstrated more than a decade ago in ovarian cancer that a large number of metabolism genes were found to be β-catenin–TCF transcriptional targets, including enzymes involved in glutamine and fatty acid metabolism (55). However, more recently, direct associations have been demonstrated for control of cancer cell metabolism by the canonical WNT pathway. In breast cancer, for example, WNT/β-catenin signaling increases aerobic glycolysis through suppression of mitochondrial respiration by reducing the transcription of the gene for cytochrome c oxidase, which is an integral enzyme of the ETC and thus essential for OXPHOS (12). Furthermore, in triple-negative breast cancer cells, WNT5B was recently shown to be able to control the expression of the OXPHOS-related genes for cytochrome c1 and the ATP synthase γ subunit through the canonical WNT pathway (15). In CRC too, an elegant study recently showed that canonical WNT signaling promotes aerobic glycolysis though increased expression of pyruvate dehydrogenase kinase 1 (PDK1), an enzyme that inhibits mitochondrion-bound pyruvate dehydrogenase (PDH) to decrease pyruvate oxidation, resulting in increased pyruvate conversion to lactate in the cytosol (Fig. 2) (14). Furthermore, the lactate transporter monocarboxylate transporter 1 (MCT-1) is also upregulated, facilitating lactate secretion, where this enhanced secretion was shown to have microenvironmental effects in tumor tissue by promoting angiogenesis (14). This suggests that the WNT/β-catenin-mediated metabolic reprogramming of cancer cells can directly affect vessel density in tumors. In fact, in other models, canonical WNT signaling has been strongly linked to tumor angiogenesis by directly regulating the expression of the proangiogenic growth factor (GF) vascular endothelial GF (56–58). Since blood vessels deliver nutrients and O2 to tissues, it is hardly surprising that metabolism and angiogenesis should be coregulated by WNT signaling. Such work has identified a key role for WNT/β-catenin signaling in the regulation of cancer cell metabolism, but how this regulation is controlled is still poorly understood and likely to be highly context dependent. Here I outline important functions of WNT/β-catenin signaling that play a role in the metabolic regulation of tumors, highlighting opportunities for future investigations. These functions include control of c-Myc expression, concomitant regulation of the WNT signaling pathways themselves by metabolic enzymes and nutrients, and cross talk with reactive oxygen species (ROS) signaling.
The c-Myc gene is a well-characterized proto-oncogene that is transcriptionally activated by canonical WNT signaling. c-Myc functions as a transcription factor by binding enhancer box sequences (CACGTG) of target genes to regulate the expression of a number of genes, many of which were historically found to be involved in cell cycle control, including those for cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors (59–62). Crucially c-Myc-mediated transcriptional changes also promote glycolysis and energy production in transformed cells (63). It is well established that WNT/β-catenin signaling transcriptionally upregulates c-Myc expression in a TCF-dependent manner (64, 65) and through an elegant study investigating a simultaneous conditional knockout of APC and c-Myc in intestinal crypts, c-Myc expression was demonstrated to be essential for the oncogenic potential of canonical WNT signaling in CRC (66). Furthermore, the WNT signaling pathway is also downstream of c-Myc through transcriptional repression of the secreted Wnt inhibitors DKK1 and SFRP-1 in cancer cells (67). This provides a positive feedback loop between WNT and c-Myc signaling, placing c-Myc as a key oncogenic component of the WNT/β-catenin signaling pathway. Myc regulates gene transcription in growing cells, where it actively controls a myriad of metabolic processes to facilitate growth and proliferation, which has been the subject of a recent review (68). Glycolysis, nucleotide synthesis, lipid synthesis, glutaminolysis, and mitochondrial bioenergetics are all controlled by Myc-driven transcriptional regulation in cancer cells, all of which are essential for biomass accumulation and genome replication in rapidly dividing cells (50, 68). Specifically, c-Myc promotes the metabolic reprogramming of cancer cells during the G1 phase of the cell cycle, inducing ROS production and activating master transcriptional regulators of cellular metabolism such as the forkhead transcription factors (FOXOs), while also promoting autophagy (69). Cross talk between the WNT and c-Myc pathways in cancer cells means that they converge to regulate not only progression through the cell cycle but also concomitant reprogramming of tumor cell metabolism. Important evidence of this is that β-catenin-mediated c-Myc expression results in upregulation in the expression of a number of rate-limiting glycolytic genes, including those for glucose transporter 1 (GLUT-1), LDH, and the M2 isoform of pyruvate kinase (PKM2; the enzyme that catalyzes the final step of glycolysis to produce ATP and pyruvate) to promote aerobic glycolysis in cancer cells (70).
