Abstract
Background:
There has been an increase in the development and use of oral health-related quality-of-life (OHRQoL) measures in the past two decades. This study aimed to assess the association between OHRQoL and clinical oral health measures, among mid-level school children in Southeast of Iran.
Materials and Methods:
A cross-sectional study was conducted on a random cluster sample of 11-13 year-old student population. Consented participants interviewed for OHRQoL measurements using Persian version of child-oral impacts on daily performances (OIDP). Oral examination was done by a trained dentist using WHO oral health assessment form, version 2011. Data were analyzed by SPSS software version 20 using Mann–Whitney and correlation tests.
Results:
A total of 400 school children participated. The overall mean of decayed missing filled teeth (DMFT) was 1.76 ± 2.4. A total of 82% of the school children presented the impact of oral problems in at least one of the eight daily performances. As DMFT increased, the OIDP score tended to increase or quality-of-life of children tended to be worse (r = 0.397, P < 0.001).
Conclusions:
The results showed a positive relation between some oral health status and quality-of-life score.
Keywords: Oral health, school children, Quality-of-life, oral impacts on daily performances
Introduction
Oral diseases like dental caries may result in pain, which in turn may lead to consequences on children’s daily life, taking time off from school or difficulty eating.1 Quality-of-life has been increasingly used as a scientific concept in the literature embracing a wide range of target groups and population as a whole.2
Measures of quality-of-life are increasingly being used to supplement clinical indicators to explore the individual’s perspectives on their health and health care, and it is an important part of assessing oral health.3
These measures, which assess “the extent to which oral conditions disrupt normal social role functioning and lead to major changes in behavior,” are known as socio-dental indicators or oral health-related quality-of-life (OHRQoL) measures. These indicators were developed to assess subjective aspects of oral health.4
Adolescent oral health is influenced by many factors; good oral health is also associated with broader social and economic determinants.
A variety of child OHRQoL instruments has been developed in the past 20 years but child version of the oral impacts on daily performances (OIDP) measure is a commonly used OHRQoL indicator.5 OHRQoL and oral health status represent two different concepts; the former putting the greatest emphasis on subjective and individual perception aspects, whereas oral health status is more closely related to objective aspects and normative assessment.6
In some researches, the association between clinical characteristics (such as caries and malocclusion) and OHRQoL have been extensively studied, for example, caries experience has been reported in a number of studies to negatively affect OHRQoL.7,8
Carvalho et al.9 found Adolescents living in an area where oral health education (OHE) and dental treatment (DT) were provided had better OHRQoL than those living in an area where only DT was provided. Raphael10 mentioned that QOL seems implicated in a wide range of adolescent health outcomes and health-related behaviors. Because OHRQoL is a condition, which influence under many factors such as oral health status, cultural, and general standard of living and also on the perception of the individual,11 therefore, this study aimed to assess the association between OHRQoL and clinical oral health measures, among mid-level school children in the city of Kerman, Southeast of Iran and also, answer this question whether the status of oral health can modify OIDP index in adolescents.
Materials and Methods
This report is the first phases of an interventional study of the effect of an OHE program using PRECEDE-PROCEED model12 on quality-of-life of children. A random sample of 400 adolescents between 11 and 13 years of age were recruited into the study through clustering of schools from both sexes, in 2012 in the city of Kerman, Southeast of Iran.
Ethical approval of the study was obtained from research ethic committee of Kerman University of Medical Sciences. School officials, children’ parents, and interviewees were briefed about the purpose and process of the study and consent was sought for questionnaire-led interviews and oral examination.
Pupils with serious medical problem and any condition influencing on their quality-of-life and also their oral health like orthodontic treatment were excluded from the study.
OHRQoL data were collected by validated Persian version of child-OIDP questionnaire.13
Initially, children were asked to fill the self-completed part of the questionnaire on 17 oral health-related problems experienced in the past 3 months. Afterward, face-to-face interviews were conducted in the school health room on the basis of the questionnaire instruction, to collect data on the impacts of oral problems, considering eight common daily performances: Eating, speaking, cleaning mouth, sleeping, emotional status, and smiling, studying and social relation. In the event that the impact on a performance was reported the severity of the impact (mild, moderate or severe) was recorded as well as its frequency.
