Abstract
Disruptive Behavior Disorders (DBD) exhibit a sex-biased prevalence rate favoring boys, and prenatal testosterone exposure appears to be part of the complex etiology of these disorders. The current study examines whether high prenatal testosterone exposure may heighten risk for DBD symptoms in males by increasing susceptibility to negative environmental conditions such as maternal nicotine and alcohol use during pregnancy. Participants were 109 three- to six-year-olds (64% male; 72% with DBD) and their 109 primary caregivers and 55 daycare providers/teachers who completed a multi-informant diagnostic procedure. A proxy of prenatal testosterone exposure, finger-length ratios, interacted with maternal report of prenatal nicotine use to predict teacher-rated hyperactivity-impulsivity during preschool, for boys, but not girls, although the three-way interaction was not significant. Prenatal testosterone interacted with prenatal alcohol exposure to predict teacher-rated hyperactivity-impulsivity and ODD symptoms differentially based on child sex (significant three-way interaction). Boys with higher levels of prenatal testosterone who were also exposed to higher levels of nicotine and alcohol during pregnancy exhibited increased hyperactivity-impulsivity during early childhood, but girls did not exhibit this same pattern. Thus, high prenatal testosterone exposure seems to increase risk for DBD symptoms particularly in males by increasing susceptibility to prenatal environmental stressors.
Keywords: Disruptive Behavior, hormones, prenatal, sex differences
1. INTRODUCTION
Disruptive Behaviors Disorders (DBD), including Oppositional-Defiant Disorder (ODD) and arguably Attention-Deficit/Hyperactivity Disorder (ADHD), the latter of which also overlaps substantially with neurodevelopmental disorders, are common and impairing childhood behavior disorders that often begin as early as preschool (Keenan & Wakschlag, 2002; Pelham, Foster, & Robb, 2007; Sheeringa, 2003). DBD exhibit a sex-biased prevalence rate, occurring approximately three times as often in boys as girls (Costello, Foley & Angold, 2006). Based on this sex-biased prevalence rate, prenatal sex hormone exposure, such as prenatal testosterone exposure, may be one viable factor that increases risk for childhood DBD.
High levels of prenatal testosterone exposure, measured indirectly using masculinized finger-length ratios, have been associated with DBD and ADHD during childhood and adulthood, as well as associated behavioral traits including low conscientiousness/effortful control, high activity levels and sensation-seeking, and high aggression (Geschwind & Galaburda, 1985; Fink et al., 2006; Fink et al., 2007; Martel et al., 2008; McFadden et al., 2005). Yet, the effect size of associations between prenatal testosterone exposure and DBD-related behaviors is in the small to medium range, suggesting that it is only part of the complex etiology of these disorders. A key remaining question concerns how prenatal testosterone exerts downstream effects on behaviors related to DBD.
Theory suggests that high levels of prenatal testosterone slow down neural development in utero in males compared to females, perhaps lengthening the prenatal period during which males (vs. females) are particularly sensitive to environmental influences (Martel et al., 2009; Morris, Jordan, & Breedlove, 2004). Thus, negative prenatal environmental influences may exert particularly negative effects on male (vs. female) fetuses because they are vulnerable to them for longer periods of time during pregnancy (Martel, in press). High maternal use of nicotine and alcohol during pregnancy are two such important prenatal environmental stressors that have been associated with DBD (Kuhn et al., 2010; Langley et al., 2007; Neuman et al., 2007). These prenatal stressors seem to exert particularly potent effects on and through interactions with the dopaminergic neurotransmission system, particularly in the prefrontal cortex, which develops slowly during the prenatal period (Goldstein & Volkow, 2002; Morris et al., 2004). Such influences on developing neural circuitry may be particularly relevant to DBD-related behaviors including executive function and traits like sensation-seeking (Huizink & Mulder, 2006, Beauchaine et al., 2009; Noland, et al., 2003). Yet, little work has examined whether males (vs. females) are particularly susceptible to these negative prenatal environmental influences on DBD due to their higher prenatal testosterone levels, as one would expect based on this theory.
Thus, one possible theory of sex differences in the prevalence rate of DBD is that high prenatal testosterone exposure may increase susceptibility to negative environmental conditions such as maternal nicotine and alcohol use during pregnancy in males (vs. females) with particular effects on DBD-related behavior (Martel, in press). The current study is the first to examine this idea empirically by evaluating interactions between prenatal testosterone exposure, prenatal environmental conditions (i.e., maternal prenatal nicotine and alcohol use), and child sex in relation to child DBD symptoms, albeit using a proxy measure of prenatal testosterone and retrospective maternal report of prenatal substance use as a preliminary first step. Hypotheses are that prenatal testosterone exposure, negative prenatal environmental circumstances (i.e., maternal prenatal nicotine and alcohol use), and child sex will interact in relation to DBD symptoms (Haslam et al., 2006). In particular, it is predicted that high prenatal testosterone exposure will be associated with increased DBD when exposure to prenatal maternal nicotine and alcohol use is high, and these effects will be specific to males (vs. females).
2. METHODS
2.1. Participants
2.1.1. Overview
Participants were 109 preschoolers between the ages of three and six (M=4.34 years, SD=1.08) and their primary caregivers (hereafter termed parents for simplicity; 67% mothers with the remaining 33% fathers+mothers together, fathers only, foster parents, or grandmothers with guardianship). As shown in Table 1, sixty four percent of the sample was male, and 36% of the sample represented an ethnic or racial minority (28% African American), coded dichotomously (0=non-Hispanic Caucasian; 1= any ethnic or racial minority). Family income ranged from below $20,000 to above $100,000 annually. Parental highest educational level ranged from grade school to doctorate, and family employment ranged from unemployed to full-time weekly.
Table 1.
