With increased chemotherapy use for malignant glioma, the paradigm for treatment and associated out-of-pocket and total medical costs continue to evolve.
Abstract
Purpose:
Approximately 18,500 persons are diagnosed with malignant glioma in the United States annually. Few studies have investigated the comprehensive economic costs. We reviewed the literature to examine costs to patients with malignant glioma and their families, payers, and society.
Methods:
A total of 18 fully extracted studies were included. Data were collected on direct and indirect costs, and cost estimates were converted to US dollars using the conversion rate calculated from the study's publication date, and updated to 2011 values after adjustment for inflation. A standardized data abstraction form was used. Data were extracted by one reviewer and checked by another.
Results:
Before approval of effective chemotherapeutic agents for malignant gliomas, estimated total direct medical costs in the United States for surgery and radiation therapy per patient ranged from $50,600 to $92,700. The addition of temozolomide (TMZ) and bevacizumab to glioblastoma treatment regimens has resulted in increased overall costs for glioma care. Although health care costs are now less front-loaded, they have increased over the course of illness. Analysis using a willingness-to-pay threshold of $50,000 per quality-adjusted life-year suggests that the benefits of TMZ fall on the edge of acceptable therapies. Furthermore, indirect medical costs, such as productivity losses, are not trivial.
Conclusion:
With increased chemotherapy use for malignant glioma, the paradigm for treatment and associated out-of-pocket and total medical costs continue to evolve. Larger out-of-pocket costs may influence the choice of chemotherapeutic agents, the economic implications of which should be evaluated prospectively.
Introduction
In 2012, approximately 1.64 million persons were diagnosed with cancer in the United States.1 Although malignant brain tumors account for only 1.4% of the total cancer cases,1 they cause permanent injury to the brain, are commonly resistant to most treatments, and result in disproportionately high morbidity.2 Current standard treatment for glioblastoma multiforme (GBM) is radiation therapy (RT) plus temozolomide (TMZ), followed by 6 to 12 months of maintenance TMZ. No standard exists for grade 3 gliomas, but treatment includes the above regimen, chemotherapy alone, or RT alone. At progression, many patients are treated with bevacizumab if they are not eligible for a clinical trial. Patients age 65 years and older receive full-dose RT plus TMZ, unless they are frail or do not tolerate RT; for those patients, short-course RT may be used, or RT may be omitted. Gliomas account for 45% to 50% of all malignant tumors of the CNS.2,3 The majority of gliomas arise from astrocytes and are classified as low-grade astrocytomas, anaplastic astrocytomas, or GBM; the latter making up more than half of all cases. Median patient survival after GBM diagnosis is less than 15 months, despite aggressive standard treatment involving tumor resection, 6 weeks of radiation with concomitant TMZ, and six cycles of adjuvant TMZ. Bevacizumab is currently indicated for the treatment of recurrent GBM, with a median overall survival of approximately 9 months.3–5
The SEER Program database reports the 5-year relative survival rate for GBM as 3.3%, but current data suggest an approximately 10% survival for patients treated with RT at 2 years and 27% for patients treated with RT and TMZ.6,7
Despite the high morbidity and mortality associated with malignant glioma, little attention has been paid in the United States to its economic costs. Reviews in Europe have focused on treatment costs associated with TMZ8,9 and carmustine implants.10 Direct medical costs have been reported in several large insurance claims database studies, but indirect costs associated with premature death or years of productive life lost need consideration. Low-grade and anaplastic gliomas, which affect adults between ages 20 and 55, exert the greatest economic impact; the overall economic impact of GBMs, in comparison, is less, as median at diagnosis is 55 years. However, with many people working beyond age 65, lost productivity for geriatric patients is becoming increasingly important.
In an age of limited resources and soaring health care costs, economic assessments of malignant gliomas, with their frequent occurrence in younger and middle-aged individuals, is particularly important. Herein, we review and summarize the literature relative to the direct and indirect costs of malignant gliomas for patients and their families, payers, and society.
Methods
We systematically searched MEDLINE, PubMed, EMBASE, and the Cochrane Library from January 1996 through March 2011. Abstracts from recent scientific meetings and reference lists of relevant reviews were hand-searched to identify additional studies. A standardized data extraction form was used, which allowed for input of information from different trials and for subgroup analysis: type of study, participants, interventions, outcomes, decision to include or exclude a study, and organizational aspects (author, year, country of origin, publication type). It also allowed for the classification or grouping of several studies with common features (eg, study quality, protocol of intervention) and included a section on reasons for exclusion of a study from review, such as methods used by the included studies, patient characteristics (eg, age), and outcomes. Studies that failed one of the checks were discarded. Only four studies fell into this category. “AND” narrowed our search by ensuring that all of our terms were in the article of interest. “OR” broadened our search to include results that contained either of the terms in the article of interest and was useful for linking synonyms. Additionally, the Physician Data Query clinical trials database and the proceedings of the 1997 to 2010 ASCO Annual Meetings were searched for reports of new or ongoing trials. Relevant articles and abstracts were selected and reviewed, and the reference lists from these sources were searched for additional trials. The quality of randomized clinical trials and nonrandomized controlled studies was assessed using standard checklists.11 We used the following search terms: economics, costs, cost-benefit analysis, cost effectiveness, cost of illness, insurance coverage/statistics and numerical data, brain cancer, and glioma. Content experts provided additional potential studies not identified by the database searches. Three investigators (K.F., J.R., and J.M.M.) independently reviewed all identified publications for inclusion using predetermined criteria. Disagreements were resolved by an independent adjudicator. No language restrictions were applied. Studies published in a language other than English were translated before consideration for inclusion. Included studies were those that looked at direct and indirect medical cost reports: direct costs are costs paid for medical goods and services related to the diagnosis and treatment of a disease; indirect costs are the estimated economic losses associated with cancer-related employment drop-out (short or long-term), decrease in productivity, lost salary, and early retirement pensions, as well as losses due to travel, food, out-of-pocket expenditures for medical care, and costs related to loss of income and expenses incurred by family members, spouses, and caregivers as a consequence of a cancer diagnosis for an individual patient. Cost estimates were converted to US dollars using the conversion rate calculated from the study's publication date and were updated to 2012 values after adjustment for inflation based on the date of publication. The Consumer Price Index for all goods and services was used for the adjustment. Inflation rates were obtained from the United States Department of Labor, Bureau of Labor Statistics Web site.