Oral examination was performed under headlight, sitting on a chair using dental mirror and WHO probe by a dentist who was trained with WHO basic oral health survey methods. WHO standard oral health assessment form, version 2011 was used to record data for presence of gingival bleeding; caries index (decayed missing filled teeth [DMFT]), fluorosis, enamel defects, dental trauma, and malocclusion.14 Bacterial plaque accumulation was recorded on the basis of standard Loe and Silness plaque index.15
In the calculation of the child-OIDP score, the frequency of the impact is multiplied by the severity of each performance as it was described on the original paper.13
The child-OIDP final scores were obtained by adding the values for the eight performances, in a scale ranging from 0 to 72. The score is multiplied by 100 and divided by 72, which results in a final score of child-OIDP from 0 to 100.13
Frequency (percentage) mean ± standard deviation and also (median) were used to summarize qualitative and quantitative variables, respectively. Data were tested for normality of distribution by Kolmogorov-Smirnov test; therefore, nonparametric Mann–Whitney U-test and Spearman’s correlation were used to assess the association of OIDP score with oral health clinical indicators. The analysis of the data was carried out using the Statistical Package SPSS version 20 (Chicago, IL, USA). The significance level was set at 0.05.
Results
A total of 400 (response rate= 99%) school children (187 boys and 213 girls) participated with total mean age 12.02 ± 0.79. The overall mean of DMFT was 1.76 ± 2.4 and 328 (82%) of subjects reported one impact related to their oral health status. The most common reported problems related to oral health were malodor (23.26%), eruption related complaints (19.44%), and losing deciduous teeth (19.27%), respectively (Figures 1 and 2).
Figure 1.
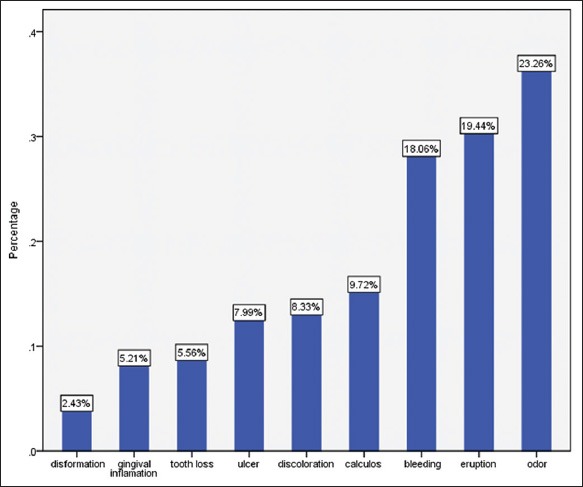
Frequency (percentage) of oral health reported problems among study participants.
Figure 2.
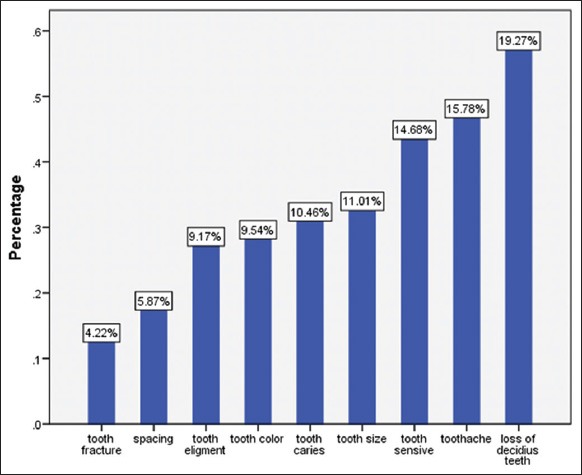
Frequency (percentage) of some other oral health reported problems among study participants.
The mean and median of OIDP score for the population were 10.2 ± 11.7 and 8, respectively. The most prevalent OIDP impacts were “difficulty brushing,” “eating,” and “smiling” with a mean of 1.47, 1.36, and 1.36, respectively. The mean prevalence of a variety of other oral impacts has been shown in Figure 3.
Figure 3.
