A VTB program positively affected the process of care for patients with HCC by improving the quality and timeliness of the multidisciplinary evaluation process.
Abstract
Purpose:
Multidisciplinary evaluation (MDE) of hepatocellular cancer (HCC) is the current standard, often provided through a tumor board (TB) forum; this standard is limited by oncology workforce shortages and lack of a TB at every institution. Virtual TBs (VTBs) may help overcome these limitations. Our study aim was to assess the impact of a regional VTB on the MDE process for patients with HCC.
Methods:
A retrospective cohort study was conducted, including patients with HCC referred to a tertiary cancer center from regional facilities (2009 to 2013). Baseline characteristics and outcomes were compared based on the referral mechanism: VTB versus subspecialty consultation (non-VTB). The primary outcome was comprehensive MDE (all required specialists present and key topics discussed). Secondary outcomes included timeliness of MDE and travel burden to complete MDE. Univariable and multivariable logistic regressions were performed to examine the association of a VTB with comprehensive MDE.
Results:
A total of 116 patients were included in the study; 48 (41.4%) were evaluated through the VTB. A higher proportion of VTB patients received comprehensive MDE (91.7% v 64.7%; P = .001); the VTB was independently associated with higher odds of accomplishing comprehensive MDE (odds ratio, 6.0; 95% CI, 1.2 to 29.9; P = .02). VTB patients completed MDE significantly faster (median, 23 v 39 days; P < .001), with lower travel burden (median, 0 v 683 miles traveled; P < .001).
Conclusion:
This VTB program positively affected the process of care for patients with HCC by improving the quality and timeliness of the MDE process, while avoiding the burden arising from travel needs. Future studies should focus on implementation of VTB programs on a wider scale.
Introduction
Hepatocellular carcinoma (HCC) is a global health problem, and its incidence and death rate continue to steadily increase.1–3 The prognosis for HCC is determined by the interaction between tumor-related factors and underlying liver disease4–6; treatment for HCC is therefore complex, with competing factors often precluding the ability to provide the best treatment and significant variability in outcomes resulting from inefficient health care delivery and substandard care.7 Evidence-based guidelines recommend the use of a multidisciplinary approach, with a minimum set of specialists, as the standard of care. Tumor boards (TBs) have emerged as a multidisciplinary forum targeted at providing this standard4,8 and have been shown to be associated with improved satisfaction, outcome, and survival of patients with HCC.9–13
Effective multidisciplinary evaluation (MDE) through a TB is dependent on local infrastructure and oncology workforce, which are not available at every institution.14 Through telemedicine applications, virtual TBs (VTBs) are a potential strategy for increasing access to specialized cancer care services at underserved facilities and facilitating appropriate guideline-driven MDE and care.15,16 We previously developed a regional VTB program within the Department of Veterans Affairs (VA) health care system to meet the specialized oncology needs of patients with cancer treated at distant sites and reported favorable results after the pilot phase of implementation.15,17 The purpose of this study was to evaluate the impact of a regional VTB program on the process of care for HCC by examining the MDE process for patients referred to specialized care from distant VA facilities.
Methods
Study Design
This was a retrospective cohort study designed to evaluate the impact of a VTB program within a regional Veterans Integrated Service Network (VISN) on the quality of care for patients with HCC. The study was approved by the Baylor College of Medicine Institutional Review Board and the Michael E. DeBakey VA Medical Center (MEDVAMC) Research and Development Committee.
Setting
A regional VTB program was implemented in September 2011 between MEDVAMC (referral institution) and nine other VA Medical Centers (VAMCs; referring institutions) within the South Central VA Health Care Network (VISN 16). VISN 16 serves more than 1.8 million veterans in an eight-state region including Florida, Alabama, Mississippi, Louisiana, Arkansas, Missouri, Oklahoma, and Texas and comprises 10 VAMCs, with heterogeneous hospital and workforce resources for cancer care. Additionally, VISN 16 encompasses the highest distribution of rural and highly rural veterans within the system (> 50%), representing the ideal environment to implement an intervention focused on improving access to care. MEDVAMC is an American College of Surgeons Commission on Cancer–accredited tertiary cancer center located in Houston, Texas, and serves as a regional and national referral center for the management of complex cancers. MEDVAMC provides highly specialized cancer services for HCC, ranging from chemotherapy and radiation therapy to interventional radiology–based therapies and complex cancer surgery (including liver resection and transplantation). An HCC-specific TB (HCC-TB) conference takes place on a weekly basis; it has standardized protocols and is staffed by the full spectrum of cancer care providers required for HCC care, including radiology, gastroenterology and hepatology, transplantation, surgical oncology (with hepatobiliary expertise), medical oncology, interventional radiology, and ancillary support staff, such as social workers, case managers, and cancer center personnel. Details regarding VTB program implementation have been previously reported.15,17 In short, the VTB program was established as a regional cancer care model for VAMCs within VISN 16. It uses the VA electronic medical record (EMR) and audiovisual teleconferencing technology to support real-time interaction between referring physicians at distant institutions and the HCC-TB forum at MEDVAMC. Patient referral processes and structure and function of the HCC-TB were standardized and disseminated regionally using a validated TB implementation framework.18
Participants and Data Collection
Patients referred to MEDVAMC from any of the nine VAMCs (referring institutions) within the VISN 16 region with an established diagnosis of HCC, confirmed by imaging (characteristic features on magnetic resonance imaging and/or computed tomography scan)19 and/or biopsy, were eligible for study inclusion (2009 to 2013). Local MEDVAMC patients with HCC were excluded. Hospital and oncology workforce resources for HCC care remained stable during the study period. Referral mechanisms to MEDVAMC included two consult types, chosen based on availability of the VTB and at the discretion of the referring physicians at distant sites: direct interfacility specialty consultation to a variety of MEDVAMC specialty-based clinics, and interfacility VTB consultation request.
