Abstract
In temporal lobe epilepsy (TLE), determining the hemispheric specialization for language before surgery is critical to preserving a patient's cognitive abilities post‐surgery. To date, the major techniques utilized are limited by the capacity of patients to efficiently realize the task. We determined whether resting‐state functional connectivity (rsFC) is a reliable predictor of language hemispheric dominance in right and left TLE patients, relative to controls. We chose three subregions of the inferior frontal cortex (pars orbitalis, pars triangularis, and pars opercularis) as the seed regions. All participants performed both a verb generation task and a resting‐state fMRI procedure. Based on the language task, we computed a laterality index (LI) for the resulting network. This revealed that 96% of the participants were left‐hemisphere dominant, although there remained a large degree of variability in the strength of left lateralization. We tested whether LI correlated with rsFC values emerging from each seed. We revealed a set of regions that was specific to each group. Unique correlations involving the epileptic mesial temporal lobe were revealed for the right and left TLE patients, but not for the controls. Importantly, for both TLE groups, the rsFC emerging from a contralateral seed was the most predictive of LI. Overall, our data depict the broad patterns of rsFC that support strong versus weak left hemisphere language laterality. This project provides the first evidence that rsFC data may potentially be used on its own to verify the strength of hemispheric dominance for language in impaired or pathologic populations. Hum Brain Mapp, 36:288–303, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: temporal lobe epilepsy, resting‐state functional connectivity, language, laterality index, hemispheric dominance
INTRODUCTION
Temporal lobe epilepsy (TLE) commonly leads to surgical treatment [Engel, 2001]. While a standard anterior temporal lobectomy (ATL) provides a successful treatment for seizure control [Englot et al., 2012], it may cause verbal impairments when the resected lobe is part of the dominant hemisphere for language. Therefore, determining the hemispheric specialization for language is critical to preserving a patient's verbal abilities following surgery [Helmstaedter, 2004]. Until now, the principal methods used have been the intracarotid amobarbital procedure (IAP) [Sharan et al., 2011; Tracy et al., 2009] and task‐based functional MRI (fMRI) [Deblaere et al., 2004]. While these procedures have good validity for determining language lateralization, each has limitations. The disadvantages of the IAP are invasiveness, patient discomfort, and cerebrovascular risks [Abou‐Khalil and Schlaggar, 2002]. These limitations have led to noninvasive alternatives such as fMRI. In turn, a major limitation of fMRI‐based language studies involves the demands placed on patients in terms of the skill level to complete the task, cooperation, and adequately following instructions. These limitations become problematic when trying to evaluate pathologic populations.
Determining language dominance is more complex in TLE than in normal population as left TLE patients have a higher likelihood of atypical language organization and lateralization, than right TLE patients or healthy participants [Hamberger and Cole, 2011]. It appears that TLE pathology more readily initiates both interhemispheric and intrahemispheric changes in language network [Brazdil et al., 2005]. Also, there is evidence that damage to the hippocampus may be a critical structure in determining if reorganization and atypical hemispheric dominance for language occurs [for review see Hamberger and Cole, 2011; Tracy and Boswell, 2008].
In recent years, functional connectivity (FC) methods have been increasingly used to reveal the integrity of cognitive networks [Damoiseaux et al., 2006; Doucet et al., 2011]. FC has also been shown to be of value in determining the impact of epilepsy on the brain activity and identifying abnormal brain networks [Bettus et al., 2009; Doucet et al., 2013b, in press; Liao et al., 2010]. In TLE, only a few studies have investigated abnormal language reorganization through this technique [Pravata et al., 2011; Waites et al., 2006]. Waites et al. [2006] suggested that using FC during a resting‐state condition was as efficient as traditional fMRI methods to reveal an abnormal language network. Indeed, the authors revealed that resting‐state functional connectivity (rsFC) emerging from language areas differed in left TLE patients relative to healthy controls, with the patients showing reduced FC. Pravata et al. [2011] investigated FC during a verb generation task in right and left epilepsy patients. They found left‐sided epilepsy was associated with a reduced FC between language areas, relative to controls. While these studies showed how FC can be used to reveal language network differences in TLE compared to control populations, they did not directly investigate the power of FC to predict lateralization of the language network (i.e., dominance).
In this study, we determined whether rsFC is a reliable predictor of hemispheric dominance for language processing in unilateral TLE patients. We chose the left inferior frontal cortex (IFC) as the principal seed region, as this area (n.b., Broca's area) is known to be robustly activated with expressive language tasks [Pang et al., 2011; Sanjuan et al., 2010]. We investigated three subregions: pars orbitalis, pars triangularis, and pars opercularis. We utilized the right IFC as a comparison region. We analyzed right and left TLE patients and healthy controls who underwent both an fMRI verb generation task and a resting‐state procedure. Verb generation fMRI paradigms have been shown to provide a reliable index of laterality [Ruff et al., 2008], correlating well with IAP results [Szaflarski et al., 2008]. Based on the verb generation task, we computed a laterality index (LI) for the resulting network. We then tested whether this LI correlated with the rsFC values emerging from each subregion of the left IFC with the rest of the brain. Lastly, we ran multiple regression analyses to test the ability of the significant rsFCs to predict the observed LIs in our samples. We hypothesized FC measures emerging from the left hemisphere would more strongly predict the LI. Moreover, we expected right and left TLE patients to differ in the pattern of predictive FC. In addition, we expected participants with a less‐lateralized LI would show higher levels of interhemispheric and right hemisphere bias connectivity than participants with strongly lateralized hemispheric dominance. The ultimate goal of this empirical undertaking is to provide evidence that rsFC data can potentially be used on its own to verify hemispheric dominance for language. Such findings would demonstrate the added value and contribution of rsFC to the standard presurgical algorithms currently utilized to determine, for instance, candidacy for ATL procedures.
MATERIAL AND METHODS
Participants: TLE Patients
Fifty‐five patients with refractory unilateral TLE (33 left‐sided and 22 right‐sided) were recruited from the Thomas Jefferson University Comprehensive Epilepsy Center. All patients were good surgical candidates for either a standard ATL or a thermal ablation of their ictal hippocampus. A combination of EEG, MRI, PET, and neuropsychological testing was used to lateralize the side of seizure focus [Sperling et al., 1992]. All patients met the following criteria: unilateral temporal lobe seizure onset through surface video/EEG recordings; normal MRI or MRI evidence of mesial temporal sclerosis (MTS) in the epileptogenic temporal lobe; concordant PET finding of hypometabolism in the ictal temporal lobe. TLE patients were excluded from the study for any of the following: previous brain surgery; extratemporal or multifocal epilepsy; medical illness with central nervous system impact other than epilepsy; contraindications to MRI; psychiatric diagnosis other than an Axis‐I Depressive Disorder; or hospitalization for any Axis I disorder listed in the Diagnostic and Statistical Manual of Mental Disorders, IV. Depressive Disorders were allowed given the high comorbidity of depression and epilepsy [Tracy et al., 2007].
Participants: Healthy Controls
Twenty‐three healthy controls were recruited to match the patient participants in age, gender, education, and handedness. All controls were free of psychiatric or neurological disorders based on health screening measures.
This study was approved by the Institutional Review Board for Research with Human Subjects at Thomas Jefferson University. All participants have provided a written informed consent. All participants were English native speakers.
