Abstract
The receptor tyrosine kinase Axl contributes to cell migration and invasion. Expression of Axl correlates with metastatic progression in cancer patients, yet the specific signaling events promoting invasion downstream of Axl are poorly defined. Herein, we report Elmo scaffolds to be direct substrates and binding partners of Axl. Elmo proteins are established to interact with Dock family guanine nucleotide exchange factors to control Rac-mediated cytoskeletal dynamics. Proteomics and mutagenesis studies reveal that Axl phosphorylates Elmo1/2 on a conserved carboxyl-terminal tyrosine residue. Upon Gas6-dependent activation of Axl, endogenous Elmo2 becomes phosphorylated on Tyr-713 and enters into a physical complex with Axl in breast cancer cells. Interfering with Elmo2 expression prevented Gas6-induced Rac1 activation in breast cancer cells. Similarly to blocking of Axl, Elmo2 knockdown or pharmacological inhibition of Dock1 abolishes breast cancer cell invasion. Interestingly, Axl or Elmo2 knockdown diminishes breast cancer cell proliferation. Rescue of Elmo2 knockdown cells with the wild-type protein but not with Elmo2 harboring Tyr-713-Phe mutations restores cell invasion and cell proliferation. These results define a new mechanism by which Axl promotes cell proliferation and invasion and identifies inhibition of the Elmo-Dock pathway as a potential therapeutic target to stop Axl-induced metastases.
INTRODUCTION
Tyro3, Axl, and Mer (TAMs) belong to a family of receptor tyrosine kinases (RTKs) characterized by an extracellular part formed by two immunoglobulin-like domains and two fibronectin type III domains followed by a transmembrane region and an intracellular tyrosine kinase module (1, 2). Like the majority of RTKs, TAMs are activated by ligands, which include the vitamin K-dependent coagulation factor-like growth arrest-specific 6 (Gas6) and protein S, in addition to the unconventionally secreted Tubby/Tubby-like proteins (3–6). While these ligands activate TAMs in a canonical manner when presented in free forms, they also bridge phosphatidylserine (PS) exposed on the outer surface of apoptotic cells, such that TAMs on phagocytes promote prompt clearance of dying cells (7–10). TAMs are also activated in a ligand-independent manner by either overexpression or transphosphorylation by other RTKs (11–13). A number of signaling pathways are activated following engagement of TAMs, including phosphatidylinositol (PI) 3-kinase/Akt, Ras/Mapk, Stat3, and Rac (14). Together, these pathways are thought to integrate Axl-induced proliferation, survival, cytoskeletal remodeling, and cell migration responses depending on the biological context (14). Moreover, the normal biological functions of TAMs are complex. Individual inactivation of TAMs in mice does not impair development, and a panel of mild defects is observed in adult animals (15–17). The most striking defect among them is blindness in Mer mutant animals arising from abnormal clearance of photoreceptor outer segments by retinal pigment epithelial cells (18). Studies of triple mutant animals lacking TAMs also revealed their role in limiting the macrophage response, and this has important consequences, such as the development of autoimmune diseases (15, 16).
Among TAMs, Axl is highly expressed in various invasive cancers (19). High expression of Axl in breast tumors associates with metastasis and poor patient outcome (20). Notably, expression levels of Axl correlate with an invasion potential of breast cancer cell lines (20), where silencing its expression or blocking its activity through a pharmacological inhibitor or blocking antibodies impairs breast cancer cell invasion (20–23). In addition, in vivo experiments suggest that downregulation of Axl in human breast cancer cells drastically blocks metastasis without considerably affecting tumor growth (20, 24). Within basal/triple-negative human breast cancer cell lines, Axl signaling promotes the expression of an epithelial-to-mesenchymal (EMT) gene signature, including the upregulation of Slug, Snail, and vimentin and the downregulation of E-cadherin, which are important for ensuring a stem cell and invasive phenotype (20, 25). Notably, the signaling pathways engaged by Axl to promote such aggressive migration and invasive behaviors remain to be fully defined, since this may uncover new targets for antimetastatic treatments.
Evolutionarily conserved Dock family guanine nucleotide exchange factors (GEFs) activate Rac or Cdc42 GTPases through a unique dock homology region 2 domain to promote cytoskeletal rearrangements (26–28). Elmo1 to -3 are autoregulated scaffold proteins that interact with Dock1 to -5 to spatiotemporally organize Rac signaling (29–32). In vivo, in mice, Dock1 and Elmo1 promote migration, engulfment of apoptotic cells, and myoblast fusion during development and adult life (reviewed in reference 33). High expression of Dock1 in breast cancer tumors correlates with a poor probability of survival for HER2+ or basal-like breast cancer patients (34). Deletion of Dock1 in the mouse mammary gland protects mice from developing lung metastasis in a model of HER2 breast cancer (34). In addition, activation of Dock1 by the platelet-derived growth factor (PDGF) receptor or the EGFRvIII receptor promotes cancer cell dissemination in distinct subclasses of gliomas and correlates with poor patient survival (35–37). Likewise, interfering with Dock1 or Elmo expression in human breast cancer cell lines impairs invasion (38, 39). These results point to Dock1 and Elmo as potentially important proteins to promote Rac1-dependent cell migration and invasion during metastasis.
Here, we present evidence that Axl orchestrates breast cancer cell invasion by phosphorylating Elmo proteins. Our results demonstrate that Elmo2 is required for Axl-induced Rac activation. We identify Tyr 713 on Elmo2, homologous to Tyr 720 in Elmo1, as the site of phosphorylation by Axl kinase, and their mutation abolishes cell invasion and proliferation. Collectively, our efforts uncover a long-sought-after signaling pathway operating downstream of Axl to promote cell invasion and proliferation.
MATERIALS AND METHODS
Antibodies.
The antibodies against the following proteins were obtained commercially: Tyro3 (C-20), Axl (C-20), Myc (9E10), green fluorescent protein (GFP) (B-2), DOCK 180 (H-4), and pY99 (sc-7020) were from Santa Cruz Biotechnology (Santa Cruz, CA); FLAG M2 and tubulin were from Sigma (St. Louis, MO); Rac1 was from Millipore (Billerica, MA); pAKTS473, AKT, pY100, and pAxlY702 were from Cell Signaling Technology (Danvers, MA); vimentin, N-cadherin, and E-cadherin were from BD Biosciences (Franklin Lakes, NJ); Elmo2 was from Novus Biologicals (Littleton, CO); glutathione S-transferase (GST) was from GE Healthcare (United Kingdom); Mer and pAxl779 were from R&D Systems (Minneapolis, MN); and Mer and Twist1 were from Abcam (Cambridge, United Kingdom). Rabbit phospho-specific polyclonal antibody against pY713 Elmo2 was custom generated using the synthetic phosphopeptide CIPKEPSSpTyrDFVYHYG as an immunogen (GenScript, Piscataway, NJ). Specificity of the pElmo2pY713 antibody was verified by dot blotting against the phosphorylated and unphosphorylated peptides.
Plasmid constructs.
pCNX2 Flag-DOCK1 was from M. Matsuda (Kyoto University, Japan). pDEST27 Tyro3 was described in reference 40. pCMVSport6 Axl was from Open Biosystems (catalog no. MHS1010-7430144). pCMVSport6 Axl kinase dead K561M was generated by site-directed mutagenesis (QuikChange; Stratagene) using primers specified in Table S3 in the supplemental material. The pcDNA3.1 Myc-Elmo1, pcDNA3.1 Myc-Elmo2, and pcDNA3.1 Myc-Elmo3 plasmids were described previously (26, 41). The tyrosine-to-phenylalanine (Y-F) mutants of Elmo1 and Elmo2 were generated by site-directed mutagenesis with the primers specified in Table S3. The Elmo1-Myc αN/PXXP mutant plasmid was described previously (32). The pGEX-4T1-Elmo1 wild type and mutants were subcloned using XhoI/BamHI from pcDNA3.1 into pGEX-4T1 (Amersham, Piscataway, NJ). pGEX-4T1-Elmo2 and pGEX-4T1-Elmo3 were subcloned using BamHI/XhoI into the pGEX-4T1 vector from pcDNA3.1.
Cell culture and transfections.
Cell lines (MDA-MB-231, Hs578T, and HEK 293T) were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin-streptomycin (Invitrogen-BRL, Carlsbad, CA) at 37°C in a 5% CO2 incubator. MDA-MB-231 and Hs578T cells were transfected with the indicated plasmids or small interfering RNAs (siRNAs) using Lipofectamine 2000 (Invitrogen). HEK 293T cells were transfected with the indicated plasmids by the calcium phosphate method. Hs578T and MDA-MB-231 cells were transfected with ON-Target SmartPool human siRNA (60 nM Elmo2, 100 nM Axl siRNA, and 200 nM Dock1 and Dock5) (Dharmacon). Control cells were transfected with 60 nM or 100 nM NON-Targeting siRNA (Dharmacon). Biochemical and cell biological studies were performed 48 to 72 h after transfection.
Kinase library screen.
