Abstract
Purpose
To determine the sensitivity of portal venous phase contrast-enhanced CT for the detection of renal stones.
Methods
This retrospective study included 97 CT examinations of the abdomen without and with intravenous contrast, including 85 (87.6%) examinations with at least one renal stone on the “gold standard” noncontrast images, as scored by a single radiologist. Three other radiologists each independently reviewed only the contrast-enhanced images from all 97 examinations and recorded all renal stones. Reviewer sensitivity for stones was categorized by stone diameter. Reviewer sensitivity and specificity for stone disease were also calculated on a per-kidney basis.
Results
The 97 cases included a total of 238 stones ≥1 mm, with a mean (±SD) of 1.2 ± 1.9 stones per kidney and a stone diameter of 3.5 ± 3.0 mm. Pooling data for the three reviewers, sensitivity for all stones was 81%; sensitivity for stones ≥2, ≥3, ≥4, and ≥5 mm was 88%, 95%, 99%, and 98%, respectively. Sensitivity for stone disease on a per-kidney basis was 94% when considering all stones; when considering only stones ≥2, ≥3, and ≥4 mm, sensitivity was 96%, 99%, and 100%, respectively. Specificity for stone disease on a per-kidney basis was 98% overall, 99% when considering only stones ≥2 mm, and 100% when considering only stones ≥3 mm. Conclusion: Contrast-enhanced CT is highly sensitive for the detection of renal stones ≥3 mm in diameter and less sensitive for smaller stones. In cases where the clinical diagnosis is uncertain and performance of a CT examination is being contemplated, intravenous contrast utilization would allow assessment for stone disease while also optimizing evaluation for other conditions.
Keywords: Renal stones, Calculi, CT, Intravenous contrast, Iodinated contrast, Emergency imaging
Flank pain with suspicion of stone disease is an extremely common cause for CT imaging in the Emergency Department (ED), accounting for greater than 20% of all ED CTs performed for non-traumatic abdominal pain [1–3]. As supported by the American College of Radiology (ACR) Appropriateness Criteria, the current imaging examination of choice in this scenario is CT of the abdomen and pelvis without intravenous contrast [4]. However, several studies have shown that the majority of these examinations are negative for stone disease, in some cases demonstrating alternative diagnoses related to the urinary tract such as infection or neoplasm, or unrelated diagnoses such as diverticulitis [3, 5–8]. While the initial clinical differential diagnosis often includes infectious, inflammatory, or neoplastic conditions which would warrant intravenous contrast administration for adequate evaluation [9], CT is generally ordered without intravenous contrast when stone disease is among the considerations. This is seemingly due to the belief that intravenous contrast would decrease sensitivity for stone disease, although we know of no studies to support such a position.
Contrast-enhanced CT is similar to unenhanced CT in demonstrating signs of urinary tract obstruction such as hydronephrosis and perinephric stranding, while often increasing sensitivity for mild obstruction by revealing an asymmetric delayed nephrogram on the side of obstruction [10, 11]. However, while ureteral stones are well outlined by the ureteral wall and periureteral fat [12, 13], it is conceivable that stones within the renal collecting system may be obscured on contrast-enhanced CT due to the surrounding enhancing renal parenchyma during the portal venous/late corticomedullary phase. Nevertheless, in our experience, even small renal stones are often seen on contrast-enhanced CT. Although controversial, many studies have shown that these seemingly incidental non-obstructing stones may be clinically relevant as a potential etiology of chronic or recurrent flank pain [14–21]. As such, our aim was to determine the sensitivity of portal venous phase contrast-enhanced CT for the detection of stones within the renal calyces and pelvis.