A prospectively maintained database was used to identify eligible patients, and additional information was collected retrospectively from the VA EMR by a trained abstractor using pre-established algorithms. Information collected included patient demographics, clinical characteristics, geographic residence (classified as urban, rural, or highly rural according to VA system based on census block population density),20 and details regarding type, quality, and time to MDE and treatment.
Measures and Outcomes
The primary outcome of interest was comprehensive MDE, defined as a composite outcome that comprised guideline-driven MDE in addition to a formal assessment of transplantation eligibility and tumor stage. Guideline-driven MDE was defined as clinical evaluation at MEDVAMC before the initiation of treatment by a team that at minimum was composed of the following clinical specialties4: radiology, hepatology, surgical oncology, interventional radiology, medical oncology, and transplantation. Formal assessment of tumor stage and transplantation eligibility were defined based on documentation of such assessment in any of the corresponding notes from specialty-based consultation reports and/or HCC-TB notes and/or VTB-based HCC-TB notes.
Secondary outcomes included timeliness of the cancer care process and patient and health care system burdens in completing guideline-driven MDE. Timeliness of the cancer care process was measured in days and included the following: time from referral to MDE completion and time from referral to treatment initiation. Patient and health care system burdens were examined by evaluating travel needs, measured by calculating the miles traveled to accomplish guideline-driven MDE using an online tool (www.zipcodes.com) to estimate the distance between the patient's residential zip code and the zip code of the evaluation site (MEDVAMC, Houston, TX).
Statistical Analysis
To evaluate the impact of the VTB program, patients were categorized by consult type (VTB v non-VTB). Referral volume was modeled as a binary variable based on the median number of HCC referrals to MEDVAMC across all referring VAMCs. The high-volume category represents VAMCs that referred ≥ nine patients with HCC to MEDVAMC during the study period. Comorbidity was described using the Charlson comorbidity index (CCI), with patients categorized into two groups (0 to 2 v ≥ 3). Malignancy was excluded in the CCI, given that this factor was present in all patients. Descriptive analyses were performed for the entire study population and compared by consult type. Baseline characteristics were compared using the t test for normally distributed variables, χ2 test for dichotomous or categorical variables, and Mann-Whitney U and Kruskal-Wallis tests for comparison of nonparametric variables. Stepwise multivariable logistic regression models were used to examine the association between VTB and comprehensive MDE, while adjusting for baseline clinical and tumor characteristics. Criteria for inclusion in the multivariable model were clinically and statistically driven (P value < .250 in univariable models of primary outcome). Miles traveled for MDE could not be adjusted for, because of collinearity between this variable and VTB evaluation. P values less than .05 were considered statistically significant. All statistical analyses were performed using the STATA statistical software package (version 12; StataCorp, College Station, TX).