One hundred eighty full-length human protein kinase cDNA clones derived from the MGC/ORFeome collection (Open Biosystems, Invitrogen) were Gateway recombined with the pDEST27 vector (Invitrogen) to generate in-frame GST kinase open reading frames (ORFs) (40). GST kinases encoded in plasmids were transfected into HEK293T and arrayed in 96-well plates. GST kinases were immobilized 24 h later on glutathione-coated plates (Pierce) whose wells were previously rinsed and equilibrated with kinase buffer (25 mM Tris [pH 7.5]), 5 mM β-glycerol phosphate, and 1 mM NaVO4) prior to adding 1 μg recombinant mouse Elmo1 substrate, 1 μg of myelin basic protein (MBP) as an internal control, and 2 μCi of [γ-32P]ATP. Reactions were carried out at 30°C for 30 min and stopped with 2× SDS sample buffer; samples were boiled before separation on SDS-PAGE gels. Phosphorylated substrates were detected by autoradiography. pGEX-4T1 Elmo constructs were transformed in BL21 cells for protein production. Exponentially growing BL21 cultures (2 to 4 liters) were induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) overnight at 25°C. Cleared lysates were prepared, and GST-Elmo1 was purified on GSTrap minicolumns using an Äktaprime Plus chromatography system. The GST tag was cleaved by incubation with thrombin, and the protease was removed by passing the sample on a HiTrap Benzamidine FF column. Sample was dialyzed against a phosphate-buffered saline solution and passed on glutathione-Sepharose 4B to remove uncleaved GST-Elmo1 and the GST moiety. GST-Elmo1 and truncated proteins, in addition to GST-Elmo2 and -3, were affinity purified on small amounts of glutathione-Sepharose 4B for small-scale pulldown (see below) (32).
Immunoprecipitation, GST fusion protein pulldowns, and Rac-GTP assays.
Cells were lysed for 10 min in 150 mM NaCl, 50 mM Tris (pH 7.5), 1% NP-40, 5 mM NaF, 1 mM Na3VO4, and 1× complete protease inhibitor (Roche, Indianapolis, IN). For immunoprecipitation, clarified cell lysates were incubated with the indicated antibodies, and the immune complexes were allowed to form for 1 h at 4°C. Protein A-agarose was added for 30 min to recover the immune complex. The beads were washed 3 times with lysis buffer, and bound proteins were analyzed by SDS-PAGE and immunoblotting. For GST fusion protein pulldowns, the GST fusion proteins were expressed in bacteria and purified on glutathione-Sepharose 4B as described above. Equal amounts of the various GST fusion proteins bound to beads were next incubated with cell extracts (500 μg of protein per condition). The in vitro kinase (IVK) assays with the GST fusion proteins and recombinant kinase domains of TAMs were carried out as describe above. The kinase domains of the human TAMs were obtained from Signal Chem (Richmond, BC, Canada). Following IVK assays, the proteins were separated by SDS-PAGE and stained with Coomassie blue, and the phosphorylated proteins were detected by autoradiography. For the Rac activation assay, Hs578T cells were treated and lysed as described previously (34). The GTP loading status of Rac was analyzed by affinity precipitation of the purified p21-binding domain of PAK protein kinase expressed as a GST fusion protein (GST-PAK-PBD) as described previously (26). Equal amounts of protein lysates or pulldowns were separated by SDS-PAGE, and Rac was detected by immunoblotting. Rac activation was quantified by densitometry analysis using the ImageJ software program (http://rsb.info.nih.gov/ij/).
Mass spectrometry.
The human GST-Tyro3 kinase expressed in HEK293T cells was purified by affinity purification and used to phosphorylate 2 μg of recombinant mouse Elmo1 by IVK assay. To produce phosphorylated Elmo1 in cells, HEK293T cells were cotransfected with human GST-Tyro3 and mouse cMyc-Elmo1. Ten milligrams of lysate was used for immunoprecipitation of Elmo1 with 10 μg of anti-c-Myc antibody (9E10) bound to protein A beads. Samples were separated by SDS-PAGE. The gel was stained with mass spectrometry-compatible Coomassie, and the band corresponding to Myc-Elmo1 was excised and destained extensively in water; in-gel digestion was then performed according to standard procedures. The peptide digestion products were extracted from the gel with an extraction buffer (1:2 [vol/vol] 5% formic acid-acetonitrile) and incubated for 15 min at 37°C. Peptides were redissolved in 0.1% trifluoroacetic acid for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis at the IRIC platform (Montreal, QC, Canada).
RT-PCR.
Total RNAs were extracted using the TRIzol reagent (Invitrogen) and treated with DNase I (Invitrogen) to remove genomic DNA. cDNAs were generated using the Superscript II reverse transcriptase (Invitrogen) and random primers (Invitrogen) as recommended by the manufacturer. The expression profiles of beta-actin, Elmo1, Elmo2, and Elmo3 were determined using specific primers, shown in Table S3 in the supplemental material.
Boyden chamber invasion assay.
Cell invasion assays were performed using 8-μm-pore Boyden chambers (Costar, Cambridge, MA) coated with 6 μl of Matrigel (BD Biosciences, San Jose, CA) dissolved in 100 μl of DMEM. Cells were detached and washed with DMEM–0.1% bovine serum albumin (BSA) as described in reference 42. One hundred thousand cells were seeded in the upper chamber in duplicate for each condition in serum-starved DMEM, and cells were allowed to invade for 16 h toward the bottom chamber containing DMEM with or without 10% FBS before fixation in 4% paraformaldehyde. Cells in the upper chambers were mechanically removed using cotton swabs. Invading cells were permeabilized with 0.2% Triton X-100 in PBS and blocked in PBS-1% BSA before staining with anti-c-Myc and anti-GFP. The membrane was isolated and mounted on a microscope slide using SlowFade Gold reagent (Invitrogen). An aliquot of the cells was lysed to verify the expression levels of the exogenous proteins and the knockdown of Elmo2 by Western blotting. GFP-positive cells and c-Myc-positive cells that had invaded to the underside were counted from 8 to 10 independent fields on each membrane (magnification, ×20).
BrdU proliferation assay.
Cell were transfected with siRNA as indicated in the figure legends and were plated on fibronectin-coated glass slips for 24 h prior to being incubated with 0.03 mg/ml BrdU at 37°C for 30 min. Cells were then fixed with 70% ethanol for 5 min, rinsed with PBS (three times), denatured with 1.5 M HCl for 30 min at room temperature, and rinsed three times with PBS for 5 min each. After incubation with PBS-1%BSA to block nonspecific staining for 60 min, cells were incubated with BrdU antibody (Cell Signaling Technology) overnight at 4°C. After three washes with PBS, cells were incubated with corresponding Alexa Fluor-conjugated secondary antibody (Invitrogen) for 2 h. The samples were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to stain the nuclei and analyzed using a Zeiss Observer.Z1 microscope. The percentage of BrdU-positive cells versus total cells was calculated in 5 different fields of each condition. The average of the percentage of BrdU-positive cells calculated for the 5 images was used for the final quantification. Values are reported as means ± standard errors of means (SEM). Statistical differences were evaluated using analysis of variance (ANOVA) followed by Bonferroni's multiple-comparison post hoc test using the Prism 6 software program (GraphPad). A P value of less than 0.05 was considered statistically significant. An aliquot of the cells was lysed to verify the expression levels of the exogenous proteins and the knockdown of Elmo2 or Axl by Western blotting.
Statistical analysis.
Statistical significance was determined using Student's t test, with P values of ≤0.05 considered significant, using the software program Prism. In all tests, two groups with one changed parameter were compared. For invasion assays, ANOVA and all pairwise multiple-comparison procedures (Holm-Sidak method) were performed (n = 6 for each condition).
RESULTS
A kinase screen uncovers Elmo proteins as direct substrates of Tyro3, Axl, and Mer receptor tyrosine kinases (TAMs).