Aside from aerobic glycolysis, c-Myc also induces glutaminolysis through induction of the solute carrier family 1 member 5 (SLC1A5) glutamine transporter and the mitochondrial glutaminase enzyme to increase glutamine uptake and its subsequent conversion to glutamate, respectively (53). Recently, β-catenin was shown to stimulate c-Myc-mediated glutamine metabolism in colon cancer cells (71). As recently suggested, canonical WNT stimulation of c-Myc provides cancer cells with the concomitant simulation of glutaminolysis and glycolysis to support increased nucleotide and fatty acid synthesis, thus driving de novo biosynthesis during proliferation (72). Therefore, transcriptional upregulation of c-Myc is a key component of canonical WNT-mediated metabolic reprogramming of cancer cell metabolism (Fig. 3).
FIG 3.
Effects of the WNT signaling network on cancer cell metabolism. Active WNT signaling is linked to alterations in cancer cell metabolism. Transcriptional changes induced through WNT-mediated stabilization of β-catenin can reprogram cancer cell metabolism, largely by increasing aerobic glycolysis. The pathway can be inhibited by AMPK at the level of DVL activity. β-Catenin–TCF/LEF gene transcription can increase enzymes that promote glycolysis (for example, PDK1, which inhibits pyruvate flux through the TCA cycle) or can increase c-Myc expression. c-Myc induces the transcription of genes for glycolysis enzymes and proteins involved in glutamine catabolism (the latter is not shown). ROS production from the mitochondrial respiratory chain can alter β-catenin fidelity for TCF/LEF and promote binding to FOXO transcription factors. FOXO gene transcription activates genes that combat oxidative stress and therefore promote cell survival. Noncanonical WNT signaling can induce Akt-mTOR activity in cancer cells by a currently undefined mechanism. Stabilization of mTORC1 promotes aerobic glycolysis through a variety of mechanisms, including induction of HIF-1α-mediated transcription of the PKM2 gene (not shown). Cross talk between the WNT/β-catenin and WNT/Akt-mTOR pathways occurs where Akt can inhibit GSK3β, which otherwise, in conjunction with AMPK, could destabilize mTORC1 expression by promoting TSC1/2 activity. This again promotes aerobic glycolysis by stabilizing the expression of both mTORC1 and β-catenin. A question mark indicates that the precise molecular mechanism remains unknown. See the text for additional details.
Metabolic enzymes, by-products, and nutrients have all been shown to regulate canonical WNT signaling, suggesting that the WNT/β-catenin pathway represents a newly discovered signaling node that can detect environmental changes in nutrient and O2 availability to subsequently control metabolic reprogramming events through gene expression changes in cancer cells. The glycolysis enzyme PKM2 is a pleiotropic protein and can also function as a transcriptional coactivator (73, 74). Nuclear translocation of PKM2 facilitates its interaction with β-catenin downstream of epidermal GF (EGF) signaling in a variety of cancer cell types to elicit β-catenin-induced transcriptional changes, resulting in c-Myc expression and subsequent upregulation in GLUT-1 and LDH (70, 73, 75). However, there is cross talk between the canonical WNT and EGF signaling pathways, as EGF–PKM2–β-catenin signaling resulted in increased expression of DKK-1 and hence suppression of canonical WNT signaling under these conditions (73). Another pivotal respiration enzyme, succinate dehydrogenase 5 (an ETC component), has been shown to antagonize β-catenin signaling in lung cancer, resulting in inhibition of transcriptional reprogramming of the WNT/β-catenin-mediated epithelial-to-mesenchymal transition and reduction of tumor metastasis (76). Thus, a number of central cellular metabolism enzymes have been identified as antagonists of canonical WNT signaling in cancer cells, perhaps to suppress potential metabolic reprogramming events.