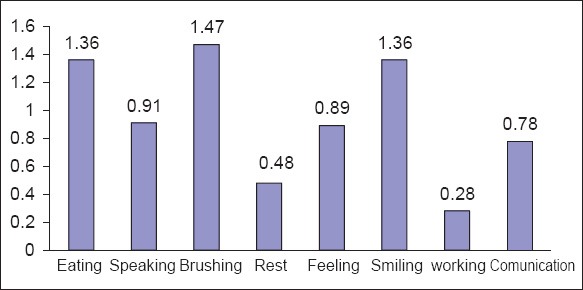
File scanned image before the initial use.
The impacts on “showing teeth while smiling” had high frequency (20.4%).
About 8% of the sample experienced a difficulty brushing impact “nearly every day” or for a spell of “more than three months” which was categorized as a severe effect (Table 1).
Table 1.
The prevalence of impacts’ severity in study subjects.
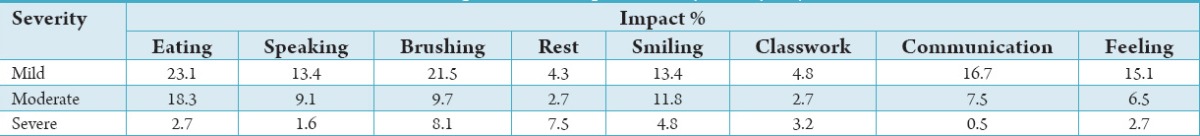
There was a positive and significant relationship between DMFT and OIDP scores (r = 0.397, P < 0.001) and also the presence of bacterial plaque and OIDP scores (r = 0.302, P < 0.001).
The presence of enamel defects, dental trauma, fluorosis, malocclusion, gingival bleeding, calculus had no significant association with OIDP separately but in the study 335 cases had at least one of the problems.
Discussion
The results described above, permit verification of the association between some oral health index, such as present or absence of plaque and subjective variables, with the child-OIDP index.
Using Spearman’s correlation coefficient, it was verified that the child-OIDP index had statistically significant association with several normative clinically observed variables, such as dental caries experience, presence of dental plaque. However, due to violation of normality, we were not able to employ a multivariate analysis to assess association of independent variables, simultaneously.
A positive correlation between DMFT and the Iranian teenagers’ quality-of-life was observed and Tubert-Jeannin et al.16 found similar association between the number of decayed teeth and the child-OIDP index which is in line with present study. However, Mtaya et al.17 did not find a statistically significant association between DMFT and child-OIDP, but they verified that the perception of the state of the teeth influenced the child-OIDP index. Furthermore, Biazevic et al.18 had a study to assess oral health status and its relationship with quality-of-life and mentioned a positive and statistically significant correlation between the highest score in the OHIP and DMFT. But they could not find associations between periodontal condition and OHIP as well as fluorosis and OHIP. About the malocclusion, Bernabé et al.19 found that malocclusion that affected the activity of “smiling” was the performance that attained the highest impact of this condition followed by “emotional state” and “social contact.” Another study verified that 24.6% of the examined adolescents presented impact on quality-of-life related to malocclusions.20
The recorded mean score of the child-OIDP index of 7.1 was similar to other studies: 8.8 in Thailand, 6.3 in France, and 7.8 in Peru but different to that 1.2 of Tanzania that had the lowest one.16,17,21,22
A research among 499 Iranian adults 20-50 year-olds showed 82.6% had experienced one or more oral impacts on their daily activities which are similar to our study, and 49.5% of impacts were reported to be of severe or very severe intensity. Eating was the performance most frequently affected (50.1%) followed by smiling (16.2%) and sleeping (11.8%).23
These frequencies were substantially lower than those found in the present study. This difference may be explained by the characteristics of the age of children and the level of understanding and sharing experiences in adults and children. A comparison of the results is partially limited because the instrument used is similar to the child-OIDP but was originally developed for adults. However, the three most common performances with impact were the same in both studies.
In the use of the child-OIDP questioner in other countries, high frequencies of impacts were found: Thailand (89.8%), France (73.2%), England (40.4%), Peru (82.0%), Tanzania (28.6%), and Brazil (88.7%).16-18,21,22,24 Another study in a similar age group reported OIDP score about 8.6 with 53% who experienced an oral problem in Tehran13 which difference with reported study may be related to the perception of children regarding oral problems. The present results of the overall impact with 82% are close with France and Peru with regard to the prevalence of impacts.