Materials and methods
Institutional review board approval was obtained for this HIPAA compliant study. The picture archiving and communication system (PACS) was searched for 85 consecutive cases of CT of the abdomen and pelvis performed without and with IV contrast between August 2010 and September 2012 meeting the following criteria: there was at least one stone measuring greater than or equal to 1 mm in diameter in either kidney, noncontrast images and portal venous phase postcontrast images (defined as contrast enhancement within the portal vein) were available, 2.5-mm-thick axial images were available for both series of images, and there was no prior partial nephrectomy (which may result in high density foci within the kidney attributable to surgical material). In order to limit potential reviewer bias [22], additional 12 cases obtained in the course of this search without renal stones were added to the group for a total of 97 cases. All CTs were performed using one of three GE LightSpeed VCT 64-slice scanners (General Electric Healthcare, Milwaukee, WI). Six of the cases meeting criteria were also found to have ureteral stones; these stones were excluded, as the focus of this study was limited to renal (calyceal or pelvic) stones.
Noncontrast images from each case served as our reference standard. They were reviewed by one author (MHS) and the location, size, and maximum Hounsfield unit (HU) attenuation value of each stone were recorded. For a calcification to be recorded as a stone, it was required to measure at least 1 mm in axial diameter and to not be typical of another type of calcification such as vascular calcification. This reviewer had access to all planes of imaging as well as the contrast-enhanced series in order to aid in the determination of the presence of stones and to distinguish them from vascular calcification, when relevant. Three other reviewers (RJD, DRD, and MS), each board certified in diagnostic radiology and fellowship trained in abdominal imaging, independently reviewed portal venous phase images from each case while blinded to the noncontrast images. Reviewers were informed that not all the examinations would include stones. Reviewers were unaware of any clinical history or results of prior imaging examinations. The location of each identified stone (i.e., right or left kidney, and image number) and its largest axial diameter were recorded. The reviewers could refer to reconstructed coronal images, but only to confirm the presence of a stone seen on the axial images. Routine use of coronal images was not required and would not be expected to increase sensitivity, especially considering that the 2.5-mm-thick axial images were thinner than the 3-mm-thick coronal images [23].
Descriptive statistics were calculated using Microsoft Excel. Other statistical analyses were performed using SPSS for Windows, Version 20 (SPSS, Inc., Chicago, IL, USA).
Spearman rank correlation test was used to test correlation between stone size and stone attenuation values. Analyses of stone detection by the reviewers were performed using five stone size categories: all stones, stones greater than or equal to 2 mm in diameter, stones greater than or equal to 3 mm in diameter, stones greater than or equal to 4 mm in diameter, and stones greater than or equal to 5 mm in diameter. On a per stone basis, Cohen’s kappa between each of the three reviewers was calculated. Strength of agreement was determined using the categories of Landis and Koch [24, 25]. For each reviewer, sensitivity for stone detection was calculated for each of the size categories. Specificity could not be calculated due to lack of a valid “true negative” category. Images recorded as containing a stone were categorized as false positives when no stone was present on the corresponding noncontrast images. Mean diameters of undetected and false-positive stones were calculated.
For each reviewer, sensitivity and specificity for stone disease on a per-kidney basis were also calculated for each of the size categories. For the purposes of this analysis, the following definitions were used: true positive = correctly detecting any stone in the kidney, true negative = correctly scoring the kidney as having no stones, false positive = recording a stone in a kidney where there was no stone in the entire kidney, and false negative = scoring a kidney as having no stones when there was indeed at least one stone.
Results
Based on review of noncontrast images, the 97 cases contained a total of 238 stones. Characteristics of the cases are detailed in Table 1 and a histogram of stone sizes is shown in Fig. 1. There was a mean (±SD) of 2.5 ± 3.2 stones per patient, with a range of 0–16. There was a mean (±SD) of 1.2 ± 1.9 stones per kidney, with a range of 0–11. Maximum stone attenuation values ranged from 114 to 1575 HU with a mean (±SD) of 549 ± 373 HU. Stone diameter ranged from 1 to 25 mm with a mean (±SD) of 3.5 ± 3.0 mm. Spearman rank correlation testing the relationship between stone size and attenuation value demonstrated a strong correlation, with a rho of 0.73. Due to this correlation and the generally more important clinical usefulness of stone size [1, 26, 27], subsequent analyses were performed using categories based on size rather than attenuation value.
Table 1.