Results
Our search yielded 32 studies; 18 systematic reviews or meta-analyses met our criteria and were fully reviewed. The 10 studies with the best fit appear in Table 1; others are discussed in the text below.
Table 1.
Estimated Direct Costs of Brain Cancer for the Most Frequently Evaluated Components of Total Direct Costs
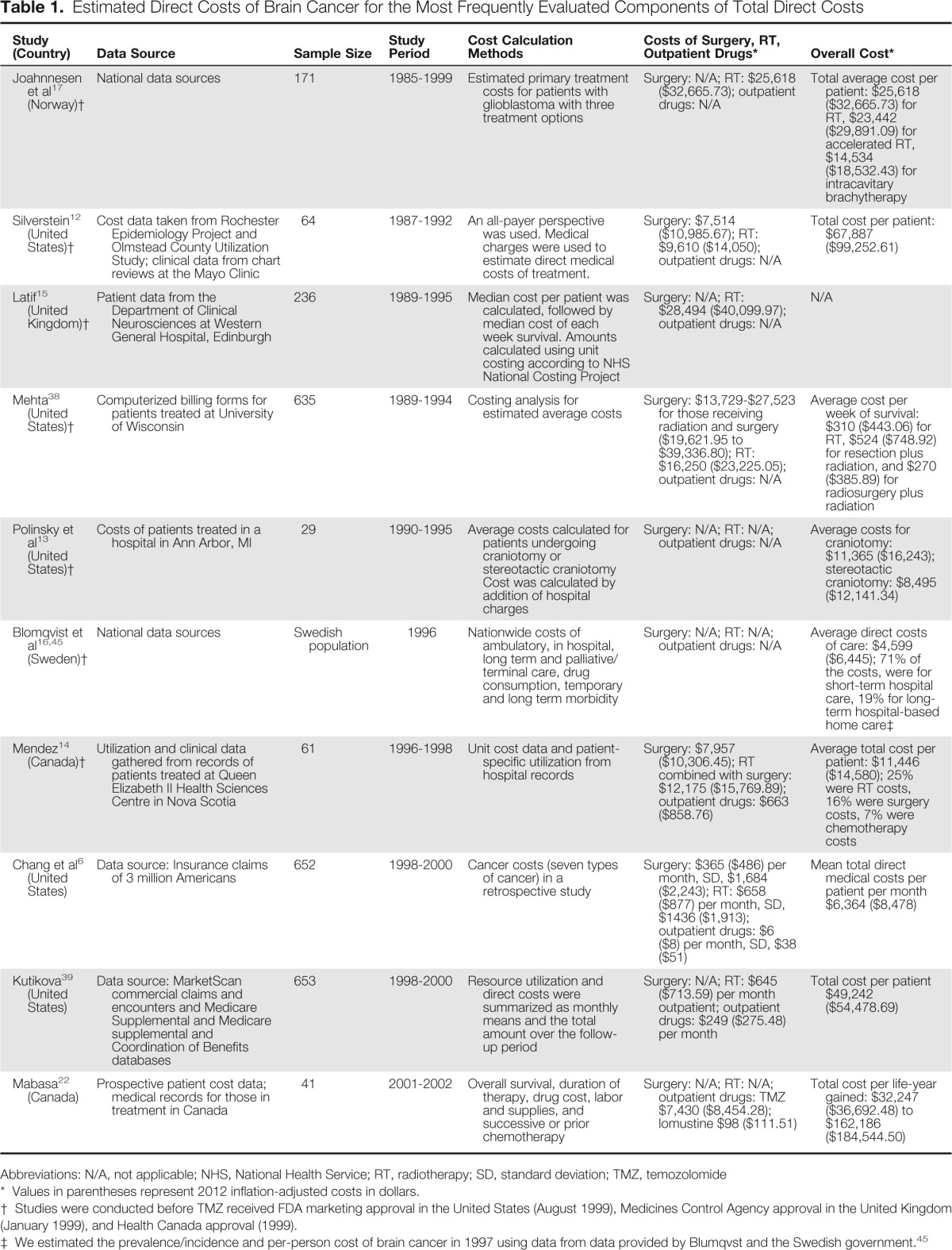
| Study (Country) | Data Source | Sample Size | Study Period | Cost Calculation Methods | Costs of Surgery, RT, Outpatient Drugs* | Overall Cost* |
|---|---|---|---|---|---|---|
| Joahnnesen et al17 (Norway)† | National data sources | 171 | 1985-1999 | Estimated primary treatment costs for patients with glioblastoma with three treatment options | Surgery: N/A; RT: $25,618 ($32,665.73); outpatient drugs: N/A | Total average cost per patient: $25,618 ($32,665.73) for RT, $23,442 ($29,891.09) for accelerated RT, $14,534 ($18,532.43) for intracavitary brachytherapy |
| Silverstein12 (United States)† | Cost data taken from Rochester Epidemiology Project and Olmstead County Utilization Study; clinical data from chart reviews at the Mayo Clinic | 64 | 1987-1992 | An all-payer perspective was used. Medical charges were used to estimate direct medical costs of treatment. | Surgery: $7,514 ($10,985.67); RT: $9,610 ($14,050); outpatient drugs: N/A | Total cost per patient: $67,887 ($99,252.61) |
| Latif15 (United Kingdom)† | Patient data from the Department of Clinical Neurosciences at Western General Hospital, Edinburgh | 236 | 1989-1995 | Median cost per patient was calculated, followed by median cost of each week survival. Amounts calculated using unit costing according to NHS National Costing Project | Surgery: N/A; RT: $28,494 ($40,099.97); outpatient drugs: N/A | N/A |
| Mehta38 (United States)† | Computerized billing forms for patients treated at University of Wisconsin | 635 | 1989-1994 | Costing analysis for estimated average costs | Surgery: $13,729-$27,523 for those receiving radiation and surgery ($19,621.95 to $39,336.80); RT: $16,250 ($23,225.05); outpatient drugs: N/A | Average cost per week of survival: $310 ($443.06) for RT, $524 ($748.92) for resection plus radiation, and $270 ($385.89) for radiosurgery plus radiation |
| Polinsky et al13 (United States)† | Costs of patients treated in a hospital in Ann Arbor, MI | 29 | 1990-1995 | Average costs calculated for patients undergoing craniotomy or stereotactic craniotomy Cost was calculated by addition of hospital charges | Surgery: N/A; RT: N/A; outpatient drugs: N/A | Average costs for craniotomy: $11,365 ($16,243); stereotactic craniotomy: $8,495 ($12,141.34) |
| Blomqvist et al16,45 (Sweden)† | National data sources | Swedish population | 1996 | Nationwide costs of ambulatory, in hospital, long term and palliative/terminal care, drug consumption, temporary and long term morbidity | Surgery: N/A; RT: N/A; outpatient drugs: N/A | Average direct costs of care: $4,599 ($6,445); 71% of the costs, were for short-term hospital care, 19% for long-term hospital-based home care‡ |
| Mendez14 (Canada)† | Utilization and clinical data gathered from records of patients treated at Queen Elizabeth II Health Sciences Centre in Nova Scotia | 61 | 1996-1998 | Unit cost data and patient-specific utilization from hospital records | Surgery: $7,957 ($10,306.45); RT combined with surgery: $12,175 ($15,769.89); outpatient drugs: $663 ($858.76) | Average total cost per patient: $11,446 ($14,580); 25% were RT costs, 16% were surgery costs, 7% were chemotherapy costs |