Demographic and Descriptive Information on Sample by Clinical Group
| M (SD) | Non-DBD (c) n=30 |
ODD (o) n=18 |
ADHD (a) n=18 |
ODD+ADHD (oa) n=43 |
|
|---|---|---|---|---|---|
| Age | 3.9(1.03) | 4.56(1.25) | 4.56(.92) | 4.47(1.05) | |
| Sex (N[% male]) | 14(46.7) | 10(55.6) | 13(72.2) | 27(62.8) | |
| Race/Ethnicity (N[% minority]) | 7(23.3) | 2(11.2) | 10(55.6) | 17(39.6)* | |
| Caucasian | 23(76.7) | 16(88.9) | 8(44.4) | 26(60.5) | |
| African American | 7(23.3) | 0(0) | 9(50) | 12(27.9) | |
| Latino | 0(0) | 0(0) | 1(5.6) | 2(4.7) | |
| American Indian | 0(0) | 1(5.6) | 0(0) | 0(0) | |
| Mixed | 0(0) | 1(5.6) | 0(0) | 3(7) | |
| Family Income (mode) | 3 | 5 | 1 | 0* | |
| Maternal Education | 4 | 6 | 4 | 4 | |
| Maternal Employment | 0,3† | 0,3† | 0 | 3 | |
| Paternal Education | 4 | 4 | 4 | 4 | |
| Paternal Employment | 3 | 3 | 3 | 3 | |
| Prenatal Testosterone | .97(.07) | .97(.09) | .95(.06) | .97(.06) | |
| Prenatal Nicotine Use | .38(.1.02) | .20(.41) | .83(1.58) | .37(1.08) | |
| Prenatal Alcohol Use | 1.24(1.98) | .60(1.6) | .28(1.18) | 1.2(1.95) | |
| ODD symptoms (P) | 2.97(3.08)1,2 | 10(6.02)1,3 | 5.83(3.28)3,4 | 11.6(7.24)2,4** | c<a<o<oa |
| ADHD symptoms (P) | 8.6(6.86)1,2,3 | 19.73(12.98)1,4 | 26.72(9.09)2,5 | 35.26(13.54)3,4,5** | c<o<a<oa |
| Inattention | 3.77(3.87)1,2,3 | 8.93(6.77)1,4 | 11.39(5.88)2,5 | 16(7.29)3,4,5** | c<o<a<oa |
| Hyper-Imp | 4.83(3.76)1,2,3 | 10.8(6.62)1,4,5 | 15.33(5.43)2,4,6 | 19.26(7.04)3,5,6** | c<o<a<oa |
| ODD symptoms (T) | 2.77(3.75)1 | 4(3.84)2 | 4.6(4.16)3 | 11.84(6.68)1,2,3** | others<oa |
| ADHD symptoms (T) | 10.08(9.11)1,2 | 7.78(7.97)3,4 | 37.6(10.16)1,3 | 36.32(8.51)2,4** | c,o<a,oa |
| Inattention | 4.15(4.26)1,2 | 3.89(3.95)3,4 | 23.2(2.95)1,3,4 | 17.95(5.75)2,4** | c,o<a, oa |
| Hyper-Imp | 5.92(5.59)1,2 | 3.89(4.17)3,4 | 14.4(8.91)1,3 | 18.39(5.41)2,4** | c,o<a,oa |
Note.
p<.05.
p<.01.
multiple modes. Subgroup differences based on chi-square or ANOVA with follow-up LSD post hoc tests indicated with like superscripts. Family income modes: 0=annual income less than $20,000, 1=between $20,000 and $40,000, 2=between $40,000 and $60,000, 3=between $60,000 and $80,000, 4=between $80,000 and $100,000, and 5=over $100,000 annually. Parental education modes: 0 for grade school, 1 for some high school, 2 for high school equivalent, 3 for high school degree, 4 for some college, 5 for associates degree, 6 for bachelors degree, 7 for masters or equivalent degree, and 8 for doctorate. Parental employment modes: 0 for unemployed, 1 for 1–19 hours part-time weekly, 2 for 20–39 hours part-time weekly, and 3 for full-time weekly; sample mode=3. Nicotine and Alcohol Use scale=0 (never used) to 5 (used daily). (P)=Parent report. (T)=Teacher report.
Based on multistage and comprehensive diagnostic screening procedures (detailed below), preschoolers were recruited into two groups: those with Disruptive Behavior Disorders (DBD; n=79), subdivided into those with ADHD-only (n=18), Oppositional-Defiant Disorder (ODD)-only (n=18), and ADHD+ODD (n=43); and children without DBD (n=30). The non-DBD group included preschoolers with minimal and subthreshold symptoms to provide a more continuous measure of symptoms, consistent with research suggesting that DBD may be better captured by continuous dimensions than categorical diagnosis and to be sensitive to the young age of the sample (Leblanc et al., 2008; Levy et al., 1997; Marcus & Barry, 2011). No siblings were included.
2.1.2. Recruitment and Identification
Participants were recruited from an urban, Southern United States community primarily through direct mailings to families with children between the ages of three and six and internet postings, as well as through advertisements in newspapers and flyers posted at doctors’ offices, community centers, daycares, and on campus bulletin boards. Two sets of advertisements were utilized; one set of advertisements targeted children between ages 3 and 6 with disruptive behavior problems and/or attention problems and a second set of advertisements targeted children between ages 3 and 6 without these types of problems. After recruitment, all families passed through a multi-gated screening process. An initial telephone screening was conducted to rule out children prescribed psychotropic medication or children with neurological impairments, mental retardation, psychosis, autism spectrum disorders, seizure history, head injury with loss of consciousness, or other major medical conditions. Only 10 families were screened out at this phase. All families screened into the study at this point completed written and verbal informed consent procedures prior to data collection and consistent with the university Institutional Review Board, the National Institute of Mental Health, American Psychiatric Association guidelines, and in compliance with national and local legislation. All families were compensated $50 for their participation.