Renal stone characteristics of selected patient cases
| Patient cases—total 97 | |
| 85 (88%) | At least one stone in either kidney |
| 12 (12%) | No stones bilaterally |
| 28 (29%) | Only left renal stone(s) |
| 28 (29%) | Only right renal stone(s) |
| 29 (30%) | Bilateral stones |
| Kidneys—total 194 | |
| 114 (59%) | Kidneys with stones |
| 80 (41%) | Kidneys without stones |
| Renal stones—total 238 | |
| 178 (75%) | ≥2 mm |
| 93 (39%) | ≥3 mm |
| 59 (25%) | ≥4 mm |
| 41 (17%) | ≥5 mm |
Fig. 1.
Histogram of renal stone sizes.
The 97 cases were reviewed independently by the three reviewers over multiple reading sessions. The kappa values between each of the three reviewers for each of the stone size categories are tabulated in Table 2. Sensitivities for each reviewer, separated by size category, are tabulated in Table 3. Sensitivities and specificities for stone disease on a per-kidney basis are tabulated in Table 4 for each reviewer and size category.
Table 2.
Kappa values between the three reviewers
| Reviewer 1 vs. reviewer 2 | Reviewer 1 vs. reviewer 3 | Reviewer 2 vs. reviewer 3 | |
|---|---|---|---|
| All stones | 0.62 | 0.58 | 0.50 |
| ≥2 mm | 0.61 | 0.58 | 0.48 |
| ≥3 mm | 0.37 | 0.52 | 0.56 |
| ≥4 mm | Undefineda | Undefineda | Undefineda |
| ≥5 mm | Undefineda | Undefineda | Undefineda |
Measures of association could not be computed, as at least one variable upon which measures of association are computed is a constant
Table 3.
Sensitivity of each reviewer for renal stones (%)
| Reviewer 1 | Reviewer 2 | Reviewer 3 | All reviewersa | |
|---|---|---|---|---|
| All stones | 81 | 86 | 76 | 81 |
| <2 mm | 62 | 68 | 52 | 61 |
| <3 mm | 73 | 79 | 64 | 72 |
| ≥2 mm | 85 | 92 | 87 | 88 |
| ≥3 mm | 92 | 97 | 96 | 95 |
| ≥4 mm | 97 | 100 | 100 | 99 |
| ≥5 mm | 95b | 100 | 100 | 98b |
Pooled data for all three reviewers
Reviewer 1 did not detect two 5-mm stones
Table 4.
Sensitivity and specificity of each reviewer for stone disease on a per-kidney basis (%)
| Reviewer 1 | Reviewer 2 | Reviewer 3 | All reviewersa | |
|---|---|---|---|---|
| All stones | ||||
| Sensitivity | 96 | 96 | 91 | 94 |
| Specificity | 98 | 100 | 98 | 98 |
| ≥2 mm | ||||
| Sensitivity | 98 | 97 | 94 | 96 |
| Specificity | 99 | 100 | 99 | 99 |
| ≥3 mm | ||||
| Sensitivity | 98 | 100 | 98 | 99 |
| Specificity | 100 | 100 | 100 | 100 |
| ≥4 mm | ||||
| Sensitivity | 100 | 100 | 100 | 100 |
| Specificity | 100 | 100 | 100 | 100 |
| ≥5 mm | ||||
| Sensitivity | 100 | 100 | 100 | 100 |
| Specificity | 100 | 100 | 100 | 100 |
Pooled data for all three reviewers
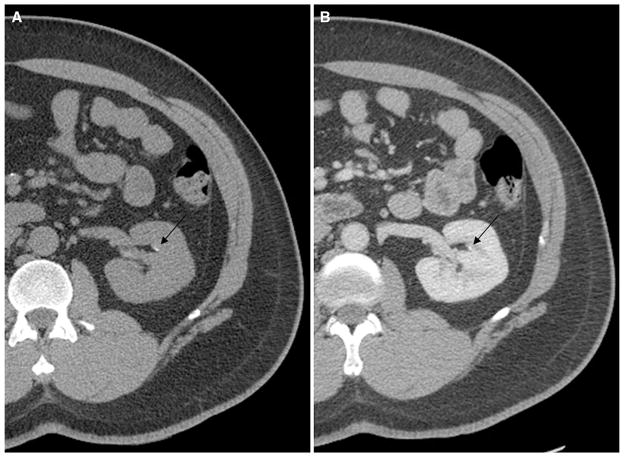
For the three reviewers, there was a combined total of 135 stones which were not detected, out of a maximum combined total of 714 stones. The mean diameter (±SD) of these undetected stones was 2.1 ± 0.75 mm with a range of 1–5 mm. Figure 2 provides an example of a 3-mm stone that was not detected on postcontrast images by one of the three reviewers. There was also a combined total of 10 false-positive stones, with a mean recorded diameter (±SD) of 1.5 ± 0.66 mm and a range of 1–2.9 mm.