| Chang et al6 (United States) | Data source: Insurance claims of 3 million Americans | 652 | 1998-2000 | Cancer costs (seven types of cancer) in a retrospective study | Surgery: $365 ($486) per month, SD, $1,684 ($2,243); RT: $658 ($877) per month, SD, $1436 ($1,913); outpatient drugs: $6 ($8) per month, SD, $38 ($51) | Mean total direct medical costs per patient per month $6,364 ($8,478) |
| Kutikova39 (United States) | Data source: MarketScan commercial claims and encounters and Medicare Supplemental and Medicare supplemental and Coordination of Benefits databases | 653 | 1998-2000 | Resource utilization and direct costs were summarized as monthly means and the total amount over the follow-up period | Surgery: N/A; RT: $645 ($713.59) per month outpatient; outpatient drugs: $249 ($275.48) per month | Total cost per patient $49,242 ($54,478.69) |
| Mabasa22 (Canada) | Prospective patient cost data; medical records for those in treatment in Canada | 41 | 2001-2002 | Overall survival, duration of therapy, drug cost, labor and supplies, and successive or prior chemotherapy | Surgery: N/A; RT: N/A; outpatient drugs: TMZ $7,430 ($8,454.28); lomustine $98 ($111.51) | Total cost per life-year gained: $32,247 ($36,692.48) to $162,186 ($184,544.50) |
Abbreviations: N/A, not applicable; NHS, National Health Service; RT, radiotherapy; SD, standard deviation; TMZ, temozolomide
Values in parentheses represent 2012 inflation-adjusted costs in dollars.
Studies were conducted before TMZ received FDA marketing approval in the United States (August 1999), Medicines Control Agency approval in the United Kingdom (January 1999), and Health Canada approval (1999).
We estimated the prevalence/incidence and per-person cost of brain cancer in 1997 using data from data provided by Blumqvst and the Swedish government.45
Direct Medical Costs
In 2004, Chang et al6 examined direct costs associated with seven types of cancer in the United States after reviewing insurance claims of three million Americans between 1998 and 2000. Brain cancer ranked fourth in mean total direct medical costs per month at $8,478, based on 652 patients. Services, specifically RT ($877 per month; standard deviation [SD], $1,913) and surgery ($486 per month; SD, $2,243) contributed most to the direct costs of brain cancer; mean cost of outpatient chemotherapy dispensing was only $8 per month (SD, $51; Table 1).
Silverstein et al12 investigated the direct costs of care for patients with anaplastic astrocytomas (AA) and GBM. This study included 64 patients diagnosed between 1987 and 1992 at the Mayo Clinic, and estimated mean and median total cost of direct medical services to be $99,253 and $91,368, respectively. Radiotherapy (RT) costs were the most expensive ($14,050), followed by imaging ($14,238).10,12 Three quarters of the charges were incurred in the initial treatment period (median of 116 days). Direct costs reached a plateau after 1 year, likely as a result of low survival rates (median survival, 323 days). Polinsky et al13 estimated the average costs for 29 patients undergoing standard craniotomy ($16,292) and 15 patients undergoing stereotactic craniotomy ($12,178) in Ann Arbor, MI. Cost was calculated by addition of hospital charges. Studies in Nova Scotia13 and the United Kingdom14 evaluated the costs of brain tumors outside of the United States.