During the second stage, parents and preschoolers attended a campus laboratory visit. Before and during the laboratory visit, diagnostic information was collected via parent and teacher/caregiver ratings. Parents completed the Kiddie Disruptive Behavior Disorders Schedule (K-DBDS; Orvaschel & Puig-Antich, 1995), a semi-structured diagnostic interview modeled after the Schedule for Affective Disorders and Schizophrenia for School-Age Children administered by a trained graduate student clinician (Leblanc et al., 2008). The K-DBDS demonstrates high test-retest reliability and high inter-rater reliability in the preschool population (Leblanc et al., 2008). In the current study, fidelity to interview procedure was determined by calculation of reliability of blind interviewer ratings of DBD symptoms on a randomly-chosen 10% of families. Inter-rater clinician agreement was adequate for symptoms (ICC=.97).
Families were mailed teacher/caregiver questionnaires one week prior to the laboratory visit and instructed to provide the questionnaires to children’s teacher, daycare provider, or babysitters who then mailed the completed questionnaires back to the university. When available (i.e., available on 50% of participating families), teacher/caregiver report on DBD symptoms was obtained via report on the Disruptive Behavior Rating Scale (DBRS; Barkley & Murphy, 2006). Response rate did not differ based on child DBD diagnostic group (χ2[3]=.59, p=.9), ethnic/racial minority status (χ2[1]=1.73, p=.19), or family income (t[97]=1.82, p=.07). Ultimately, clinical diagnoses and groupings were determined by the Principal Investigator, a licensed clinical psychologist, after a review of parent ratings on the K-DBDS and (when available) teacher/caregiver ratings on the DBRS, consistent with current best practice guidelines for current diagnosis (Pelham et al., 2005).
2.2. Measures
2.2.1. Symptom Counts
Parent and teacher/caregiver reports on symptoms were available via the Disruptive Behavior Rating Scale (DBRS; Barkley & Murphy, 2006), which assesses symptoms using a 0 to 3 scale for a more continuous dimension. Symptom domain scores were calculated as sums of scores within each diagnostic subdomain (ODD symptoms, inattentive ADHD symptoms, hyperactive-impulsive ADHD symptoms). The DBRS has high internal consistency ranging from .78 to .96 in the preschool age range (Pelletier et al., 2006). All scales for parent and teacher/caregiver report on the DBRS had high internal reliability (all alphas > .92) in the current sample. Teacher ratings were returned for approximately half of the sample (N=55). Primary analyses were conducted using teacher report on the DBRS to minimize shared source variance and to constrain the number of analyses conducted (and hence decrease Type I error).
2.2.2. Prenatal Testosterone Exposure
Prenatal hormone levels were measured indirectly via finger length ratios. Although these ratios exhibit well-established associations with prenatal hormone levels, the mechanisms of these effects remain unexplained, making it a somewhat controversial measure of such influences (Brown et al., 2002; Cohen-Bendahan et al., 2005; Lutchmaya et al. 2004; McIntyre, 2006; Zheng & Cohn, 2011). Levels of prenatal testosterone measured via routine amniocentesis have been found to be significantly correlated with finger length ratios (McIntyre, 2006), and women with Congenital Adrenal Hyperplasia, or women exposed to high levels of adrenal androgens in utero, have more masculine finger length ratios (Manning et al., 1998; Phelps, 1952). However, it should be noted that geographic, racial, and ethnic differences in finger-length ratios have been found (Loehlin et al., 2006; Manning et al., 2000; 2004; Phelps, 1952; Terrance et al., 2000), and the mechanisms of these effects remain poorly understood.
In the current study, finger length ratio measurements were obtained using an electronic caliper. All fingers (i.e., 2D [index finger], 3D [middle finger], 4D [ring finger], 5D [little finger]) are measured on the palm side of the hand, from the connection to the palm to the tip of the finger. Interrater reliability was satisfactory, computed via correlations between independent measurements of finger-lengths on approximately ten percent of the sample (ICC=.83–.99). As recommended, ratios were computed for each pair of fingers (e.g., 2D/4D; Brown et al., 2002; Cohen-Bendahan et al., 2005). Right 2D:4D was emphasized in the current study since it is the most well-established and best-replicated in regard to human behavioral sex differences (Cohen-Bendahan et al., 2005). Smaller finger-length ratios (i.e., 4D slightly longer than 2D) are considered indicative of higher exposure to prenatal testosterone, and this continuous measure was utilized in study analyses.
2.2.3. Prenatal Environmental Factors
A thorough and confidential background questionnaire was administered to participants by trained study staff that asked structured questions about alcohol, nicotine, and other recreational drug use during pregnancy, including if use occurred and, if so, during which months of pregnancy use occurred, the frequency with which average use occurred, and the prenatal month during which heaviest use occurred. It should be noted that there was no external validation of the veracity of these reports, consistent with other work done in this area (see D’Onofrio et al., 2007; Knopik et al., 2006; Neuman et al., 2007). Analyses emphasized the frequency with which use occurred for both alcohol and nicotine. This variable ranged from 0 (never used) to 5 (used daily).
2.3. Statistical Analysis
Analyses were conducted using Mplus (Muthen & Muthen, 2012) which allows for the statistical control of non-normality and outliers through the use of robust maximum likelihood estimation and utilizes full information likelihood estimation (i.e., FIML or direct fitting), a method of directly fitting models to raw data without imputing values to address any missingness (Burchinal & Neebe, 2006; Curran, West, & Finch, 1996). Hierarchical regressions were conducted with main effects entered at step 1, two-way interactions entered at step 2, and the three-way interactions entered at step 3, per recommendations (Baron & Kenny, 1986; Dearing & Hamilton, 2006; Ellis et al., 2011; Kraemer et al., 2001). Power was adequate (.76) to detect a medium to large effect size (r = .4) in a sample of 55 participants; power was adequate (.8) to detect a medium effect size (r = .3), but not a small effect size, in 109 participants.
3. RESULTS
As shown in Table 1, child age and sex did not significantly differ between diagnostic groups (all p>.1). However, child race/ethnicity and family income significantly differed between the four diagnostic groups (p<.05). Specifically, there were more racial/ethnic minorities within the ADHD and ADHD+ODD diagnostic groups compared to the ODD and non-DBD groups, and family income was lower in the ADHD and ODD+ADHD groups compared to the ODD and non-DBD groups. Therefore, child race/ethnicity and family income was covaried in all main study analyses. Bivariate correlations between parent and teacher DBD symptom ratings within domain were all significant and at least in the moderate range (r range from .52 to .59, all p<.01).