Fig. 2.
A 47-year-old man with bilateral flank pain. Non-contrast (A) and postcontrast (B) CT images demonstrate a 3-mm non-obstructing calyceal stone in the left kidney. This stone was not detected on the postcontrast series of images by one of the three reviewers.
Discussion
It has become a standard practice in today’s ED for CTs to be performed without intravenous contrast when urinary tract stone disease is a major consideration. While supported by the ACR Appropriateness Criteria, this strategy has a major practical disadvantage in that the symptoms of the majority of these patients are not actually due to stone disease, but rather to a different urinary tract or non-urinary tract condition which may clinically mimic stone disease [3, 5–8, 28]. The lack of intravenous contrast in these cases may decrease the sensitivity or degree of confidence for various diagnoses. For example, in cases where there is perinephric stranding without a stone or ureteral dilatation, contrast would be very useful to differentiate between a passed stone and other pathology such as pyelonephritis, renal infarct, or renal vein thrombosis [6, 7, 28–30]. Neoplasms such as renal cell or urothelial carcinoma, which may be subtle or occult on noncontrast CT [31], can also present with flank pain and hematuria and initially may be mistaken for stone disease [28]. The diagnosis of many gynecologic, gastrointestinal, pancreaticobiliary, and vascular conditions which may clinically mimic renal colic would also benefit from the administration of intravenous contrast [28]. Furthermore, there is no literature to support the dogma that intravenous contrast would limit the evaluation of stone disease. In fact, intravenous contrast may actually help by revealing a delayed nephrogram due to urinary tract obstruction [10, 11]. Excretory phase images can also help in the distinction between a ureteral stone and a phlebolith, as recommended by the ACR Appropriateness Criteria for uncertain cases [1, 4, 32].
In our sample, the overall sensitivity for all renal stones on contrast-enhanced CT was 81%. As would be expected, sensitivity generally correlated positively with size, with a sensitivity of 95% for stones measuring at least 3 mm in diameter, and 99% for stones with a diameter of at least 4 mm. (Note that overall sensitivity for stones at least 5 mm in diameter was slightly lower at 98%, which was due to the impact of one reviewer’s failure to detect two 5-mm stones within a single kidney. This was likely a recording error, considering that the kidney referred to contained a total of 9 stones.) Sensitivity for smaller stones was much lower, at 72% for stones <3 mm in diameter and 61% for stones <2 mm in diameter.
Overall sensitivity for stone disease on a per-kidney basis was higher, at 94% for all stones, 99% for stones measuring at least 3 mm in diameter, and 100% once stone diameter reached 4 mm. The overall higher sensitivity reflects the fact that kidneys afflicted by nephrolithiasis often contain multiple stones, increasing the likelihood that at least one stone would be detected.
As manifested by the much lower sensitivities of the <2 and <3 mm stone categories, most of the undetected stones in our series were relatively small, with a mean diameter of 2.1 mm. However, many very small stones could still be detected on the contrast-enhanced images. Figure 3 provides an example of one such stone.
Fig. 3.