Blomqvist et al15 reported direct costs of care for brain tumors in Sweden in 1996 as $68.0 million per million population (estimated average direct costs = $6,445); of these costs, 71% were for short-term hospital care, 19% for long-term hospital-based home care, and 3% for ambulatory care/drugs. Johannesen et al16 collected data on primary treatment costs for patients with glioblastoma with three treatment options in Oslo, Norway from 1985 to 1999. They estimated an average cost of $32,764 for 58 patients treated with RT, $29,596 for 75 patients treated with accelerated RT, and $23.408 for 41 patients treated with intracavitary brachytherapy.
Indirect Costs
The National Brain Tumor Foundation assessed the financial impact of brain tumors using an online survey from 277 patients and 224 caregivers.17 Ninety-one percent of respondents had medical insurance. Despite being out of active treatment, 34% of respondents and 54% of caregivers reported out-of-pocket costs of more than $271 each month. For 5% of respondents and 27.2% of caregivers, out-of-pocket costs were more than $1,900 each month. In all categories assessed, caregiver out-of-pocket expenses were greater than those of the patients. Medications accounted for the greatest out-of-pocket medical cost, whereas meals, transportation to treatment, telephone bills, housing, and consumer goods accounted for the greatest nonmedical costs.17
The National Brain Tumor Foundation 2006 survey found that 91% of patients with brain tumors were employed prediagnosis versus 33% postdiagnosis.17 For caregivers, 16% discontinued employment, and 62% decreased their hours or took time off. Decreased household income was reported by 48% of respondents, and families reported decreased spending overall.17
Blomqvist et al15 estimated that the total indirect costs (sick leave, early retirement, and mortality) amounted to $197.7 million for the Swedish population ($22.5 million per million). These indirect costs accounted for the vast majority (74%) of the total cost of illness ($101,058 per patient) in 1996. Mortality for patients younger than 65 years accounted for 73.1% of the indirect costs. Early retirement pensions cost $378.4 million in lost production (19.2% of indirect costs), and temporary morbidity (sickness leave) cost $15.5 million (7.7% of indirect costs).15
Chemotherapeutic Treatment and Cost Effectiveness
The carmustine wafer received US Food and Drug Administration (FDA) approval in September 1996 and February 2003 for recurrent malignant gliomas and newly diagnosed malignant gliomas, respectively. Rogers et al performed a cost analysis of carmustine wafers, estimating a cost effectiveness of $115,458 per quality-adjusted life-year (QALY); $50,000 per QALY is often considered the upper limit of cost-effectiveness, although in this trial $30,000 was used.17,18
Three years after initial FDA approval of the carmustine wafer, TMZ, an oral chemotherapeutic agent, was approved by the FDA for recurrent AA and in 2005 for newly diagnosed GBM. TMZ is now part of the standard of care for patients with newly diagnosed GBM. In 2001, Dinnes et al published a report on the cost effectiveness of TMZ in recurrent malignant glioma, highlighting increase in progression-free survival (PFS), but not overall survival19 (Table 2).
Table 2.
Cost Effectiveness of Chemotherapy for Patients With Primary Brain Tumors
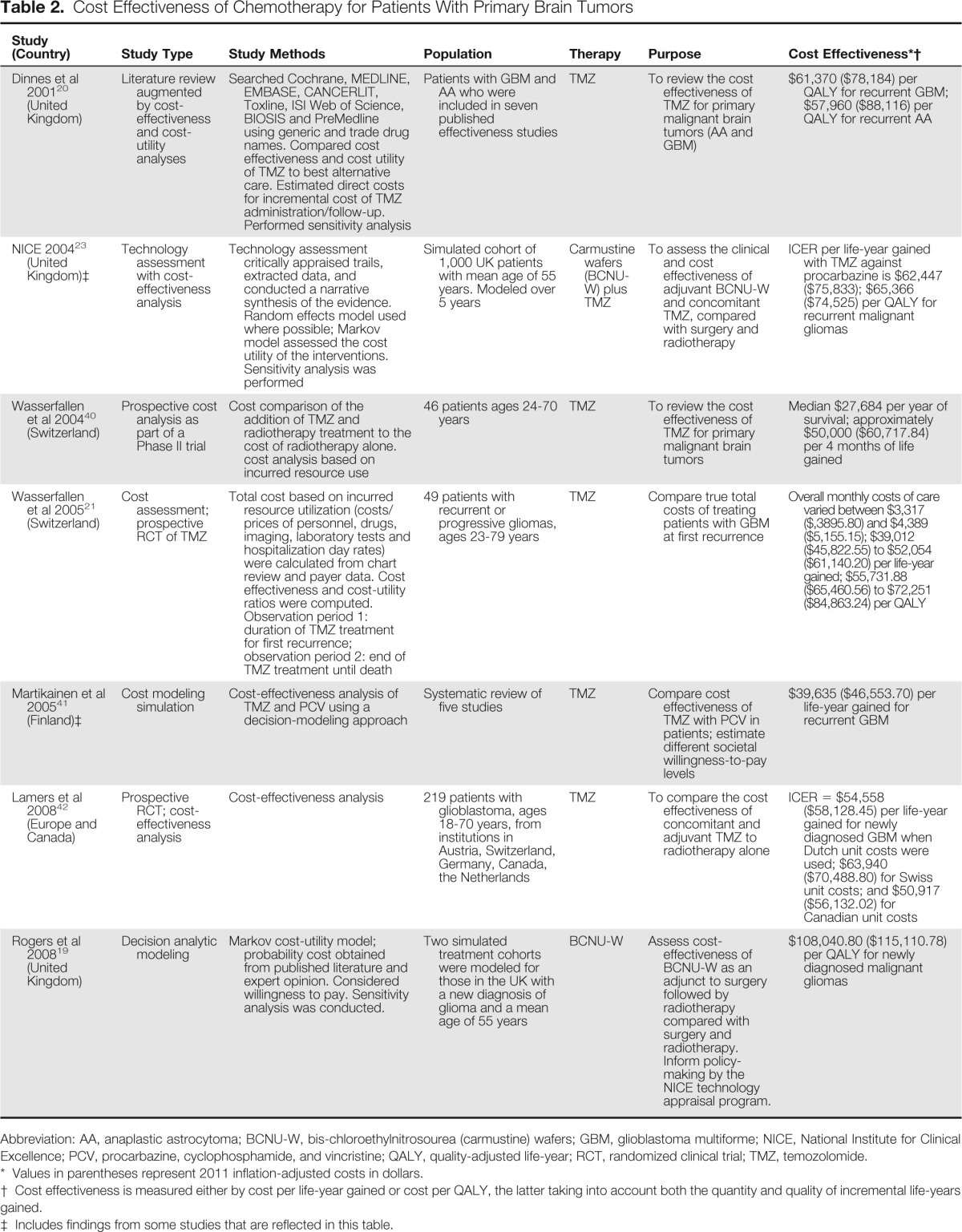
| Study (Country) | Study Type | Study Methods | Population | Therapy | Purpose | Cost Effectiveness*† |
|---|---|---|---|---|---|---|
| Dinnes et al 200120 (United Kingdom) | Literature review augmented by cost-effectiveness and cost-utility analyses | Searched Cochrane, MEDLINE, EMBASE, CANCERLIT, Toxline, ISI Web of Science, BIOSIS and PreMedline using generic and trade drug names. Compared cost effectiveness and cost utility of TMZ to best alternative care. Estimated direct costs for incremental cost of TMZ administration/follow-up. Performed sensitivity analysis | Patients with GBM and AA who were included in seven published effectiveness studies | TMZ | To review the cost effectiveness of TMZ for primary malignant brain tumors (AA and GBM) | $61,370 ($78,184) per QALY for recurrent GBM; $57,960 ($88,116) per QALY for recurrent AA |