MRI Data Acquisition
All participants underwent Magnetic Resonance Imaging on a 3‐T X‐series Philips Achieva clinical MRI scanner (Amsterdam, The Netherlands) using an 8‐channel head coil. A total of 5 min of a resting‐state condition was collected as well as a verb generation task to provide a measure of language hemispheric lateralization.
Anatomical and functional acquisitions were similar for all participants. Regarding the resting‐state condition, the participants were instructed to remain still, keep their eyes closed but not fall asleep throughout the scan. Single shot echoplanar gradient echo imaging sequence acquiring T2* signal was used with the following parameters: 120 volumes, 34 axial slices acquired parallel to the AC‐PC line, TR = 2.5 s, TE = 35 ms, FOV = 256 mm, 128 × 128 data matrix isotropic voxels, flip angle=90°. The in‐plane resolution was 2 × 2 mm2 and the slice thickness was 4 mm. Regarding the verb generation task, participants were instructed to covertly generate an action word in response to a viewed object noun presented on a screen. Each word was presented for 2 s, within a 30‐second block. These blocks were alternated with passive viewing of a central stimulus (#####) in epochs of 30 s for a total of 5 min. The scanning parameters were similar to those described for the resting‐state condition, except for the slice thickness which was 3 mm and a number of axial slices of 36. A training session was conducted before entering the scanner to ensure that the instructions were understood. After the task, all participants reported that they were able to complete the task as instructed.
Prior to collection of the functional images, T1‐weighted images were collected using an MPRage sequence (180 slices, 256 × 256 isotropic voxels; TR = 640 ms, TE = 3.2 ms, FOV = 256 mm, flip angle = 8°) in positions identical to the functional scans to provide an anatomical reference. The in‐plane resolution for each T1 slice was 1 mm3. Each EPI imaging series started with three discarded scans to allow for T1 signal stabilization.
Preprocessing Analyses
Verb generation and resting‐state fMRI data were preprocessed in the same way using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8), except for the last steps (see later). Slice timing correction was used to adjust for variable acquisition time over slices in a volume, with the middle slice used as reference. Next, a six‐parameter variance cost function rigid body affine registration was used to realign all images within a session to the first volume. Motion regressors were computed and later used as regressors of no interest. To maximize mutual information, coregistration between functional scans and the MNI305 (Montreal Neurological Institute) template was carried out using six iterations and resampled with a 7th‐Degree B‐Spline interpolation. Functional images were then normalized and warped into standard space (MNI305) to allow for signal averaging across subjects. We utilized the standard normalization method in SPM8. All normalized images were smoothed by convolution with a Gaussian kernel, with a full width at half maximum of 8 mm in all directions. For the resting‐state data only, sources of spurious variance were removed through linear regression: six parameters obtained by rigid body correction of head motion, the cerebrospinal fluid, and white matter signals. Finally, the resting‐state data were also temporally filtering in the band [0.008–0.1] Hz [Cordes et al., 2001].
Regarding the verb generation task, individual maps were computed for the contrast “verb generation‐control” at the threshold P < 0.001 uncorrected (T = 3.17). The six parameters obtained by rigid body correction of head motion were added in the model as covariates of no‐interest.
Five TLE patients' resting‐state data (3 left and 2 right) were excluded from further analyses because of high artifacts (such as motion). Therefore, second‐level resting‐state analyses were done on 30 left TLE and 20 right TLE patients.
Verb Generation Analysis
Group analysis
Unthresholded individual networks emerging from the verb generation task were entered into a second‐level random‐effects analyses (one‐way ANOVA) to determine the network at the group level as well as the differences between the experimental groups. A height threshold was set at P < 0.05 (family wise error corrected) for the group network and P < 0.0001 (uncorrected, cluster>15 voxels) for the comparison between the groups. The spatial extent threshold was chosen based on the expected number of voxels per cluster.
LI computation
Using the thresholded activation map (P < 0.001 uncorrected, T > 3.17), individual LIs were computed using the LI toolbox available in SPM8 [Wilke and Schmithorst, 2006]. To exclude voxels not specifically related to language function, we used an inclusive mask containing left and right inferior frontal cortices, left and right middle and superior temporal cortices. The spatial definition of these regions was taken from the normalized template available in the automated anatomical labeling (AAL) atlas [Tzourio‐Mazoyer et al., 2002]. LI values ranged between −1 and +1, where −1 indicated left‐sided lateralization.
Definition of the Seed Regions
The IFC was subdivided into three independent regions, based on the AAL atlas (Supporting Information Fig. 1). These seed regions were created in MNI standard space. Consequently, a total of six seed regions were then computed (three per hemisphere). These included the pars orbitalis, pars triangularis, and pars opercularis subregions. The seed regions were applied to both patients and controls, as all the participants had normalized functional data. Finally, data analyses within each patient included calculation of the mean signal time course in each seed region.
Figure 1.
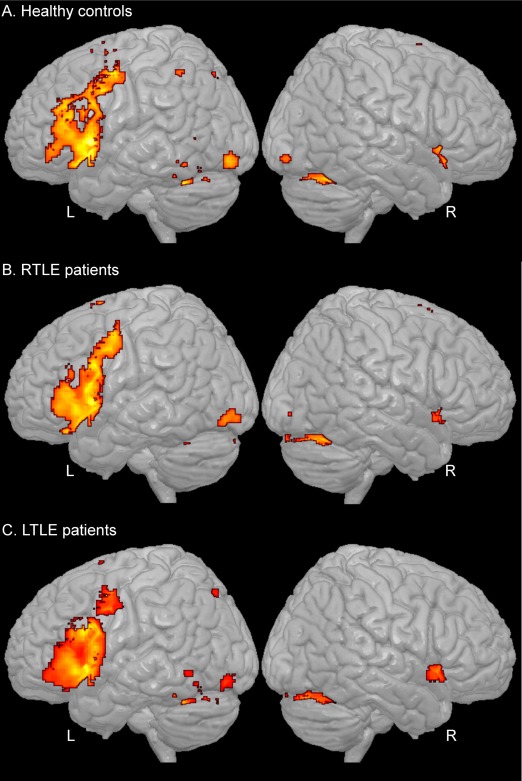
Network resulted from the verb generation task (P < 0.05, FWE corrected) for the healthy controls (A), right TLE patients (B), and left TLE patients (C). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
RsFC Computation
For each individual, a correlation map was produced by extracting the time course from each seed region and then computing the correlation between that time course and the time course from all other brain voxels. Next, these maps were submitted to a Fisher r‐to‐Z transformation. All further analyses were conducted on these transformed data.
Correlation Between rsFC and LI
For each seed separately, the LI was included as a continuous covariate in the one‐way ANOVA design, including the individual rsFC maps as the dependent variables and the experimental groups (left, right TLE, controls) as a main factor. We investigated regions positively or negatively covarying with LI, at a height threshold set at P < 0.001 (uncorrected), with a spatial extent threshold chosen based on the expected voxels per cluster (>20 voxels).
Regression Analyses to Predict LI
Based on the results from the correlation analyses, we extracted the individual correlation values for each region showing a significant relation with LI, for each group and each seed separately. We computed linear regression analyses to determine and compare regional rsFC values for their ability to predict LI. Independent analyses were done for each seed and each group. The regression model was considered significant at P < 0.008 (applying a Bonferroni correction, this yielded an effective alpha of P < 0.05 (0.05/6 seeds). All statistics (outside SPM) were computed using the software IBM® SPSS® v19.