Mitochondria are an important source of ROS, produced from complexes I and III of the respiratory chain during OXPHOS, as previously reviewed (77). ROS levels in cancer cells can directly affect the transcriptional activity of β-catenin, as ROS has been shown to displace the interaction of β-catenin–TCF4 to facilitate preferential interaction of β-catenin with the FOXO3a transcription factor (78). In breast cancer, shifting β-catenin binding from TCF to FOXO3a altered cell fate from a proliferative, cancer stem cell phenotype to a more differentiated state, reducing pluripotency and tumorigenesis (78). Indeed, oxidative stress can activate canonical WNT signaling in a range of cell types (including neuroblastoma cells) upstream of β-catenin at the level of DVL. DVL interaction with the thioredoxin family protein nucleoredoxin inhibits DVL activity yet can be augmented by H2O2 treatment, which activates canonical WNT signaling independently of extracellular WNT stimulation (79). Hence, oxidative stress can regulate the canonical WNT pathway to alter transcriptional changes in cancer cells.
In addition to metabolic enzymes and by-products, nutrients have also been shown to regulate β-catenin activity in cancer cells. For example, glucose increases β-catenin acetylation and nuclear accumulation to activate gene expression from WNT target genes in response to simultaneous glucose and WNT ligand stimulation (80). It is logical, then, that nutrient-sensing signaling pathways should regulate canonical WNT signaling, such as AMP-activated protein kinase (AMPK). AMPK is a key energy sensor of intracellular AMP/ATP ratios and can, when AMP is high, inhibit anabolic metabolism while simultaneously activating catabolic pathways such as fatty acid oxidation and glucose metabolism (81). Indeed, activated AMPK can inhibit WNT/β-catenin signaling in cervical cancer cells through reduction of DVL activity (82), suggesting that nutrient sensors can modulate canonical WNT signaling to alter the metabolic reprogramming of cancer cells. Taken together, the above studies have highlighted a variety of mechanisms by which the WNT/β-catenin signaling pathway is integrated into the transcriptional reprogramming of tumor metabolism.
NONCANONICAL WNT SIGNALING AND CANCER CELL METABOLISM
Until now I have focused on the WNT/β-catenin pathway as a regulator of cancer cell metabolism, yet the noncanonical WNT pathways can also control the metabolic reprogramming of cancer cells, suggesting that the WNT signaling pathways can function as a network in this process. The major mechanism by which the β-catenin-independent WNT pathways regulate cancer cell metabolism is through cross talk with Akt signaling. The Akt-mammalian target of rapamycin (mTOR) signaling pathway provides potent control over metabolic reprogramming during tumorigenesis by regulating nutrient uptake and allocating carbon and nitrogen to anabolic pathways to support de novo macromolecular synthesis (83). Akt activates a multitude of downstream effector molecules, with the serine/threonine kinase, mTOR, being a key regulator of protein synthesis, cell growth, and cellular metabolism. mTOR signaling increases aerobic glycolysis in cancer cells by increasing GLUT expression and stimulating glycolytic enzyme activity, including that of hexokinase and phosphofructose kinase (two essential enzymes of the glycolysis pathway) (50). mTOR is regulated by Akt-mediated inactivation of the mTOR upstream regulator tuberous sclerosis complex (TSC), where TSC functions by blocking the GTPase Rheb (Ras homology enriched in brain), freeing Rheb to directly activate mTOR complex 1 (mTORC1), which contains mTOR (84).