3.1. Associations Among Prenatal Testosterone Exposure, Prenatal Nicotine Exposure, Prenatal Alcohol Exposure, and Child DBD Symptoms
Based on bivariate correlations (shown in Table 2), there were no significant associations between prenatal testosterone, nicotine, or alcohol exposure and teacher-rated child DBD symptoms (all p>.4). A lack of main effects suggests the possibility of interactive effects because if children differ in their sensitivity to prenatal stressors based on prenatal testosterone exposure, these contrasting effects in different groups of children might result in a lack of significant main effects. Non-Hispanic white children were exposed to more prenatal alcohol and exhibited fewer inattentive symptoms; lower family income was associated with ethnic/racial minority status and higher prenatal nicotine use.
Table 2.
Correlation Table
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1 Prenatal Testosterone | 1 | |||||||
| 2 Prenatal Nicotine | −.13 | 1 | ||||||
| 3 Prenatal Alcohol | −.02 | .12 | 1 | |||||
| 4 Child Inattention | −.1 | −.01 | −.13 | 1 | ||||
| 5 Child Hyperactivity | −.05 | .09 | .02 | .73** | 1 | |||
| 6 Child ODD | −.13 | .01 | .08 | .48** | .74** | 1 | ||
| 7 Racial/Ethnic minority status | .004 | −.15 | −.25* | .38* | .22 | .19 | 1 | |
| 8 Family Income | .09 | −.30** | .04 | −.12 | −.11 | −.11 | −.33** | 1 |
Note.
p<.05.
p<.01. Child symptoms are teacher-rated. Racial/ethnic minority status: 0=non-Hispanic Caucasian; 1=any ethnic or racial minority.
3.2. Interactions Among Prenatal Testosterone Exposure, Child Sex, and Prenatal Environmental Influences
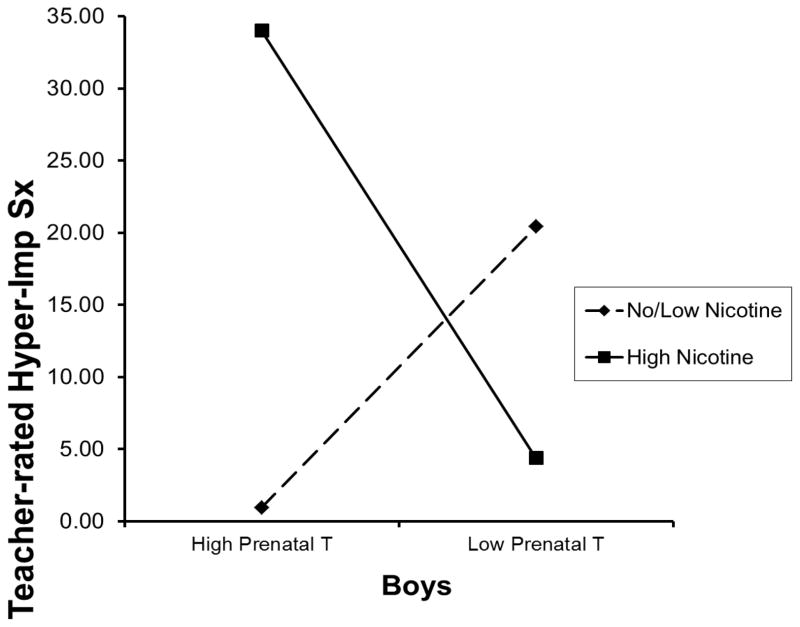
Based on multivariate regression analyses, shown in Table 3, covarying child race/ethnicity and family income, prenatal testosterone exposure interacted with prenatal nicotine use to predict teacher-rated hyperactivity-impulsivity during preschool, for boys (t=−2.06, p=.05), but not girls (t=−1.35, p=.2), although the three-way interaction was not significant (t=−.52, p=.61). This sex effect was also present for ADHD inattentive symptoms (t=−2.16, p=.05 for males; t=−1.61, p=.13 for females), for not for ODD symptoms (p≥.4 for males and females). As shown in Figure 1, boys exposed to higher prenatal testosterone and increased nicotine use during the prenatal period exhibited higher hyperactivity-impulsivity during preschool. This effect was largely absent for girls.
Table 3.
Regression Table of Interactions between Child Sex, Prenatal Testosterone, and Prenatal Substance Exposure in Relation to Teacher- and Parent-Rated Child Symptoms
| DV: | Hyper-Imp Teacher/parent n=55/N=109 | Inattention Teacher/parent | ODD Teacher/parent-report |
|---|---|---|---|
| IV (B: unstandardized beta coefficients): | |||
| Step 1 | |||
| Sex | −1.11/−.20 | −1.39/2.94 | −1.99/.39 |
| Prenatal T | 1.01/−14.89 | −7.30/−14.09 | −2.49/2.13 |
| Nicotine | .73/.23 | .52/.79 | −.85/.38 |
| Step 2 | |||
| Sex x Prenatal T | −5.50/−8.98 | 12.27/4.93 | 24.18/4.04 |
| Sex x Nicotine | 1.12/1.16 | 4.71/4.77 | 1.38/4.04 |
| Nicotine x Prenatal T | −155.31*/10.89 | −135.88*/8.58 | −57.72/4.01 |
| Step 3 | |||
| Sex x Prenatal T x Nicotine | −96.57/71.18 | −230.10/−13.65 | −5.29/56.90 |
|
| |||
| Step 1 | |||
| Sex | −1.11/−.20 | −1.39/2.94 | −1.99/.39 |
| Prenatal T | 1.01/−14.89 | −7.30/−14.09 | −2.49/2.13 |
| Alcohol | .41/.02 | .31/.61 | −1.10/.39 |
| Step 2 | |||
| Sex x Prenatal T | 31.36/−17.14 | 42.68/−2.07 | 39.33/6.15 |
| Sex x Alcohol | −2.50/−2.57** | −3.53*/−2.19* | −.89/−1.11 |
| Alcohol x Prenatal T | 2.12/1.19 | 2.32/.94 | .38/.43 |
| Step 3 | |||
| Sex x Prenatal T x Alcohol | 17.52*/6.81 | 11.34/9.65 | 12.64*/.37 |
Note.
p<.05.
p<.01. Covariates of family income and racial/ethnic minority status not shown. Prenatal T=Prenatal testosterone. Hyper-imp=Hyperactivity-impulsivity.