RESULTS
Behavioral
The three groups did not differ by age, handedness nor gender (Table 1). The right and left TLE groups did not differ by age at seizure onset, illness duration, number of anti‐epileptic drugs, nor full‐scale IQ. Within the left TLE patients, 15 (45%) showed MTS. Within the right TLE patients, six patients showed MTS (27%). This difference was not significant between the groups (P > 0.1).
Table 1.
Clinical and demographic characteristics of the experimental groups
| Right TLE | Left TLE | Controls | |
|---|---|---|---|
| N (Females) | 22 (8) | 33 (15) | 23 (6) |
| Age (M ± SD) | 38.8 ± 12.8 | 40.1 ± 12.5 | 41.2 ± 11.5 |
| Right‐handers | 19 (86%) | 29 (88%) | 16 (70%) |
| Duration of epilepsy (years) | 16.4 ± 13.9 | 17.8 ± 15.6 | — |
| Age at epilepsy onset | 21.4 ± 9.3 | 23.7 ± 11.5 | — |
| Seizure type | CPS: 8 (36%) | CPS: 11 (33%) | — |
| CPS/SPS: 3 (14%) | CPS/SPS: 2 (6%) | — | |
| CPS w/GSa: 9 (41%) | CPS w/GSa: 6 (18%) | — | |
| CPS/rare GSb: 2 (9%) | CPS/rare GSb: 14 (42%) | ||
| Presence of MTS | 6 (27%) | 15 (45%) | — |
| Full scale IQ | 95.9 ± 11.9 | 91.0 ± 14.4 | — |
| Laterality index for language network | −0.59 ± 0.32 | −0.62 ± 0.28 | −0.59 ± 0.29 |
Abreviations: CPS=Complex Partial Seizures; SPS=Simple Partial Seizures; GS=Generalized Tonic‐Clonic Seizures; MTS=Mesial Temporal sclerosis.
CPS is the primary seizure type; GS is the second type, with more than 10 in lifetime.
rare GS = 10 or less GS in lifetime.
Verb Generation fMRI
Based on the verb generation fMRI task, individual LI values were computed. No significant differences were evident between the groups, indicating a general equivalence in the level of left hemisphere language dominance (LI = −0.59 ± 0.29 for controls, −0.59 ± 0.32 for right TLE, −0.62 ± 0.28 for left TLE). However, the distribution of scores revealed that, within each group, one participant was an outlier (LI > mean + 2 SD), showing a LI superior to zero, suggestive of non‐left hemisphere dominance (Supporting Information Fig. 2). Therefore, to maintain a normal distribution of the LI values within each group, the three outliers were excluded from further analyses.
Figure 2.
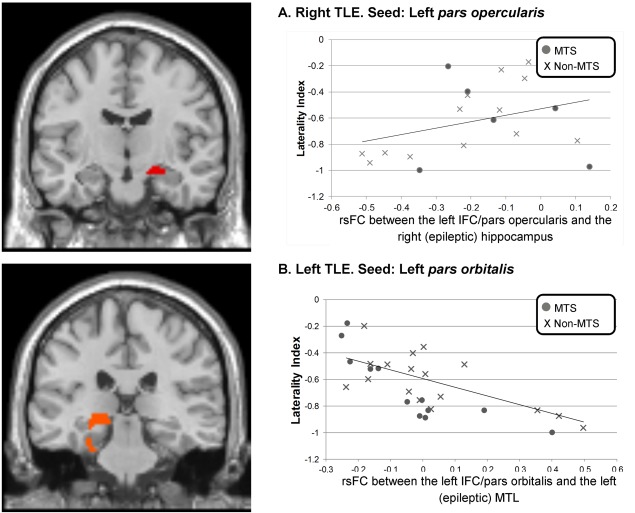
Significant relations between rsFC and LI between the left IFC and each epileptic mesial temporal lobe, in the right (A) and left (B) TLE patients. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
For each group, the resulted network was computed (Fig. 1). Across all groups, the major cluster was located in the left IFC. This activation covered areas in each subpart of the IFC, namely the pars orbitalis, pars triangularis, and pars opercularis, extending to the precentral gyrus. To a lesser degree, a small contralateral cluster was observed in the right IFC, mainly in the pars orbitalis region. Also, a large bilateral medial frontal cluster was revealed in the supplementary motor area (SMA). The three groups showed bilateral activation in the cerebellum. Finally, a subcortical cluster was revealed in the left thalamus. The controls also showed activation in the left inferior parietal cortex.
When comparing the network between the groups, we did not observe significant differences between the right and left TLE patients. However, relative to controls, each patient group displayed significant differences (Table 2). The control group consistently demonstrated higher activation in a cluster located in the left inferior parietal cortex, relative to both TLE groups. Specific to the left TLE group, the control group had higher activation in three left frontal clusters. Finally, within the left TLE patients, we compared the patients with and without MTS (15 vs. 17). No differences were found. We did not compute this comparison for the right TLE patients because of the limited number of subjects with MTS (6 of 21).
Table 2.
Activation differences between the three experimental groups for the verb generation fMRI task
| T | k | x | y | z | |
|---|---|---|---|---|---|
| Right TLE > Controls | |||||
| R Caudate | 4.99 | 18 | 4 | 8 | −6 |
| L Precuneus | 4.71 | 180 | −2 | −54 | 26 |
| Controls > Left TLE | |||||
| L Mid Fr | 5.51 | 88 | −44 | 32 | 30 |
| L Precentral | 5.35 | 100 | −44 | 8 | 38 |
| L Inf Parietal | 4.55 | 31 | −36 | −56 | 48 |
| L Precentral | 4.54 | 30 | −42 | −6 | 44 |
| Controls > Right TLE | |||||
| L Inf Parietal | 4.58 | 44 | −38 | −52 | 48 |
| Left TLE > Controls | |||||
| L PCC | 5.39 | 392 | −2 | −52 | 26 |
| R Precuneus | 5.36 | 2 | −60 | 22 | |
| L MPFC | 5.17 | 62 | −8 | 58 | 0 |
Abbreviations: Fr: Frontal cortex, Inf: Inferior, L: Left, Mid: Middle, MPFC: Medial prefrontal cortex, PCC: Posterior cingular cortex, R: Right.
Correlation Analyses Between LI and rsFC
We tested whether any of the rsFC values emerging from each subregion of the left IFC covaried with the LI (Table 3). Note, a positive relation between LI and rsFC indicates a more weakly left hemisphere language dominant pattern in association with increased rsFC. Conversely, a negative relation indicates stronger left hemisphere language dominance in association with higher rsFC.
Table 3.