Results
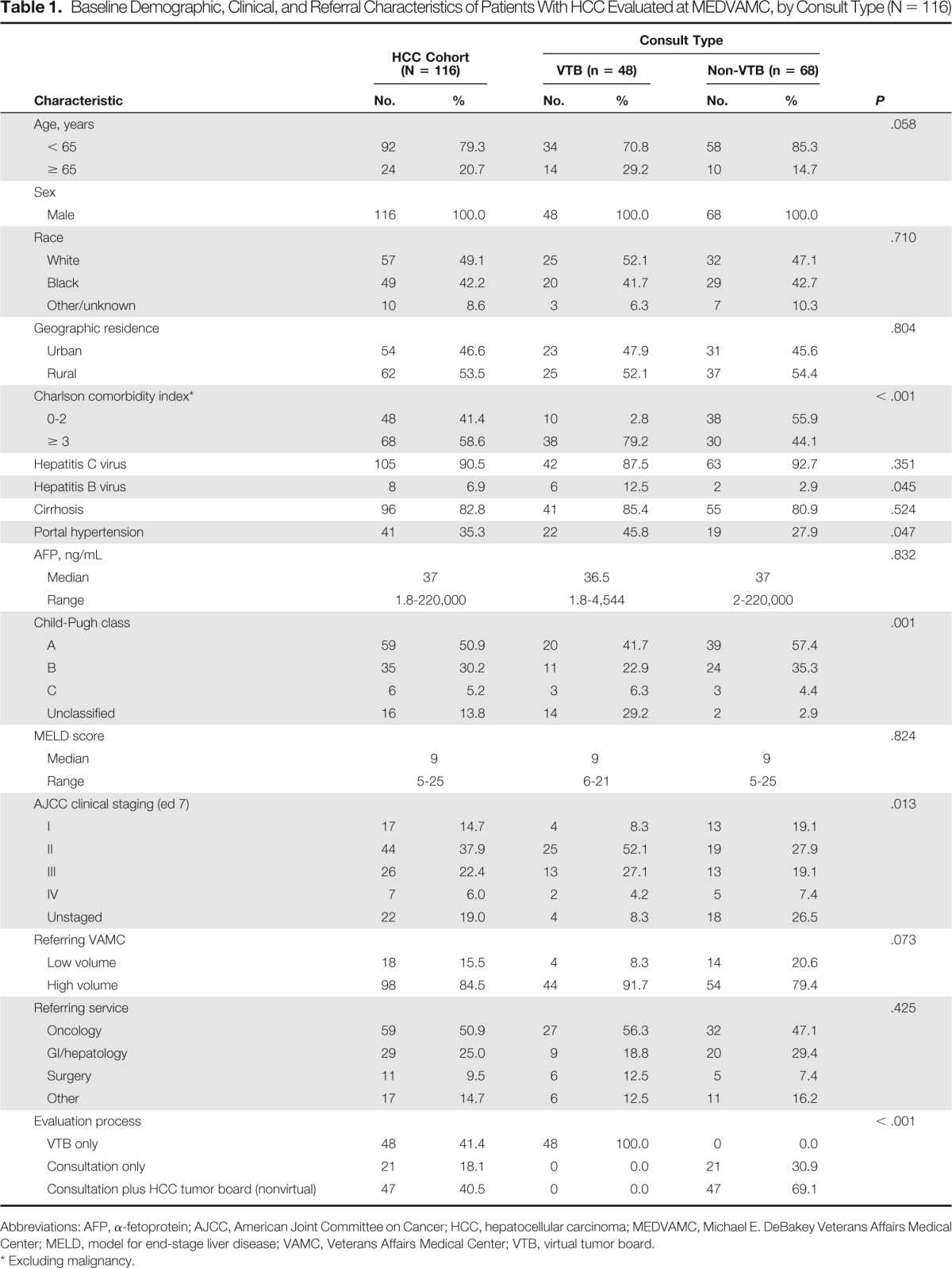
A total of 116 patients were referred to MEDVAMC and were included in the study; 48 were evaluated through the VTB program (41%), and the remaining 68 were evaluated through non-VTB interfacility consultation. Baseline sociodemographic and clinical characteristics, as well as information regarding the referral process, are listed and compared by consult type in Table 1. Notably when compared with those referred to MEDVAMC through interfacility specialty consultation, those referred via the VTB program were older (age ≥ 65 years, 29.2% v 14.7%; P = .05), had a higher degree of comorbidity (CCI ≥ 3, 79.2% v 44.1%; P < .001), and were more likely to have portal hypertension (45.8% v 27.9%; P = .04).
Table 1.
Baseline Demographic, Clinical, and Referral Characteristics of Patients With HCC Evaluated at MEDVAMC, by Consult Type (N = 116)
| Characteristic | HCC Cohort (N = 116) |
Consult Type |
P | ||||
|---|---|---|---|---|---|---|---|
| VTB (n = 48) |
Non-VTB (n = 68) |
||||||
| No. | % | No. | % | No. | % | ||
| Age, years | .058 | ||||||
| < 65 | 92 | 79.3 | 34 | 70.8 | 58 | 85.3 | |
| ≥ 65 | 24 | 20.7 | 14 | 29.2 | 10 | 14.7 | |
| Sex | |||||||
| Male | 116 | 100.0 | 48 | 100.0 | 68 | 100.0 | |
| Race | .710 | ||||||
| White | 57 | 49.1 | 25 | 52.1 | 32 | 47.1 | |
| Black | 49 | 42.2 | 20 | 41.7 | 29 | 42.7 | |
| Other/unknown | 10 | 8.6 | 3 | 6.3 | 7 | 10.3 | |
| Geographic residence | .804 | ||||||
| Urban | 54 | 46.6 | 23 | 47.9 | 31 | 45.6 | |
| Rural | 62 | 53.5 | 25 | 52.1 | 37 | 54.4 | |
| Charlson comorbidity index* | < .001 | ||||||
| 0-2 | 48 | 41.4 | 10 | 2.8 | 38 | 55.9 | |
| ≥ 3 | 68 | 58.6 | 38 | 79.2 | 30 | 44.1 | |
| Hepatitis C virus | 105 | 90.5 | 42 | 87.5 | 63 | 92.7 | .351 |
| Hepatitis B virus | 8 | 6.9 | 6 | 12.5 | 2 | 2.9 | .045 |
| Cirrhosis | 96 | 82.8 | 41 | 85.4 | 55 | 80.9 | .524 |
| Portal hypertension | 41 | 35.3 | 22 | 45.8 | 19 | 27.9 | .047 |
| AFP, ng/mL | .832 | ||||||