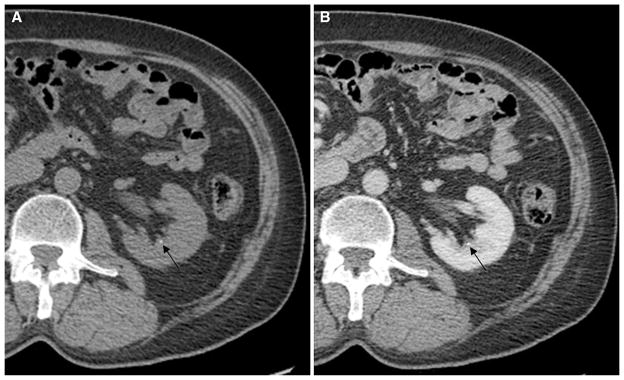
A 69-year-old man with microscopic hematuria. Noncontrast (A) and postcontrast (B) CT images demonstrate a 1.1-mm non-obstructing calyceal stone in the left kidney (arrows). This stone was detected on the postcontrast series of images by all three reviewers.
Overall specificity for stone disease on a per-kidney basis was also very high at 98% for all stones and 100% for stones greater than 3 mm in diameter. Ten false-positive stones, all of which were recorded at less than 3 mm in diameter, may in some cases have corresponded to foci of vascular enhancement mistaken for tiny stones.
The clinical relevance of non-obstructing stones is subject to ongoing debate [14]. While some may dismiss these stones as incidental findings; many studies have shown that these stones can be a cause of symptoms, often chronic and resulting in multiple ED visits and CT scans, with relief of pain after treatment [14–21]. It has been theorized that small seemingly non-obstructing renal stones may cause symptoms due to mucosal irritation, intermittent calyceal obstruction, or obstruction of the collecting ducts in the case of papillary stones [21]. In the absence of another explanation for a patient’s symptoms, such stones should be considered possibly significant and urologic consultation may be warranted.
There are other instances when the ability to detect non-obstructing renal stones may hold clinical significance. For example, when a passed ureteral stone is a consideration for a patient’s symptoms, the detection of a renal stone would lend support to a stone-related etiology over other possible causes such as urinary tract infection or neoplasm [33]. In other cases, renal stones may be inherently significant as a marker of metabolic disease, potentially even altering management in patients with hyperparathyroidism [33, 34]. It should be noted, however, that the large number and very small size of stones often present in patients with medullary nephrocalcinosis might sometimes cause them to be unnoticed or misattributed to vascular enhancement on contrast-enhanced CT, due to the seemingly homogenous and symmetric appearance of the kidney.
In cases where stone disease is the primary consideration, avoidance of the potential nephrotoxic effects of intravenous contrast is another reason that noncontrast scanning may be favored. However, recently reported studies which included control subjects have shown that currently used intravenous contrast has limited if any risk for nephrotoxicity in most patients [35–38]. As such, patients with good renal function should not be denied intravenous contrast in cases when it may benefit their diagnostic workup. While a recent study has shown that intravenous contrast administration for CT does entail a higher radiation dose to some organs [39], the decreased likelihood of requiring a repeat examination with intravenous contrast should far outweigh that consideration.
Our study had several limitations. First, the focus of this study was limited to non-obstructing stones within the renal collecting system and did not include stones obstructing the ureter, which are usually of greater concern in the emergency setting. Even small ureteral stones are certainly more problematic than renal stones and are likely to present with acute symptoms. Although intravenous contrast should theoretically facilitate detection of obstructing ureteral stones [10, 11], data to prove that such stones can indeed be detected on contrast-enhanced CT would be useful to reassure clinicians that intravenous contrast will not hinder diagnosis of obstructive ureteral stone disease. Still, as stated above, we chose to focus our investigation on renal stones which abut enhancing renal parenchyma and could hypothetically go undetected. Our data demonstrates that for most stones, particularly above 3–4 mm in diameter, this is actually not a concern.
A limitation of our study design is that only a single reviewer served to establish the presence and location of stones on the noncontrast series of images for each examination. In designing the study, we felt that the detection of stones within the kidney on noncontrast images was a fairly straightforward task with a binary answer that would not require corroboration by multiple reviewers.