WNT controls mTOR signaling to affect cancer cell growth and tumor metabolism. In murine hyperplastic mammary tissue, WNT1 overexpression induces mTOR signaling. Regulation occurs at the level of GSK3β, which (in concert with AMPK), phosphorylates and directly activates TSC; thus, WNT-mediated GSK3β inhibition stimulates mTORC1 activity (85). Such β-catenin-independent WNT signaling via the mTOR pathway likely represents a conserved and general mechanism for noncanonical WNT regulation of glucose metabolism, as WNT3A-induced increases in aerobic glycolysis during osteoblast differentiation are dependent on mTORC activation yet independent of β-catenin (48). Likewise, in the context of prostate cancer cells, the WNT coreceptor LRP6 increases aerobic glycolysis in a β-catenin-independent manner by directly activating Akt-mTORC1 signaling (86). Furthermore, my colleagues and I have found that WNT5A, signaling by the Ca2+/WNT signaling pathway in melanoma cells, also increases aerobic glycolysis, mediated by the Akt-mTORC1 signaling module (13). Collectively, this work suggests that WNT/Akt-mTOR signaling is an important step in controlling cancer cell metabolism in a variety of tumor types. Although most of this evidence centers around β-catenin-independent mechanisms, Akt also promotes β-catenin signaling in cancer cells to increase aerobic glycolysis (87), suggesting that β-catenin signaling is downstream of Akt activity, presumably through Akt-mediated inhibition of GSK3β (88). Thus, the WNT signaling network also has the capacity to regulate cancer cell metabolism through cross talk with Akt-mTOR signaling.
FUTURE PERSPECTIVE
This review demonstrates that there now exists an emergent body of evidence identifying the WNT signaling network as a regulator of cancer cell metabolism. Given that existing findings show that the canonical WNT pathway regulates changes in metabolic activities of cancer cells but is itself also regulated by the cellular metabolism machinery (including metabolic enzymes, by-products, nutrients, and nutrient-sensing pathways; Fig. 3) suggests that WNT/β-catenin signaling represents a critical node in the regulation of central metabolism in tumors. Hence, WNT signaling could provide cancer cells with metabolic flexibility, allowing them to respond to changes in the tumor microenvironment and alter their metabolic status accordingly.
Current research on this topic, however, is still in its infancy and much work is needed to determine the precise molecular mechanisms involved in the metabolic regulation of WNT signaling in differing tumor types. A lot of the molecular characterization to date has been done using cancer cell lines and xenograft models. While these offer convenient systems for identifying possible mechanisms of WNT-mediated metabolic regulation, such models are limited when studying cellular metabolism, not least in part because they do not recapitulate the tumor microenvironment, which provides nongenetic contributions to cancer cell metabolism through temporal gradients in pH, O2, and nutrient availability within solid tumors (89, 90). Other cancer models, such as immunocompetent, genetically engineered mouse models of tumors (with the introduction of genetic alterations of the WNT pathways) or patient-derived tumor xenograft models that retain the same characteristics as the donor tumor, offer more sophisticated systems by which to study the role of WNT signaling in tumor metabolism. This will allow us to understand the complex molecular processes involved at a fundamental level.
Furthermore, the clinical implications of the findings highlighted in this article have yet to be understood. My colleagues and I have found that WNT5A signaling alters carbohydrate metabolism with directly opposite effects on breast cancer and melanoma cells, increasing OXPHOS in the former and aerobic glycolysis in the latter (13), which correlate with reduced and enhanced survival of patients, respectively. So, although most of the WNT signaling pathways studied to date result in enhanced aerobic glycolysis in cancer cells (Fig. 3), our work shows that this is clearly not always the case. As the net cellular response to WNT stimulation is ultimately determined by context-specific WNT network interactions (43, 44), our observation raises the intriguing possibility that distinct, context-dependent metabolic reprogramming of cancer cells by the WNT signaling network poses a direct effect on patient outcome. This represents a potentially hugely important direction for future investigations.