| Median | 37 | 36.5 | 37 | ||||
| Range | 1.8-220,000 | 1.8-4,544 | 2-220,000 | ||||
| Child-Pugh class | .001 | ||||||
| A | 59 | 50.9 | 20 | 41.7 | 39 | 57.4 | |
| B | 35 | 30.2 | 11 | 22.9 | 24 | 35.3 | |
| C | 6 | 5.2 | 3 | 6.3 | 3 | 4.4 | |
| Unclassified | 16 | 13.8 | 14 | 29.2 | 2 | 2.9 | |
| MELD score | .824 | ||||||
| Median | 9 | 9 | 9 | ||||
| Range | 5-25 | 6-21 | 5-25 | ||||
| AJCC clinical staging (ed 7) | .013 | ||||||
| I | 17 | 14.7 | 4 | 8.3 | 13 | 19.1 | |
| II | 44 | 37.9 | 25 | 52.1 | 19 | 27.9 | |
| III | 26 | 22.4 | 13 | 27.1 | 13 | 19.1 | |
| IV | 7 | 6.0 | 2 | 4.2 | 5 | 7.4 | |
| Unstaged | 22 | 19.0 | 4 | 8.3 | 18 | 26.5 | |
| Referring VAMC | .073 | ||||||
| Low volume | 18 | 15.5 | 4 | 8.3 | 14 | 20.6 | |
| High volume | 98 | 84.5 | 44 | 91.7 | 54 | 79.4 | |
| Referring service | .425 | ||||||
| Oncology | 59 | 50.9 | 27 | 56.3 | 32 | 47.1 | |
| GI/hepatology | 29 | 25.0 | 9 | 18.8 | 20 | 29.4 | |
| Surgery | 11 | 9.5 | 6 | 12.5 | 5 | 7.4 | |
| Other | 17 | 14.7 | 6 | 12.5 | 11 | 16.2 | |
| Evaluation process | < .001 | ||||||
| VTB only | 48 | 41.4 | 48 | 100.0 | 0 | 0.0 | |
| Consultation only | 21 | 18.1 | 0 | 0.0 | 21 | 30.9 | |
| Consultation plus HCC tumor board (nonvirtual) | 47 | 40.5 | 0 | 0.0 | 47 | 69.1 | |
Abbreviations: AFP, α-fetoprotein; AJCC, American Joint Committee on Cancer; HCC, hepatocellular carcinoma; MEDVAMC, Michael E. DeBakey Veterans Affairs Medical Center; MELD, model for end-stage liver disease; VAMC, Veterans Affairs Medical Center; VTB, virtual tumor board.
Excluding malignancy.
Comprehensive MDE
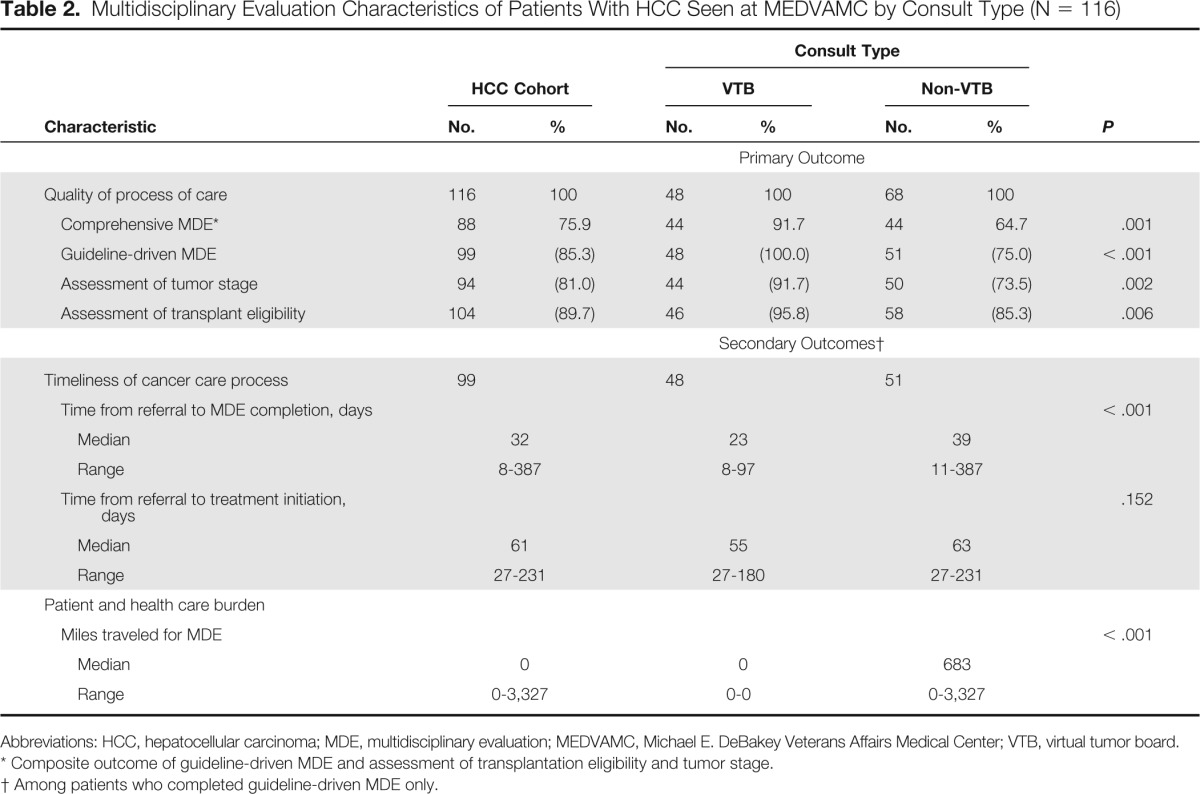
In all, 88 patients (75.9%) completed comprehensive MDE, with a significantly higher proportion in the VTB compared with the non-VTB group (91.7% v 64.7%; P = .001). Similarly, when compared with non-VTB patients, those evaluated through the VTB program had higher rates of guideline-driven MDE (100% v 75%; P < .001) as well as staging (91.7% v 73.5%; P = .002) and transplantation eligibility (95.8% v 85.3%; P = .02) assessment (Table 2).