Another limitation inherent to our study design relates to the fact that the three reviewers were all aware of the purpose of this study and were presumably meticulous in inspecting the images for stones, possibly identifying some which they may not have otherwise seen in a routine clinical review. Still, the study included 12 completely negative cases (i.e., no stones bilaterally) and overall 80 of 194 kidneys without stones, which we hoped would diminish the bias to “overcall” stones and more closely mimic normal reading conditions. Also, despite this limitation, our results do demonstrate that it is possible to detect renal stones on a contrast-enhanced examination if one reviews the images thoroughly, as one certainly should in a clinical scenario of urinary tract-related symptoms, or when there is no other identifiable cause of abdominal pain.
While all the reviewers were fellowship trained in abdominal imaging, most radiologists who interpret abdominal CTs on a regular basis, such as on-call in the ED, are likely to have comparable competency in detecting renal stones. Likewise, while all of our CTs were performed using a 64-slice GE VCT scanner, most modern CT scanners which can generate 2.5-mm-thick images should provide data of comparable quality for the purposes of renal stone detection.
A potential limitation in the generalizability of our results relates to variability in the precise phase of imaging. All the cases selected for this series included imaging in the portal venous phase of imaging, usually corresponding to the late corticomedullary or early nephrographic phase of renal enhancement. Some cases may have had a slightly earlier phase of renal arterial enhancement (possibly increasing sensitivity for stones) or a slightly later nephrographic phase. We believe that this minor variability, which is related to technique as well as patient cardiac and renal function, likely reflects the expected variation at most centers. However, the use of a test injection or a technical error resulting in excretory phase imaging would certainly severely limit sensitivity for renal stones, and such cases were not evaluated in our study.
In conclusion, this study demonstrates that contrast-enhanced CT is highly sensitive in the detection of renal calyceal and pelvic stones measuring at least 3 mm in diameter, though somewhat less so for smaller stones. Consequently, when there are reasonable diagnostic considerations aside from urinary tract stone disease, our results would support the use of intravenous contrast, as it would aid in the diagnosis of other conditions while not compromising evaluation for renal stones 3 mm in diameter and larger. Considering the current increased focus on limiting medical radiation, and new data suggesting a lower than previously believed risk profile of modern contrast agents [35–38], such an approach of utilizing a single contrast-enhanced study to evaluate for all forms of abdominal disease would seem prudent and warrants validation with a prospective study.
Acknowledgments
Funding for H.W.C.—This publication was supported in part by the CTSA Grant UL1RR025750, KL2RR025749, and TL1RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
References
- 1.Kambadakone AR, Eisner BH, Catalano OA, Sahani DV. New and evolving concepts in the imaging and management of urolithiasis: urologists’ perspective. Radiographics. 2010;30(3):603–623. doi: 10.1148/rg.303095146. [DOI] [PubMed] [Google Scholar]
- 2.Rosen MP, Siewert B, Sands DZ, et al. Value of abdominal CT in the emergency department for patients with abdominal pain. Eur Radiol. 2003;13(2):418–424. doi: 10.1007/s00330-002-1715-5. [DOI] [PubMed] [Google Scholar]
- 3.Abujudeh HH, Kaewlai R, McMahon PM, et al. Abdominopelvic CT increases diagnostic certainty and guide management decisions: a prospective investigation of 584 patients in a large academic medical center. AJR. 2011;196(2):238–243. doi: 10.2214/AJR.10.4467. [DOI] [PubMed] [Google Scholar]
- 4.American College of Radiology. Acute onset flank pain—suspicion of stone disease. [Accessed 29 July 2013];ACR Appropriateness Criteria®. 2011 http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/AcuteOnsetFlankPainSuspicionStoneDisease.pdf.