ACKNOWLEDGMENTS
I thank Richard Mithen (Institute of Food Research, United Kingdom) for useful discussions about this article. Apologies go to any colleagues whose work was not cited because of space constraints.
This work was supported by Royal Society grants.
REFERENCES
- 1.Nusse R, Varmus HE. 1982. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31:99–109. [DOI] [PubMed] [Google Scholar]
- 2.Kanazawa A, Tsukada S, Sekine A, Tsunoda T, Takahashi A, Kashiwagi A, Tanaka Y, Babazono T, Matsuda M, Kaku K, Iwamoto Y, Kawamori R, Kikkawa R, Nakamura Y, Maeda S. 2004. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am J Hum Genet 75:832–843. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo YF, Xiong DH, Shen H, Zhao LJ, Xiao P, Guo Y, Wang W, Yang TL, Recker RR, Deng HW. 2006. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet 43:798–803. doi: 10.1136/jmg.2006.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. 2006. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 5.Mani A, Radhakrishnan J, Wang H, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. 2007. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behari J, Li H, Liu S, Stefanovic-Racic M, Alonso L, O'Donnell CP, Shiva S, Singamsetty S, Watanabe Y, Singh VP, Liu Q. 2014. β-Catenin links hepatic metabolic zonation with lipid metabolism and diet-induced obesity in mice. Am J Pathol pii:S0002-9440(14)00509-4. doi: 10.1016/j.ajpath.2014.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elghazi L, Gould AP, Weiss AJ, Barker DJ, Callaghan J, Opland D, Myers M, Cras-Meneur C, Bernal-Mizrachi E. 2012. Importance of β-catenin in glucose and energy homeostasis. Sci Rep 2:693. doi: 10.1038/srep00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori H, Prestwich TC, Reid MA, Longo KA, Gerin I, Cawthorn WP, Susulic VS, Krishnan V, Greenfield A, Macdougald OA. 2012. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest 122:2405–2416. doi: 10.1172/JCI63604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeve D, Seo J, Suh JM, Stenesen D, Tang W, Berglund ED, Wan Y, Williams LJ, Lim A, Martinez MJ, McKay RM, Millay DP, Olson EN, Graff JM. 2012. Wnt signaling activation in adipose progenitors promotes insulin-independent muscle glucose uptake. Cell Metab 15:492–504. doi: 10.1016/j.cmet.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrlund A, Mejhert N, Lorente-Cebrian S, Astrom G, Dahlman I, Laurencikiene J, Ryden M. 2013. Characterization of the Wnt inhibitors secreted frizzled-related proteins (SFRPs) in human adipose tissue. J Clin Endocrinol Metab 98:E503–8. doi: 10.1210/jc.2012-3416. [DOI] [PubMed] [Google Scholar]
- 11.Gauger KJ, Bassa LM, Henchey EM, Wyman J, Bentley B, Brown M, Shimono A, Schneider SS. 2013. Mice deficient in sfrp1 exhibit increased adiposity, dysregulated glucose metabolism, and enhanced macrophage infiltration. PLoS One 8:e78320. doi: 10.1371/journal.pone.0078320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SY, Jeon HM, Ju MK, Kim CH, Yoon G, Han SI, Park HG, Kang HS. 2012. Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res 72:3607–3617. doi: 10.1158/0008-5472.CAN-12-0006. [DOI] [PubMed] [Google Scholar]
- 13.Sherwood V, Chaurasiya SK, Ekstrom EJ, Guilmain W, Liu Q, Koeck T, Brown K, Hansson K, Agnarsdottir M, Bergqvist M, Jirstrom K, Ponten F, James P, Andersson T. 2013. WNT5A-mediated β-catenin-independent signalling is a novel regulator of cancer cell metabolism. 35:784–794. Carcinogenesis doi: 10.1093/carcin/bgt390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pate KT, Stringari C, Sprowl-Tanio S, Wang K, TeSlaa T, Hoverter NP, McQuade MM, Garner C, Digman MA, Teitell MA, Edwards RA, Gratton E, Waterman ML. 2014. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J 33:1454–1473. doi: 10.15252/embj.201488598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Perez AA, Fujie S, Warden C, Li J, Wang Y, Yung B, Chen YR, Liu X, Zhang H, Zheng S, Liu Z, Ann D, Yen Y. 2014. Wnt modulates MCL1 to control cell survival in triple negative breast cancer. BMC Cancer 14:124. doi: 10.1186/1471-2407-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clevers H, Nusse R. 2012. Wnt/β-catenin signaling and disease. Cell 149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Clevers H. 2006. Wnt/β-catenin signaling in development and disease. Cell 127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Valenta T, Hausmann G, Basler K. 2012. The many faces and functions of β-catenin. EMBO J 31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawano Y, Kypta R. 2003. Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 20.Fearon ER, Vogelstein B. 1990. A genetic model for colorectal tumorigenesis. Cell 61:759–767. [DOI] [PubMed] [Google Scholar]