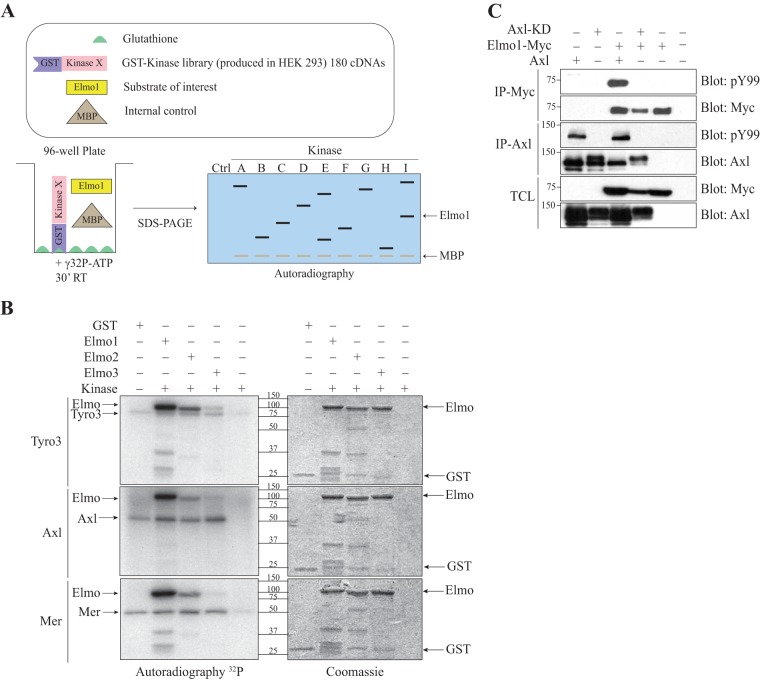
Dock1 is activated by phosphorylation to promote cell migration and invasion (34–37). Previously, we reported that mRNA expression levels of Dock1 correlate with poor patient outcome in HER+ and basal/triple-negative breast cancer subtypes (34). Because Elmo proteins are bound to Dock1 and regulate Rac signaling (43), we aimed to identify novel regulators of the Elmo/Dock1 complex by carrying out a screen designed to uncover kinases that could phosphorylate Elmo1. To this end, a panel of 180 GST-tagged human kinases, composed of representative members of each kinase subfamily, was expressed in HEK293T cells as previously reported (40) (see Table S1 in the supplemental material for a full list of kinases). Following cell lysis, each GST kinase was recovered in a glutathione-coated well as depicted in Fig. 1A. To carry out in vitro kinase (IVK) assays, immobilized kinases were mixed with recombinant purified Elmo1, myelin basic protein (MBP), and [γ-32P]ATP (Fig. 1A). Seven putative candidate kinases that phosphorylate Elmo1 were identified, including five Ser/Thr kinases (Pftaire1, Camkk2, Dclk1, Prpf4b, and Ttbk2) and two tyrosine kinases (Blk and Tyro3) (see Table S2). A secondary screen of selected candidates revealed that GST-tagged Camkk2 and Pftaire1 cannot phosphorylate Elmo1; instead, they comigrated with recombinant Elmo1, and their autophosphorylation led us to conclude that they were false positives (not shown). Although we have not retested Blk's ability to phosphorylate Elmo1, another Src family kinase, Hck, has been reported to do that efficiently (44). Instead, we chose to further study Elmo1 phosphorylation by the RTKs of the TAM family (including Tyro3, Axl, and Mer) due to their involvement in biological processes similarly controlled by Elmo/Dock1, including cell migration, cell invasion, and phagocytosis of apoptotic cells (2, 45).
FIG 1.
The TAM receptors phosphorylate Elmo proteins. (A) Schematic overview of the kinase screen. Elmo1 is the substrate of interest, and MBP1 is used as a positive control. (B) Elmo is phosphorylated in vitro by the TAMs. An in vitro kinase assay was performed, where 2 μg of GST-Elmo proteins were incubated with 0.05 μg of the kinase domains of Tyro3, Axl and Mer, and [γ-32P]ATP. Expression of the proteins was analyzed by Coomassie staining and the phosphorylation by autoradiography. (C) Elmo1 phosphorylation in cells is dependent on Axl catalytic activity. Lysates of HEK293T cells transfected with the indicated plasmids were immunoprecipitated with an antibody against the Myc epitope (Elmo1) and with an antibody against Axl. The phosphorylation and expression levels of Elmo1 and Axl were analyzed via immunoblotting with anti-Myc and anti-Axl antibodies, respectively.
Since Axl and Mer were not part of the initial screen, we extended our analyses to test if, as seen for Tyro3, they could phosphorylate Elmo proteins, indicating this as a conserved feature of TAMs. We conducted IVK assays using purified recombinant kinase domains of Tyro3, Axl, and Mer to test their ability to directly phosphorylate the Elmo1 to -3 proteins. We found that all three TAMs preferentially phosphorylate Elmo1 and Elmo2, yet phosphorylation of Elmo2 seems to be less than that of Elmo1 (Fig. 1B). We also found that full-length TAMs but not kinase-dead mutants phosphorylate recombinant Elmo1 in vitro (see Fig. S1A in the supplemental material). To confirm these results in cells, we coexpressed TAMs with Myc-Elmo1 and examined the phosphorylation status of immunoprecipitated Myc-Elmo1 using an antiphosphotyrosine antibody. In a cellular context, we similarly found that TAMs but not their kinase-dead mutants promote tyrosine phosphorylation of Myc-Elmo1 (Fig. 1C; see also Fig. S1B and C). Collectively, these results establish Elmo1 and Elmo2 as previously unidentified direct substrates of TAMs and raise the question of whether TAMs could exploit the Elmo/Dock1 complex to promote migration and invasion.
TAMs phosphorylate two tyrosine residues on Elmo1/2.
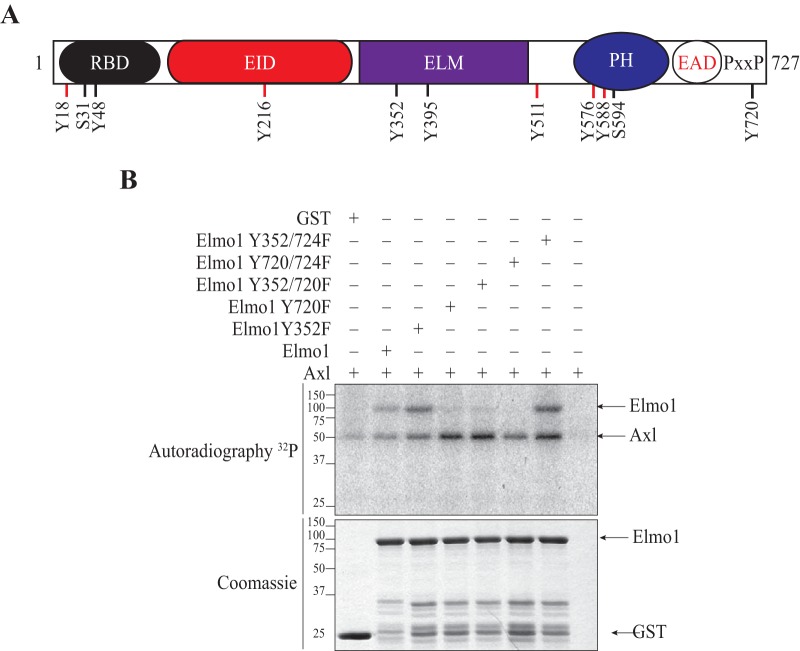
To gain mechanistic insights into the effect of tyrosine phosphorylation of Elmo proteins, a proteomics approach was used to map the tyrosine residues of Elmo1 targeted by TAMs. First, in vitro-phosphorylated Elmo1 was obtained by mixing bacterially produced and purified Elmo1 with Tyro3 immunoprecipitated from HEK293T lysates and cold ATP (as done for Fig. S1A in the supplemental material). Second, cellular phosphorylated Myc-Elmo1 was generated by coexpression with Tyro3 and immunoprecipitation with an anti-Myc antibody (as done for Fig. S1B). Both phosphorylated Elmo1 samples were subjected to proteomics analysis, and 9 phosphorylated tyrosine sites were identified (18, 48, 216, 352, 395, 511, 576, 588, and 720) (Fig. 2A). We also included in our analysis Tyr 724 of Elmo1 as a residue potentially phosphorylated by TAMs, wince we could not rule it out from the mass spectrometry spectrum that identified Tyr 720. We generated single Tyr-to-Phe mutants of Elmo1 for each site identified by proteomics and narrowed our focus to Tyr 720 as the major site targeted by Axl by performing IVK assays (Fig. 2B). The residual phosphorylation on the Elmo1 Tyr 720 mutant was attributable to Tyr 724, since the 720/724 double mutant failed to become phosphorylated upon incubation with Axl (Fig. 2B). In contrast, mutation of Tyr 352 did not affect the residual phosphorylation signal observed for the Tyr 720 mutant (Fig. 2B). Tyr 720 is highly conserved between the Elmo1 and Elmo2 but not Elmo3 proteins (see Fig. S1D), explaining the differential phosphorylation of Elmo family proteins by TAMs observed in Fig. 1B. Together, these results demonstrate that TAMs specifically phosphorylate Elmo1 on Tyr 720/724.
FIG 2.
Elmo1 is phosphorylated on tyrosines 720 and 724 by TAM receptors. (A) Elmo1 phosphorylation sites identified by mass spectrometry. Lysates of HEK293T cells were transfected with c-Myc–Elmo1 and were either subjected to an in vitro kinase assay with GST-Tyro3 or cotransfected with GST-Tyro3 and subjected to immunoprecipitation of c-Myc–Elmo1. Sites on Elmo1 phosphorylated by Tyro3 were identified by mass spectrometry. Sites depicted in red were identified by both experiments, whereas the sites depicted in black were identified only in the c-Myc–Elmo1 immunoprecipitation. RBD, Ras-binding domain; EID, Elmo inhibitory domain; ELM, Elmo domain; PH, atypical pleckstrin homology domain; EAD, Elmo autoregulatory domain; PxxP, proline-rich region. (B) Elmo1 is phosphorylated at tyrosines 720 and 724. An in vitro kinase assay was performed, where 3 μg of the GST-Elmo1 protein or Y-F mutants were incubated with 0.025 μg of the Axl kinase domain and [γ-32P]ATP. Expression of the proteins were analyzed by Coomassie staining and phosphorylation by autoradiography.