Regions showing significant relations with LI, for each seed in the left IFC
| Left IFC seed | Correlation analyses | Regression analyses | ||||||
|---|---|---|---|---|---|---|---|---|
| Relation with LI | T | k | x | y | z | Adj. R2 | P‐val | |
| A‐Pars opercularis | Adj. Beta | P‐val | ||||||
| Right TLE | 0.456 | 0.007 | ||||||
| R PCC | + | 4.12 | 74 | 10 | −36 | 20 | n.s | n.s |
| R PCC | + | 3.95 | 12 | −26 | 20 | |||
| R Hippocampus | + | 3.84 | 56 | 26 | −10 | −16 | 0.782 | 0.009 |
| L PHG | + | 3.9 | 33 | −16 | 6 | −28 | n.s | n.s |
| N/A | − | N/A | ||||||
| Left TLE | 0.419 | 0.001 | ||||||
| R precentral | + | 4.03 | 53 | 32 | −18 | 48 | 0.419 | 0.022 |
| L Insula | − | 4 | 55 | −28 | 20 | 6 | n.s | n.s |
| L Sup Tp | − | 3.92 | 32 | −56 | −10 | −2 | n.s | n.s |
| Controls | 0.464 | 0.002 | ||||||
| R Sup Fr | + | 3.94 | 32 | 20 | −12 | 78 | n.s | n.s |
| L SMA | + | 3.84 | 29 | −10 | 18 | 50 | 0.473 | 0.038 |
| L Orb Mid Fr | − | 3.68 | 26 | −44 | 48 | 0 | n.s | n.s |
| B‐Pars triangularis | ||||||||
| Right TLE | 0.557 | 0.003 | ||||||
| L PHG | + | 4.36 | 144 | −16 | 6 | −24 | n.s | n.s |
| L Lingual | − | 4.23 | 48 | −6 | −62 | 6 | n.s | n.s |
| L Calcarine | − | 3.82 | 47 | −14 | −58 | 16 | n.s | n.s |
| L Mid Occip | − | 3.8 | 28 | −30 | −78 | 12 | −0.723 | 0.006 |
| Left TLE | 0.477 | <0.001 | ||||||
| R precentral | + | 4.26 | 175 | 28 | −22 | 64 | n.s | n.s |
| R precentral | + | 3.72 | 28 | −16 | 70 | |||
| R SMA | + | 4.03 | 36 | 6 | −2 | 76 | n.s | n.s |
| R PCL | + | 3.85 | 117 | 6 | −20 | 70 | n.s | n.s |
| L PCL | + | 3.75 | −4 | −22 | 72 | |||
| L PCL | + | 3.67 | −2 | −30 | 76 | |||
| L Insula | − | 4.28 | 88 | −34 | 10 | −8 | −0.371 | 0.026 |
| L Putamen | − | 3.77 | −30 | 8 | 0 | |||
| L Putamen | − | 3.56 | −30 | 6 | 10 | |||
| Controls | n.s | n.s | ||||||
| R IFC, pars triangularis | + | 3.94 | 27 | 40 | 32 | 12 | ||
| N/A | − | |||||||
| C‐Pars orbitalis | ||||||||
| Right TLE | 0.798 | <0.001 | ||||||
| N/A | + | |||||||
| L Mid Tp | − | 4.38 | 536 | −50 | −60 | 10 | n.s | n.s |
| L Mid Tp | − | 3.84 | −48 | −48 | 10 | |||
| L Mid Tp | − | 3.8 | −50 | −60 | 22 | |||
| L Mid Occip | − | 4.18 | 94 | −40 | −78 | 34 | n.s | n.s |
| L Precuneus | − | 3.91 | 53 | −8 | −54 | 14 | n.s | n.s |
| L Orb MPFC | − | 3.88 | 54 | −4 | 66 | −14 | n.s | n.s |
| L Precuneus | − | 3.74 | 21 | −12 | −52 | 50 | −0.915 | <0.001 |
| R Sup Fr | − | 3.69 | 32 | 22 | 38 | 54 | 0.554 | 0.007 |
| Left TLE | 0.6 | <0.001 | ||||||
| R SMA | + | 5.66 | 1343 | 10 | −18 | 74 | n.s | n.s |
| R Sup Fr | + | 5.3 | 24 | −12 | 72 | |||
| R Thalamus | + | 4.93 | 14 | −24 | 66 | |||
| R SMA | + | 4.41 | 144 | 4 | 8 | 60 | n.s | n.s |
| L precentral | + | 3.82 | 44 | −20 | −16 | 68 | n.s | n.s |
| L Sup Fr | + | 3.45 | −14 | −8 | 74 | |||
| R SMA | + | 3.78 | 31 | 14 | 6 | 48 | n.s | n.s |
| L PHG | − | 4.91 | 622 | −26 | −34 | −20 | n.s | n.s |
| L fusiform | − | 4.47 | −30 | −40 | −12 | |||
| L hippocampus | − | 4.06 | −16 | −34 | −2 | |||
| L precuneus | − | 4.58 | 203 | −10 | −54 | 14 | n.s | n.s |
| R Vermis | − | 3.99 | 2 | −56 | 6 | |||
| R fusiform | − | 3.89 | 60 | 30 | −38 | −16 | n.s | n.s |
| Controls | 0.436 | 0.007 | ||||||
| R Sup Fr | + | 4.4 | 147 | 20 | 34 | 48 | n.s | n.s |
| Orb MPFC | + | 3.99 | 24 | 0 | 60 | −22 | n.s | n.s |
| L Sup Fr | + | 3.68 | 32 | −16 | 40 | 34 | n.s | n.s |
| L SMG | − | 3.89 | 57 | −52 | −38 | 30 | −0.467 | 0.046 |
Linear regression results on LI within each IFC seed and experimental group on the right side.
In the last 2 columns, bold italic values are indicative of the adjusted R 2 and P value of the whole regression model (within the experimental group and seed of interest). For significant models (P < .008), adjusted beta, and P‐values are indicated for regions that significantly predict the LI (P < 0.05).
Abbreviations: n.s: nonsignificant (P > 0.05); Fr: Frontal cortex; L: Left; IFC: Inferior frontal cortex; Mid: Middle; MPFC: Medial prefrontal cortex; Occip: Occipital cortex; Orb: Orbital; Par: Parietal cortex; PCC: Posterior cingular cortex; PCL: Paracentral lobule; PHG: Parahippocampal gyrus; R: Right; SMA: Supplementary motor area; SMG: Supramarginal gyrus; Sup: Superior; Tp: Temporal cortex.
Seed: Left IFC, pars opercularis
Within the right TLE, only positive relationships with LI were evident (Table IIIA). In detail, rsFC between the seed and the right posterior cingular cortex (PCC), the left parahippocampal gyrus (PHG), and also the right (epileptogenic) hippocampus (Fig. 2A) were all found to be positively related to the LI. Said differently, increased rsFC between the seed and these three regions was associated with a less strongly left‐lateralized language network.
Regarding the significant effect involving the right epileptogenic hippocampus, we examined the role played by MTS (Fig. 2A). The low sample size precluded formal statistical analysis of the MTS group, however, for the group without MTS, the positive relationship remained significant (r = 0.66, P = 0.018).
Within the left TLE group, the rsFC between the left seed and the right precentral gyrus was positively correlated with LI (Fig. 3A, left panel), and the rsFC between the seed and the left insula and left superior temporal cortex was negatively related with LI.
Figure 3.
