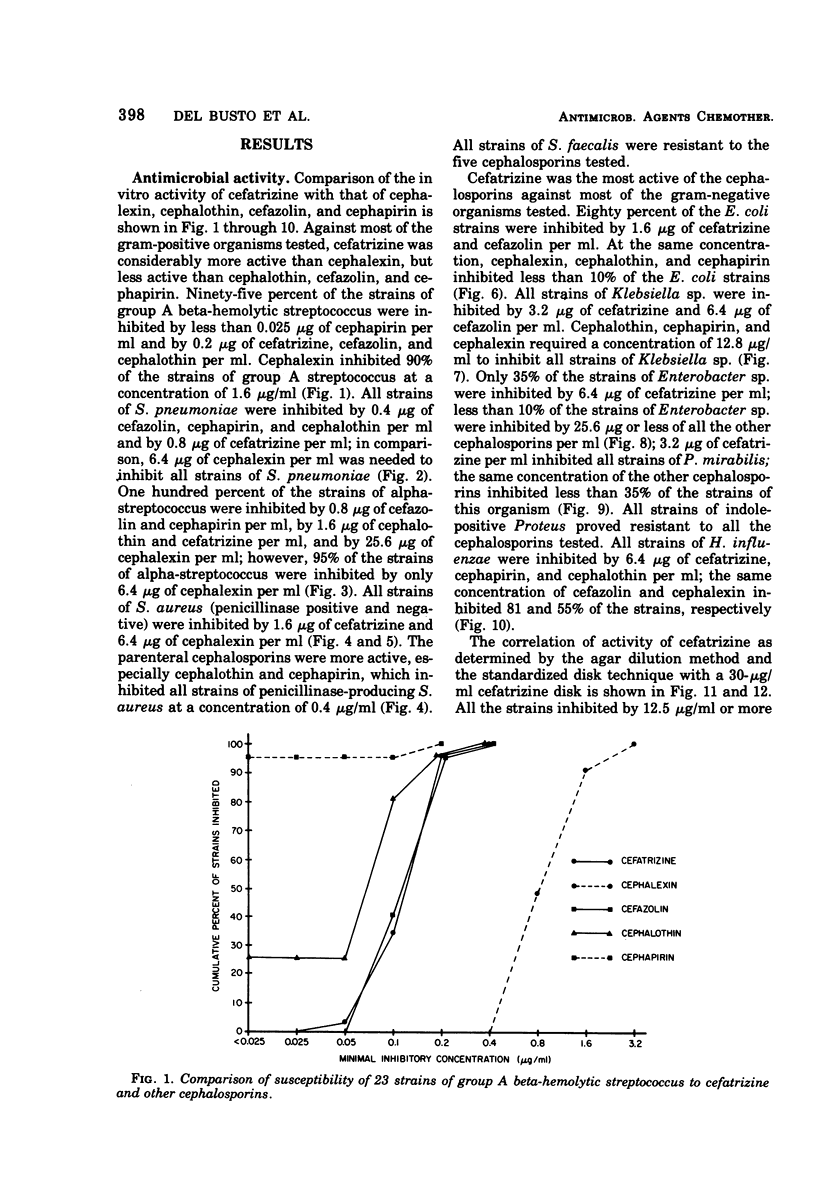
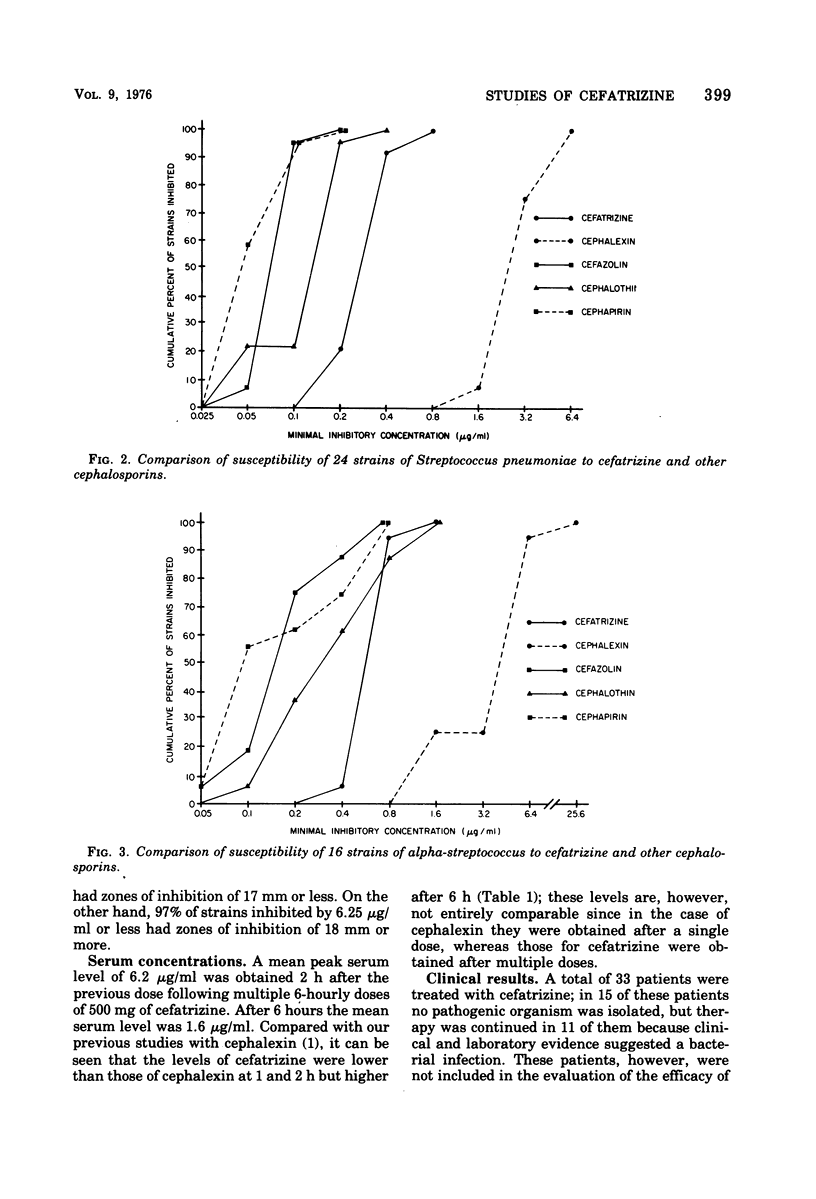
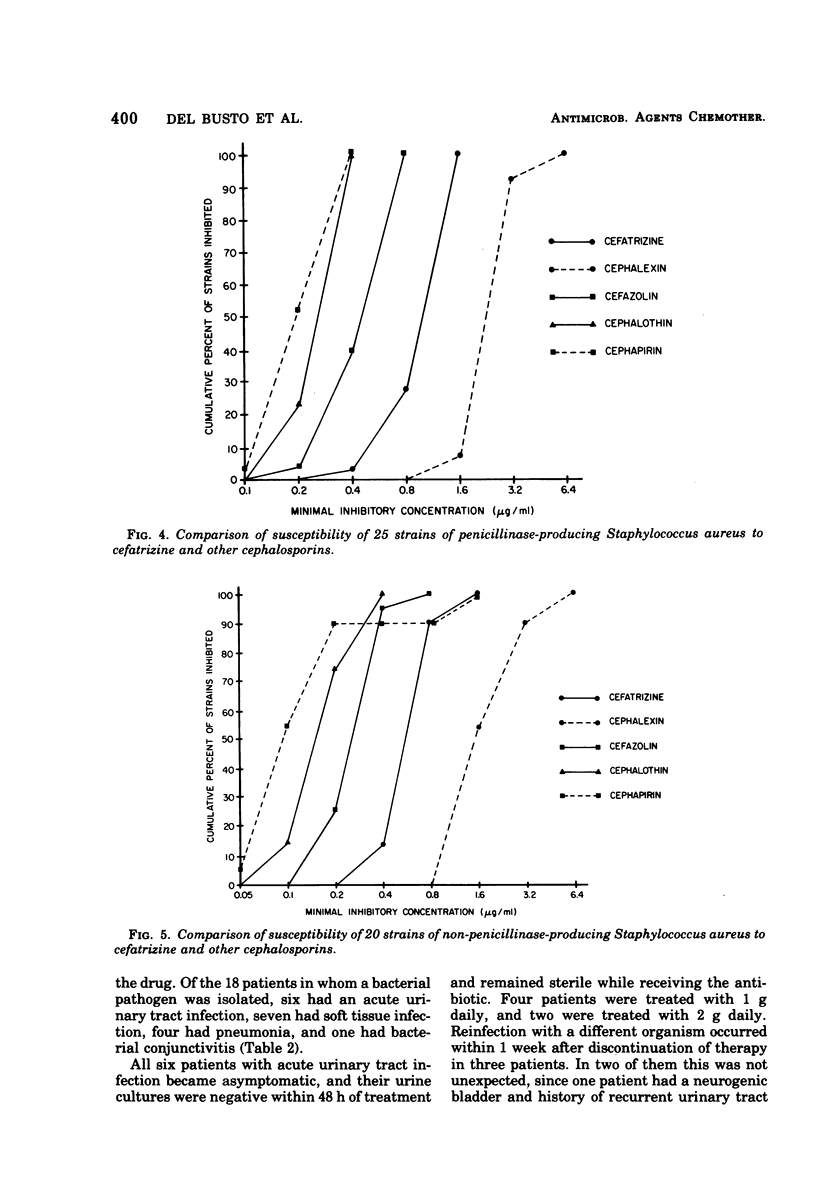
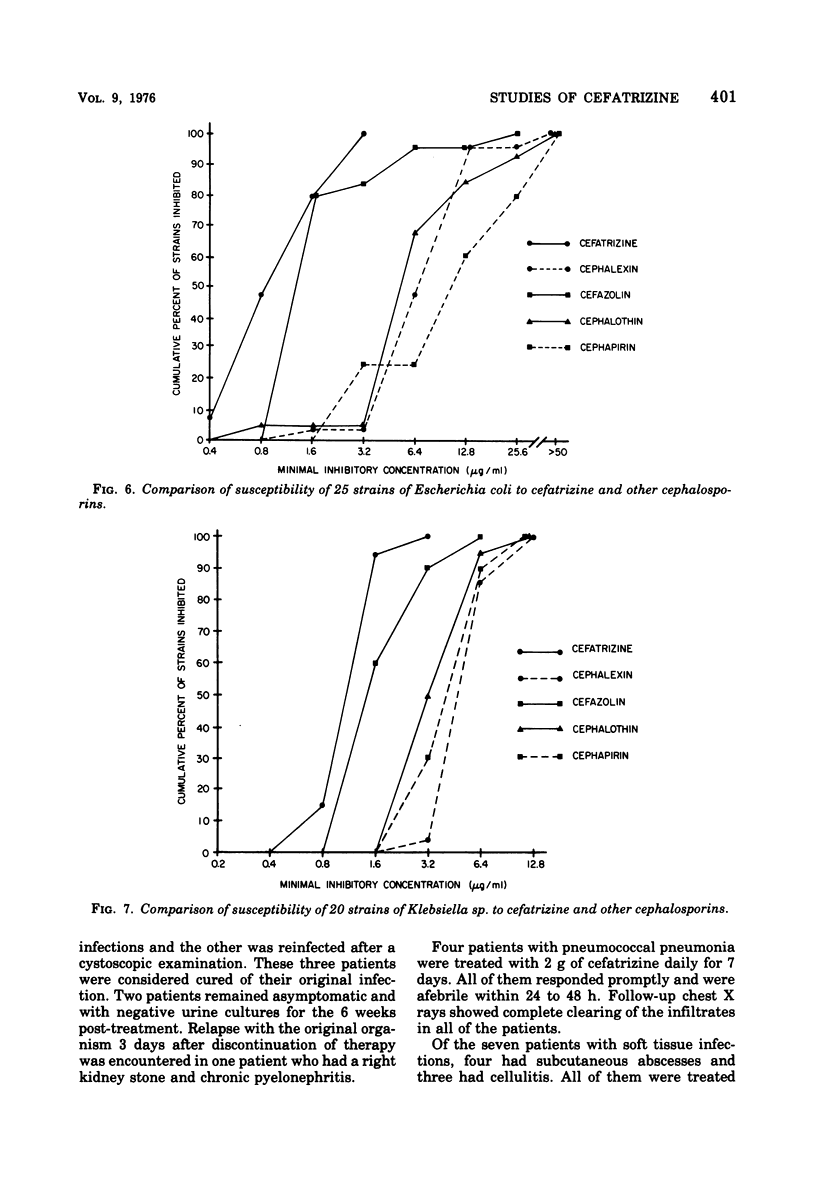
Abstract
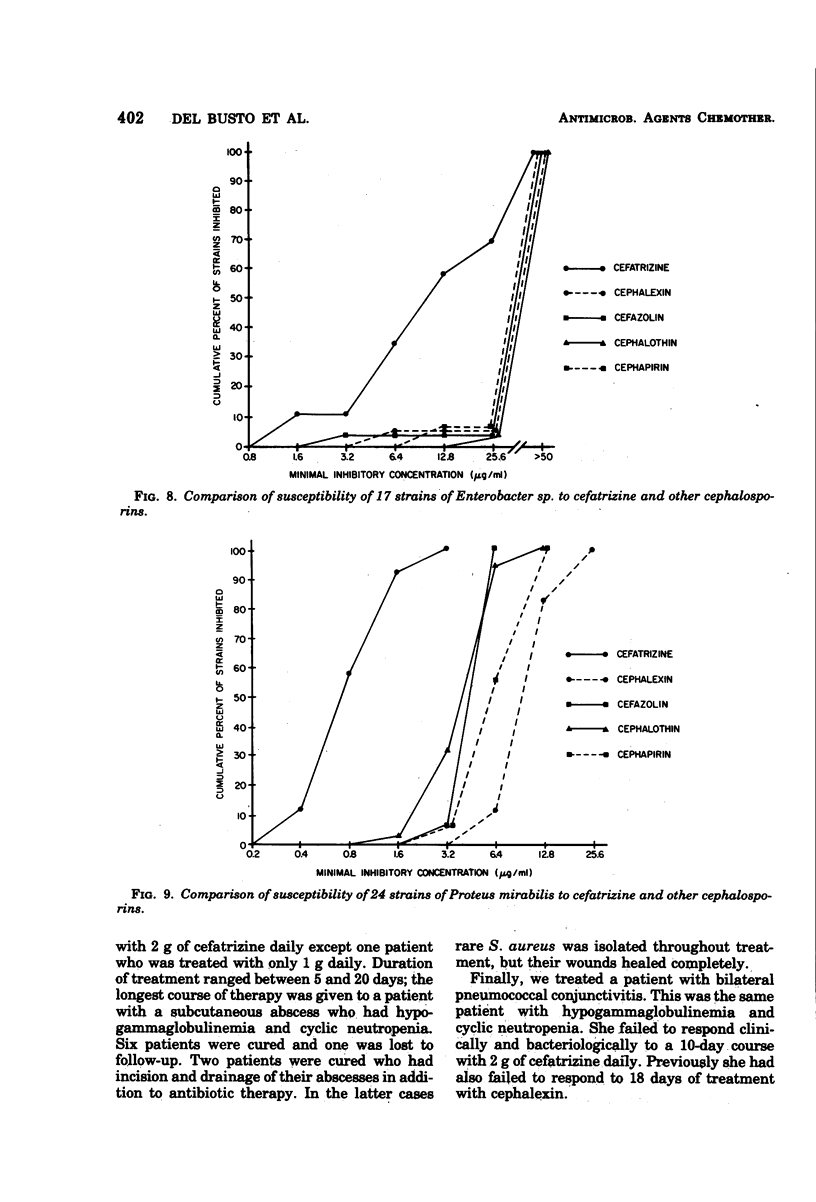
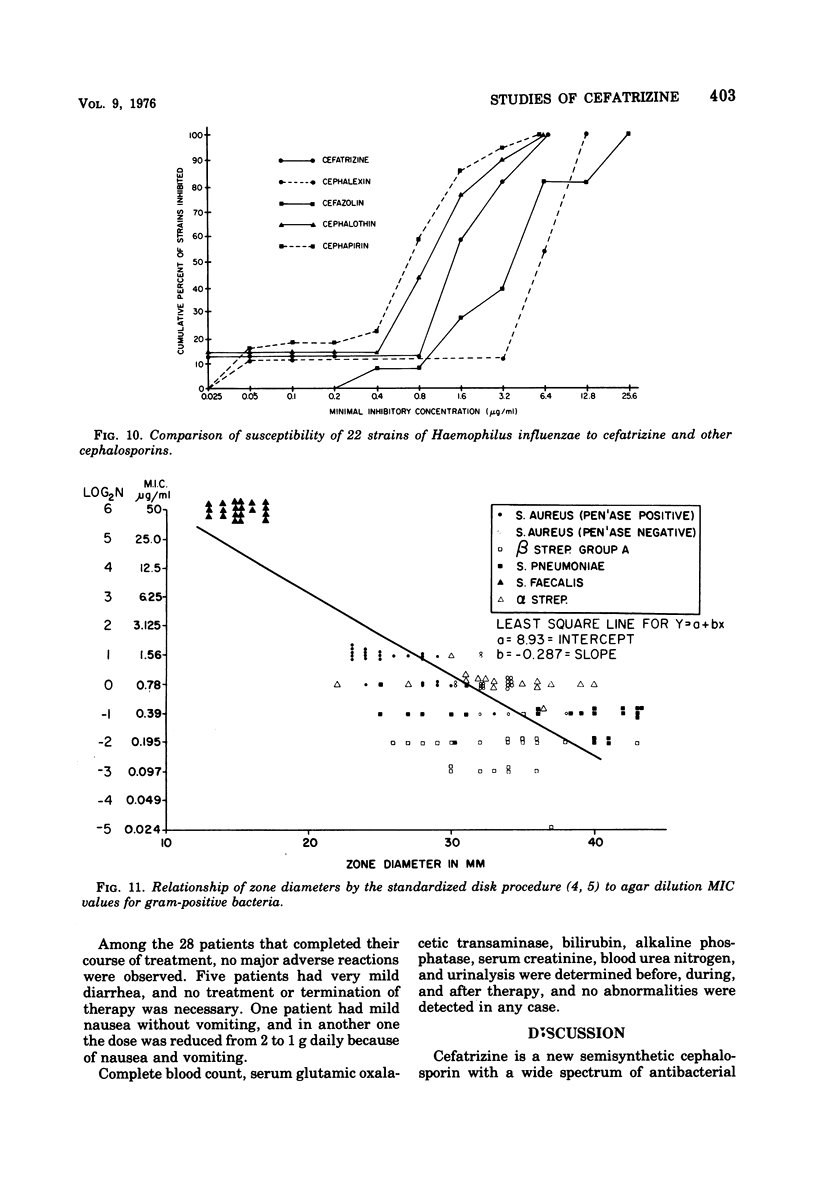
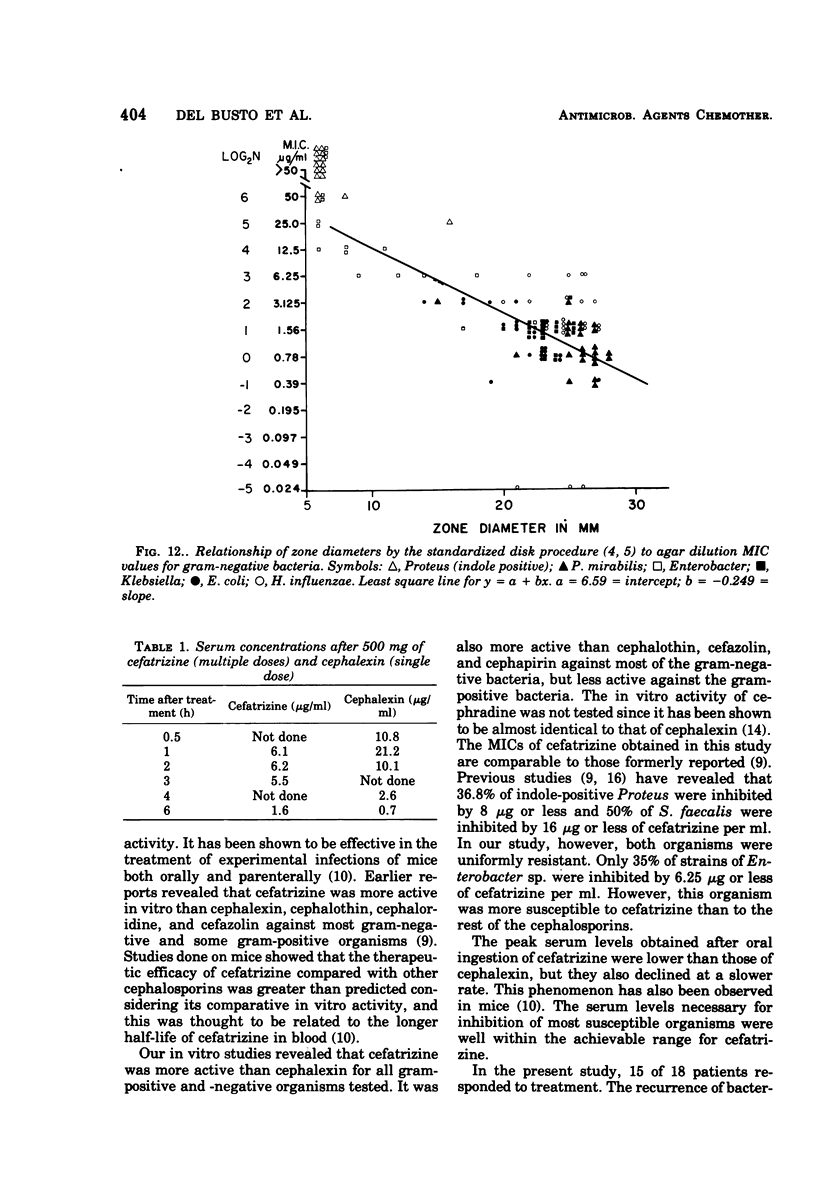
Cefatrizine, a new oral semisynthetic cephalosporin, was evaluated in vitro and in the treatment of 18 patients with acute urinary tract infection, pneumonia, and soft tissue infection. In vitro, it was more active than cephalexin for gram-positive and gram-negative