Axl interacts with and phosphorylates Elmo proteins.
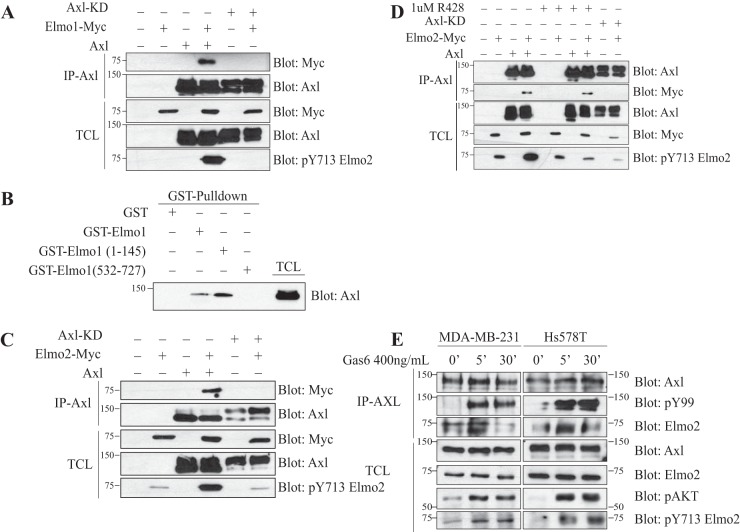
We investigated whether Elmo recognition by Axl could involve the formation of a physical RTK-substrate complex. In cells coexpressing Axl and Myc-Elmo1, we found that Myc-Elmo1 specifically coimmunoprecipitated with Axl (Fig. 3A). However, the binding of Myc-Elmo1 to Axl was lost when kinase-dead Axl was immunoprecipitated. We raised a phospho-specific antibody against Tyr 720 of Elmo1 (Tyr 713 in Elmo2) to monitor the phosphorylation of this site in cells (see Fig. S2 in the supplemental material for antibody characterization). As shown in Fig. 3A, coexpression of Axl with Myc-Elmo1 promoted the phosphorylation of Tyr 720, and this increase was not observable with kinase-dead Axl. To further validate the interaction between Elmo and Axl, we performed in vitro binding assays using GST Elmo1 fusion proteins and found that full-length Elmo1 and the N terminus of Elmo1 (amino acids [aa] 1 to 495) but not the C terminus of Elmo1 (aa 532 to 727) were able to bind to Axl (Fig. 3B). We also tested if Elmo2 was a binding partner of Axl and found that it coimmunoprecipitated with Axl but not the kinase-dead mutant when the two proteins were coexpressed in HEK293T cells (Fig. 3C). In addition, by monitoring Elmo2 phosphorylation on Tyr 713 (Tyr 720 in Elmo1) (see Fig. S1D) with our phospho-specific antibody, we similarly found that Axl but not the kinase-dead mutant promoted phosphorylation of this site (Fig. 3C). We next used a pharmacological inhibitor against, Axl R428 (22), to investigate whether inhibiting the kinase activity would be sufficient to abrogate Elmo2 phosphorylation and binding to Axl. Treatment of HEK293T cells expressing Axl and Myc-Elmo2 with R428 prevented Elmo2 phosphorylation on Tyr 713 but surprisingly did not inhibit the interaction of Axl with Elmo2 (Fig. 3D).
FIG 3.
Elmo modulation by Axl is dependent on Axl's catalytic activity. (A and C) Axl that is wild type and not kinase dead interacts with and phosphorylates Elmo1 (A) and Elmo2 (C). Lysates of HEK293T cells transfected with the indicated plasmids were coimmunoprecipitated with an antibody against Axl. The coprecipitation and expression levels of the Axl proteins and Elmo1 (A) or Elmo2 (C) were analyzed via immunoblotting with anti-Myc (Elmo) and anti-Axl antibodies. (B) Interaction between Elmo1 and Axl is mediated by the N terminus of Elmo1. Lysates from transfected HEK293T cells with Axl were incubated with 3 μg of the GST-Elmo1 full-length protein or fragments. The binding of Axl to Elmo1 fragments and the expression levels were analyzed via immunoblotting with anti-Axl antibody. (D) Inhibition of Axl activity with R428 abolishes Elmo2 phosphorylation by Axl. Transfected HEK293T cells with the indicated plasmids were serum starved prior to treatment with either dimethyl sulfoxide (DMSO) or a 1 μM concentration of the Axl inhibitor R428 for 1 h. Lysates were coimmunoprecipitated with an antibody against Axl. The coprecipitation and expression levels of the Axl proteins and Elmo2 was analyzed via immunoblotting with anti-Myc (Elmo2) and anti-Axl antibodies, respectively. Protein expression and Elmo2 phosphorylation were analyzed by immunoblotting with anti-Myc, anti-Axl, and anti-pY713 Elmo2. (E) Axl interacts with and phosphorylates endogenous Elmo2 in basal breast cancer cell lines. MDA-MB-231 and Hs578T cells were treated with 400 ng/ml of Gas6 for the indicated time points and then were subjected to coimmunoprecipitation of Axl using anti-Axl antibody. The precipitation of Elmo2 by Axl was detected by immunoblotting with anti-Elmo2 antibody. The protein expression levels were also analyzed via immunoblotting with anti-pAKT, anti-pY99, anti-Elmo2, anti-Axl, and anti-pY713 Elmo2 antibodies.
In an effort to understand if adaptor proteins could facilitate coupling of Elmo2 to Axl, we mutated Tyr residues in Axl (at positions 779, 821, and 866), known to be involved in binding the Src homology 2 (SH2) adaptor proteins Grb2 and PI 3 kinase (46, 47), to Phe and found that this did not abrogate Axl-Elmo2 association (see Fig. S3 in the supplemental material). From these results, it is still unclear how Elmo2 is recruited to the Axl receptor, and this remains to be investigated.
Moreover, we found that Elmo2 is the only Elmo family member expressed in the MDA-MB-231 and Hs578T basal breast cancer cell lines (48) (see Fig. S4A in the supplemental material). We also observed that Hs578T cells expressed only Axl, whereas MDA-MB-231 expressed both Axl and Mer (see Fig. S4B). Tyro3 was not expressed in either cell line. Using these basal breast cancer cell line models, we next investigated if endogenous Axl can phosphorylate and bind Elmo2. To this end, we treated serum-starved MDA-MB-231 cells with recombinant Gas6 to activate Axl. As expected, immunoprecipitation of Axl revealed that it becomes globally phosphorylated on Tyr residues following 5- and 30-min treatments with Gas6, suggesting that the RTK is activated (Fig. 3E). An increase in Akt phosphorylation, a known target of Axl, confirmed the activation of downstream signaling following Gas6 treatments (Fig. 3E; see also Fig. S4C). We also found that endogenous Elmo2 in MDA-MB-231 and Hs578T cells coprecipitated minimally with Axl at the basal state, and this interaction was enhanced transiently at 5 min after Gas6 treatment (Fig. 3E). Blotting of total cell lysates with Elmo2 pTyr 713 phospho-specific antibody revealed an increase in Elmo2 phosphorylation at this site after 5 and 30 min of Gas6 treatments (Fig. 3E). We found that Axl is the major kinase promoting Gas6 signaling, since siRNA-mediated knockdown of Axl completely prevented Akt phosphorylation after stimulation with Gas6 (see Fig. S4C). Identical results were observed in MDA-MB-231 cells. Collectively, these data demonstrate that Axl phosphorylates and interacts with Elmo2 in invasive breast cancer cells.
Elmo2 is required for Axl-induced Rac activation.