| NICE 200423 (United Kingdom)‡ | Technology assessment with cost-effectiveness analysis | Technology assessment critically appraised trails, extracted data, and conducted a narrative synthesis of the evidence. Random effects model used where possible; Markov model assessed the cost utility of the interventions. Sensitivity analysis was performed | Simulated cohort of 1,000 UK patients with mean age of 55 years. Modeled over 5 years | Carmustine wafers (BCNU-W) plus TMZ | To assess the clinical and cost effectiveness of adjuvant BCNU-W and concomitant TMZ, compared with surgery and radiotherapy | ICER per life-year gained with TMZ against procarbazine is $62,447 ($75,833); $65,366 ($74,525) per QALY for recurrent malignant gliomas |
| Wasserfallen et al 200440 (Switzerland) | Prospective cost analysis as part of a Phase II trial | Cost comparison of the addition of TMZ and radiotherapy treatment to the cost of radiotherapy alone. cost analysis based on incurred resource use | 46 patients ages 24-70 years | TMZ | To review the cost effectiveness of TMZ for primary malignant brain tumors | Median $27,684 per year of survival; approximately $50,000 ($60,717.84) per 4 months of life gained |
| Wasserfallen et al 200521 (Switzerland) | Cost assessment; prospective RCT of TMZ | Total cost based on incurred resource utilization (costs/prices of personnel, drugs, imaging, laboratory tests and hospitalization day rates) were calculated from chart review and payer data. Cost effectiveness and cost-utility ratios were computed. Observation period 1: duration of TMZ treatment for first recurrence; observation period 2: end of TMZ treatment until death | 49 patients with recurrent or progressive gliomas, ages 23-79 years | TMZ | Compare true total costs of treating patients with GBM at first recurrence | Overall monthly costs of care varied between $3,317 ($,3895.80) and $4,389 ($5,155.15); $39,012 ($45,822.55) to $52,054 ($61,140.20) per life-year gained; $55,731.88 ($65,460.56) to $72,251 ($84,863.24) per QALY |
| Martikainen et al 200541 (Finland)‡ | Cost modeling simulation | Cost-effectiveness analysis of TMZ and PCV using a decision-modeling approach | Systematic review of five studies | TMZ | Compare cost effectiveness of TMZ with PCV in patients; estimate different societal willingness-to-pay levels | $39,635 ($46,553.70) per life-year gained for recurrent GBM |
| Lamers et al 200842 (Europe and Canada) | Prospective RCT; cost-effectiveness analysis | Cost-effectiveness analysis | 219 patients with glioblastoma, ages 18-70 years, from institutions in Austria, Switzerland, Germany, Canada, the Netherlands | TMZ | To compare the cost effectiveness of concomitant and adjuvant TMZ to radiotherapy alone | ICER = $54,558 ($58,128.45) per life-year gained for newly diagnosed GBM when Dutch unit costs were used; $63,940 ($70,488.80) for Swiss unit costs; and $50,917 ($56,132.02) for Canadian unit costs |
| Rogers et al 200819 (United Kingdom) | Decision analytic modeling | Markov cost-utility model; probability cost obtained from published literature and expert opinion. Considered willingness to pay. Sensitivity analysis was conducted. | Two simulated treatment cohorts were modeled for those in the UK with a new diagnosis of glioma and a mean age of 55 years | BCNU-W | Assess cost-effectiveness of BCNU-W as an adjunct to surgery followed by radiotherapy compared with surgery and radiotherapy. Inform policy-making by the NICE technology appraisal program. | $108,040.80 ($115,110.78) per QALY for newly diagnosed malignant gliomas |
Abbreviation: AA, anaplastic astrocytoma; BCNU-W, bis-chloroethylnitrosourea (carmustine) wafers; GBM, glioblastoma multiforme; NICE, National Institute for Clinical Excellence; PCV, procarbazine, cyclophosphamide, and vincristine; QALY, quality-adjusted life-year; RCT, randomized clinical trial; TMZ, temozolomide.
