Abstract
Background:
Matrix metalloproteinases (MMP)-8 is increased in smokers with chronic periodontitis (CP). The levels of MMP-9 have not been assessed in the periodontal tissues of smokers. Therefore, the aim of this study is to estimate the concentrations of MMP-8 and -9 in the gingival crevicular fluid (GCF) of smokers and non-smokers with CP.
Materials and Methods:
Plaque, bleeding on probing, probing pocket depth, and attachment level were assessed in 60 subjects who were equally divided into three groups. Group A consisted of smokers with CP, Group B - non-smokers with CP and Group C - periodontally healthy subjects. The concentrations of MMP-8 and -9 were determined from GCF samples by using enzyme-linked immunosorbent assay.
Results:
A highly significant difference (P < 0.0001) was found in the MMP-9 concentration between the Groups A (27.56 ± 2.87 ng/ml) and B (17.16 ± 1.74 ng/ml) and between Groups A and C (14.13 ± 2.87 ng/ml). A significant difference (P < 0.002) was also found between the Groups B and C. A highly significant difference (P < 0.0001) in MMP-8 concentration was found between the A (68.96 ± 20.53 ng/ml) and C (7.05 ± 0.75 ng/ml) and also between Groups B (56.97 ± 12.70 ng/ml) and C. The difference in MMP-8 concentration between A and B was not statistically significant.
Conclusion:
Our findings of significantly elevated MMP-9 than MMP-8 from the diseased sites in smokers imply that MMP-9 is more significantly related to the increased periodontal destruction seen among smokers with CP.
Keywords: Chronic periodontitis, gingival crevicular fluid, matrix metalloproteinases-8, matrix metalloproteinases-9, smoking
Introduction
Matrix metalloproteinases (MMPs) represent a superfamily of proteases acting not only in physiological development and tissue remodeling, but also in pathological tissue destruction.1 Pathogens in microbial dental plaque are capable of stimulating host cells to increase their MMP release, which is considered among the indirect mechanisms of tissue destruction seen during periodontitis.2 MMPs are secreted in latent forms that can be activated by microbial-derived proteases or host inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and reactive oxygen species.3 MMPs can collectively degrade almost all components of extracellular matrix and basement membrane and their excess activity in the periodontium leads to periodontal tissue destruction.
MMP-8 (collagenase-2) is synthesized by differentiating granulocytes in the bone marrow and stored in specific granules of circulating neutrophils. However, the other cellular sources are sulcular epithelial cells, gingival and periodontal ligament fibroblasts, monocytes/macrophages, and plasma cells.4 MMP-8 is detected as a latent pro-enzyme in the gingival crevicular fluid (GCF) of shallow pockets, but in deep periodontal pockets it is converted to the active form.5 MMP-9, a gelatinase is secreted mainly by neutrophils and it is capable of degrading denatured interstitial collagens, gelatins, laminin, elastin, fibronectin and also collagens Type IV and Type VII.6 During active and progressing phases of periodontitis, MMP-8 and -9 levels in the GCF are significantly elevated.7
Smoking is a major risk factor as well as a modifier for the progression of periodontal disease.8 Chronic smokers present with an altered response of specific MMPs-8 and -9. MMP-8 expression is higher in the periodontal tissue of smokers than non-smokers that indicate that smoking modulates the expression of MMP-8 in periodontal tissues.9 The levels of MMP-9 have not been assessed from the GCF smokers with periodontitis. The current assessment of this association could be extremely significant in our understanding of the “raison d’ etre” for the increased levels of periodontal breakdown in smokers, as well as to potentially serve as a biomarker for impending periodontal breakdown amongst these patients.
This study which evaluated the levels of MMP-8 and -9, in smokers and non-smokers with periodontitis, was performed to assess the relationship between the expression levels of MMP-8 and -9 in the GCF.
Materials and Methods
Study population
Sixty subjects were selected from the patients who attended the clinics of the Department of Periodontics of SRM dental college, for the management of their dental and oral conditions. Subjects were classified into three groups - Group A (smokers with chronic periodontitis [CP]), Group B (non-smokers with CP) and Group C (clinically healthy periodontium) based on the smoking status and periodontal parameters. The study protocol was approved by the Ethical Committee of SRM University. All participants gave written informed consent in accordance with the Helsinki Declaration. The dental, as well as medical histories, were obtained from the patient. Pregnant and lactating women, patients with chronic inflammatory systemic diseases and patients with acute infections were excluded from the study. Systemically healthy patients between 18 and 45 years who had not received any antibiotic therapy and periodontal therapy in the last 6 months and having a minimum of 20 teeth (excluding third molars) with not more than two teeth missing in each quadrant were included in the study.