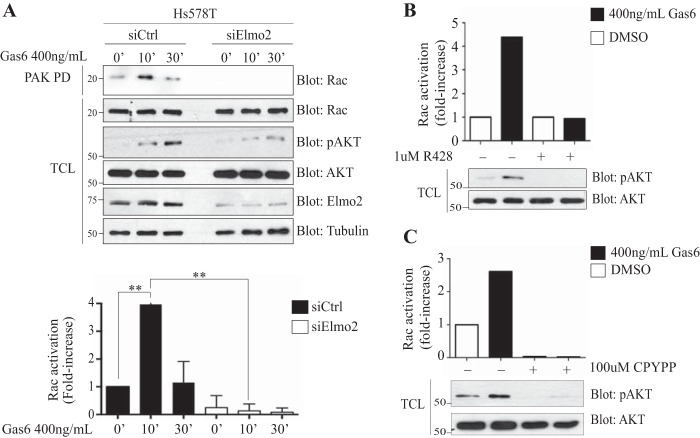
Axl promotes neuron migration by activating Rac (49). We investigated if Elmo2 functions as a scaffold protein to connect Axl to the Rac GEF Dock1 in breast cancer cells. Immunoprecipitation of Flag-Dock1 revealed formation of a multiprotein complex with Axl (see Fig. S5A in the supplemental material). Surprisingly, and in addition to Elmo, Dock1 also appears to make its own contacts with Axl, since this interaction was neither enhanced nor reduced by the expression of wild-type Myc-Elmo1 or a Myc-Elmo1 mutant (αN/PXXP) that is not able to bind Dock1 (see Fig. S5C). Interestingly, Dock1 might contribute to guiding Elmo for phosphorylation by Axl, since the phosphorylation of the Elmo1 mutant (αN/PXXP), which is not interacting with Dock1, is decreased (see Fig. S5C). We also found that mutating Tyr 713 to Phe in Elmo2 did not impact the formation of an Elmo2/Dock1 complex (see Fig. S5A). This was also observed when Elmo1 was coexpressed, where the wild type and an Elmo1 protein carrying a mutation at Tyr 720 or Tyr 720/724 bound similarly to Axl and Dock1 (see Fig. S5B). Because Rac is a key molecule in promoting cell migration, we therefore investigated the possibility that Axl employs the Elmo-Dock1 complex to promote Rac activation in invasive breast cancer cells. To test this, serum-starved Hs578T cells were stimulated with Gas6, and Rac-GTP loading was monitored by affinity precipitation with a GST PAK-PBD fusion protein. Treatment of Hs578T cells with Gas6 induced Rac activation that peaked at 10 min (Fig. 4A). To confirm that Gas6 mediates Rac activation under these conditions through Axl, we found that treatment of Hs578T cells with R428 prevented Rac activation (Fig. 4B). In addition, interfering with Elmo2 expression by using siRNA or with Dock1 GEF activity using the small-molecule inhibitor CPYPP (50) prevented Gas6-induced Rac activation (Fig. 4A to C). It is noteworthy that depletion of Elmo2 by siRNA in the MDA-MB-231 cell line led to a partial decrease in expression of Dock1 and the closely related member Dock5, and this could explain at least in part the decrease in Rac activation observed following Gas6 treatment in Elmo2-depleted cells (see Fig. S6A). We reproducibly found that downregulation of Elmo2 and inhibition of Dock1 GEF activity impaired maximal activation of Akt following Gas6 treatment (Fig. 4A to C), suggesting that Elmo2-Dock1-Rac-GTP might be involved in stimulating a PI 3-kinase.
FIG 4.
Rac activation in Hs578T cells is Axl and Elmo2 dependent. (A) Elmo2 is required for Rac activation upon Gas6 stimulation. Hs578T cells transfected with 60 nM NON-Targeting or ON-Target SmartPool Elmo2 siRNA prior to being treated with 400 ng/ml of Gas6 at the indicated time points were assayed for Rac activation by precipitation of Rac with the purified p21-binding domain of PAK protein kinase expressed as a GST fusion protein (GST-PAK-PBD) (n = 6). The amount of Rac in pulldowns and in total cell lysates (TCL) was detected by immunoblotting with an anti-Rac antibody. Expression levels of the various proteins and equal loading of Rac in all samples were analyzed by immunoblotting of the TCL using anti-Rac, antitubulin, anti-Elmo2, anti-pAKT, and anti-AKT. (B and C) Axl and Dock1 inhibition reduces Rac activation upon Gas6 stimulation. Hs578T cells were treated with 1 μM R428 (B) or with 100 μM CPYPP (C) for 1 h followed by 400 ng/ml Gas6 for 20 min. Rac activation was assayed by precipitation with the purified p21-binding domain of PAK protein kinase expressed as a GST fusion protein (GST-PAK-PBD) (n = 5). The amount of Rac in pulldowns and in total cell lysates (TCL) was quantified by the software program Image J. Expression levels of the various proteins were analyzed by immunoblotting of the TCL using anti-pAKT and anti-AKT. Data are shown as means ± SD; **, P < 0.0001; one-way ANOVA.
Axl promotes the expression of epithelial-to-mesenchymal transition (EMT) markers in invasive breast cancer cells (20). Rac signaling is also found to contribute to the maintenance of the mesenchymal and stem cell phenotype of cancer cells (51, 52). Therefore, we investigated the possibility of Elmo and Dock1 (or Dock5) playing a role downstream of Axl in promoting a mesenchymal phenotype in MDA-MB-231 cells via Rac activation either by suppressing the expression of E-cadherin or inducing the expression of mesenchymal markers. To test this, we inhibited Axl and Dock GEF activity using R428 and CPYPP, respectively (see Fig. S6B in the supplemental material). In addition, we blocked the expression of Axl, Elmo2, Dock1, and Dock5 using a siRNA approach (see Fig. S6A). The expression of the epithelial marker E-cadherin was not rescued either by blocking the expression of Axl, Dock1, Dock5, and Elmo2 or by inhibiting their activity (see Fig. S6A and B). Interestingly, the expression of the mesenchymal marker vimentin was significantly reduced by the knockdown of Axl, Elmo2, and Dock1 expression and by inhibiting Dock GEF activity in MDA-MB-231 cells (see Fig. S6A and B). However, knockdown of Dock5 and R428 treatment was not able to reduce vimentin expression levels (see Fig. S6A and B). Collectively, these results uncover the Elmo2-Dock1 complex as a key signaling module for Rac activation and in promoting the expression of mesenchymal markers downstream of Axl in invasive breast cancer cells.
Phosphorylation of Elmo2 on Tyr 713 is required for cell invasion and cell proliferation.
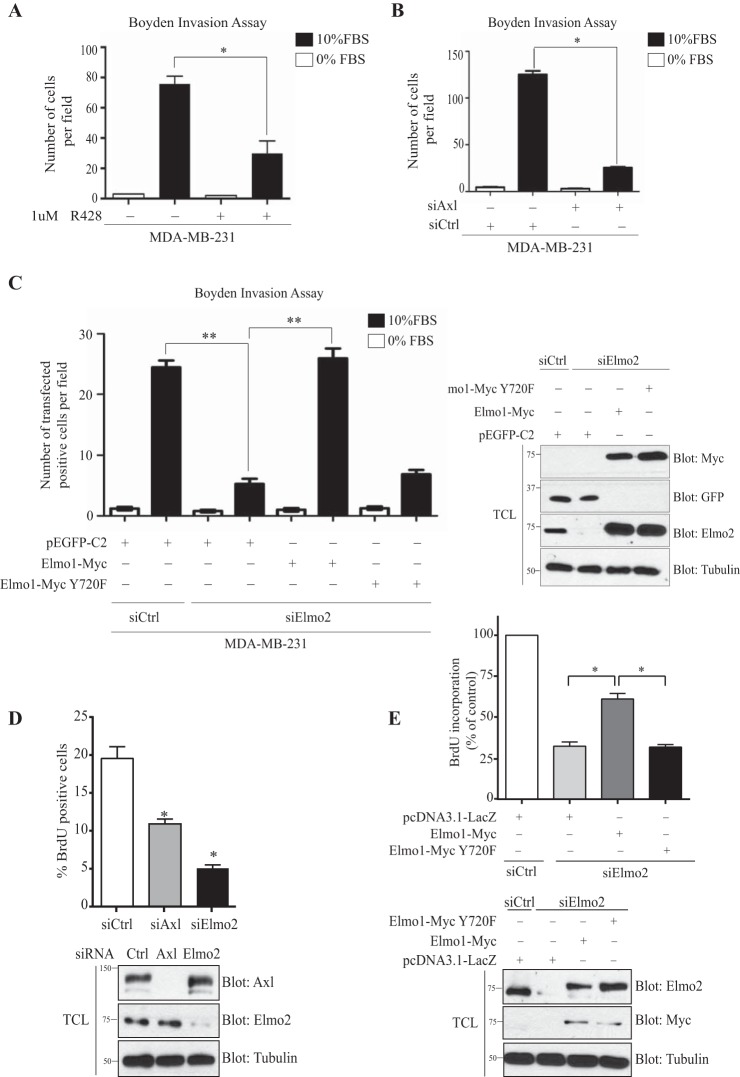
We aimed to define if phosphorylation of Elmo2 by Axl is a required signaling event to promote cell invasion. We first confirmed previous observations suggesting that MDA-MB-231 and Hs578T cells invade through Matrigel in an Axl-dependent manner (20, 22, 53). We treated MDA-MB-231 cells with R428 or siRNA against Axl and found that both treatments blocked cell invasion (Fig. 5A and B). We next assayed the role of Elmo2 in breast cancer cell invasion by a siRNA approach. Knockdown of Elmo2 robustly inhibited migration of MDA-MB-231 cells across a Matrigel barrier (Fig. 5C). In an effort to determine if phosphorylation of Elmo is important for cell invasion, we performed rescue experiments in Elmo2 knockdown cells with a construct encoding either Myc-Elmo1 wild-type or a Myc-Elmo1 Y720F mutant. We reexpressed Elmo1, since the exogenous Myc-Elmo2 was difficult to express in Elmo2 siRNA-treated cells (the human Elmo2 SmartPool siRNA also targets murine mRNA). We also previously reported that biological functions of Elmo1 and Elmo2 in myoblast fusion are interchangeable (54). We observed that expression of Myc-Elmo1 in Elmo2 knockdown cells completely restored the invasion to an extent comparable to that for cells expressing a control siRNA (Fig. 5C). In contrast, reexpression of Myc-Elmo1 Y720F in Elmo2 knockdown cells failed to reestablish cell invasion despite its expression being identical to that of the wild-type protein (Fig. 5C).