Values in parentheses represent 2011 inflation-adjusted costs in dollars.
Cost effectiveness is measured either by cost per life-year gained or cost per QALY, the latter taking into account both the quantity and quality of incremental life-years gained.
Includes findings from some studies that are reflected in this table.
Wasserfallen et al found mean treatment costs for 49 patients treated with TMZ for first or second relapse after RT to be $31,274, accounting for an average of 61% of the total costs of care, with the acquisition cost of TMZ being 76% of the total drug cost.20 The mean cost associated with first relapse was $8,131, and the total cost of TMZ treatment (mean of 5.4 cycles) was $17,847. From the completion of TMZ treatment to relapse, progression, or death, the average cost per month was $4,389. Median post-treatment survival was 3.6 months, and mean cost per patient was $15,804. After relapse, 13 patients started continuous TMZ (42 days on and 14 days off) for a cost of $4,696. In seven patients, TMZ was continued with other drugs for a cost of $5,942, and in six patients TMZ was discontinued and other drugs were given, for a cost of $5,114. Mean survival in patients who received continuous TMZ alone (12.6 months) or TMZ with other drugs (12.0 months) was longer than for patients who received no treatment (8.2 months) or other drugs only (6.7 months). Because continuous TMZ was administered to patients with better prognoses, these patients had longer survival. On the basis of a 95% CI and the calculation methods used by the authors, the average cost per life-year gained ranged from $39,012 (2011 inflation-adjusted cost: $45,822.55) to $52,054 ($61,140.20). The cost per QALY ranged from $55,731.88 ($65,460.56) to $72,251 ($84,863.24). The authors felt these were acceptable costs even though they exceed the generally accepted cost-effectiveness threshold20 (Table 2). Wasserfeld et al also reported that use of TMZ as concomitant and adjuvant treatment until disease recurrence represents an eight-fold increase in cost compared with standard RT alone.
In Canada, Mabasa et al compared cost of drug therapy (lomustine or TMZ) in patients with a diagnosis of AA and GBM who experienced a first relapse and had not received other drugs as part of a regimen or as adjuvant therapy.21 (Table 1) On the basis of TMZ's higher cost and lack of additional clinical benefit, lomustine was found to be a more cost-effective treatment strategy. However, the authors noted that in British Columbia, TMZ was the treatment of choice for recurrent malignant gliomas because of its clinically perceived benefit relative to the health-related quality of life of those patients and its safety record.21
On the basis of the cost-effectiveness analysis of these and other similar studies, the National Institute for Clinical Excellence (NICE) in the United Kingdom recommended TMZ treatment consideration for patients for whom first-line chemotherapy with other agents had failed and whose life expectancy exceeded 12 weeks at treatment initiation. The NICE guidance estimates the cost per progression-free week to be $1,955 for GBM and $1,368 for AA.22 The cost per life-year gained was estimated at $78,185 for both GBM and AA.3
Discussion
The available data suggest that cost of care is significant in the treatment of patients with malignant gliomas. Both carmustine wafers and TMZ have contributed substantially to the increased per-patient costs for brain tumor treatment, and further increase is anticipated with bevacizumab approval. For newly diagnosed GBM, new extended-dose trials for TMZ showed a benefit over procarbazine, but insufficient to receive approval in the United States.23–25 Preliminary data highlight potential survival benefits in a subset of glioblastoma patients with methylated methylguanine-methyltransferase (MGMT) gene promoters26; the MGMT repair enzyme has the ability to revert the DNA damage induced by alkylating agents like TMZ, thus silencing of the gene increases the effectiveness of TMZ therapy.
In 2009, the FDA granted accelerated approval for bevacizumab as monotherapy for glioblastoma with progressive disease after prior therapy.27–29 Administration of bevacizumab in combination with irinotecan or other chemotherapeutic agents for recurrent GBM has resulted in monthly cost far greater than that of TMZ. For patients treated with bevacizumab alone on a 10 mg/kg dose biweekly, the cost is approximately $10,000 to $20,000 per month (as calculated by Epocrates; Athenahealth, Princeton, NJ) or $120,000 to $240,000 per year for a patient weighing 70 kg, excluding other combination chemotherapies, other associated costs of infusion, loss of work, and other indirect costs such as parking. Patients can receive this drug for up to 12 to 24 months, with resultant increase in health-related costs.30 Continued use of bevacizumab with RT and TMZ, despite lack of current efficacy data, contributes additional costs.
In March 2009, intravenous TMZ received approval from the FDA and European Commission for the same indications as those of its oral formulation, and in April 2009, the Centers for Medicare and Medicaid Services granted a preliminary Healthcare Common Procedure Coding System code for TMZ 1 mg injection.31 The costs for patients treated with intravenous TMZ might be similar to those of bevacizumab, especially if given in combination with RT, but as noted could have a higher coverage rate than oral TMZ as a result of incidental costs noted above. In light of findings from the ASCO reports of AVAglio and RTOG Study I, it is unlikely that bevacizumab will be used for upfront treatment of GBM, but it may continue to be used at progression only.32 Intravenous TMZ use will likely be restricted to a select group of patients, such as those unable to swallow the capsules.
Economic analyses provide useful information for decision making; the cost-effectiveness ratio provides a measure of the efficiency of each intervention being considered in producing an additional QALY, thereby allowing for comparison of alternative approaches or therapies. Economists tend to agree that indirect cost of illness is essential to consider when addressing cost of malignant brain tumors. However, economic analytic methods have not yet been perfected, particularly with regard to how certain indirect costs should be categorized and calculated. The US Public Health Service (PHS) has articulated a standard method for undertaking cost-effectiveness analysis, recommending calculation of the incremental cost-effectiveness ratio (ICER) and use QALY, which reflects both quantity and quality of life.32a,32b The Public Health Service Task Force also recommends that one consider how an intervention affects all costs relative to a disease, including costs that patients incur and overall health care expenditures.33 Lost wages and productivity are implicitly included in this perspective. However, Nyman34 identifies issues in the construction of QALYs and between the measurement of productivity costs and the societal perspective.