All patients in the smokers and non-smokers group with CP showed clinically detectable attachment loss and bone loss characterized by periodontal pocket formation and/or gingival recession with changes in density and height of subjacent alveolar bone as detected on the intraoral periapical radiographs. Each patient had at least one site with probing pocket depth (PPD) of ≥6 mm were diagnosed as CP in accordance with the clinical criteria stated in the consensus report of the World Workshop in periodontitis.10
Periodontal status evaluation
Clinical parameters assessed for the study were plaque index (PlI), sulcular bleeding index (SBI), bleeding on probing (BOP), PPD and clinical attachment level. The periodontal parameters were assessed at six sites per tooth excluding third molars. BOP (deemed positive if it occurred within 15 s after periodontal probing) and visible plaque accumulation were recorded dichotomously by visual examination. All measurements were performed by a single calibrated examiner using a Williams periodontal probe. GCF samples were obtained using extra-sulcular method before initiation of any periodontal intervention. The total levels of MMP-8 and -9 in GCF were measured using a commercially available enzyme-linked immunosorbent assay (Quantikine®; R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions.
MMP-8 and -9 analysis
All reagents and samples were brought to room temperature before use and prepared for the assay.150 µl of assay diluents RD1-52 was added to each well, followed by 50 µl of standard or sample to each well. The plate was covered and incubated for 2 h at room temperature (18-25°C) and was gently tapped to ensure thorough mixing. The cover was removed, and plate was washed four times using the wash buffer. After the last wash, the plate was inverted and blotted against clean paper towels. 200 µl of MMP-8 or -9 conjugate was added to all the wells.
The plate was then securely covered with a plate sealer and incubated for 1 h at room temperature. The plate was washed 4 times followed by the addition of 200 µl of substrate solution to each well. It was then incubated for 30 min at room temperature and protected from light. The optical density values for the plate were monitored, and the substrate reaction stopped before positive wells were no longer properly readable. The enzyme-substrate reaction was stopped quickly by pipetting 50 µl of stop solution to each well. The color in the wells changed from blue to yellow. The optical density of each well was determined within 30 min, using a microplate reader set to 450 nm as the primary wavelength. The levels of MMP-8 in the samples were estimated using the standard curve. The same procedure was followed for determining the levels of MMP-9 in the GCF samples using a standard curve. MMP-8 and -9 concentrations were obtained in ng/ml.
Statistical analysis
Mean and standard deviation was estimated from the sample for each study group. Mean values were compared among different study groups by using Kruskal–Wallis one-way ANOVA and then post-hoc (Tukey HSD) test. Spearman rank correlation analysis was done to assess the relationship between various study parameters. In the present study, P < 0.05 was considered as the level of significance. The statistical software SPSS version 17 was used for the analysis of the data.
RESULTS
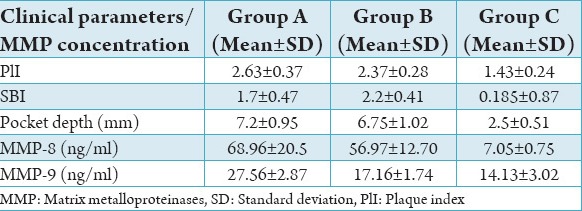
The comparison of mean values of PlI, sulcular bleeding index, pocket depth, MMP-8 and -9 among the three study groups are shown in Table 1.
Table 1.
Comparison of mean values of clinical parameters, MMP-8 and MMP-9 concentrations among the three study groups.
PlI
There was no statistical significant difference (P < 0.025) found in the PlI between Groups A and B. A very high statistical significant difference (P < 0.0001) was found between Groups A and C and also between Groups B and C.
Sulcular bleeding index
A statistically highly significant difference (P < 0.0001) was found between the study groups.
PPD
No statistical difference (P < 0.230) in the PPD between Groups A and B, but a statistically high significant difference (P < 0.0001) was found between Groups A and C and also between Groups B and C.
MMP-8 concentration
The mean MMP-8 concentration was 68.96 ± 20.53 ng/ml in Group A, 56.97 ± 12.70 ng/ml in Group B and 7.05 ± 0.75 ng/ml in Group C. A statistically mild significant difference (P < 0.023) was found in the MMP-8 concentration between Groups A and B, and statistically high significant difference (P < 0.0001) was found between Groups A and C and between Groups B and C (Table 2).
Table 2.
Comparison of the levels of MMP-8 between the study groups.

MMP-9 concentration
The mean MMP-9 concentration was 27.56 ± 2.87 ng/ml in Group A, 17.16 ± 1.74 ng/ml in Group B and 14.13 ± 3.02 ng/ml in Group C. A statistically highly significant difference (P < 0.0001) was found in the MMP-9 concentration between Groups A and B, between Groups A and C and a statistically significant difference (P < 0.002) was also found between Groups B and C (Table 3).
Table 3.
Comparison of the levels of MMP-9 between the study groups.