According to a special impact of daily activities, “difficulty brushing” was the activity with the highest impact in this research using the Child-OIDP. The second activity with higher impact was “eating” that was the activity with the highest impact in all researches. “Cleaning mouth,” similarly to what occurred in France, England, Peru, and Tanzania, while in the Thailand was “enjoying the contact of other people.”16,17,21,22,25
In the present study, the presence of oral impacts was associated with dental caries, as well as with, bleeding gums, and also with malocclusion. It could be noticed that the impacts measured through the Child-OIDP index are expressed more by perceived oral health subjective variables than by clinical indices. Mtaya et al.17 found similar results verifying that the clinical indices, such as caries index and oral hygiene, did not remain statistically significant in the multivariate regressions when subjective variables were analyzed.
Although clinical variables such as DMFT and the presence of bacterial biofilm were found statistically associated in the logistic and multinomial regression models, this result was similar in the present work. According to these results, it was believed that OHRQoL indicators can be associated to complement the other oral health indicators, but cannot be the only source of epidemiological data in oral health.
The association of the measure of OHRQoL with clinical oral health indicators allows its utilization in studies of oral health needs’ assessment. The complementary role of the socio-dental indicators for children partly has been known.26
The literature refers to the indices of OHRQoL are advisable that used together with the oral health indicators can benefit the dental services planning, because the use of oral health needs alone, usually, overestimate the patients’ needs. Moreover, the socio-dental indicators can be used to prioritize DT in situations involving a lack of resources. Under this reasoning, if there is no impact on quality-of-life, there is no need for immediate clinical intervention, and the patient can be directed to an OHE program.26
Conclusion
Considering the obtained results, it can be concluded that the association between dental caries and the child-OIDP index is an evidence of the impact of this condition on the quality-of-life of school children. Furthermore, there is a need for researches involving representative population and longitudinal studies assessing the effects of treatment or changes over time.
Acknowledgment
The authors wish to sincere thanks to all participants to the study and vice chancellor of research of Kerman Medical University for financial support of the study. Many thanks also go to Dr. Fereshte Emrani for her contribution to the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None
References
- 1.Tsakos G, Allen PF, Steele JG, Locker D. Interpreting oral health-related quality of life data. Community Dent Oral Epidemiol. 2012;40(3):193–200. doi: 10.1111/j.1600-0528.2011.00651.x. [DOI] [PubMed] [Google Scholar]
- 2.Felce D. Defining and applying the concept of quality of life. JIntellect Disabil Res. 1997;41:126–35. doi: 10.1111/j.1365-2788.1997.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 3.Streiner D, Norman G. Practical Guide to their Development and Use. 2nd ed. Ch. 5. NewYork: Oxford University Press; 2000. Health Measurement Scales; pp. 307–24. [Google Scholar]
- 4.Slade GD. Measuring Oral Health and Quality of Life, North Carolina: Department of Dental Ecology, School of Dentistry, University of North Carolina. 1997 [Google Scholar]
- 5.Gherunpong S, Sheiham A, Tsakos G. Asociodental approach to assessing children's oral health needs: integrating an oral health-related quality of life (OHRQoL) measure into oral health service planning. Bull World Health Organ. 2006;84(1):36–42. doi: 10.2471/blt.05.022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Research on oral health and the quality of life – A critical overview. Community Dent Health. 2008;25:130–1. [PubMed] [Google Scholar]
- 7.Foster Page LA, Thomson WM, Jokovic A, Locker D. Validation of the Child Perceptions Questionnaire (CPQ 11-14) JDent Res. 2005;84(7):649–52. doi: 10.1177/154405910508400713. [DOI] [PubMed] [Google Scholar]