Figure 1.
Prenatal Testosterone by Prenatal Nicotine Use Interaction in Relation to Teacher-rated Preschool Hyperactivity-Impulsivity in Boys
Note. Hyper-Imp Sx=Hyperactivity-impulsivity symptoms. T=testosterone.
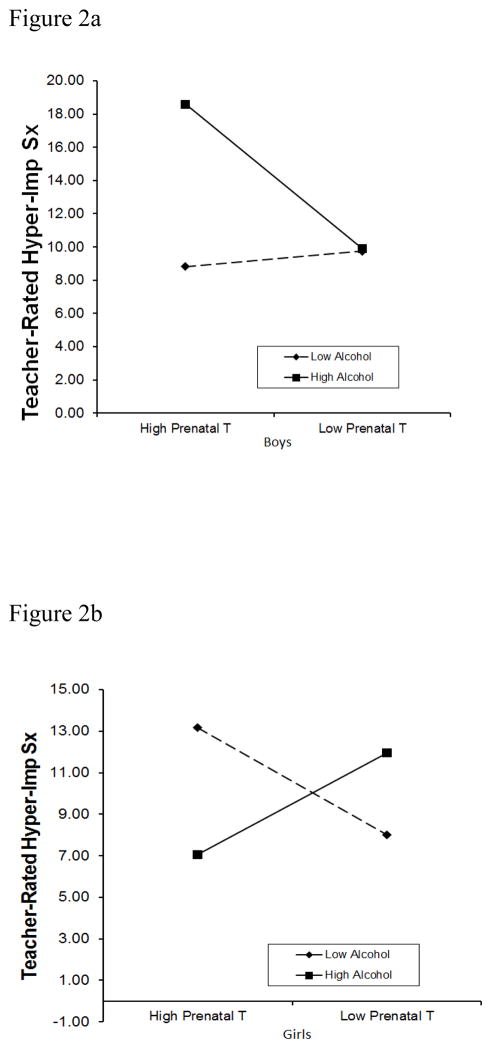
Using the same analytic strategy, prenatal testosterone interacted with prenatal alcohol exposure to predict teacher-rated hyperactivity-impulsivity differentially based on child sex (three-way interaction t=−2.49, p=.02). The three-way interaction was also significant for ODD symptoms (t=2.25, p=.03), but not inattention (p=.25). As shown in Figures 2a and 2b, boys who were exposed to higher levels of prenatal testosterone and alcohol during pregnancy exhibited increased hyperactivity-impulsivity during early childhood, but girls did not exhibit this same pattern.
Figure 2.
Figure 2a. Sex by Prenatal Testosterone by Prenatal Alcohol Use Interaction in Relation to Teacher-rated Preschool Hyperactivity-Impulsivity: Boys
Figure 2b. Sex by Prenatal Testosterone by Prenatal Alcohol Use Interaction in Relation to Teacher-rated Preschool Hyperactivity-Impulsivity: Girls
Note. Hyper-Imp Sx=Hyperactivity-impulsivity symptoms. T=testosterone.
None of the three-way interactions were significant in predicting parent-rated ADHD or ODD symptoms (p range .53–.98). However, there were significant sex by prenatal alcohol exposure interactions predicting parent-rated ADHD symptoms (i.e., hyperactivity-impulsivity and inattention; p<.05).
4. DISCUSSION
The current study examined one possible mechanism by which prenatal testosterone exposure might increase risk for DBD differentially in boys, namely by increasing male susceptibility to environmental stressors such as prenatal nicotine and alcohol use during pregnancy. Results of the current study were consistent with this idea; a proxy measure of prenatal testosterone exposure seemed to interact with maternal retrospective report of prenatal stressors differentially based on child sex to increase risk for early teacher-rated childhood hyperactivity-impulsivity. Study results suggest that males who were exposed to higher levels of prenatal testosterone and higher maternal alcohol (and, to a lesser extent, nicotine) use during pregnancy exhibited increased hyperactivity-impulsivity during early childhood, but females did not exhibit this pattern. Further, while effects of prenatal nicotine use appeared relatively specific to hyperactivity-impulsivity, effects of prenatal alcohol use generalized across child hyperactivity-impulsivity and ODD symptoms.
These results are in line with the idea that high prenatal testosterone exposure may make males more sensitive to prenatal environmental stressors, compared to females, potentially by slowing down fetal growth (Martel et al., 2009; Morris, Jordan, & Breedlove, 2004). Higher prenatal testosterone levels may function to increase sensitivity to early environmental conditions in males in order to calibrate a phenotype so that it will be well-suited for coping with these early childhood environmental conditions (Ellis et al., 2012; Geary, 2010; Martel, in press). Thus, males with high prenatal testosterone levels may be particularly sensitive to prenatal cues suggesting a harsh and/or unpredictable childhood environment with resulting neurobiological effects on dopaminergic neurotransmission and prefrontal cortex function, developing late during the prenatal period (Martel, in press; Morris et al., 2004; Goldstein & Volkow, 2002). These neurobiological effects, in turn, instantiate DBD behaviors and associated traits, including executive dysfunction, sensation-seeking, and aggression (Beauchaine et al., 2009; Huizink et al., 2006; Noland et al, 2003).