- 21.Stewart DJ. 1 July 2014. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst 106:djt356. doi: 10.1093/jnci/djt356. [DOI] [PubMed] [Google Scholar]
- 22.Laurent-Puig P, Zucman-Rossi J. 2006. Genetics of hepatocellular tumors. Oncogene 25:3778–3786. doi: 10.1038/sj.onc.1209547. [DOI] [PubMed] [Google Scholar]
- 23.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. 2000. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 24.Zurawel RH, Chiappa SA, Allen C, Raffel C. 1998. Sporadic medulloblastomas contain oncogenic β-catenin mutations. Cancer Res 58:896–899. [PubMed] [Google Scholar]
- 25.Palacios J, Gamallo C. 1998. Mutations in the β-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res 58:1344–1347. [PubMed] [Google Scholar]
- 26.Bao R, Christova T, Song S, Angers S, Yan X, Attisano L. 2012. Inhibition of tankyrases induces axin stabilization and blocks Wnt signalling in breast cancer cells. PLoS One 7:e48670. doi: 10.1371/journal.pone.0048670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anastas JN, Moon RT. 2013. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 28.Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL, Moon RT. 2009. Activated Wnt/β-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A 106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horvath LG, Henshall SM, Lee CS, Kench JG, Golovsky D, Brenner PC, O'Neill GF, Kooner R, Stricker PD, Grygiel JJ, Sutherland RL. 2005. Lower levels of nuclear β-catenin predict for a poorer prognosis in localized prostate cancer. Int J Cancer 113:415–422. doi: 10.1002/ijc.20599. [DOI] [PubMed] [Google Scholar]
- 30.Dejmek J, Safholm A, Kamp Nielsen C, Andersson T, Leandersson K. 2006. Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells. Mol Cell Biol 26:6024–6036. doi: 10.1128/MCB.02354-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheldahl LC, Park M, Malbon CC, Moon RT. 1999. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol 9:695–698. [DOI] [PubMed] [Google Scholar]
- 32.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. 2000. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem 275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 33.Choi SC, Han JK. 2002. Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Dev Biol 244:342–357. doi: 10.1006/dbio.2002.0602. [DOI] [PubMed] [Google Scholar]
- 34.Huang T, Xie Z, Wang J, Li M, Jing N, Li L. 2011. Nuclear factor of activated T cells (NFAT) proteins repress canonical Wnt signaling via its interaction with Dishevelled (Dvl) protein and participate in regulating neural progenitor cell proliferation and differentiation. J Biol Chem 286:37399–37405. doi: 10.1074/jbc.M111.251165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. 2002. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 417:295–299. doi: 10.1038/417295a. [DOI] [PubMed] [Google Scholar]
- 36.Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. 2003. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/β-catenin signaling. Mol Cell Biol 23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallingford JB. 2012. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol 28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 38.Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, Greene ND, Copp AJ. 2007. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development 134:789–799. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lejeune S, Huguet EL, Hamby A, Poulsom R, Harris AL. 1995. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin Cancer Res 1:215–222. [PubMed] [Google Scholar]
- 40.Dejmek J, Dejmek A, Safholm A, Sjolander A, Andersson T. 2005. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res 65:9142–9146. doi: 10.1158/0008-5472.CAN-05-1710. [DOI] [PubMed] [Google Scholar]