bacteria. It was also more active than cephalothin, cefazolin, and cephapirin against most of the gram-negative bacteria but less active against the gram-positive bacteria. Of the patients treated with cefatrizine, only one failed to respond. This patient had pneumococcal conjunctivitis and hypogammaglobulinemia and neutropenia. The mean peak serum level after multiple 6-hourly doses of 500 mg was 6.2 μg/ml. The serum levels of cefatrizine necessary for inhibition of most susceptible organisms were well within the achievable range. The drug was well tolerated, and no renal, hepatic, or hematological toxicity was detected.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox F., Jr, Quinn E. L., Gatmaitan B. G., Peterson N. Cephalexin. In vitro and in vivo studies. Henry Ford Hosp Med J. 1970 Summer;18(2):91–98. [PubMed] [Google Scholar]
- Griffith R. S., Black H. R. Cephalexin. Med Clin North Am. 1970 Sep;54(5):1229–1244. [PubMed] [Google Scholar]
- Leitner F., Buck R. E., Misiek M., Pursiano T. A., Price K. E. BL-S 640, a cephalosporin with a broad spectrum of antibacterial activity: properties in vitro. Antimicrob Agents Chemother. 1975 Mar;7(3):298–305. doi: 10.1128/aac.7.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner F., Chisholm D. R., Tsai Y. H., Wright G. E., Deregis R. G., Price K. E. BL-S640, a cephalosporin with a broad spectrum of antibacterial activity: bioavailability and therapeutic properties in rodents. Antimicrob Agents Chemother. 1975 Mar;7(3):306–310. doi: 10.1128/aac.7.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison M. E., Johnson W. D., Thornhill T. S., Kaye D. Clinical and in vitro evaluation of cephalexin. A new orally administered cephalosporin antibiotic. JAMA. 1969 Sep 1;209(9):1331–1336. [PubMed] [Google Scholar]
- Perkins R. L., Glontz G. E., Saslaw S. Cephaloglycin: crossover absorption studies and clinical evaluation. Clin Pharmacol Ther. 1969 Mar-Apr;10(2):244–249. doi: 10.1002/cpt1969102244. [DOI] [PubMed] [Google Scholar]
- Saslaw S. Cephalosporins. Med Clin North Am. 1970 Sep;54(5):1217–1228. [PubMed] [Google Scholar]
- Scholand J. F., Hodges G. R., Fass R. J., Saslaw S. Clinical evaluation of cephradine, a new oral cephalosporin. Am J Med Sci. 1974 Feb;267(2):111–116. doi: 10.1097/00000441-197402000-00005. [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C., Bannister T., Glotzbecker C. Susceptibility of clinical isolates of Enterobacteriaceae to BL-S640, a new oral cephalosporin. Antimicrob Agents Chemother. 1975 Mar;7(3):381–385. doi: 10.1128/aac.7.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein L., Kaplan K. The cephalosporins. Microbiological, chemical, and pharmacological properties and use in chemotherapy of infection. Ann Intern Med. 1970 May;72(5):729–739. doi: 10.7326/0003-4819-72-5-729. [DOI] [PubMed] [Google Scholar]