Table 2.
Multidisciplinary Evaluation Characteristics of Patients With HCC Seen at MEDVAMC by Consult Type (N = 116)
| Characteristic | HCC Cohort |
Consult Type |
P | ||||
|---|---|---|---|---|---|---|---|
| VTB |
Non-VTB |
||||||
| No. | % | No. | % | No. | % | ||
| Primary Outcome | |||||||
| Quality of process of care | 116 | 100 | 48 | 100 | 68 | 100 | |
| Comprehensive MDE* | 88 | 75.9 | 44 | 91.7 | 44 | 64.7 | .001 |
| Guideline-driven MDE | 99 | (85.3) | 48 | (100.0) | 51 | (75.0) | < .001 |
| Assessment of tumor stage | 94 | (81.0) | 44 | (91.7) | 50 | (73.5) | .002 |
| Assessment of transplant eligibility | 104 | (89.7) | 46 | (95.8) | 58 | (85.3) | .006 |
| Secondary Outcomes† | |||||||
| Timeliness of cancer care process | 99 | 48 | 51 | ||||
| Time from referral to MDE completion, days | < .001 | ||||||
| Median | 32 | 23 | 39 | ||||
| Range | 8-387 | 8-97 | 11-387 | ||||
| Time from referral to treatment initiation, days | .152 | ||||||
| Median | 61 | 55 | 63 | ||||
| Range | 27-231 | 27-180 | 27-231 | ||||
| Patient and health care burden | |||||||
| Miles traveled for MDE | < .001 | ||||||
| Median | 0 | 0 | 683 | ||||
| Range | 0-3,327 | 0-0 | 0-3,327 | ||||
Abbreviations: HCC, hepatocellular carcinoma; MDE, multidisciplinary evaluation; MEDVAMC, Michael E. DeBakey Veterans Affairs Medical Center; VTB, virtual tumor board.
Composite outcome of guideline-driven MDE and assessment of transplantation eligibility and tumor stage.
Among patients who completed guideline-driven MDE only.
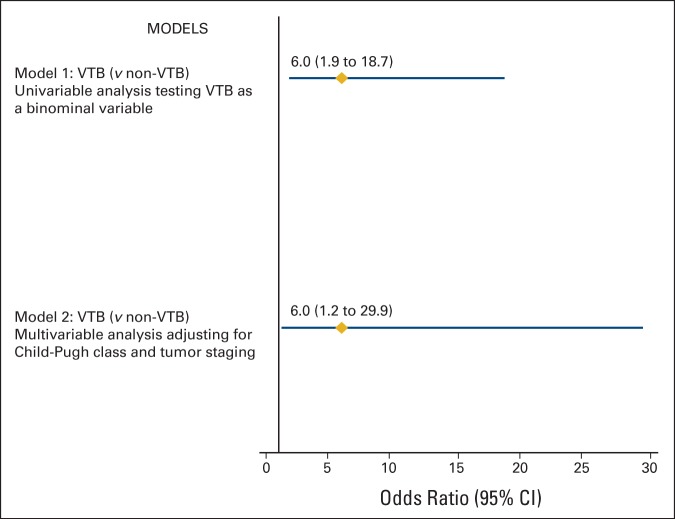
Logistic regression analysis showed that evaluation through the VTB program was associated with significantly greater odds of completing comprehensive MDE in univariable analysis (odds ratio, 6.0; 95% CI, 1.9 to 18.7; P = .001) and after adjusting for important covariates (odds ratio, 6.0; 95% CI, 1.2 to 29.9; P = .02; Figure 1).