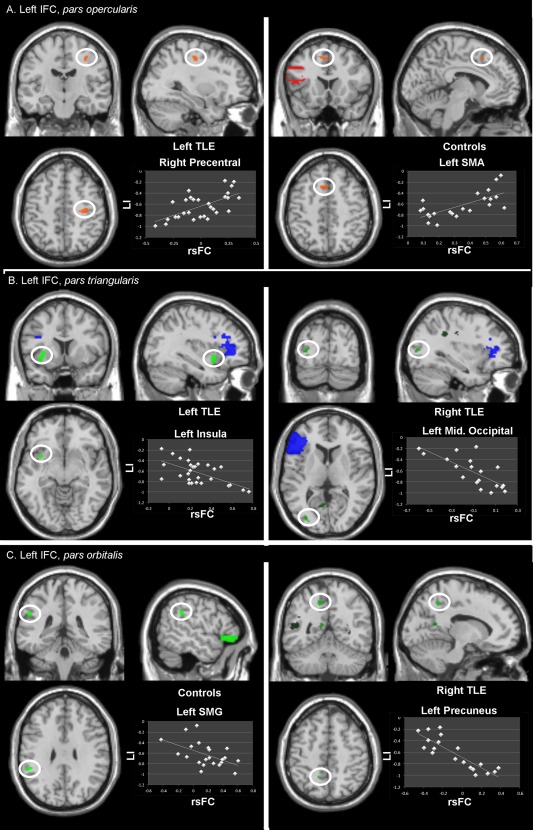
Display of the regions and their significant (predictive) relation between rsFC and LI, using the left IFC (A) pars opercularis, (B) pars triangularis, and (C) pars orbitalis as a seed. The region of interest is highlighted in the white circle; the seed region is shown in red (A), blue (B), or green (C). The images are seen in neurological orientation (the left side is the left hemisphere). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Within the controls, the seed bore a positive relation with LI involving two clusters in the right superior frontal cortex and the left SMA (Fig. 3A, right panel), respectively; and a negative relation with LI involving a cluster in the left orbital middle frontal cortex.
Lastly, we checked if these significant effects described in the patient groups reflected abnormal rsFC values in comparison to our controls. None of the above cited correlations were significantly different between the patient and control groups.
Seed: Left IFC, pars triangularis
For the right TLE group, we observed a positive relation with LI and rsFC involving the left (non epileptic) PHG (Table IIIB). In contrast, negative relations with LI were found, involving three clusters in the left occipital cortex (Fig. 3B, right panel).
The left TLE group showed positive relations between LI and rsFC of clusters localized in the frontal cortex (right precentral, right SMA and paracentral lobule, bilaterally). In contrast, rsFC between the left seed and a cluster covering the left insula (Fig. 3B, left panel) and putamen was negatively related to LI, suggesting that higher rsFC between these left‐sided regions reflects stronger left‐hemisphere dominance for language.
For the controls, the only cluster's rsFC positively related to LI was located in the contralateral homologous right pars triangularis region of the IFC. This indicates that a higher rsFC between the two IFCs reflects a less strongly left‐lateralized recruitment for language. No negative relation with LI was found for the controls.
None of these rsFC/LI associations were significantly different between the patient and control groups.
Seed: Left IFC, pars orbitalis
The right TLE patients demonstrated negative relations only, mostly involving left‐sided regions located in the middle temporal cortex, the middle occipital cortex, the precuneus (Fig. 3C, right panel) and the orbital medial prefrontal cortex (Table IIIC). One region in the right superior frontal cortex was also found.
In the left TLE group, we found three clusters involving the right SMA, as well as, to a lesser degree, a left‐sided contralateral cluster involving the precentral gyrus and the superior frontal cortex, clusters positively related to LI. In contrast, negative relations with LI mostly were revealed involving left‐sided clusters: a large cluster involving left mesial temporal lobe (MTL; epileptogenic PHG, fusiform gyrus, and hippocampus; Fig. 2B) as well as a cluster in the left precuneus. To a lesser degree, a smaller cluster was found in the right fusiform cortex. As a subanalysis, we investigated the effects of MTS in the left TLE group on the relation involving the left MTL cluster (Fig. 2B). The presence or absence of MTS did not change the relation; however, the correlation was stronger for the left TLE patients with than without MTS (MTS patients: r = −0.89, P < 0.001; non‐MTS patients: r = −0.57, P = 0.01).
For the controls, the major effects observed in relation to LI were positive, involving right and left superior frontal clusters. One negative relation with LI was found with the left supramarginal gyrus (Fig. 3C, left panel).
None of the rsFC involved in the above cited effects with LI were significantly different between the patient and control groups.
Overall, the left pars orbitalis seed produced the most significant associations with LI, regardless of the groups. Nevertheless, some major differences were highlighted between the groups. While the controls showed that significant relationships with LI only engaged lateral neocortical regions, each patient group demonstrated the capacity of their own epileptogenic MTL to correlate with the degree of left hemispheric specialization. Lastly, we revealed a clear difference in the direction of the correlation, depending on the hemisphere: a negative relation with LI (e.g., increased left hemisphere specialization) was mostly associated with higher intra‐left hemispheric rsFC; in contrast, a positive relation with LI (e.g., reduced left hemispheric dominance) was mainly associated with higher interhemispheric rsFC (rsFC between the seed and almost exclusively right‐sided regions) (Fig. 4A).
Figure 4.
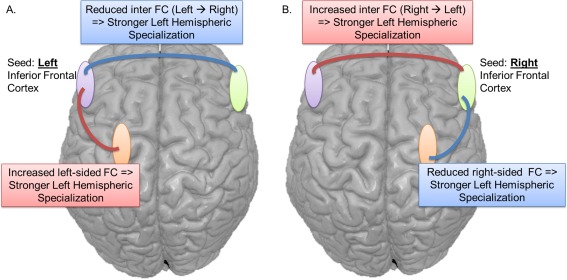
Schematic depiction of the rsFC relations associated with stronger left hemispheric specialization for language, shown in separate panels for the left‐ (A) and right‐ (B) sided seeds. Note, weaker hemispheric specialization (weaker laterality) is associated with the inverse rsFC patterns emerging from the seeds. Importantly, both our left and right TLE patients displayed these general patterns. Blue lines/boxes represent reduced rsFC with stronger specialization. Red lines/boxes represent higher rsFC with stronger specialization. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Right IFC seeds
As a control, the right IFC's subparts were also investigated as seeds (Table 4, Supporting Information Fig. 3). While the pars opercularis region was the seed generating most of the significant effects for the left TLE and the control groups (Table IVA), the pars orbitalis region was generating the most effects for the right TLE group (Table IVC). In contrast, the pars triangularis region yielded the lowest number of effects, for the three groups (Table IVB).
Table 4.