- 8.Do LG, Spencer A. Oral health-related quality of life of children by dental caries and fluorosis experience. J Public Health Dent. 2007;67(3):132–9. doi: 10.1111/j.1752-7325.2007.00036.x. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho JC, Rebelo MA, Vettore MV. The relationship between oral health education and quality of life in adolescents. Int J Paediatr Dent. 2013;23(4):286–96. doi: 10.1111/ipd.12006. [DOI] [PubMed] [Google Scholar]
- 10.Raphael D, Rukholm E, Brown I, Hill-Bailey P, Donato E. Toronto: Center for Health Promotion of Univ; 1996. The Quality of Life Profile-Adolescent Version: Background, Description, and Initial Validation. [DOI] [PubMed] [Google Scholar]
- 11.McGrath C, Bedi R. Population based norming of the UK oral health related quality of life measure (OHQoL-UK) Br Dent J. 2002;193(9):521–4. doi: 10.1038/sj.bdj.4801616. [DOI] [PubMed] [Google Scholar]
- 12.Green L, Kreuter M. 2nd edition. Mountain View: Mayfield Publishing Co; 1991. Health Promotion Planning. [Google Scholar]
- 13.Kavand G, Younesian F, Atefeh SS, Dorri M, Akbarzadeh Baghban AR, Khoushnevisan MH. Oral health related quality of life among Iranian children: Part i-validity, reliability, prevalence and severity assessment of daily impact factors. JDent Sch. 2010;27(4):187–196. [Google Scholar]
- 14.World Health Organization. Basic Methods. 4th ed. Geneva: World Health Organization; 1997. Oral Health Survey. [Google Scholar]
- 15.Loe H, Silness J. Periodontal disease in pregnancy. I. prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 16.Tubert-Jeannin S, Pegon-Machat E, Gremeau-Richard C, Lecuyer MM, Tsakos G. Validation of a French version of the Child-OIDP index. Eur J Oral Sci. 2005;113(5):355–62. doi: 10.1111/j.1600-0722.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 17.Mtaya M, Astrøm AN, Tsakos G. Applicability of an abbreviated version of the Child-OIDP inventory among primary school children in Tanzania. Health Qual Life Outcomes. 2007;5:40. doi: 10.1186/1477-7525-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biazevic MG, Rissotto RR, Michel-Crosato E, Mendes LA, Mendes MO. Relationship between oral health and its impact on quality of life among adolescents. Braz Oral Res. 2008;22(1):36–42. doi: 10.1590/s1806-83242008000100007. [DOI] [PubMed] [Google Scholar]
- 19.Bernabé E, Flores-Mir C, Sheiham A. Prevalence, intensity and extent of Oral Impacts on Daily Performances associated with self-perceived malocclusion in 11-12-year-old children. BMC Oral Health. 2007;7:6. doi: 10.1186/1472-6831-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernabé E, Tsakos G, Messias de Oliveira C, Sheiham A. Impacts on daily performances attributed to malocclusions using the condition-specific feature of the oral impacts on daily performances index. Angle Orthod. 2008;78(2):241–7. doi: 10.2319/030307-111.1. [DOI] [PubMed] [Google Scholar]
- 21.Gherunpong S, Tsakos G, Sheiham A. The prevalence and severity of oral impacts on daily performances in Thai primary school children. Health Qual Life Outcomes. 2004;2:57. doi: 10.1186/1477-7525-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernabé E, Sheiham A, Tsakos G. Acomprehensive evaluation of the validity of Child-OIDP: Further evidence from Peru. Community Dent Oral Epidemiol. 2008;36(4):317–25. doi: 10.1111/j.1600-0528.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- 23.Mohebbi SZ, Sheikhzadeh S, Batebi A, Bassir SH. Oral impacts on daily performance in 20- to 50-yearolds demanding dental care in Tehran, Iran: Association with clinical findings and self-reported health. Oral Health Prev Dent. 2014;12(1):29–36. doi: 10.3290/j.ohpd.a31217. [DOI] [PubMed] [Google Scholar]
- 24.Castro Rde A, Portela MC, Leão AT, de Vasconcellos MT. Oral health-related quality of life of 11- and 12-year-old public school children in Rio de Janeiro. Community Dent Oral Epidemiol. 2011;39(4):336–44. doi: 10.1111/j.1600-0528.2010.00601.x. [DOI] [PubMed] [Google Scholar]
- 25.Yusuf H, Gherunpong S, Sheiham A, Tsakos G. Validation of an English version of the Child-OIDP index, an oral health-related quality of life measure for children. Health Qual Life Outcomes. 2006;4:38. doi: 10.1186/1477-7525-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gherunpong S, Tsakos G, Sheiham A. Asociodental approach to assessing dental needs of children: Concept and models. Int J Paediatr Dent. 2006;16(2):81–8. doi: 10.1111/j.1365-263X.2006.00701.x. [DOI] [PubMed] [Google Scholar]