FIG 5.
Cell invasion and proliferation of MDA-MB-231 cells is Elmo2 and Axl dependent. (A and B) Axl activity or expression inhibition reduces cell invasion. Serum-starved MDA-MB-231 cells treated with 1 μM R428 (A) or transfected with 100 nM NON-Targeting or ON-Target SmartPool Axl siRNA (B) were detached and placed in the upper compartment of a Boyden chamber. Cells were allowed to invade through the Matrigel for 16 h. The invasion assay was performed in triplicate, and data are shown as means ± SEM; *, P < 0.001; one-way ANOVA. (C) The Elmo1 phosphorylation site Tyr 720 is required for cell invasion in MDA-MB-231 cells. Serum-starved MDA-MB-231 cells transfected with 60 nM NON-Targeting or ON-Target SmartPool Elmo2 siRNA and rescued 24 h later with either 1 μg GFP or 1 μg Elmo1 wild type or 2 μg Elmo1 Y720F mutant were detached and placed in the upper compartment of a Boyden chamber. Cells were allowed to invade through the Matrigel for 16 h and then were fixed and stained with anti-Myc and anti-GFP. GFP- and Myc-positive cells that invaded the Matrigel to the underside of the membrane were counted from photographs taken at magnification ×20. The invasion assay was performed in triplicate, and data are shown as mean ± SD; **, P < 0.0001; one-way ANOVA. Expression levels of the transfected proteins for invasion assays were analyzed by immunoblotting cell lysates with anti-Elmo2, anti-Myc, anti-GFP, and antitubulin antibodies, as indicated. (D and E) Axl and Elmo1 phosphorylation site Tyr 720 are required for cell proliferation in MDA-MB-231 cells. Cells transfected with 100 nM NON-Targeting, 100 nM ON-Target SmartPool Axl siRNA, or 60 nM ON-Target SmartPool Elmo2 siRNA were detached 24 h later and plated on fibronectin-coated glass slips. Transfected MDA-MB-231 cells with 60 nM NON-Targeting or ON-Target SmartPool Elmo2 siRNA were rescued 24 h later with either 1 μg pcDNA3.1-LacZ or 1 μg Elmo1 wild type or 2 μg Elmo1 Y720F mutant prior to being plated on fibronectin-coated glass slips 24 h later (E). Cells were allowed to grow for 24 h and then were stained with BrdU for 30 min. The BrdU-positive cells were counted, and the percentage of BrdU-positive cells versus total cells stained for DAPI were calculated in 5 different fields of each condition. Three experiments were performed, and the percentage of BrdU-positive cells was calculated for each experiment and used for the final quantification. For rescue experiments (E), BrdU incorporation was expressed as a percentage of decrease relative to control (n = 3). Values are reported as means ± SEM. Expression levels of the transfected proteins for proliferation assays were analyzed by immunoblotting cell lysates with anti-Elmo2, anti-Myc, anti-Axl, and antitubulin antibodies, as indicated.
We next aimed to define if Elmo, i.e., Rac signaling, downstream of Axl contributes to the proliferation of these cells. We assayed proliferation by BrdU staining and found that knockdown of Axl or Elmo2 robustly inhibited the proliferation of MDA-MB-231 cells (Fig. 5D). To determine if Elmo phosphorylation is important for proliferation, we performed rescue experiments as mentioned above. We observed that expression of Myc-Elmo1 in Elmo2 knockdown cells partially restored the proliferation of these cells (Fig. 5E). In contrast, expression of Myc-Elmo1 Y720F in Elmo2 knockdown cells failed to reestablish cell proliferation despite its expression being identical to that of the wild-type protein (Fig. 5E). Globally, our findings demonstrate a central role for Axl-mediated phosphorylation of Elmo2 in promoting proliferation and invasion of basal breast cancer cells.
DISCUSSION
Axl is a potent promoter of invasion and metastasis in experimental models, and its expression correlates with a poor outcome for breast cancer patients. Therefore, defining the molecular pathways by which this RTK promotes invasion is essential in order to interfere with downstream signaling. We report here a previously unrecognized molecular mechanism by which Axl uses Elmo scaffold proteins to signal to the Rac pathway to promote cell invasion. Despite identification of many Axl-interacting proteins, Elmo1 and Elmo2 may be the first identified bona fide direct substrates of this RTK, and we show that their interaction with Axl and phosphorylation on carboxyl-terminal tyrosine residues is essential for invasion of basal breast cancer cells. Early studies exploiting an epidermal growth factor receptor (EGFR)-Axl chimeric protein had identified unknown proteins of approximately 45 and 80 kDa that became robustly tyrosine phosphorylated in response to epidermal growth factor (EGF) stimulation (46). Elmo proteins are approximately 80 kDa in mass and may represent the proteins observed in that study. In addition, early studies in Drosophila melanogaster demonstrated that Myoblast City, the fly orthologue of mammalian DOCK1, acts downstream of the receptor tyrosine kinase PDGF/vascular endothelial growth factor (VEGF) receptor to promote the Rac-dependent migration of border cells (55). In connection with our study, we present evidence that the Elmo/Dock1 complex likewise acts as a signaling mediator downstream of receptor tyrosine kinases of the TAM family receptors which are not found in Caenorhabditis elegans or Drosophila.
Our knockdown of Elmo2, in parallel to rescue assays with Elmo1, demonstrates that Elmo2 is required for basal breast cancer cell invasion and proliferation. Likewise, a recent study demonstrated that stable knockdown of Elmo2 prevents metastasis of MDA-MB-231 cells to lungs in experimental tail vein assays (39). However, we find here that rescuing Elmo2 with Elmo1 lacking Y720 completely prevented cell invasion. These data point to a critical role for this residue in transmitting signaling. Exactly how phosphorylation of Elmo2 promotes cell invasion is not fully understood. We previously reported that Elmo proteins are regulated by intramolecular interactions that prevent aberrant Rac signaling (31). In particular, expression of Elmo with mutations maintaining it in an open conformation can increase migration in cells and biological activity in vivo (31, 56). Through the use of a conformational state biosensor for Elmo2 that we previously described (31), we failed to detect changes in Elmo2 conformation when it enters into a complex with, and is phosphorylated by, Axl (data not shown). Another mechanism could be that the tyrosine-phosphorylated residue Y713 in Elmo2 becomes a docking site for other signaling molecules, but we deemed this hypothesis unlikely because bioinformatics analysis conflicts with these sites being strong candidates for SH2 or PTB domain-containing proteins. Recent studies also demonstrated that phosphorylation of Dock1 on serine and tyrosine residues can increase Rac binding and GEF activity (34–37). One hypothesis is that this phosphorylation site on Elmo can transmit signals to Dock1 and enhance its GEF activity, such as relieving Dock1 from its autoinhibited state, which may explain why we found the Elmo/Dock1 complex stable whether or not the proteins are phosphorylated.
Moreover, guanine nucleotide exchange factors for Rho GTPases operating downstream of Axl have not been previously described. They have been neglected as signaling intermediates that are important in defining the mechanism whereby this RTK promotes metastasis. Through an interaction with Elmo2 or potentially through another mechanism, we report here that Axl can form a complex with the Rac regulator Dock1. We demonstrated, using the CPYPP small-molecule inhibitor, that Dock1, a member of the Dock-A subfamily (Dock1, Dock2, and Dock5), mediates Axl-induced Rac activation. Because this inhibitor also targets hematopoietic cell-specific Dock2 and ubiquitous Dock5, we cannot rule out that Dock5 is also recruited to Axl via an interaction with Elmo2, since it is also expressed in basal breast cancer cells. Similarly, Dock4, which belongs to the Dock-B subfamily (Dock3 and Dock4), is another broadly expressed Elmo-binding GEF that may contribute to Axl signaling, since it has been shown recently to promote MDA-MB-231 cell migration through activation of Rac (57). In the case of the Mer TAM family member, it has been reported to recruit the classical GEF Vav1 for activating Rac during engulfment of damaged photoreceptors (58). Interestingly, Mer can also recruit Dock1, but in this context through an interaction with the scaffolding protein p130Cas and the adaptor protein CrkII, to promote engulfment of apoptotic cells (8). We did not investigate if CrkII can complex with tyrosine-phosphorylated Axl through its SH2 domain; if that was the case, CrkII or other SH2/SH3 adaptors could also cooperate with Elmo proteins to facilitate the recruitment of Dock GEFs to the RTK.