Figure 1.
Logistic regression models evaluating association between virtual tumor boards (VTBs) and comprehensive multidisciplinary evaluation (N = 116).
Secondary Outcomes
For patients completing guideline-driven MDE (n = 99), the time from referral to MDE completion was significantly longer for patients evaluated through interfacility consultation when compared with those evaluated through the VTB program (median, 39 [range, 11 to 387] v 23 days [range, 8 to 97]; P < .001). Also, the time from referral to treatment initiation was longer for patients evaluated through interfacility consultation, although this did not reach statistical significance (median, 63 [range, 27 to 231] v 55 days [range, 27 to 180]; P = .152). Finally, when comparing travel burden, patients evaluated through the non-VTB mechanism required a significantly higher number of travel miles to complete guideline-driven MDE than those evaluated by the VTB (median, 683 [range, 0 to 3,327] v 0 miles traveled; P < .001; Table 2).
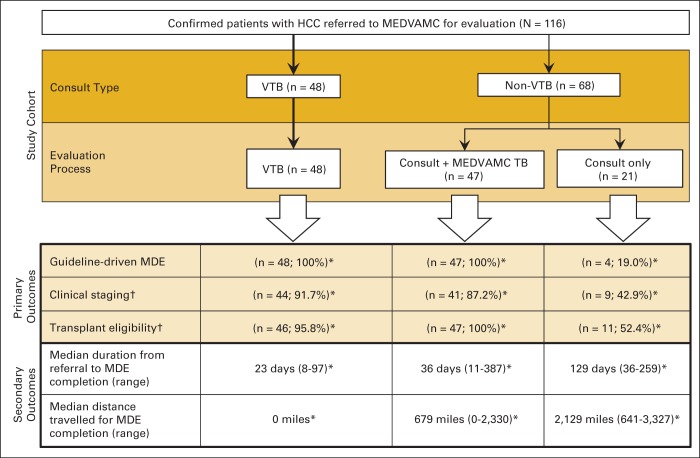
Appendix Figure A1 (online only) is a flowchart of the different trajectories experienced by study patients based on referral mechanism (consult type) and the outcomes experienced by each corresponding group. Interestingly, we observed that among patients referred through interfacility specialty consultation (non-VTB), a subgroup of patients were evaluated by the MEDVAMC HCC-TB after the first specialty consultation took place; this group of patients experienced high rates of comprehensive MDE completion, including guideline-driven MDE and staging and transplantation eligibility assessment, which were in the same range as those observed for patients evaluated through the VTB program. However, despite these similarities in the quality of MDE, differences in the timeliness of MDE process completion and in travel needs persisted, with significantly improved outcomes observed in the VTB group (P < .001).
Discussion
HCC remains a leading cause of cancer mortality in the United States, with incidence rates tripling over the past three decades.1,21 Despite being endorsed as a critical model in the management of HCC,4,8 multidisciplinary-based care is rarely accomplished during the process of care for HCC.6,7 Telemedicine applications, such as the VTB program described in this study, can increase access to comprehensive MDE at institutions lacking the necessary workforce and/or infrastructure to appropriately evaluate patients with complex cancers. The findings from this study are significant, because it specifically examined the impact of a regional VTB program on the MDE process for patients with HCC within an integrated health care system. We found that patients with HCC referred from distant VA facilities who were evaluated through the VTB program experienced improved quality in the process of care, represented by having higher rates of comprehensive MDE completion over a significantly shorter period of time. Furthermore, this standard of care was accomplished with a significant decrease in the burdens to patients and the health care system as a whole, represented by the ability to completely eliminate any travel needs during the MDE process.
The increased proportion of patients completing guideline-driven MDE observed among those evaluated through the VTB program and the overall high rate of patients completing this standard (100%) are noteworthy—higher than those reported by other studies12,22—and reflect the magnitude of the impact on cancer care derived from an organized VTB regional program. A retrospective cohort study using the VA Hepatitis C Clinical Case Registry in 2013 reported that only 31% of patients with HCC were evaluated by a surgeon or oncologist7; another analysis using the SEER-Medicare database over a similar time period revealed that only 39% of patients with HCC were evaluated by ≥ three HCC cancer care specialists.6 The perfect guideline-driven MDE rate observed in patients evaluated through the VTB program reported in this study is the result of the establishment of a guideline-based process as part of the VTB implementation process, grounded on a validated framework for TB implementation, which minimizes program barriers,23,24 and is congruent with the high provider acceptance (satisfaction and confidence) and use rates associated with the program, which we previously reported.15 It should also be recognized, however, that among patients evaluated outside the VTB program, there was a relatively high guideline-driven MDE rate observed (75%), which was largely the result of the support provided by the HCC-TB to evaluate the majority of these patients; approximately 70% of non-VTB patients in our study received HCC evaluation through a combination of specialty-based clinics and the HCC-TB (Appendix Fig A1, online only). Fewer than 20% of non-VTB patients who received HCC evaluation exclusively through specialty-based clinic consultation received guideline-driven MDE, which is more consistent with the findings cited earlier from previous studies.12,22 These findings highlight the key role of TBs in providing MDE for HCC. This is particularly true in our study, where the HCC-TB was created under the premise of having the full spectrum of specialists required for HCC care; the ability of our program to accomplish this standard was in great part driven by the standardization of HCC-TB structure and processes.