- 5.Smith RC, Verga M, McCarthy S, Rosenfield AT. Diagnosis of acute flank pain: value of unenhanced helical CT. AJR. 1996;166(1):97–101. doi: 10.2214/ajr.166.1.8571915. [DOI] [PubMed] [Google Scholar]
- 6.Chen MY, Zagoria RJ, Saunders HS, Dyer RB. Trends in the use of unenhanced helical CT for acute urinary colic. AJR. 1999;173:1447–1450. doi: 10.2214/ajr.173.6.10584780. [DOI] [PubMed] [Google Scholar]
- 7.Tamm EP, Silverman PM, Shuman WP. Evaluation of the patient with flank pain and possible ureteral calculus. Radiology. 2003;228(2):319–329. doi: 10.1148/radiol.2282011726. [DOI] [PubMed] [Google Scholar]
- 8.Dalrymple NC, Verga M, Anderson KR, et al. The value of unenhanced helical computerized tomography in the management of acute flank pain. J Urol. 1998;159:735–740. [PubMed] [Google Scholar]
- 9.American College of Radiology. Appropriateness criteria—diagnostic imaging topics. [Accessed 29 July 2013];ACR Appropriateness Criteria®. 2013 http://www.acr.org/Quality-Safety/Appropriateness-Criteria/Diagnostic.
- 10.Saunders H, Dyer R, Shifrin R, et al. The CT nephrogram: implications for evaluation of urinary tract disease. Radiographics. 1995;15:1069–1085. doi: 10.1148/radiographics.15.5.7501851. [DOI] [PubMed] [Google Scholar]
- 11.Cheng PM, Moin P, Dunn MD, Boswell WD, Duddalwar VA. What the radiologist needs to know about urolithiasis: part 2–CT findings, reporting, and treatment. AJR. 2012;198(6):W548–W554. doi: 10.2214/AJR.11.8462. [DOI] [PubMed] [Google Scholar]
- 12.Smith RC, Verga M, Dalrymple N, McCarthy S, Rosenfield AT. Acute ureteral obstruction: value of secondary signs on helical unenhanced CT. AJR. 1996;167:1109–1113. doi: 10.2214/ajr.167.5.8911160. [DOI] [PubMed] [Google Scholar]
- 13.Heneghan JH, Dalrymple NC, Verga M, Rosenfield AT, Smith RC. Soft-tissue “rim” sign in the diagnosis of ureteral calculi with use of unenhanced helical CT. Radiology. 1997;202:709–711. doi: 10.1148/radiology.202.3.9051021. [DOI] [PubMed] [Google Scholar]
- 14.Furlan A, Federle MP, Yealy DM, Averch TD, Pealer K. Nonobstructing renal stones on unenhanced CT: a real cause for renal colic? AJR. 2008;190:W125–W127. doi: 10.2214/AJR.07.2922. [DOI] [PubMed] [Google Scholar]
- 15.Andersson L, Sylvén M. Small renal caliceal calculi as a cause of pain. J Urol. 1983;130:752–753. doi: 10.1016/s0022-5347(17)51440-8. [DOI] [PubMed] [Google Scholar]
- 16.Brandt B, Ostri P, Lange P, Kvist Kristensen J. Painful caliceal calculi. The treatment of small nonobstructing caliceal calculi in patients with symptoms. Scand J Urol Nephrol. 1993;27:75–76. doi: 10.3109/00365599309180418. [DOI] [PubMed] [Google Scholar]
- 17.Mee SL, Thuroff JW. Small caliceal stones: is extracorporeal shock wave lithotripsy justified? J Urol. 1988;139:908–910. doi: 10.1016/s0022-5347(17)42712-1. [DOI] [PubMed] [Google Scholar]
- 18.Brannen GE, Bush WH, Lewis GP. Caliceal calculi. J Urol. 1986;135:1142–1145. doi: 10.1016/s0022-5347(17)46018-6. [DOI] [PubMed] [Google Scholar]
- 19.Lau PC, Norman RW. When is ESWL of small calyceal stones indicated? Can J Urol. 1997;4:413–415. [PubMed] [Google Scholar]
- 20.Schiff SF, Dretler SP. Extracorporeal shock wave lithotripsy. J Endourol. 1988;2:31–34. [Google Scholar]
- 21.Jura YH, Lahey S, Eisner BH, Dretler SP. Ureteroscopic treatment of patients with small, painful, non-obstructing renal stones: the small stone syndrome. Clin Nephrol. 2013;79(1):45–49. doi: 10.5414/CN107637. [DOI] [PubMed] [Google Scholar]