Previous studies have shown that high Axl expression in breast cancer patients is correlated with poor patient survival (20). Similarly, the Axl ligand Gas6 has been shown to be a target for overexpression and amplification in breast cancer (59). However, some studies have shown that upregulation of Axl in breast cancer cells led to an increase in Axl activity independently of Gas6 binding, confirming the constitutive activation of Axl in these cells (25). Because we detect Axl phosphorylation at basal levels prior to Gas6 stimulation in serum-starved cells in MDA-MB-231, our data suggest that Axl in basal breast cancer cells is constitutively active and may act independently of its ligand, which may be the reason why we observe an Elmo2/Axl complex at basal levels prior to Gas6 stimulation.
Our data highlight a previously unsuspected role of Axl and Elmo in proliferation of invasive breast cancer cells. Previous studies did not detect a reduction in proliferation upon knockdown of Axl and Elmo expression using short-hairpin RNA (shRNA) (20, 39). A transient siRNA approach may have not allowed enough time for alternative pathways to rescue proliferation, which allowed us to identify a role for Elmo tyrosine site Y713 in promoting proliferation. Similarly, we showed in another study that Dock1-null mammary tumors' growth was reduced compared to that of Dock1-wild-type mammary tumors, indicating a role for Dock1 in promoting cell proliferation (34).
Furthermore, it remains unclear at what step of breast cancer progression Axl is contributing. Knockout mouse models looking at this important question are missing. Recent data highlight that Axl expression is important in basal breast cancer cells to maintain a stem cell-like phenotype (20, 25). In part, this is done through the expression of transcription factors that maintain a mesenchymal phenotype, such as Snail and Slug. Our results revealing a role for Dock/Elmo proteins in EMT is novel and potentially unique for basal breast cancer cells. In a previous study, we found Dock1 in vivo not to be required for mesenchymal transition of cardiomyocytes (60). Likewise, deletion of Dock1 in HER2 breast cancer tumors was found to alter interferon gene expression but not EMT gene expression (34). The pathophysiological importance of Vimentin expression by Elmo/Dock proteins remains to be fully explored in basal breast cancer cells. It will also be important to verify in vivo if Axl contributes to stem cell maintenance and if its role in sustaining epithelial-to-mesenchymal transition is directly linked to invasion.
Altogether, these results led us to propose that Axl may hijack the Rac activator Elmo/Dock complex to phosphorylate Elmo and promote cell invasion and cell proliferation. It also identifies inhibition of the Elmo-Dock pathway as a potential therapeutic target to stop Axl-induced cell proliferation, invasion, and metastases.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to R. Birge (Rutgers New Jersey Medical School) for the gift of Mer plasmids. We thank members of the Côté lab for helpful discussions. We recognize the technical support of A. Pelletier, C. Julien, C. Meunier, and M. Al-Azzabi. We thank E. Boneil of the mass spectrometry platform of IRIC (Montreal, Canada) for his assistance in mapping phosphorylation sites.
This work was funded by a Quebec Breast Cancer Foundation grant to J.-F.C. and J.-P.G; A.A.-T. was supported in part by the Programmes de Biologie Moléculaire funds from the Université de Montréal and was a recipient of a Ph.D. studentship from Emmanuel-Triassi-IRCM. R.G. was a recipient of an M.Sc. studentship from Wisent-IRCM. J.-F.C. is a recipient of a Senior Investigator Award from the FRQ-S. R.A.S. holds the Canada Research Chair in Apoptotic Signaling.
We have no conflict of interest to declare.
J.-F.C. designed the research; A.A.-T., R.G., R.C., R.A.S., and J.-F.C. performed the research; A.A.-T., R.G., R.C., Y.F., R.A.S., J.-P.G., and J.-F.C. analyzed the data; J.-F.C. wrote the manuscript with contributions from all authors.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00764-14.
REFERENCES
- 1.O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R III, Le Beau MM, Earp HS, Liu ET. 1991. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol 11:5016–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linger RM, Keating AK, Earp HS, Graham DK. 2008. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, Masiakowski P, Ryan TE, Tobkes NJ, Chen DH, DiStefano PS, Long GL, Basilico C, Goldfarb MP, Lemke G, Glass DJ, Yancopoulos GD. 1995. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 4.Caberoy NB, Zhou Y, Li W. 2010. Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. EMBO J 29:3898–3910. doi: 10.1038/emboj.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. 1996. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 6.O'Bryan JP, Fridell YW, Koski R, Varnum B, Liu ET. 1995. The transforming receptor tyrosine kinase, Axl, is post-translationally regulated by proteolytic cleavage. J Biol Chem 270:551–557. doi: 10.1074/jbc.270.2.551. [DOI] [PubMed] [Google Scholar]
- 7.Hall MO, Obin MS, Heeb MJ, Burgess BL, Abrams TA. 2005. Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res 81:581–591. doi: 10.1016/j.exer.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Singh S, Georgescu MM, Birge RB. 2005. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci 118:539–553. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
- 9.Caberoy NB, Maiguel D, Kim Y, Li W. 2010. Identification of tubby and tubby-like protein 1 as eat-me signals by phage display. Exp Cell Res 316:245–257. doi: 10.1016/j.yexcr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano T, Ishimoto Y, Kishino J, Umeda M, Inoue K, Nagata K, Ohashi K, Mizuno K, Arita H. 1997. Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J Biol Chem 272:29411–29414. doi: 10.1074/jbc.272.47.29411. [DOI] [PubMed] [Google Scholar]
- 11.Bellosta P, Costa M, Lin DA, Basilico C. 1995. The receptor tyrosine kinase ARK mediates cell aggregation by homophilic binding. Mol Cell Biol 15:614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burchert A, Attar EC, McCloskey P, Fridell YW, Liu ET. 1998. Determinants for transformation induced by the Axl receptor tyrosine kinase. Oncogene 16:3177–3187. doi: 10.1038/sj.onc.1201865. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, Halsey W, Sathe GM, Martin AM, Gilmer TM. 2009. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res 69:6871–6878. doi: 10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- 14.Fridell YW, Jin Y, Quilliam LA, Burchert A, McCloskey P, Spizz G, Varnum B, Der C, Liu ET. 1996. Differential activation of the Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. Mol Cell Biol 16:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemke G, Lu Q. 2003. Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol 15:31–36. doi: 10.1016/S0952-7915(02)00016-X. [DOI] [PubMed] [Google Scholar]
- 16.Lu Q, Lemke G. 2001. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp HS, Goff SP, Lemke G. 1999. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 18.D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. 2000. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 19.Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, Wu CW. 2005. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia 7:1058–1064. doi: 10.1593/neo.05640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT, Micklem DR, Akslen LA, Glackin C, Lorens JB. 2010. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A 107:1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland SJ, Powell MJ, Franci C, Chan EW, Friera AM, Atchison RE, McLaughlin J, Swift SE, Pali ES, Yam G, Wong S, Lasaga J, Shen MR, Yu S, Xu W, Hitoshi Y, Bogenberger J, Nor JE, Payan DG, Lorens JB. 2005. Multiple roles for the receptor tyrosine kinase axl in tumor formation. Cancer Res 65:9294–9303. doi: 10.1158/0008-5472.CAN-05-0993. [DOI] [PubMed] [Google Scholar]
- 22.Holland SJ, Pan A, Franci C, Hu YM, Chang B, Li WQ, Duan M, Torneros A, Yu JX, Heckrodt TJ, Zhang J, Ding PY, Apatira A, Chua J, Brandt R, Pine P, Goff D, Singh R, Payan DG, Hitoshi Y. 2010. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res 70:1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 23.Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, Weimer R, Wu Y, Pei L. 2010. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 29:5254–5264. doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J, Kallop D, Ludlam MJ, Pei L. 2009. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene 28:3442–3455. doi: 10.1038/onc.2009.212. [DOI] [PubMed] [Google Scholar]
- 25.Asiedu MK, Beauchamp-Perez FD, Ingle JN, Behrens MD, Radisky DC, Knutson KL. 2014. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene 33:1316–1324. doi: 10.1038/onc.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cote JF, Vuori K. 2002. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci 115:4901–4913. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- 27.Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. 2002. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol 4:574–582. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 28.Meller N, Irani-Tehrani M, Kiosses WB, Del Pozo MA, Schwartz MA. 2002. Zizimin1, a novel Cdc42 activator, reveals a new GEF domain for Rho proteins. Nat Cell Biol 4:639–647. doi: 10.1038/ncb835. [DOI] [PubMed] [Google Scholar]
