Introduction
Treatment with tissue plasminogen activator (tPA) is standard of care treatment of acute ischemic stroke. Various risk factors have been associated with the development of intracranial hemorrhage (ICH) following thrombolysis, but there is no literature on the use of tPA in patients with known vasculitis who develop acute ischemic stroke. We present a case of ICH after tPA administration in a patient with known Churg-Strauss Syndrome (CSS) or eosinophilic granulomatosis with polyangiitis and discuss the implications of thrombolysis in patients with vasculitis.
Case Report
A 53 year old right-handed man with a past medical history of well controlled hyperlipidemia, negative anti-neutrophil cytoplasmic antibodies (ANCA) CSS characterized by asthma, eosinophilia, nail-fold infarcts and peripheral neuropathy presented acutely with dysarthria, left facial weakness and partial sensory loss over the left upper extremity. CSS was diagnosed eleven years earlier based on the clinical presentation and with a skin biopsy that demonstrated vasculitis. There was no history of hypertension or diabetes. His disease has not been well controlled as he had pulmonary symptoms and his eosinophil count before admission was 14% therefore he was continued on prednisone 5 mg daily, methotrexate 15 mg weekly. His other medication included simvastatin 40 mg nightly. The patient was not receiving antiplatelet or nonsteroidal anti-inflammatory drugs. He had a remote 12 pack-year smoking history. Serologic studies were significant for an eosinophil count of 12%, C-reactive protein (CRP) of 4.6 mg/L, and an elevated fibrinogen level of 547 mg/dL; otherwise, laboratory values were normal, including coagulation profiles, platelet count and chemistries. Non-contrast head computed tomography (CT) showed a chronic lacunar infarct in the right caudate nucleus, but no evidence of intracranial hemorrhage, early infarction or edema (Figure 1). His initial National Institutes of Health Stroke Scale (NIHSS) score was four. Intravenous (IV) tPA was administered 130 minutes after the onset of symptoms. One hour after the start of tPA infusion, his level of consciousness declined and he developed a dense left hemiparesis, vomited and required intubation for airway protection. A repeat head CT showed an 8 × 3 cm intraparenchymal hemorrhage in the deep white matter of the right hemisphere with 6 mm of right-to-left shift of the septum pellucidum and intraventricular hemorrhage involving both lateral as well as the third and fourth ventricles. The patient was given cryoprecipitate, phenytoin and a continuous nicardipine infusion to keep mean arterial blood pressure less than 100. Hydrocortisone 50 mg IV every 6 hours was given, and his scheduled dose of methotrexate was held. The patient underwent decompressive hemi-craniectomy the following day, and subsequently showed gradual neurologic improvement. Magnetic resonance imaging (MRI) revealed an area of restricted diffusion representing an ischemic stroke in the right anterior centrum semiovale with interval evolution of the right basal ganglia hemorrhage with associated mass effect. There were no findings on CT angiography of the head and neck to suggest central nervous system (CNS) vasculitis or large vessel disease. Transthoracic echocardiography with agitated saline contrast, carotid duplex imaging and cardiac telemetry were unrevealing of a source of embolism. Serological investigations were unremarkable, including a negative ANCA, anti-cardiolipin antibodies, beta-2 glycoprotein antibody, anti-nuclear antibody, extractable nuclear antigens, rheumatoid factor and methylenetetrahydrofolate reductase mutation. Three weeks after admission, he had fluent speech with a right gaze preference, left hemi-neglect, left central seventh nerve palsy, a dense left hemiparesis and complete sensory loss on the left. He was discharged on antiplatelet and a prednisone taper. Three months later he was readmitted for pneumonia and was found to have a 2 × 1 cm right basal ganglia hemorrhage and a 4 × 2 cm right parieto-occipital hemorrhage. MRI showed multiple small sub-centimeter foci of restricted diffusion in the bilateral cerebellar hemispheres, right occipital lobe and right temporal lobe with corresponding prolonged T2/FLAIR signal intensity consistent with ischemic strokes. Magnetic resonance angiography of the brain was normal. Conventional cerebral arteriography did not reveal evidence of vasculitis. Cerebrospinal fluid examination revealed normal cell counts and protein. The serum eosinophil count peaked at 26%. Brain biopsy was inconclusive due to the small amount of tissue available for analysis. A head CT obtained 13 months after the initial event showed extensive chronic changes, including regions of encephalomalacia involving the right cerebral peduncle, midbrain, basal ganglia, frontal, parietal and temporal lobes.