Discussion
In this study, we analyzed the GCF levels of MMP-8, and -9, in smokers and non-smokers with CP patients in an attempt to explain possible mechanisms of the detrimental effects of smoking on periodontal tissues. Tobacco smokers are reported to exhibit an increased susceptibility for development and progression of periodontal diseases.11,12 Accordingly, we hypothesized that the significantly increased susceptibility of tobacco smokers to the development and progression of periodontitis may be due, in part, to tobacco-induced increases in circulating levels of MMP-8, and -9.
Smokers tend to exhibit excess production of inflammatory mediators, such as IL-6, IL-8, TNF-α and increased neutrophil-derived proteolytic enzymes which could contribute to periodontal destruction. Because MMPs are responsible for the degradation of all extracellular matrix proteins, precise information about their presence, amounts, and activities is thought to be very important for the in-depth knowledge and characterization of different forms of periodontal disease and for determination of active destruction. MMPs-8 and -9 participate in the degradation of Type I, III, and Type IV collagen including basement membrane that are essential for maintaining the integrity of periodontal connective tissue. Assessment of collagenase or gelatinase levels in GCF during periodontal inflammation effectively reflects the degree of the pathological periodontal collagen metabolism and may be of diagnostic value.13
MMP-8 is one of the central biomarkers in the connective tissue breakdown of periodontitis.1,2 and it has been found to be a potential candidate as a diagnostic marker.3 Periodontal pockets at risk for irreversible tissue destruction indicates repeatedly elevated MMP-8 levels.3 MMP-8 expression in periodontal tissue was higher in smokers than in non-smokers,9 which is in accordance to the current study which showed increased MMP-8 levels in the GCF of smokers than in non-smokers with CP. MMP-9 also participates significantly in periodontal tissue destruction.14,15 Higher levels of MMP-9 have been found in GCF of patients with periodontitis, with levels decreasing following conventional periodontal treatment.15,16
MMP-9 is involved in bone resorption, playing a role in the subsequent digestion of denatured collagen I after being cleaved by collagenase.17 Although many studies18,19 have evolved to establish a relation between MMP-8 and -9 and the extent of periodontal diseases, there are no studies to date that evaluate the levels of MMP -9 in cohorts representing smokers. Knowing the fact that MMPs -8 and -9 are the major metalloproteinases that take part in connective tissue destruction in periodontal disease, the current study was performed to estimate and evaluate the possible role of these MMPs in periodontal disease in both smokers and non-smokers compared to the healthy cohorts devoid of periodontal disease.
The results of the current study showed that the mean MMP-8 concentrations were higher in smokers with CP when compared with non-smokers with CP and healthy patients. However, this increase in levels seen amongst smokers was not statistically significant (P < 0.023). This in accordance with the earlier studies conducted by Söder20,21 which identified that MMP-8 concentration was not significantly increased among smokers. A statistically high significant difference (P < 0.0001) was present between smokers with CP on comparison with healthy cohorts.
The GCF levels of MMP-9 in Group A and Group B was significantly elevated than healthy cohorts. However on comparing the MMP- 9 levels between patients in Groups A and B, a statistically high significant difference (P < 0. 0001) was noted. This shows that smokers with CP had increased levels of MMP-9 than the non-smokers with CP. This higher level of MMP-9 in the GCF of patients with CP indicates that MMP-9 may be a significant factor that modulates the increased periodontal breakdown that is evident amongst smokers. This study concludes that GCF levels of MMP-9 could be a very significant marker for periodontal breakdown.
This current study, as many as others has not found a significant difference between the levels of MMP-8 in smokers and non-smokers with CP, indicating that it may not be a significant marker of periodontal breakdown.20,21 This study that was carried out to identify the difference in the capacity of smokers and non-smokers to elicit markers of connective tissue breakdown a necessary feature required prior to the development of attachment loss. This study proposes that MMP-9 would be a more significant marker for impending periodontal attachment loss and more specifically in smokers. This will need to be borne out by longitudinal studies that correlate the levels of MMP 9 with ensuing attachment loss.
Further directions in the search for a clinically relevant biomarker will require that studies are conducted longitudinally to identify whether the MMP is in its active or inactive form for such assays to be more significant.
Conclusion
Our present finding of significantly higher GCF concentrations of MMP-9 in smokers with CP patients than their non-smokers and healthy cohort counterparts provides further support for the smoking associated risk for increased periodontal destruction.