Assessment of tumor staging and transplantation eligibility is a critical aspect of the MDE process. In our study, patients evaluated through the VTB platform had higher assessment rates for tumor stage (91.7%) and transplantation eligibility (95.8%), and overall, patients evaluated through VTB were 6× more likely to receive comprehensive MDE. It is well established that MDE through a TB forum significantly improves staging accuracy25 and ensures that correct management decisions and guideline-driven recommendations are made for patients with cancer.25,26 Our study focused on measures examining the quality of the process of care, and although the relatively small sample size limited our ability to examine the effect on treatment for each corresponding stage or liver function category, we feel that improving the quality of the MDE process provides the ideal framework to consider all relevant factors when determining the best treatment approach for each individual patient.
Several studies have previously shown distance to be a negative predictor for health services use among patients with cancer.22–24 Patients evaluated through the VTB completed MDE in a timely and convenient manner. On the contrary, non-VTB patients evaluated through specialty-based interfacility clinic consultation completed guideline-driven MDE over a longer period of time and experienced the inconvenience and cost derived from significant travel needs (Table 2; Appendix Fig A1, online only). These findings support that beyond the benefits of the TB forum, the VTB program has the added benefit of being more convenient, facilitating timely MDE and circumventing travel burdens (and decreasing costs) for patients and the health care system.
Findings from our study must be interpreted within the study limitations. These findings are limited to a VA practice setting and are not necessarily reproducible in other health care settings. However, VA and non-VA facilities within the same geographic area exhibit similar practice patterns of health care use.27 Additionally, our study involved the veteran population, in which the majority of patients are white and male and have a higher comorbidity burden compared with the general US population, thus potentially limiting the generalizability of the results. Because of nonrandom allocation to the VTB program, the study was subject to selection bias, although this bias was minimized by adjusting for the relevant differences between the two groups in relation to the primary outcome. Finally, considering the recent implementation of the HCC-VTB, we could not provide information on long-term patient outcomes, including overall survival, between the study groups.
Despite the noted limitations, this study offers a unique perspective on the potential benefits of a VTB program in the MDE process of care for patients with HCC. We found a positive impact of the VTB program characterized by improved quality and timeliness of the MDE process, along with an easing of the commonly associated patient and health care system burdens arising from travel needs.28 Furthermore, this VTB program represents an efficient way of improving access to care for patients with HCC across a large geographic region. Subsequent studies should focus on implementation of the VTB program on a wider scale within and outside the VA system and on exploring strategies to further facilitate timely treatment initiation after MDE.
Acknowledgment
Supported by the Office of Rural Health–Veterans Integrated Service Network 16 Clinical Systems Program, Telehealth, and Rural Access Program (Grant No. P00494-S1) and in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Center for Innovations in Quality, Effectiveness and Safety (Grant No. CIN 13-413). These sources had no role in the preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs of the US Government or Baylor College of Medicine. Presented at the 38th Annual Meeting of the Association of VA Surgeons, April 8, 2014.
Appendix
Figure A1.