Figure 1.
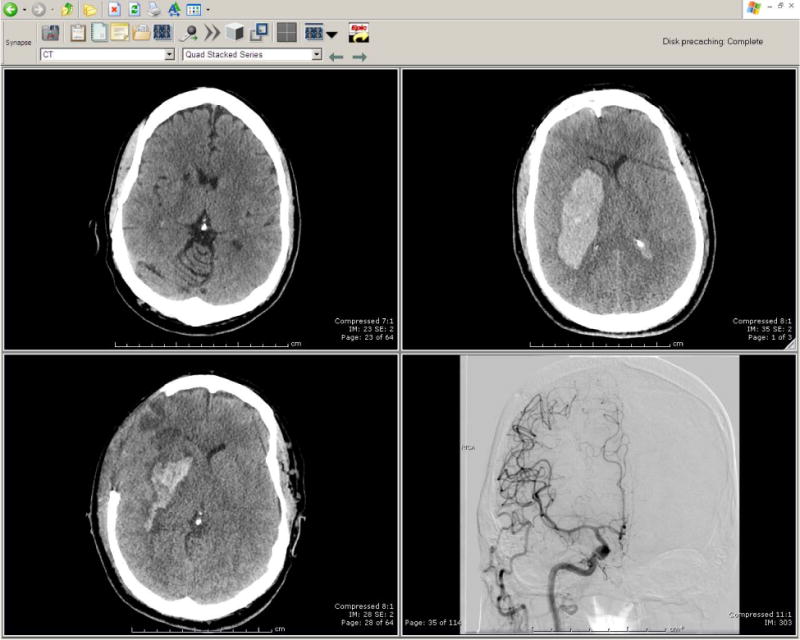
Head Computed Tomography and conventional cerebral arteriography
Non-contrast head CT at presentation with slurred speech left sided facial droop and sensory loss showed chronic lacunar infarct in the right caudate nucleus (A). CT head obtained one hour after the start of tPA administration, revealing a right subcortical acute intraparenchymal hemorrhage with ventricular extension (B), and one day after hemicraniectomy (C). Conventional cerebral arteriography demonstrated no clinical evidence of CNS vasculitis (D).
Discussion
Churg–Strauss syndrome (CSS) is a rare necrotizing and granulomatous vasculitis of small vessels. It is characterized clinically by a prodromal phase with chronic sinusitis, bronchial asthma, pulmonary infiltrates and eosinophilia, followed by a vasculitic phase with generalized multisystemic disease. To our knowledge, thrombolysis and the subsequent neurologic course has not previously been described in patients with CSS presenting with acute ischemic stroke. Although CSS rarely involves the central nervous system1, ischemic stroke complicated by intracerebral hemorrhage is a devastating complication. This case provides a rare and important opportunity to consider the pathophysiology of stroke in small vessel vasculitides and its implication for IV tPA use. The criteria for diagnosis of CSS are asthma, marked eosinophilia (eosinophil count > 10%), history of allergy, mononeuropathy or polyneuropathy, non-fixed pulmonary infiltrates, paranasal sinus abnormalities, and extravascular eosinophils2. Organ biopsy is not always helpful. Our patient fulfilled the diagnostic criteria for CSS by exhibiting elevated eosinophil counts and asthma. The pathogenesis of ischemic stroke in CSS is multifactorial, particularly in our young patient with hyperlipidemia as his only stroke risk factor. In CSS, ANCA antibodies can induce the release of free radicals and granule enzymes from neutrophils leading to vascular inflammation, making the endothelial lining prothrombotic and proadhesive3. CSS also involves an inflammatory arteriopathy with granulomatous fibrinoid necrosis of the vascular media. Cytokines can stimulate fibrinogen synthesis and platelet aggregation. In addition, our patient had eosinophilia at the time of initial presentation. Eosinophilic cationic protein (ECP) has been shown to bind to Hageman factor in vitro and subsequently activate the intrinsic coagulation pathway3. ECP could interfere with the anticoagulant activity of endogenous heparan sulphate. Together, these factors may promote fibrinogenesis and endothelial cell activation, contributing to the development of ischemic stroke. In a case series of CSS and thrombosis, it was observed that high levels of fibrinogen and von Willebrand factor (vWF) predated thrombosis3. A study of 114 consecutive children diagnosed with primary angiitis or secondary CNS vasculitis demonstrated an elevated CRP level in 26%, increased erythrocyte sedimentation rate in 57%, abnormal MRI in 91%, abnormal cerebral angiography in 33% and vasculitis on brain biopsy in 75%. vWF levels can mirror disease activity2. These