- 41.Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, Hancox RA, Fletcher A, Saldanha GS. 2008. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res 14:5825–5832. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- 42.Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. 2006. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 66:10439–10448. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 43.Kestler HA, Kuhl M. 2008. From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc Lond B Biol Sci 363:1333–1347. doi: 10.1098/rstb.2007.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Amerongen R, Nusse R. 2009. Towards an integrated view of Wnt signaling in development. Development 136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 45.Metallo CM, Vander Heiden MG. 2013. Understanding metabolic regulation and its influence on cell physiology. Mol Cell 49:388–398. doi: 10.1016/j.molcel.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sethi JK, Vidal-Puig A. 2010. Wnt signalling and the control of cellular metabolism. Biochem J 427:1–17. doi: 10.1042/BJ20091866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, Elledge SJ. 2010. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev 24:1507–1518. doi: 10.1101/gad.1924910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. 2013. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab 17:745–755. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warburg O. 1956. On the origin of cancer cells. Science 123:309–314. [DOI] [PubMed] [Google Scholar]
- 50.Vander Heiden MG, Cantley LC, Thompson CB. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. 2007. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A 104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. 2008. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. 2009. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menendez JA, Lupu R. 2007. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz DR, Wu R, Kardia SL, Levin AM, Huang CC, Shedden KA, Kuick R, Misek DE, Hanash SM, Taylor JM, Reed H, Hendrix N, Zhai Y, Fearon ER, Cho KR. 2003. Novel candidate targets of beta-catenin/T-cell factor signaling identified by gene expression profiling of ovarian endometrioid adenocarcinomas. Cancer Res 63:2913–2922. [PubMed] [Google Scholar]
- 56.Zhang X, Gaspard JP, Chung DC. 2001. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res 61:6050–6054. [PubMed] [Google Scholar]
- 57.Qu B, Liu BR, Du YJ, Chen J, Cheng YQ, Xu W, Wang XH. 2014. Wnt/β-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol Lett 7:1175–1178. doi: 10.3892/ol.2014.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z, Sun B, Qi L, Li Y, Zhao X, Zhang D, Zhang Y. 10 June 2014. Dickkopf-1 expression is downregulated along colorectal adenoma-carcinoma sequence and correlates with reduced microvessel density and VEGF expression. Histopathology doi: 10.1111/his.12474. [DOI] [PubMed] [Google Scholar]
- 59.Rudolph B, Saffrich R, Zwicker J, Henglein B, Muller R, Ansorge W, Eilers M. 1996. Activation of cyclin-dependent kinases by Myc mediates induction of cyclin A, but not apoptosis. EMBO J 15:3065–3076. [PMC free article] [PubMed] [Google Scholar]
- 60.Leone G, DeGregori J, Sears R, Jakoi L, Nevins JR. 1997. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature 387:422–426. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 61.Galaktionov K, Chen X, Beach D. 1996. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature 382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 62.Daksis JI, Lu RY, Facchini LM, Marhin WW, Penn LJ. 1994. Myc induces cyclin D1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycle. Oncogene 9:3635–3645. [PubMed] [Google Scholar]
- 63.Gordan JD, Thompson CB, Simon MC. 2007. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. 1998. Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512. [DOI] [PubMed] [Google Scholar]