Concerns exist among economists about including a lost productivity measure in an ICER calculation because the measure may appear in both the numerator and denominator. Patients may include a value for their lost productivity when valuing their quality of life (denominator), and the economist may include a lost productivity measure in the cost (numerator). Productivity loss measures are further complicated by the availability of two accepted calculation methods: human capital and friction capital. These methods are likely to produce different valuation because the former considers lost earnings of the worker from the date of diagnosis to potential date of retirement; the latter assumes that the position will be filled in a certain period of time and that productivity will return to normal over a period of time, resulting in lower costs. 35,36
Limitations
The cost data were derived from a variety of sources, countries, and health care systems, making comparisons and generalizability difficult. The studies also presented costs that may have been based on charges, which, as Finkler notes, do not necessarily reflect true economic costs.37
Summary
As treatment paradigms evolve for gliomas, the direct costs related to treating brain tumors become more outdated. Estimates of the economics of brain tumors in the current era are needed. These studies should include detailed assessments of direct and indirect costs, including empirical data on out-of-pocket expenses. There is a need for an updated comprehensive study of the costs borne by patients with glioma, using a design that is specifically geared toward estimating these costs. Several authors have explored the variation in the methodologies used in cost of illness studies and the benefits and limitations of these methods.43–45 A comprehensive longitudinal study, assessing the economic costs for patients with malignant gliomas, is needed.
Acknowledgment
Supported by National Cancer Institute Grant No. 5K01CA134554-05 (J.M.M.).
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Jeffrey Raizer, Genentech (C) Stock Ownership: None Honoraria: None Research Funding: Jeffrey Raizer, Genentech Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
Author Contributions
Conception and design: Jeffrey J. Raizer, Karen F. Fitzner, Charles Bennett, Sean A. Grimm, June M. McKoy
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012. www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf. [Google Scholar]
- 2.Huse JT, Holland EC. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10:319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Central Brain Tumor Registry of the United States. 2011. [Accessed June 18, 2014]. www.cbtrus.org/reports/reports.html.
- 5.Adamson C, Kanu OO, Mehta AI, et al. Glioblastoma multiforme: A review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–1083. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 6.Chang S, Long SR, Kutikova L, et al. Estimating the cost of cancer: Results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J Clin Oncol. 2004;22:3524–3530. doi: 10.1200/JCO.2004.10.170. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009 May;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 8.Crott R. The economics of temozolomide in brain cancer. Expert Opin Pharmacother. 2007;8:1923–1929. doi: 10.1517/14656566.8.12.1923. [DOI] [PubMed] [Google Scholar]
- 9.Uyl-de Groot CA, Stupp R, van der Bent M. Cost-effectiveness of temozolomide for the treatment of newly diagnosed glioblastoma multiforme. Expert Rev Pharmacoecon Outcomes Res. 2009;9:235–241. doi: 10.1586/erp.09.15. [DOI] [PubMed] [Google Scholar]
- 10.Garside R, Pitt M, Anderson R, et al. The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: A systematic review and economic evaluation. Health Technol Assess. 2007;11:iii–iv. ix–221. doi: 10.3310/hta11450. [DOI] [PubMed] [Google Scholar]
- 11.The CONSORT Group. CONSORT 2010 Checklist. Checklist of information to include when reporting randomized trial. www.consort-statement.org/resources/downloads.
- 12.Silverstein MD, Cascino TL, Harmsen WS. High-grade astrocytomas: Resource use, clinical outcomes, and cost of care. Mayo Clin Proc. 1996;71:936–944. doi: 10.1016/S0025-6196(11)63766-X. [DOI] [PubMed] [Google Scholar]
- 13.Polinsky MN, Geer CP, Ross DA. Stereotaxy reduces cost of brain tumor resection. Surg Neurol. 1997;48:542–550. doi: 10.1016/s0090-3019(97)00365-0. discussion 550-551. [DOI] [PubMed] [Google Scholar]
- 14.Mendez I, Jacobs P, MacDougall A, et al. Treatment costs for glioblastoma multiforme in Nova Scotia. Can J Neurol Sci. 2001;28:61–65. doi: 10.1017/s0317167100052574. [DOI] [PubMed] [Google Scholar]
- 15.Latif AZ, Signorini D, Gregor A, et al. The costs of managing patients with malignant glioma at a neuro-oncology clinic. Br J Neurosurg. 1998;12:118–122. doi: 10.1080/02688699845230. [DOI] [PubMed] [Google Scholar]
- 16.Blomqvist P, Lycke J, Strang P, et al. Brain tumours in Sweden 1996: Care and costs. J Neurol Neurosurg Psychiatry. 2000;69:792–798. doi: 10.1136/jnnp.69.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johannesen TB, Norum J, Lote K, et al. A cost-minimising analysis of standard radiotherapy and two experimental therapies in glioblastoma. Radiother Oncol. 2002;62:227–231. doi: 10.1016/s0167-8140(01)00495-9. [DOI] [PubMed] [Google Scholar]
- 18.Patterson H. Nobody can afford a brain tumor. The financial impacts of brain tumors on patients and families: A summary of findings: National Brain Tumor Foundation. 2007. www.braintumor.org/./Nobody_Can_Afford_a_Brain_Tumor.pdf.