- 29.Grimsley CM, Kinchen JM, Tosello-Trampont AC, Brugnera E, Haney LB, Lu M, Chen Q, Klingele D, Hengartner MO, Ravichandran KS. 2004. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J Biol Chem 279:6087–6097. doi: 10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- 30.Hiramoto K, Negishi M, Katoh H. 2006. Dock4 is regulated by RhoG and promotes Rac-dependent cell migration. Exp Cell Res 312:4205–4216. doi: 10.1016/j.yexcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Patel M, Margaron Y, Fradet N, Yang Q, Wilkes B, Bouvier M, Hofmann K, Cote JF. 2010. An evolutionarily conserved autoinhibitory molecular switch in ELMO proteins regulates Rac signaling. Curr Biol 20:2021–2027. doi: 10.1016/j.cub.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komander D, Patel M, Laurin M, Fradet N, Pelletier A, Barford D, Cote JF. 2008. An alpha-helical extension of the ELMO1 pleckstrin homology domain mediates direct interaction to DOCK180 and is critical in Rac signaling. Mol Biol Cell 19:4837–4851. doi: 10.1091/mbc.E08-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurin M, Cote JF. 2014. Insights into the biological functions of Dock family guanine nucleotide exchange factors. Genes Dev 28:533–547. doi: 10.1101/gad.236349.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurin M, Huber J, Pelletier A, Houalla T, Park M, Fukui Y, Haibe-Kains B, Muller WJ, Cote JF. 2013. Rac-specific guanine nucleotide exchange factor DOCK1 is a critical regulator of HER2-mediated breast cancer metastasis. Proc Natl Acad Sci U S A 110:7434–7439. doi: 10.1073/pnas.1213050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng H, Hu B, Vuori K, Sarkaria JN, Furnari FB, Cavenee WK, Cheng SY. 2013. EGFRvIII stimulates glioma growth and invasion through PKA-dependent serine phosphorylation of Dock180. Oncogene 33:2504–2512. doi: 10.1038/onc.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng H, Hu B, Jarzynka MJ, Li Y, Keezer S, Johns TG, Tang CK, Hamilton RL, Vuori K, Nishikawa R, Sarkaria JN, Fenton T, Cheng T, Furnari FB, Cavenee WK, Cheng SY. 2012. Phosphorylation of dedicator of cytokinesis 1 (Dock180) at tyrosine residue Y722 by Src family kinases mediates EGFRvIII-driven glioblastoma tumorigenesis. Proc Natl Acad Sci U S A 109:3018–3023. doi: 10.1073/pnas.1121457109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng H, Hu B, Liu KW, Li Y, Lu X, Cheng T, Yiin JJ, Lu S, Keezer S, Fenton T, Furnari FB, Hamilton RL, Vuori K, Sarkaria JN, Nagane M, Nishikawa R, Cavenee WK, Cheng SY. 2011. Activation of Rac1 by Src-dependent phosphorylation of Dock180(Y1811) mediates PDGFRalpha-stimulated glioma tumorigenesis in mice and humans. J Clin Invest 121:4670–4684. doi: 10.1172/JCI58559. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Smith HW, Marra P, Marshall CJ. 2008. uPAR promotes formation of the p130Cas-Crk complex to activate Rac through DOCK180. J Cell Biol 182:777–790. doi: 10.1083/jcb.200712050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Yang L, Fu H, Yan J, Wang Y, Guo H, Hao X, Xu X, Jin T, Zhang N. 2013. Association between Galphai2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis. Nat Commun 4:1706. doi: 10.1038/ncomms2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jansson D, Ng AC, Fu A, Depatie C, Al Azzabi M, Screaton RA. 2008. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc Natl Acad Sci U S A 105:10161–10166. doi: 10.1073/pnas.0800796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel M, Chiang TC, Tran V, Lee FJ, Cote JF. 2011. The Arf family GTPase Arl4A complexes with ELMO proteins to promote actin cytoskeleton remodeling and reveals a versatile Ras-binding domain in the ELMO proteins family. J Biol Chem 286:38969–38979. doi: 10.1074/jbc.M111.274191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cote JF, Motoyama AB, Bush JA, Vuori K. 2005. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol 7:797–807. doi: 10.1038/ncb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel M, Pelletier A, Cote JF. 2011. Opening up on ELMO regulation: new insights into the control of Rac signaling by the DOCK180/ELMO complex. Small GTPases 2:268–275. doi: 10.4161/sgtp.2.5.17716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoyama N, deBakker CD, Zappacosta F, Huddleston MJ, Annan RS, Ravichandran KS, Miller WT. 2005. Identification of tyrosine residues on ELMO1 that are phosphorylated by the Src-family kinase Hck. Biochemistry 44:8841–8849. doi: 10.1021/bi0500832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. 2011. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther 10:1763–1773. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 46.Braunger J, Schleithoff L, Schulz AS, Kessler H, Lammers R, Ullrich A, Bartram CR, Janssen JW. 1997. Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene 14:2619–2631. doi: 10.1038/sj.onc.1201123. [DOI] [PubMed] [Google Scholar]
- 47.Weinger JG, Gohari P, Yan Y, Backer JM, Varnum B, Shafit-Zagardo B. 2008. In brain, Axl recruits Grb2 and the p85 regulatory subunit of PI3 kinase; in vitro mutagenesis defines the requisite binding sites for downstream Akt activation. J Neurochem 106:134–146. doi: 10.1111/j.1471-4159.2008.05343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margaron Y, Fradet N, Cote JF. 2013. ELMO recruits actin cross-linking family 7 (ACF7) at the cell membrane for microtubule capture and stabilization of cellular protrusions. J Biol Chem 288:1184–1199. doi: 10.1074/jbc.M112.431825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nielsen-Preiss SM, Allen MP, Xu M, Linseman DA, Pawlowski JE, Bouchard RJ, Varnum BC, Heidenreich KA, Wierman ME. 2007. Adhesion-related kinase induction of migration requires phosphatidylinositol-3-kinase and ras stimulation of rac activity in immortalized gonadotropin-releasing hormone neuronal cells. Endocrinology 148:2806–2814. doi: 10.1210/en.2007-0039. [DOI] [PubMed] [Google Scholar]
- 50.Nishikimi A, Uruno T, Duan X, Cao Q, Okamura Y, Saitoh T, Saito N, Sakaoka S, Du Y, Suenaga A, Kukimoto-Niino M, Miyano K, Gotoh K, Okabe T, Sanematsu F, Tanaka Y, Sumimoto H, Honma T, Yokoyama S, Nagano T, Kohda D, Kanai M, Fukui Y. 2012. Blockade of inflammatory responses by a small-molecule inhibitor of the Rac activator DOCK2. Chem Biol 19:488–497. doi: 10.1016/j.chembiol.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Yang WH, Lan HY, Huang CH, Tai SK, Tzeng CH, Kao SY, Wu KJ, Hung MC, Yang MH. 2012. RAC1 activation mediates Twist1-induced cancer cell migration. Nat Cell Biol 14:366–374. doi: 10.1038/ncb2455. [DOI] [PubMed] [Google Scholar]
- 52.Childs G, Segall JE. 2012. Twists and turns of invasion. Nat Cell Biol 14:337–339. doi: 10.1038/ncb2477. [DOI] [PubMed] [Google Scholar]
- 53.Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L, Szabadkai I, Daub H, Keri G, Ullrich A. 2008. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res 68:1905–1915. doi: 10.1158/0008-5472.CAN-07-2661. [DOI] [PubMed] [Google Scholar]
- 54.Hamoud N, Tran V, Croteau LP, Kania A, Cote JF. 2014. G-protein coupled receptor BAI3 promotes myoblast fusion in vertebrates. Proc Natl Acad Sci U S A 111:3745–3750. doi: 10.1073/pnas.1313886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. 2001. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107:17–26. doi: 10.1016/S0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 56.Liu ZC, Odell N, Geisbrecht ER. 2013. Drosophila importin-7 functions upstream of the Elmo signaling module to mediate the formation and stability of muscle attachments. J Cell Sci 126:5210–5223. doi: 10.1242/jcs.132241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi M, Harada K, Negishi M, Katoh H. 2014. Dock4 forms a complex with SH3YL1 and regulates cancer cell migration. Cell Signal 26:1082–1088. doi: 10.1016/j.cellsig.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 58.Mahajan NP, Earp HS. 2003. An SH2 domain-dependent, phosphotyrosine-independent interaction between Vav1 and the Mer receptor tyrosine kinase: a mechanism for localizing guanine nucleotide-exchange factor action. J Biol Chem 278:42596–42603. doi: 10.1074/jbc.M305817200. [DOI] [PubMed] [Google Scholar]
- 59.Mc Cormack O, Chung WY, Fitzpatrick P, Cooke F, Flynn B, Harrison M, Fox E, Gallagher E, Goldrick AM, Dervan PA, Mc Cann A, Kerin MJ. 2008. Growth arrest-specific gene 6 expression in human breast cancer. Br J Cancer 98:1141–1146. doi: 10.1038/sj.bjc.6604260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanematsu F, Hirashima M, Laurin M, Takii R, Nishikimi A, Kitajima K, Ding G, Noda M, Murata Y, Tanaka Y, Masuko S, Suda T, Meno C, Cote JF, Nagasawa T, Fukui Y. 2010. DOCK180 is a Rac activator that regulates cardiovascular development by acting downstream of CXCR4. Circ Res 107:1102–1105. doi: 10.1161/CIRCRESAHA.110.223388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.