- 65.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. 2002. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111:241–250. doi: 10.1016/S0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 66.Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR. 2007. Myc deletion rescues Apc deficiency in the small intestine. Nature 446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 67.Cowling VH, D'Cruz CM, Chodosh LA, Cole MD. 2007. c-Myc transforms human mammary epithelial cells through repression of the Wnt inhibitors DKK1 and SFRP1. Mol Cell Biol 27:5135–5146. doi: 10.1128/MCB.02282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dang CV. 2013. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med 3:a014217. doi: 10.1101/cshperspect.a014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dang CV, Le A, Gao P. 2009. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang WW, Zheng YH, Xia Y, Ji HT, Chen XM, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Liu ZM. 2012. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol 14:1295–1304. doi: 10.1038/Ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu H, Li Z, Yang P, Zhang L, Fan Y, Li Z. 2014. PKM2 depletion induces the compensation of glutaminolysis through β-catenin/c-Myc pathway in tumor cells. Cell Signal 26:2397–2405. doi: 10.1016/j.cellsig.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 72.Thompson CB. 2014. Wnt meets Warburg: another piece in the puzzle? EMBO J 33:1420–1422. doi: 10.15252/embj.201488785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z. 2011. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature 480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL. 2011. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu Z, Huang L, Zhang T, Yang F, Xie L, Liu J, Song S, Miao P, Zhao L, Zhao X, Huang G. 2013. PIM2 phosphorylates PKM2 and promotes glycolysis in cancer cells. J Biol Chem 288:35406–35416. doi: 10.1074/jbc.M113.508226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J, Gao L, Zhang H, Wang D, Wang M, Zhu J, Pang C, Wang C. 2013. Succinate dehydrogenase 5 (SDH5) regulates glycogen synthase kinase 3β–β-catenin-mediated lung cancer metastasis. J Biol Chem 288:29965–29973. doi: 10.1074/jbc.M113.450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy MP. 2009. How mitochondria produce reactive oxygen species. Biochem J 417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, St Clair D, Hung MC, Evers BM, Zhou BP. 2013. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Funato Y, Michiue T, Asashima M, Miki H. 2006. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-β-catenin signalling through dishevelled. Nat Cell Biol 8:501–508. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 80.Chocarro-Calvo A, Garcia-Martinez JM, Ardila-Gonzalez S, De la Vieja A, Garcia-Jimenez C. 2013. Glucose-induced β-catenin acetylation enhances Wnt signaling in cancer. Mol Cell 49:474–486. doi: 10.1016/j.molcel.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 81.Winder WW, Hardie DG. 1999. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277:E1–10. [DOI] [PubMed] [Google Scholar]
- 82.Kwan HT, Chan DW, Cai PC, Mak CS, Yung MM, Leung TH, Wong OG, Cheung AN, Ngan HY. 2013. AMPK activators suppress cervical cancer cell growth through inhibition of DVL3 mediated Wnt/β-catenin signaling activity. PLoS One 8:e53597. doi: 10.1371/journal.pone.0053597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zoncu R, Efeyan A, Sabatini DM. 2011. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sengupta S, Peterson TR, Sabatini DM. 2010. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. 2006. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 86.Tahir SA, Yang G, Goltsov A, Song KD, Ren C, Wang J, Chang W, Thompson TC. 2013. Caveolin-1-LRP6 signaling module stimulates aerobic glycolysis in prostate cancer. Cancer Res 73:1900–1911. doi: 10.1158/0008-5472.CAN-12-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim MS, Chang X, LeBron C, Nagpal JK, Lee J, Huang Y, Yamashita K, Trink B, Ratovitski EA, Sidransky D. 2010. Neurofilament heavy polypeptide regulates the Akt–β-catenin pathway in human esophageal squamous cell carcinoma. PLoS One 5:e9003. doi: 10.1371/journal.pone.0009003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 89.Tredan O, Galmarini CM, Patel K, Tannock IF. 2007. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 90.Cantor JR, Sabatini DM. 2012. Cancer cell metabolism: one hallmark, many faces. Cancer Discov 2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]