These ideas are consistent with evolutionary developmental theory, particularly sexual selection (Geary, 2010) and differential susceptibility (Ellis et al., 2012). Rather than exerting direct effects on the developing fetus, prenatal environmental stressors may provide clues that are particularly important for male fetuses that the environment is unpredictable and/or harsh, thus suggesting that DBD-related behaviors and associated traits (e.g., sensation-seeking) are going to be the most adaptive strategies for successful mating and reproduction in this environment since these behaviors facilitate male-male competition for mates (Geary, 2010; Martel, in press). Testosterone may instantiate this effect particularly in males (vs. females) by operating above a particular cutoff that may be most evident in males. Future work should examine this idea empirically and evaluate whether males are differentially susceptible to positive environmental conditions (e.g., proper nutrition) during pregnancy in such a way as to decrease DBD risk. This type of work has important implications for the development of prevention strategies, suggesting that pregnant women, particularly those expecting boys, may especially benefit from refraining from use of alcohol and nicotine during pregnancy (Becker et al., 2008; Bennett, Bendersky, & Lewis, 2007).
Notably, results were significant using measures that did not contain shared source variance (i.e., when utilizing teacher report of symptoms and parent report of prenatal stressor exposure), although, interestingly, results did not hold using parent-report of symptoms. This might be because ADHD symptoms can manifest differently based on the situational context (APA, 2013; De Los Reyes & Kazdin, 2005; Dirks et al., 2012). It is possible that parents who used higher levels of alcohol and nicotine use during pregnancy may be less objective raters of their children’s inattention and hyperactivity-impulsivity compared to teachers (Bennett et al., 2007; Knopik et al., 2006). However, it is important to note that we cannot rule out the possibility that teacher findings may be due to a Type I error; therefore, it will be important to replicate these effects. Interestingly, interactive effects of prenatal nicotine and alcohol use differed somewhat. While effects of maternal prenatal nicotine use were specific to hyperactivity-impulsivity, effects of maternal prenatal alcohol use generalized across hyperactivity-impulsivity and oppositionality. These results might be due to the somewhat different neurobiological effects of nicotine and alcohol (Koob, 2000; Lavliolette & van der Kooy, 2004; Markou, 2008).
However, it should be noted that these effects could be due to a third variable such as later environmental influences or common genetic influences (D’Onofrio et al., 2007). Parental psychopathology particularly merits attention in future work as it might influence later child behavioral outcomes through genetic transmission, prenatal smoking and alcohol use, postnatal parenting behaviors, and evocative effects of child temperament on parenting (Harold et al., 2013; Johnston et al., 2012; Knopik et al., 2006; Massey & Compton, 2013; Mills-Koonce et al., 2007). Further, race/ethnicity and family income and socioeconomic status, as well as other sociodemographic factors influencing child outcomes, merit attention in future work (Martel, 2013; Miller et al., 2009; Samuel et al., 1997). That is, low family income, race/ethnicity, low maternal education, or maternal psychopathology may account for prenatal smoking and testosterone effects on child disruptive behavior (Martel, 2013). We did not find ethnic or racial differences in finger-length ratios as is common (Loehlin et al., 2006; Manning et al., 2000; 2004; Phelps, 1952; Terrance et al., 2000); this might be because our sample was very diverse and that, in combination with the small sample size, may have limited power to detect effects. Prenatal substance use was measured retrospectively which might have made reporting biases more likely; a study limitation is that there was no independent validation of this retrospective report. Current study findings suggest the utility of investment in approaches that allow for prospective measurement of substance use during pregnancy and longitudinal follow-up of offspring. The current study relied on an indirect proxy measure of prenatal testosterone levels, finger-length ratios, which – although well-validated—is controversial and has limitations including substantial ethnic/racial differences, as well as unclear mechanisms (Brown et al., 2002; Cohen-Bendahan et al., 2005; Lutchmaya et al. 2004; Zheng & Cohn, 2011). Finally, the small sample size and limited statistical power was a salient limitation. Based on the current study’s preliminary findings using such a measure, future work could invest in attempts to measure prenatal testosterone levels directly, perhaps via amniocentesis (van de Beek, 2004).
4.1. Conclusions
Overall, study results suggest the possibility that prenatal testosterone exposure may interact with prenatal stressors differentially based on child sex to increase risk for early childhood DBD symptoms, particularly hyperactivity-impulsivity. Prospective work beginning during pregnancy is needed to critically evaluate the idea that prenatal testosterone exposure operates on DBD-related behaviors by increasing male susceptibility to negative prenatal environmental influences.
Supplementary Material
Highlights.
Disruptive Behavior Disorders (DBD) have a sex-biased prevalence rate.
Mechanisms of these sex differences remain unknown.
Analyses suggest that finger-length ratios interact with prenatal stressors in boys.
Prenatal testosterone may increase sensitivity to prenatal stressors in boys with DBD.
Acknowledgments
This research was supported by National Institute of Health and Human Development Grant 5R03 HD062599-02 to M. Martel. The sponsor had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. There are no known conflicts of interests for either of the authors. We are indebted to the families who made this study possible.