- 22.Sica GT. Bias in research studies. Radiology. 2006;238(3):780–789. doi: 10.1148/radiol.2383041109. [DOI] [PubMed] [Google Scholar]
- 23.Memarsadeghi M, Schaefer-Prokop C, Prokop M, et al. Unenhanced MDCT in patients with suspected urinary stone disease: do coronal reformations improve diagnostic performance? AJR. 2007;189(2):W60–W64. doi: 10.2214/AJR.07.2199. [DOI] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 25.Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003;228(2):303–308. doi: 10.1148/radiol.2282011860. [DOI] [PubMed] [Google Scholar]
- 26.Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR. 2002;178(1):101–103. doi: 10.2214/ajr.178.1.1780101. [DOI] [PubMed] [Google Scholar]
- 27.Preminger GM, Tiselius HG, Assimos DG, et al. 2007 guideline for the management of ureteral calculi. J Urol. 2007;178(6):2418–2434. doi: 10.1016/j.juro.2007.09.107. [DOI] [PubMed] [Google Scholar]
- 28.Rucker CM, Menias CO, Bhalla S. Mimics of renal colic: alternative diagnoses at unenhanced helical CT. Radiographics. 2004;24(suppl 1):S11–S28. doi: 10.1148/rg.24si045505. [DOI] [PubMed] [Google Scholar]
- 29.Krinsky G. Unenhanced helical CT in patients with acute flank pain and renal infarction: the need for contrast material in selected cases. AJR. 1996;167:282–283. doi: 10.2214/ajr.167.1.8659408. [DOI] [PubMed] [Google Scholar]
- 30.Talner L, Vaughan M. Nonobstructive renal causes of flank pain: findings on noncontrast helical CT (CT KUB) Abdom Imaging. 2003;28:210–216. doi: 10.1007/s00261-001-0188-3. [DOI] [PubMed] [Google Scholar]
- 31.Dyer R, DiSantis DJ, McClennan BL. Simplified imaging approach for evaluation of the solid renal mass in adults. Radiology. 2008;247:331–342. doi: 10.1148/radiol.2472061846. [DOI] [PubMed] [Google Scholar]
- 32.Spencer BA, Wood BJ, Dretler SP. Helical CT and ureteral colic. Urol Clin N Am. 2000;27(2):231–241. doi: 10.1016/s0094-0143(05)70253-6. [DOI] [PubMed] [Google Scholar]
- 33.Berkenblit R, Hoenig DM, Lerer D, Moses M, Minsky L. Comparison of 0.625-mm source computed tomographic images versus 5-mm thick reconstructed images in the evaluation for renal calculi in at-risk patients. J Endourol. 2013;27(2):238–241. doi: 10.1089/end.2012.0157. [DOI] [PubMed] [Google Scholar]
- 34.Bilezikian JP, Potts JT, Jr, Fuleihan G-H, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353–5361. doi: 10.1210/jc.2002-021370. [DOI] [PubMed] [Google Scholar]
- 35.McDonald RJ, McDonald JS, Bida JP, et al. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology. 2013;267(1):106–118. doi: 10.1148/radiol.12121823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald JS, McDonald RJ, Comin J, et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119–128. doi: 10.1148/radiol.12121460. [DOI] [PubMed] [Google Scholar]
- 37.Davenport MS, Khalatbari S, Dillman JR, et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94–105. doi: 10.1148/radiol.12121394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newhouse JH, RoyChoudhury A. Quantitating contrast medium-induced nephropathy: controlling the controls. Radiology. 2013;267(1):4–8. doi: 10.1148/radiol.13122876. [DOI] [PubMed] [Google Scholar]
- 39.Amato E, Salamone I, Naso S, et al. Can contrast media increase organ doses in CT examinations? A clinical study. AJR. 2013;200:1288–1293. doi: 10.2214/AJR.12.8958. [DOI] [PubMed] [Google Scholar]