- 19.Rogers G, Garside R, Mealing S, et al. Carmustine implants for the treatment of newly diagnosed high-grade gliomas: A cost-utility analysis. Pharmacoeconomics. 2008;26:33–44. doi: 10.2165/00019053-200826010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Dinnes J, Cave C, Huang S, et al. The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: A rapid and systematic review. Health Technol Assess. 2001;5:1–73. doi: 10.3310/hta5130. [DOI] [PubMed] [Google Scholar]
- 21.Wasserfallen JB, Ostermann S, Leyvraz S, et al. Cost of temozolomide therapy and global care for recurrent malignant gliomas followed until death. Neuro-oncol. 2005;7:189–195. doi: 10.1215/S1152851704000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mabasa VH, Taylor SC. Re-evaluation of the cost effectiveness of temozolomide for malignant gliomas in British Columbia. J Oncol Pharm Pract. 2006;12:105–111. doi: 10.1177/1078155206069161. [DOI] [PubMed] [Google Scholar]
- 23.National Institute for Clinical Excellence. Guidance on the use of temozolomide for the treatment of recurrent malignant glioma (brain cancer) www.nice.org.uk/guidance/TA23/?c=91496.
- 24.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan RB, Raizer JJ, Malkin MG, et al. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro Oncol. 2002;4:39–43. doi: 10.1215/15228517-4-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry J, Mason W, Belanger K, et al. The temozolomide RESCUE study: A phase II trial of continuous (28/28) dose-intense temolozomide (TMZ) after progression on conventional 5/28 day TMS in patients with recurrent malignant glioma. J Clin Oncol. 2008;(suppl):26. abstr 2010. [Google Scholar]
- 27.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: A pilot study. J Clin Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 29.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 30.Kreisl TN. Chemotherapy for malignant gliomas. Semin Radiat Oncol. 2009;19:150–154. doi: 10.1016/j.semradonc.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 31. Reference deleted.
- 32.Gilbert MR. First-line bevacizumab in combination with standard of care not recommended for newly diagnosed glioblastoma. ASCO Daily News. Virtual Meeting Clip. 2013. Jun 14, http://am.asco.org/first-line-bevacizumab-combination-standard-care-not-recommended-newly-diagnosed-glioblastoma.
- 32a.Gold MR, Siegel JE, Russell LB, et al. New York, NY: Oxford University Press; 1996. Cost-Effectiveness in Health and Medicine: Report of the Panel on Cost-Effectiveness in Health and Medicine. [Google Scholar]
- 32b.Torrance GW, Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care. 1989;5:559–575. doi: 10.1017/s0266462300008461. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Medicare and Medicaid Services. Bridging the coverage gap. 2006. www.cms.gov/PrescriptionDrugCovGenIn/01a_bridgingthegap.asp#TopOfPage.
- 34.United States Department of Veterans Affairs. Introduction to cost-effectiveness analysis (CEA) United States. www.herc.research.va.gov/methods/cea.asp.
- 35.Nyman JA. Productivity costs revisited: Toward a new US policy. Health Econ. 2012;21:1387–1401. [Google Scholar]
- 36.Berger ML, Murray JF, Xu J, et al. Health and Productivity Management Center: Alternative valuations of work loss and productivity. www.acoem.org/Page3Column.aspx?PageID=7351&id=1340. [DOI] [PubMed]
- 37.Finkler SA. The distinction between cost and charges. Ann Intern Med. 1982;96:102–109. doi: 10.7326/0003-4819-96-1-102. [DOI] [PubMed] [Google Scholar]
- 38.Mehta M, Noyes W, Craig B, et al. A cost-effectiveness and cost-utility analysis of radiosurgery vs. resection for single-brain metastases. Int J Radiat Oncol Biol Phys. 1997;39:445–454. doi: 10.1016/s0360-3016(97)00071-0. [DOI] [PubMed] [Google Scholar]
- 39.Kutikova L, Bowman L, Chang S, et al. Utilization and cost of health care services associated with primary malignant brain tumors in the United States. J Neurooncol. 2007;81:61–65. doi: 10.1007/s11060-006-9197-y. [DOI] [PubMed] [Google Scholar]
- 40.Wasserfallen JB, Ostermann S, Pica A, et al. Can we afford to add chemotherapy to radiotherapy for glioblastoma multiforme? Cost-identification analysis of concomitant and adjuvant treatment with temozolomide until patient death. Cancer. 2004;101:2098–2105. doi: 10.1002/cncr.20619. [DOI] [PubMed] [Google Scholar]
- 41.Martikainen JA, Kivioja A, Hallinen T, et al. Economic evaluation of temozolomide in the treatment of recurrent glioblastoma multiforme. Pharmacoeconomics. 2005;23:803–815. doi: 10.2165/00019053-200523080-00006. [DOI] [PubMed] [Google Scholar]
- 42.Lamers LM, Stupp R, van den Bent MJ, et al. Cost-effectiveness of temozolomide for the treatment of newly diagnosed glioblastoma multiforme: A report from the EORTC 26981/22981 NCI-C CE3 Intergroup Study. Cancer. 2008;112:1337–1344. doi: 10.1002/cncr.23297. [DOI] [PubMed] [Google Scholar]
- 43.Akobundu E, Ju J, Blatt L, et al. Cost-of-illness studies: A review of current methods. Pharmacoeconomics. 2006;24:869–890. doi: 10.2165/00019053-200624090-00005. [DOI] [PubMed] [Google Scholar]
- 44.Clabaugh G, Ward MM. Cost-of-illness studies in the United States: A systematic review of methodologies used for direct cost. Value Health. 2008;11:13–21. doi: 10.1111/j.1524-4733.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 45.The National Board of Health and Welfare, Socialstyrelsen. Centre for Epidemiology Statistics–Health and Diseases: Cancer incidence in Sweden 1997. Chapter 6. Table B: Selected population data for Sweden 1997, by sex. Chapter 7. The number of persons with cancer diagnosed for the first time in 1997. www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/12255/1999-42-8_9942008.pdf.