Regions showing significant relations with LI, for each seed in the right IFC
| Right IFC seed | Correlation analyses | Regression analyses | ||||||
|---|---|---|---|---|---|---|---|---|
| Relation with LI | T | k | x | y | z | Adj. R2 | P‐val | |
| A‐Pars opercularis | Adj. Beta | P‐val | ||||||
| Right TLE | n.s | n.s | ||||||
| N/A | + | |||||||
| R Mid Occip | − | 4.5 | 485 | 34 | −86 | 20 | ||
| R Mid Occip | − | 4.18 | 32 | −96 | 12 | |||
| Left TLE | 0.79 | <0.001 | ||||||
| R Sup Fr | + | 4.25 | 144 | 18 | 26 | 52 | 0.35 | 0.012 |
| R Mid Fr | + | 4.01 | 34 | 18 | 60 | |||
| R Sup Fr | + | 3.92 | 18 | 26 | 42 | |||
| R Hippocampus | + | 3.84 | 80 | 32 | −10 | −22 | n.s | n.s |
| R PHG | + | 3.41 | 28 | −8 | −30 | |||
| R Fusiform | + | 3.36 | 38 | −6 | −28 | |||
| R PHG | + | 3.79 | 32 | 24 | −24 | −26 | n.s | n.s |
| L Cerebellum | + | 3.66 | 58 | −10 | −46 | −20 | n.s | n.s |
| L Cerebellum | + | 3.54 | −6 | −56 | −18 | |||
| R Inf Par | − | 4.63 | 214 | 32 | −48 | 52 | n.s | n.s |
| R postcentral | − | 4.29 | 36 | −44 | 64 | |||
| R Cerebellum | − | 4.48 | 145 | 26 | −78 | −18 | n.s | n.s |
| R Inf Occip | − | 3.74 | 34 | −80 | −12 | |||
| R Sup Occip | − | 4.43 | 303 | 28 | −72 | 42 | n.s | n.s |
| R Sup Occip | − | 4.24 | 26 | −70 | 22 | |||
| R Sup Occip | − | 3.8 | 24 | −64 | 28 | |||
| R Mid Occip | − | 4.33 | 80 | 34 | −92 | 6 | n.s | n.s |
| L Sup Fr | − | 4.33 | 118 | −18 | 68 | 24 | n.s | n.s |
| L Mid Fr | − | 4.22 | −34 | 54 | 30 | |||
| L Mid Fr | − | 4.31 | 64 | −42 | 54 | 6 | n.s | n.s |
| L IFC/pars triang. | − | 4.07 | −50 | 46 | 6 | |||
| L Mid Fr | − | 4 | 65 | −34 | 62 | 16 | −0.44 | 0.014 |
| R precentral | − | 3.95 | 24 | 28 | −16 | 58 | n.s | n.s |
| Controls | 0.744 | <0.001 | ||||||
| R Sup Fr | + | 4.38 | 67 | 18 | −14 | 76 | n.s | n.s |
| L SMA | + | 4.17 | 136 | −6 | −10 | 70 | n.s | n.s |
| R SMA | + | 3.84 | 2 | −14 | 78 | |||
| R SMA | + | 3.68 | 4 | 0 | 70 | |||
| L postcentral | + | 3.84 | 45 | −60 | −14 | 46 | n.s | n.s |
| L postcentral | + | 3.41 | −52 | −16 | 54 | |||
| R Rolandic Oper | + | 3.81 | 41 | 62 | −4 | 10 | n.s | n.s |
| R precentral | + | 3.65 | 27 | 32 | −10 | 52 | n.s | n.s |
| L Sup Tp | + | 3.55 | 22 | −56 | −22 | 12 | n.s | n.s |
| L Mid Fr | − | 3.79 | 21 | −42 | 52 | 6 | −0.538 | 0.001 |
| B‐Pars triangularis | ||||||||
| Right TLE | n.s | n.s | ||||||
| N/A | + | |||||||
| R Sup Occip | − | 4.77 | 807 | 28 | −92 | 24 | ||
| R Calcarin | − | 4.51 | 16 | −70 | 20 | |||
| R Sup Occip | − | 4.23 | 16 | −88 | 28 | |||
| R PCL | − | 4.55 | 114 | 12 | −38 | 52 | ||
| R MCC | − | 3.49 | 16 | −30 | 46 | |||
| Left TLE | 0.486 | <0.001 | ||||||
| N/A | + | |||||||
| L IFC/pars triangularis | − | 4.31 | 28 | −48 | 46 | 4 | −0.487 | 0.004 |
| L Mid Fr | − | 3.81 | 29 | −30 | 60 | 16 | −0.345 | 0.036 |
| Controls | ||||||||
| N/A | + | 0.347 | 0.002 | |||||
| R Mid Occip | − | 4.11 | 20 | 24 | −88 | 10 | −0.615 | 0.002 |
| C‐Pars orbitalis | ||||||||
| Right TLE | n.s | n.s | ||||||
| L cerebellum | + | 3.6 | 56 | −24 | −56 | −24 | ||
| L cerebellum | + | 3.56 | −20 | −68 | −18 | |||
| L cerebellum | + | 3.45 | −28 | −64 | −22 | |||
| R Sup Occip | − | 4.44 | 206 | 28 | −92 | 26 | ||
| R Mid Occip | − | 4.27 | 28 | −84 | 18 | |||
| R Mid Occip | − | 3.86 | 40 | −84 | 22 | |||
| R Cuneus | − | 3.85 | 159 | 10 | −88 | 26 | ||
| R Calcarin | − | 3.71 | 12 | −68 | 18 | |||
| R Cuneus | − | 3.56 | 16 | −76 | 22 | |||
| L Cuneus | − | 3.75 | 36 | −8 | −82 | 22 | ||
| Left TLE | ||||||||
| N/A | + | |||||||
| N/A | − | |||||||
| Controls | 0.423 | 0.001 | ||||||
| L Sup Fr | + | 4.36 | 69 | −18 | 36 | 46 | 0.671 | 0.001 |
| L Sup Fr | + | 3.36 | −24 | 40 | 38 | |||
| N/A | − | |||||||
Linear regression results on LI within each IFC seed and experimental group on the right side.
In the last 2 columns, bold italic values are indicative of the adjusted R 2 and P value of the whole regression model (within the experimental group and seed of interest). For significant models (P < .008), adjusted beta and P‐values are indicated for regions that significantly predict the LI (P < 0.05).
Abbreviations: n.s: nonsignificant (P > 0.05); Fr: Frontal cortex; L: Left; Inf: Inferior; IFC: Inferior frontal cortex; MCC: Middle cingular cortex; Mid: Middle; Occip: Occipital cortex; Par: Parietal cortex; PCC: Posterior cingular cortex; PCL: Paracentral lobule; PHG: Parahippocampal gyrus; R: Right; SMA: Supplementary motor area; Sup: Superior; Tp: Temporal cortex.
Regarding the left TLE and the pars opercularis seed, the significant effects mostly involved right‐sided regions (8 of the 12). The larger effects were negative and involved every lobe (Table IVA). Of note, rsFC between the right pars opercularis and the right (non‐epileptic) MTL was found as positively correlated with LI, indicating that stronger rsFC between these right‐sided regions reflected a weaker left hemispheric specialization. In contrast, with the same seed, the control group mostly showed positive effects associated with bilateral regions in the motor cortex. No significant effect was revealed in their MTL. Finally, for the right TLE group and the pars orbitalis seed, the regions involved were localized posteriorly in the occipital lobe and in the cerebellum, for the negative and positive effects, respectively (Table IVC).