Footnotes
Source of Support: Nil
Conflict of Interest: None
References
- 1.Sorsa T, Tjäderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10(6):311–8. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 2.Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, et al. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38(5):306–21. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 3.Mäntylä P, Stenman M, Kinane D, Salo T, Suomalainen K, Tikanoja S, et al. Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. J Periodontal Res. 2006;41(6):503–12. doi: 10.1111/j.1600-0765.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- 4.Wahlgren J, Maisi P, Sorsa T, Sutinen M, Tervahartiala T, Pirilä E, et al. Expression and induction of collagenases (MMP-8 and -13) in plasma cells associated with bone-destructive lesions. J Pathol. 2001;194(2):217–24. doi: 10.1002/path.854. [DOI] [PubMed] [Google Scholar]
- 5.Uitto VJ, Overall CM, McCulloch C. Proteolytic host cell enzymes in gingival crevice fluid. Periodontol. 2000;31:77–104. doi: 10.1034/j.1600-0757.2003.03106.x. [DOI] [PubMed] [Google Scholar]
- 6.Mäkelä M, Salo T, Uitto VJ, Larjava H. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: Cellular origin and relationship to periodontal status. J Dent Res. 1994;73(8):1397–406. doi: 10.1177/00220345940730080201. [DOI] [PubMed] [Google Scholar]
- 7.Kinney JS, Ramseier CA, Giannobile WV. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann NY Acad Sci. 2007;1098:230–51. doi: 10.1196/annals.1384.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winn DM. Tobacco use and oral disease. J Dent Educ. 2001;65(4):306–12. [PubMed] [Google Scholar]
- 9.Liu KZ, Hynes A, Man A, Alsagheer A, Singer DL, Scott DA. Increased local matrix metalloproteinase-8 expression in the periodontal connective tissues of smokers with periodontal disease. Biochim Biophys Acta. 2006;1762(8):775–80. doi: 10.1016/j.bbadis.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Bergström J, Eliasson S, Dock J. Exposure to tobacco smoking and periodontal health. J Clin Periodontol. 2000;27(1):61–8. doi: 10.1034/j.1600-051x.2000.027001061.x. [DOI] [PubMed] [Google Scholar]
- 12.Mullally BH. The influence of tobacco smoking on the onset of periodontitis in young persons. Tob Induc Dis. 2004;2(2):53–65. doi: 10.1186/1617-9625-2-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HY, Cox SW, Eley BM, Mäntylä P, Rönkä H, Sorsa T. Matrix metalloproteinase-8 levels and elastase activities in gingival crevicular fluid from chronic adult periodontitis patients. J Clin Periodontol. 2000;27(5):366–9. doi: 10.1034/j.1600-051x.2000.027005366.x. [DOI] [PubMed] [Google Scholar]
- 14.Sorsa T, Ding YL, Ingman T, Salo T, Westerlund U, Haapasalo M, et al. Cellular source, activation and inhibition of dental plaque collagenase. J Clin Periodontol. 1995;22(9):709–17. doi: 10.1111/j.1600-051x.1995.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 15.Ingman T, Tervahartiala T, Ding Y, Tschesche H, Haerian A, Kinane DF, et al. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J Clin Periodontol. 1996;23(12):1127–32. doi: 10.1111/j.1600-051x.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 16.Westerlund U, Ingman T, Lukinmaa PL, Salo T, Kjeldsen L, Borregaard N, et al. Human neutrophil gelatinase and associated lipocalin in adult and localized juvenile periodontitis. J Dent Res. 1996;75(8):1553–63. doi: 10.1177/00220345960750080601. [DOI] [PubMed] [Google Scholar]
- 17.Hill PA, Reynolds JJ, Meikle MC. Osteoblasts mediate insulin-like growth factor-I and -II stimulation of osteoclast formation and function. Endocrinology. 1995;136(1):124–31. doi: 10.1210/endo.136.1.7828521. [DOI] [PubMed] [Google Scholar]
- 18.Rai B, Kharb S, Jain R, Anand SC. Biomarkers of periodontitis in oral fluids. J Oral Sci. 2008;50(1):53–6. doi: 10.2334/josnusd.50.53. [DOI] [PubMed] [Google Scholar]
- 19.Kumar MS, Vamsi G, Sripriya R, Sehgal PK. Expression of matrix metalloproteinases (MMP-8 and -9) in chronic periodontitis patients with and without diabetes mellitus. J Periodontol. 2006;77(11):1803–8. doi: 10.1902/jop.2006.050293. [DOI] [PubMed] [Google Scholar]
- 20.Söder B. Neutrophil elastase activity, levels of prostaglandin E2, and matrix metalloproteinase-8 in refractory periodontitis sites in smokers and non-smokers. Acta Odontol Scand. 1999;57(2):77–82. doi: 10.1080/000163599428940. [DOI] [PubMed] [Google Scholar]
- 21.Söder B, Jin LJ, Wickholm S. Granulocyte elastase, matrix metalloproteinase-8 and prostaglandin E2 in gingival crevicular fluid in matched clinical sites in smokers and non-smokers with persistent periodontitis. J Clin Periodontol. 2002;29(5):384–91. doi: 10.1034/j.1600-051x.2002.290502.x. [DOI] [PubMed] [Google Scholar]