Study flowchart delineating trajectory of patients referred to Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC; referral center) for hepatocellular carcinoma (HCC) evaluation (N = 116) by referral mechanism (consult type) and corresponding outcome. MDE, multidisciplinary evaluation; TB, tumor board; VTB, virtual tumor board. (*) P < .001 for comparisons of outcomes. (†) Quality of MDE; guideline-driven MDE, defined as MDE by team that at minimum was composed of following clinical specialties: radiology, hepatology, surgical oncology, interventional radiology, medical oncology, and transplantation before initiation of treatment.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: Aanand D. Naik, Daniel A. Anaya
Financial support: Daniel A. Anaya
Administrative support: Diana L. Castillo
Provision of study materials or patients: Diana L. Castillo, Daniel A. Anaya
Collection and assembly of data: Aitua Salami, Gala M. Barden, Diana L. Castillo, Mina Hanna, Daniel A. Anaya
Data analysis and interpretation: Aitua Salami, Diana L. Castillo, Nancy J. Petersen, Jessica A. Davila, Aanand D. Naik, Daniel A. Anaya
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml
Aitua C. Salami
No relationship to disclose
Gala M. Barden
No relationship to disclose
Diana L. Castillo
No relationship to disclose
Mina Hanna
No relationship to disclose
Nancy J. Petersen
No relationship to disclose
Jessica A. Davila
No relationship to disclose
Aanand D. Naik
No relationship to disclose
Daniel A. Anaya
No relationship to disclose
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: An update. Ann Intern Med. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers. www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PMC free article] [PubMed]
- 5.Barone C, Koeberle D, Metselaar H, et al. Multidisciplinary approach for HCC patients: Hepatology for the oncologists. Ann Oncol. 2013;24(suppl 2):ii15–ii23. doi: 10.1093/annonc/mdt053. [DOI] [PubMed] [Google Scholar]
- 6.Hyder O, Dodson RM, Nathan H, et al. Referral patterns and treatment choices for patients with hepatocellular carcinoma: A US population-based study. J Am Coll Surg. 2013;217:896–906. doi: 10.1016/j.jamcollsurg.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: Effect of patient and nonpatient factors. Hepatology. 2013;57:1858–1868. doi: 10.1002/hep.26287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright FC, De Vito C, Langer B, et al. Multidisciplinary cancer conferences: A systematic review and development of practice standards. Eur J Cancer. 2007;43:1002–1010. doi: 10.1016/j.ejca.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs medical center improves survival. HPB (Oxford) 2008;10:405–411. doi: 10.1080/13651820802356572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Cleave J, Devine P, Odom-Ball P. Multidisciplinary care of hepatocellular carcinoma. Cancer Pract. 1999;7:302–308. doi: 10.1046/j.1523-5394.1999.76007.x. [DOI] [PubMed] [Google Scholar]
- 12.Gish RG, Lencioni R, Di Bisceglie AM, et al. Role of the multidisciplinary team in the diagnosis and treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2012;6:173–185. doi: 10.1586/egh.11.105. [DOI] [PubMed] [Google Scholar]
- 13.Cohen GS, Black M. Multidisciplinary management of hepatocellular carcinoma: A model for therapy. J Multidiscip Healthc. 2013;6:189–195. doi: 10.2147/JMDH.S41206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouvier AM, Bauvin E, Danzon A, et al. Place of multidisciplinary consulting meetings and clinical trials in the management of colorectal cancer in France in 2000. Gastroenterol Clin Biol. 2007;31:286–291. doi: 10.1016/s0399-8320(07)89375-4. [DOI] [PubMed] [Google Scholar]
- 15.Marshall CL, Petersen NJ, Naik AD, et al. Implementation of a regional virtual tumor board: A prospective study evaluating feasibility and provider acceptance. Telemed J E Health. 2014;20:705–711. doi: 10.1089/tmj.2013.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson MM, Irwin T, Lowry T, et al. Development of a virtual multidisciplinary lung cancer tumor board in a community setting. J Oncol Pract. 2013;9:e77–e80. doi: 10.1200/JOP.2013.000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barden GM, Naik AD, Davila JA, et al. Using implementation methodology to develop a virtual tumor board program regionally. Ann Surg Oncol. 2014:21. (abstr S135) [Google Scholar]
- 18.Look Hong NJ, Gagliardi AR, Bronskill SE, et al. Multidisciplinary cancer conferences: Exploring obstacles and facilitators to their implementation. J Oncol Pract. 2010;6:61–68. doi: 10.1200/JOP.091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Radiology. Quality and Safety Resources: Liver Imaging Reporting and Data System. www.acr.org/Quality-Safety/Resources/LIRADS.
- 20.US Department of Veterans Affairs. Rural Health 2014. www.ruralhealth.va.gov/about/rural-veterans.asp.
- 21.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264.e1–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burge FI, Lawson BJ, Johnston GM, et al. A population-based study of age inequalities in access to palliative care among cancer patients. Med Care. 2008;46:1203–1211. doi: 10.1097/MLR.0b013e31817d931d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24:390–399. doi: 10.1111/j.1748-0361.2008.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junn JC, Kim IA, Zahurak ML, et al. Multidisciplinary service utilization pattern by advanced head and neck cancer patients: A single institution study. Int J Otolaryngol. 2012;2012:628578. doi: 10.1155/2012/628578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies AR, Deans DA, Penman I, et al. The multidisciplinary team meeting improves staging accuracy and treatment selection for gastro-esophageal cancer. Dis Esophagus. 2006;19:496–503. doi: 10.1111/j.1442-2050.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 26.Abraham NS, Gossey JT, Davila JA, et al. Receipt of recommended therapy by patients with advanced colorectal cancer. Am J Gastroenterol. 2006;101:1320–1328. doi: 10.1111/j.1572-0241.2006.00545.x. [DOI] [PubMed] [Google Scholar]
- 27.Ashton CM, Petersen NJ, Souchek J, et al. Geographic variations in utilization rates in Veterans Affairs hospitals and clinics. N Engl J Med. 1999;340:32–39. doi: 10.1056/NEJM199901073400106. [DOI] [PubMed] [Google Scholar]
- 28.Birkmeyer JD, Siewers AE, Marth NJ, et al. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290:2703–2708. doi: 10.1001/jama.290.20.2703. [DOI] [PubMed] [Google Scholar]