data provide insight to how these values might be used to evaluate disease activity in CSS. CNS vasculitis can lead to intracerebral haemorrhage. Vasculitis weakens vessel walls, or can cause aneurysm formation4. The histological hallmark of CNS vasculitis is a predominantly mononuclear cell (lymphocytes, plasma cells, and histiocytes) infiltrate in and around small (200 to 500 μm diameter) leptomeningeal and intracerebral vessels4. This process may account for higher hemorrhagic conversion rates in patients with vascular inflammation. A Finnish group reported a patient with vasculitis who presented with acute ischemic stroke who without thrombolysis, four days later developed an expansive right-sided intracerebral hemorrhage. Due to remarkable eosinophilia (30–42%), elevated p-ANCA, and response to immunomodulaty therapy, a diagnosis of CSS with probable secondary CNS vasculitis was made5. Intracerebral hemorrhage is a major cause of morbidity and death in patients with CSS1. Fatal intraventricular and subarachnoid hemorrhage has been reported in one pathologically proven CSS patient. The exact mechanism of CSS-associated ICH is uncertain. In a post-cardiac thrombolysis CSS patient with subsequent intracerebral hemorrhage, the brain at autopsy showed evidence of multiple small thin-walled leptomeningeal vessels containing intramural lymphocytic and monocytic infiltrates. No fibrinoid necrosis was identified.
Conclusion
In conclusion, taken together, these data argue that although CSS has never been regarded as an absolute contraindication to IV tPA administration, it could be a relative contraindication for tPA in CSS patients with acute stroke. Considering the high likelihood of secondary hemorrhagic conversion in such patients who present with ischemic stroke, the use of thrombolysis is controversial and should be regarded with extreme caution in the absence of other risk factors for stroke. Biomarkers such as vWF, fibrinogen, and eosinophil counts need to be investigate further to establish the degree of relevance of such biomarkers in this patient population.
References
- 1.Wolf J, Bergner R, Mutallib Swt, et al. Neurologic complications of Churg-Strauss syndrome – a prospective monocentric study. Eur J Neurol. 2010 Apr;17(4):582–88. doi: 10.1111/j.1468-1331.2009.02902.x. [DOI] [PubMed] [Google Scholar]
- 2.Masi AT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis) Arthritis Rheum. 1990;33:1094–00. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 3.Ames PR, Roes L, et al. Case report thrombosis in Churg Strauss syndrome: Beyond vasculitis? British Journal of Rheumatology. 1996;35:1181–83. doi: 10.1093/rheumatology/35.11.1181. [DOI] [PubMed] [Google Scholar]
- 4.West SG. Central nervous system vasculitis. Current Rheumatology Reports. 2003;5(2):116–27. doi: 10.1007/s11926-003-0039-z. [DOI] [PubMed] [Google Scholar]
- 5.Sairanen T, Kanerva M, Valanne L, et al. Churg-Strauss Syndrome as an Unusual Aetiology of Stroke with Haemorrhagic Transformation in a Patient with No Cardiovascular Risk Factors. Case Rep Neurol. 2011 Jan-Apr;3(1):32–38. doi: 10.1159/000323214. [DOI] [PMC free article] [PubMed] [Google Scholar]