Regression Analyses
For each experimental group and each IFC seed, we took the significant regions' shown by the above rsFC analyses to be related to LI, and sought to determine the relative importance of each FC in predicting LI. This also allowed us to estimate the overall, predictive power of all the regions' FC in terms of estimating LI (Tables 3 and IV, last two columns). For the left TLE group, the regression involving the right pars opercularis seed produced the most predictive regression (adjusted R 2 = 0.791, P < 0.001; other regressions produced an adjusted R 2 ≤ 0.60). However, in this model, only 2 of the 12 clusters were significantly predicting LI. They were located in the right (ipsilateral) superior frontal cortex (standardized β = 0.35, P = 0.012, Supporting Information Fig. 3A left panel) and in the left (contralateral) middle frontal cortex (standardized β = −0.44, P = 0.014). In contrast, for the right TLE group, the regression involving the left pars orbitalis seed produced the most predictive model (adjusted R 2 = 0.798, P = 0.002; the other regressions at ≤0.55). Two clusters were significant, located in the left (ipsilateral) precuneus (standardized β = −0.92, P < 0.001, Fig. 3C right panel) and the right (contralateral) superior frontal cortex (standardized β = 0.55, P = 0.007). Of note, none of the models involving the right‐sided seeds were significantly predicting LI for the right TLE patients. Lastly, in the controls, all the regressions produced adjusted R 2 values of 0.46 or lower with the exception of the right pars opercularis seed (adjusted R 2 = 0.744, P < 0.001) with only one significant cluster in the left (contralateral) middle frontal cortex (standardized β = −0.54, P = 0.001, Supporting Information Fig. 3A right panel). Overall, these results reveal consistent findings relative to the correlation analyses previously described, however, these regression analyses gave more precision regarding the best regions to use to predict hemispheric specialization, mostly localized in the lateral frontal cortex.
DISCUSSION
The present fMRI study examined the power of rsFC emerging from the IFC to predict the degree of hemispheric dominance for language processing in TLE and healthy populations. Using the IFC's subdivisions as seeds, we demonstrated that each of our experimental groups' LIs were predicted by rsFC involving a distinct set of brain regions, with such FC measures showing sufficient reliability in predicting LI to suggest that rsFC analysis may play a role in verifying hemispheric dominance for language.
Our first result concerns the language network emerging from the verb generation task. The large majority of patients in our sample were left hemisphere dominant. The localization of the language network in each of our experimental groups was consistent with previous findings, primarily involving anterior clusters located in the left inferior frontal and medial frontal cortices [Pang et al., 2011; Sanjuan et al., 2010]. While the LI did not differ between patients and controls, we found some regional differences between the groups, with the controls displaying more extensive, or additional, activation in left‐sided regions. Our analyses did not reveal atypical recruitment of regions in either TLE group, relative to controls. Such results suggest that atypical reorganization of the language network was an infrequent occurrence in our sample. For instance, we observed that less than 4% of our sample was right‐hemisphere dominant. This contrasts with previous studies, which have reported higher rates of right hemisphere dominance (15% in [Rausch and Walsh, 1984]). It is important to note that such findings have been described as strongly related to the age at seizure onset, with earlier age (typically, before age 5) associated with stronger tendencies toward reorganization than when seizures appear later in life [Hamberger and Cole, 2011; Springer et al., 1999]. In our TLE patients, the first seizure episode, on average, occurred during adulthood (mean age = 23 ± 11, minimum age = 2.5; only three patients having an onset below age 5), decreasing the likelihood for functional reorganization.
Despite this, our rsFC data point to subtle functional reorganization of the language network in our TLE groups. Indeed, we found each of our TLE groups, relative to controls, displayed a unique set of regions predicting the LI of the language network. Thus, we believe our current findings speak to the power of FC in detecting subtle differences in cognitive network configuration, differences that are not available through standard task‐based fMRI investigations [Doucet et al., 2013a; Waites et al., 2006].
Regarding the rsFC analyses, the controls' data show that most of the significant regions correlated with LI were located in the frontal cortex, bilaterally. In contrast, in our TLE groups, one major finding in the correlation analyses pointed to an association between LI and the epileptogenic MTL. More specifically, increased rsFC between the left (epileptogenic) MTL/fusiform gyrus and the left pars orbitalis region was associated with stronger left hemispheric specialization in left TLE patients. In contrast, in right TLE patients, reduced rsFC between the right (epileptogenic) hippocampus and the left pars opercularis region was related to stronger left‐hemispheric specialization. Importantly, this latter FC measure was also a significant predictor of LI in the regression analysis run for this seed. These results support the notion that the epileptogenic hippocampus likely plays a role in determining hemispheric language lateralization in TLE patients [Hamberger and Cole, 2011; Tracy and Boswell, 2008; Tracy and Shah, 2008]. The absence of such a relation involving the MTL in controls indicates that in the presence of healthy hippocampi, MTL resting‐state activity may bear little relation to hemisphere language lateralization. Regarding the non‐epileptic MTL, we also found a significant relation with LI in each patient group. That is, a positive correlation was found between the left IFC/pars opercularis and the left (non‐epileptic) MTL in the right TLE group. A similar pattern involving the same contralateral seed and structures (non‐epileptic MTL) was found in the left TLE group. In contrast, the normal controls showed no evidence of IFC connectivity with either mesial or lateral temporal lobe to predict LI. In light of the above findings for the MTL, it is important to note that each patient group showed that both epileptic and non‐epileptic MTLs were able to either correlate with or predict hemispheric dominance for language. This finding implicating a role for both MTLs in the expression of language dominance is consistent with our prior work [Tracy and Boswell, 2008]. The right TLE showed increased FC between the left IFC and both right and left MTLs in association with a less lateralized language network. We suspect this may reflect strong abnormal rsFC between the two MTLs [Doucet et al., 2013b]. In contrast, the left TLE group displayed the pattern previously described (i.e., higher intra‐left hemispheric and lower intra‐right hemispheric rsFC in association with increased left lateralization).
We also found other cortical regions whose rsFC covaried with LI in each group. These effects involved regions of medial frontal cortex (premotor/motor cortex) whose primary functionality has related to motor and movement processing. It is important to point out that this same medial frontal region was clearly active in each group during our verb generation task. This implies that the motor processing at work serves language production, perhaps involving covert speech or imagining motor activity [Owen et al., 2006], keeping in mind that our language task did invoke the generation of action words. In addition, specific to the controls, we found that increased rsFC between the two IFC pars triangularis was associated with a more weakly left‐lateralized LI profile. Thus, the contralateral frontal involvement we see may result from attempts at recruitment of other regions to mediate language and maintain functioning. Importantly, this frontal rsFC data also suggest that this connectivity subserves language processing and its strength influences the distribution of the language network across the two hemispheres, that is, plays a role in hemispheric dominance.
Another important aspect of our results involves differences emerging from the subdivisions of the IFC. Each subregion yielded a unique pattern of results depending on the side of TLE pathology. The left pars orbitalis area most consistently demonstrated associations with LI, regardless of the group. Both right and left pars triangularis seeds produced the fewest number of LI associations. Such findings are consistent with language‐related fMRI studies demonstrating that each subregion of the IFC mediates distinct aspects of language processing [Vigneau et al., 2006, 2011]. Furthermore, our results are also supported by a recent structural study finding that the fibers from the arcuate fasciculus—key white matter tract to connect Broca's and Wernicke's areas—do not originate from the pars triangularis region [Brown et al., 2014]. Overall, our results clearly indicate that future resting‐state investigations of hemispheric dominance for language need to consider the separate contributions of these IFC subregions.