Footnotes
Conflict of Interest Statement: There are no known conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle M. Martel, Psychology Department, University of Kentucky
Bethan A. Roberts, Psychology Department, University of Kentucky
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, Hines M. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychological Science. 2009;20:144–148. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T, Klein D, Crowell S, Derbidge C, Gatzke-Kopp L. Multifinality in the development of personality disorders: A biology × sex × environment interaction model of antisocial and borderline traits. Development & Psychopathology. 2009;21:735–770. doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR. Attention-Deficit Hyperactivity Disorder: A clinical workbook. 3. New York: The Guilford Press; 2006. [Google Scholar]
- Baron R, Kenny D. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Becker K, El-Faddagh M, Schmidt MH, Esser G, Laucht M. Interaction of dopamine transpoter genotype with prenatal smoke exposure on ADHD symptoms. The Journal of Pediatrics. 2008;152:263–269. doi: 10.1016/j.jpeds.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. Journal of Developmental & Behavioral Pediatrics. 2007;28(6):467–472. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- Brown WM, Hines M, Fane BA, Breedlove SM. Masculinized finger length patterns in human males and females with Congenital Adrenal Hyperplasia. Hormones & Behavior. 2002;42:380–386. doi: 10.1006/hbeh.2002.1830. [DOI] [PubMed] [Google Scholar]
- Burchinal M, Neebe E. Best practices in quantitative methods for developmentalists: I. Data management: Recommended practices. Monographs of the Society for Research in Child Development. 2006;71:9–23. [Google Scholar]
- Cohen-Bendahan C, van de Beek C, Berenbaum S. Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neuroscience & Biobehavioral Review. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Costello E, Foley D, Angold A. 10-year research update review: The epidemiology of child and adolescent psychiatric disorders: II. Developmental epidemiology. Journal of American Academy of Child & Adolescent Psychiatry. 2006;45:8–25. doi: 10.1097/01.chi.0000184929.41423.c0. [DOI] [PubMed] [Google Scholar]
- Curran P, West S, Finch J. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychological Methods. 1996;1:16–29. [Google Scholar]
- Dearing E, Hamilton L. Best practices in quantitative methods for developmentalists: V. Contemporary advances and classic advice for analyzing mediating and moderating variables. Monographs of the Society for Research in Child Development. 2006;71:88–104. doi: 10.1111/j.1540-5834.2006.07103001.x. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Kazdin AE. Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychological Bulletin. 2005;131:483–509. doi: 10.1037/0033-2909.131.4.483. [DOI] [PubMed] [Google Scholar]
- Dirks MA, De Los Reyes A, Briggs-Gowan M, Cella D, Wakschlag LS. Embracing not erasing contextual variability in children’s behavior- theory and utility in the selection and use of methods and informants in developmental psychopathology. Journal of Child Psychology & Psychiatry. 2012;53(5):558–574. doi: 10.1111/j.1469-7610.2012.02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio B, Van Hulle C, Waldman I, Rodgers J, Rathouz P, Lahey B. Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Archives of General Psychiatry. 2007;64:1296–1304. doi: 10.1001/archpsyc.64.11.1296. [DOI] [PubMed] [Google Scholar]
- Ellis B, Boyce W, Belsky J, Bakermans-Kranenburg M, Van Ijzendoorn M. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development & Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Ellis B, Del Giudice M, Dishion TJ, Figueredo AJ, Gray P, Griskevicius V, Hawley PH, Jacobs WJ, James J, Volk AA, Wilson D. The evolutionary basis of risky adolescent behavior: Implications for science, policy, and practice. Developmental Psychology. 2012;48:598–623. doi: 10.1037/a0026220. [DOI] [PubMed] [Google Scholar]
- Fink B, Manning J, Williams J, Podmore-Nappin C. The 2nd to 4th digit ratio and developmental psychopathology in school-aged children. Personality & Individual Differences. 2007;42:369–379. [Google Scholar]
- Fink B, Neave N, Laughton K, Manning J. Second to fourth digit ratio and sensation seeking. Personality & Individual Differences. 2006;41:1253–1262. [Google Scholar]
- Geary D. Male, Female: The Evolution Of Human Sex Differences. 2. Washington, DC US: American Psychological Association; 2010. [Google Scholar]
- Geschwind N, Galaburda A. Cerebral lateralization: Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Archives of Neurology. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiology basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold GT, Leve LD, Barrett D, Elam K, Neiderhiser JM, Natsuaki MN, et al. Biological and rearing mother influences on child ADHD symptoms: Revising the developmental interface between nature and nurture. Journal of Child Psycology and Psychiatry. 2013;54(10):1038–1046. doi: 10.1111/jcpp.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam N, Williams B, Prior M, Haslam R, Graetz B, Sawyer M. The latent structure of attention-deficit/hyperactivity disorder: A taxonomic analysis. Australian & New Zealand Journal of Psychiatry. 2006;40:639–647. doi: 10.1080/j.1440-1614.2006.01863.x. [DOI] [PubMed] [Google Scholar]
- Huizink A, Mulder E. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience & Biobehavioral Review. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Johnston C, Mash EJ, Miller N, Ninowski JE. Parenting in adults with attention-deficit/hyperactivity disorder (ADHD) Clinical Psychology Review. 2012;32(4):215–228. doi: 10.1016/j.cpr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Wakschlag L. Can a valid diagnosis of disruptive behavior disorder be made in preschool children? American Journal of Psychiatry. 2002;159:351–358. doi: 10.1176/appi.ajp.159.3.351. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, et al. Maternal alcohol use disorder and offspring ADHD: Disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine. 2006;36(10):1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Sumon SA, Walker Q. The emergence of gonadal hormone influences on dopaminergic function during puberty. Hormones & Behavior. 2010;58:122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. Neurobiology of addiction: Toward the development of new therapies. Annuals of the New York Academy of Sciences. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Kraemer H, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Langley K, Holmans PA, van den Bree MBM, Thapar A. Effects of low birth weight, maternal smoking during pregnancy and social class on the phenotypic manifestations of Attention Deficit Hyperactivity Disorder and associated antisocial behavior: Investigation in a clinical sample. BMC Psychiatry. 2007;26 doi: 10.1186/1471-244X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette S, van der Kooy D. The neurobiology of nicotine addiction: Bridging the gap from molecules to behaviour. Nature Reviews Neuroscience. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Boivin M, Dionne G, Brendgen M, Vitaro F, Tremblay RE, Pérusse D. The development of hyperactive-impulsive behaviors during the preschool years: The predictive validity of parental assessments. Journal of Abnormal Child Psychology. 2008;36:977–987. doi: 10.1007/s10802-008-9227-7. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay D, McStephen M, Wood C. Attention-deficit hyperactivity disorder: A category or a continuum? Genetic analysis of a large-scale twin study. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Loehlin JC, McFadden D, Medland SE, Martin NG. Population differences in finger-length ratios: Ethnicity or latitude? Archives of Sexual Behavior. 2006;35(6):739–742. doi: 10.1007/s10508-006-9039-1. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning J. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Human Development. 2004;77:23–28. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Manning JT, Barley L, Walton J, Lewis-Jones DI, Trivers RL, Singh D, et al. The 2nd:4th ratio, sexual dimorphism, population differences, and reproductive success: Evidence for sexually antagonistic genes? Evolution and Human Behavior. 2000;21(3):163–183. doi: 10.1016/s1090-5138(00)00029-5. [DOI] [PubMed] [Google Scholar]
- Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Human Reproduction. 1998;13:3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- Manning JT, Stewart A, Bundred PE, Trivers RL. Sex and ethnic differences in 2nd to 4th digit ratio of children. Early Human Development. 2004;80(2):161–168. doi: 10.1016/j.earlhumdev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Marcus D, Barry T. Does attention-deficit/hyperactivity disorder have a dimensional latent structure? A taxometric analysis. Journal of Abnormal Psychology. 2011;120:427–442. doi: 10.1037/a0021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A. Neurobiology of nicotine dependence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM. Individual differences in Attention-Deficit/Hyperactivity Disorder symptoms and associated executive dysfunction and traits: Sex, ethnicity, and family income. American Journal of Orthopsychiatry. 2013;83(2,3):165–175. doi: 10.1111/ajop.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM. Sexual selection and sex differences in the prevalence of developmental psychopathology: Childhood externalizing and adolescent internalizing disorders. Psychological Bulletin. doi: 10.1037/a0032247. in press. [DOI] [PubMed] [Google Scholar]
- Martel MM, Gobrogge KL, Breedlove SM, Nigg JT. Masculinized finger-length ratios of boys, but not girls, are associated with Attention-Deficit/Hyperactivity Disorder. Behavioral Neuroscience. 2008;122:273–281. doi: 10.1037/0735-7044.122.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Klump K, Nigg JT, Breedlove SM, Sisk CL. Potential hormonal mechanisms of Attention-Deficit/Hyperactivity Disorder and Major Depressive Disorder: A new perspective. Hormones & Behavior. 2009;55:465–479. doi: 10.1016/j.yhbeh.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SH, Compton MT. Psychological differences between smokers who spontaneously quit during pregnancy and those who do not: A review of observational studies and directions for future research. Nicotine and Tobacco Research. 2013;15(2):307–319. doi: 10.1093/ntr/nts142. [DOI] [PubMed] [Google Scholar]
- McFadden D, Westhafer JG, Pasanen EG, Carlson C, Tucker DM. Physiological evidence of hypermasculinization in boys with the inattentive type of Attention-Deficit/Hyperactivity Disorder (ADHD) Clinical Neuroscience Research. 2005;5:233–245. [Google Scholar]
- McIntyre MH. The use of digit ratios as a marker for perinatal androgen action. Reproductive Biology & Endocrinology. 2006;4 doi: 10.1186/1477-7827-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Nigg JT, Miller RL. Attention deficit hyperactivity disorder in African American children: What can be concluded from the past ten years? Clinical Psychology Review. 2009;29:77–86. doi: 10.1016/j.cpr.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper CB, Gariepy JL, Blair C, Garrett-Peters P, Cox MJ. Bidirectional genetic and environmental influences on mother and child behavior: The family system as the unit of analyses. Development and Psychopathology. 2007;19(4):1073–1087. doi: 10.1017/S0954579407000545. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide, Fourth Edition. Los Angeles, CA: Muthen & Muthen; 1998–2012. [Google Scholar]
- Morris J, Jordan C, Breedlove S. Sexual differentiation of the vertebrate nervous system. Nature Neuroscience. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Neuman R, Lobos E, Reich W, Henderson C, Sun L, Todd R. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biological Psychiatry. 2007;61:1320–1328. doi: 10.1016/j.biopsych.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Noland JS, Singer LT, Arendt RE, Minnes S, Short EJ, Bearer CF. Executive functioning in preschool-age children prenatally exposed to alcohol, cocaine, and marijuana. Alcoholism and Clinical and Experimental Research. 2003;27:647–656. doi: 10.1097/01.ALC.0000060525.10536.F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic, 5th Version. Ft. Lauderdale, FL: Nova University; 1995. [Google Scholar]
- Pelham W, Fabiano G, Massetti G. Evidence-based assessment of Attention Deficit Hyperactivity Disorder in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:449–476. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- Pelham W, Foster E, Robb J. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. Journal of Pediatric Psychology. 2007;32:711–727. doi: 10.1093/jpepsy/jsm022. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Collett B, Gimpel G, Crowley S. Assessment of disruptive behaviors in preschoolers: Psychometric properties of the Disruptive Behavior Disorders Rating Scale and School Situations Questionnaire. Journal of Psychoeducational Assessment. 2006;24:3–18. [Google Scholar]
- Phelps VR. Relative index finger length as a sex-influenced trait in man. American Journal of Human Genetics. 1952;4:72–89. [PMC free article] [PubMed] [Google Scholar]
- Samuel VJ, Curtis S, Thornell A, George P, Taylor A, Brome DR, Biederman J, Faraone SV. The unexplored void of ADHD and African American research: A review of the literature. Journal of Attention Disorders. 1997;1(4):197–207. [Google Scholar]
- Sheeringa M. Research diagnostic criteria for infants and preschool children: The process and empirical support. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:1504–1512. doi: 10.1097/01.chi.0000091504.46853.0a. [DOI] [PubMed] [Google Scholar]
- Terrance J, Williams ME, Pepitone SE, Christensen BM, Cooke AD, Huberman NJ, et al. Finger length ratios and sexual orientation. Nature. 2000;404(6777):455–456. doi: 10.1038/35006555. [DOI] [PubMed] [Google Scholar]
- Van de Beek C, Thijssen JHH, Cohen-Kettenis PT, van Goozen SHM, Buitelaar JK. Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: What is the best source of information to investigate the effects of fetal hormonal exposure? Hormones and Behavior. 2004;46:663–669. doi: 10.1016/j.yhbeh.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Cohn MJ. Developmental basis of sexually dimorphic digit ratios. PNAS. 2011;108:16289–16294. doi: 10.1073/pnas.1108312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.