Our regression results allowed us to further clarify the broad patterns of regional connectivity emerging from the IFC that are most predictive of laterality. As can be seen in Tables 3 and IV, for both our TLE groups (note adjusted R 2 values), it is the connectivity emerging from the contralateral seed that is most predictive of laterality. This appears to suggest that the connectivity involving the non‐epileptic hemisphere is a more robust predictor of language lateralization in TLE. With respect to left TLE, this finding may reflect a shift toward right‐sided representation of language skills, a compensatory pattern of reorganization previously reported in the literature [Hamberger and Cole, 2011]. However, it should be noted that the right‐sided seeds were also the better predictors for the controls, suggesting that the left TLE patients are merely demonstrating a normative not a reorganized pattern. The exact reason for this right hemisphere involvement is unclear, but it certainly adds to the literature verifying that nondominant hemisphere structures play a role in language networks, and may even be crucial to maintaining the bias and imbalance for the left‐lateralized processing that generates hemispheric dominance patterns. In the setting of right TLE and left hemisphere language dominance (as is the case in our sample), the same adaptive forces seeking to maintain language are not at work, leaving the left IFC, and left hemisphere connectivity more broadly, as the best predictor of laterality.
These data provide strong confirmation of FC differences between right and left TLE, something our lab and others have argued previously [Doucet et al., 2013b; Pereira et al., 2010]. Note, our task fMRI data, involving a verb generation procedure, primarily activated frontal not temporal lobe structures, consistent with the expressive nature of the task. However, our resting‐state data demonstrate that stronger FC between the left IFC and left temporal cortex was associated with stronger left‐lateralization for language in our TLE patients. In this sense, we believe our data demonstrate how resting‐state can provide a broad window for capturing cortical involvement in language tasks, making clear that temporal regions, often considered to primarily mediate language comprehension, are connected to anterior language regions, and that the strength of such frontotemporal FC relationships influences the strength of left hemisphere language laterality.
Lastly, but importantly, our data depict the broad patterns of frontal lobe connectivity that support strong versus weak left hemisphere language laterality (Fig. 4). Stronger left hemisphere dominance is supported by strong intrahemispheric and low interhemispheric FC emerging from the left IFC. Weak left hemisphere dominance is supported by strong intrahemispheric and low interhemispheric FC emerging from the right IFC.
A major limitation of this study was that we were unable to recruit enough right‐hemisphere language dominant subjects to analyze and compare the ability of FC data to predict LI's in that group. Thus, our data and interpretations apply more strictly to individuals who are left hemisphere dominant for language. Yet, we did obtain significant rsFC relations with LI, when using both the left and right IFCs as seeds, in each experimental group. This may suggest LI/rsFC relationships emerging from the right IFC (the homologous Broca's area) in right hemisphere dominant individuals will be comparable to those we report in our left hemisphere dominant groups (n.b.: stronger intrahemispheric within the right hemisphere and weaker intrahemispheric within the left hemisphere in association with stronger right hemisphere dominance). However, further investigations are needed to test this hypothesis. Sample size limitations also hampered our ability to test for differences between patients with and without MTS.
It is important to also note that we focused our analyses on only one expressive language task, namely verb generation task, to operationalize a key “language network.” Therefore, it is possible that our task produces a LI that is biased towards frontal lobe contributions toward language processing, and does not sufficiently capture the contribution of posterior language systems involving the temporal lobes, the area where resective surgery occurs. It is important to note that posterior/superior temporal lobe activation (Wernicke's area) is commonly present for the verb generation task we used [Fiez et al., 1996]. It is clear that other language tasks emphasizing different components of language processing (e.g., activating strictly phonemic, syntactic, or semantic processes, or focusing more on posterior temporal, receptive language networks), may generate alternate laterality patterns, and produce different rsFC/LI relationships. We certainly acknowledge that our LI calculation may not capture temporal lobe contributions fully, and, therefore, may be missing important components that contribute to the variance in language dominance for this e epilepsy surgery population. We agree with others [Gaillard et al., 2002; Thivard et al., 2005] that an optimized fMRI may need to involve more than one language paradigm to gain convergent validity when determining laterality patterns in an individual. Unfortunately, we did not have enough participants with a second language task, assessing more posterior language systems, to incorporate such convergent data in our current results. However, previous studies have demonstrated that verb generation is one of the most reliable language tasks to predict hemispheric dominance and the location of Broca's area [Harrington et al., 2006; Ruff et al., 2008], suggesting that we have utilized a valid LI.
Lastly, it should be noted that other available methods for computing a LI may possess greater reliability (see [Wilke and Schmithorst, 2006] for an example of bootstrap analysis). In this study, we found high correlations (0.84 or higher, depending on the group) between the LIs computed with the bootstrap and our method, suggesting that it is likely that the findings would have remained similar if we had chosen a bootstrap method.
This study shows for the first time that rsFC emerging from the IFC can be an effective predictor of the strength of hemispheric dominance for language processing, in patients suffering from TLE as well as healthy participants. While we did not reveal strong signs of atypical reorganization within the left‐lateralized network in TLE patients, compared to controls, we were able to demonstrate that the relation between rsFC and LI is unique for each experimental group. Importantly, we showed that a specific set of regions (IFC) can be used to predict the laterality of the language network, using solely rsFC data. More specifically, our results demonstrated that both strong intrahemispheric and low interhemispheric rsFCs, when seeding the left hemisphere, are related to higher left hemispheric specialization (e.g., more lateralized network). This was true in the setting of both left and right TLE pathology.
Overall, our data suggest that, even at rest, FC within and between the hemispheres expresses a specific brain functional organization directly related to language processing. While other areas need to be investigated for the predictive value (e.g., Wernicke's area), our results demonstrate that it is possible to efficiently predict language laterality using only a limited set of regions located in the IFC (i.e., our Region of Interest (ROI) seeds). Therefore, we believe that rsFC might eventually be used as a potential biomarker of language specialization in neurological populations such as TLE. In this context, we believe that a next step would be to develop, in larger samples, indices of FC asymmetry between the right and left hemispheres, indices that would capture the balance between the two hemispheres, and test how these would be predictive of hemispheric specialization.
We believe our results open up a new window for determining language dominance through the use of rsFC emerging from a major language hub or module (namely, Broca's area). Such data can be collected with only a five‐minute scanning period. rsFC methods have an advantage over task‐based fMRI methods as they allow for more careful individual analysis of the role played by particular regional connections in the determination of language dominance. This study provides the first evidence that rsFC is able to determine the degree of left‐sided language specialization in healthy and neurologic participants. We hope that future investigations will be able to generalize our findings to both right and left hemisphere dominant populations, bringing into clinical practice the advantage rsFC offers in terms of testing impaired patient populations who cannot otherwise cooperate or respond effectively to the demands of either fMRI or IAP.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors thank Dr. Karol Osipowicz for his participation in the data acquisition. None of the authors have any conflict of interest to disclose.
REFERENCES
- Abou‐Khalil B, Schlaggar BL (2002): Is it time to replace the Wada test? Neurology 59:160–161. [DOI] [PubMed] [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort Gouny S, Soulier E, Laguitton V, PJ Cozzone, P Chauvel, JP Ranjeva, F Bartolomei (2009): Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp 30:1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazdil M, Chlebus P, Mikl M, Pazourkova M, Krupa P, Rektor I (2005): Reorganization of language‐related neuronal networks in patients with left temporal lobe epilepsy—An fMRI study. Eur J Neurol 12:268–275. [DOI] [PubMed] [Google Scholar]
- Brown EC, Jeong JW, Muzik O, Rothermel R, Matsuzaki N, Juhasz C, et al. (2014): Evaluating the arcuate fasciculus with combined diffusion‐weighted MRI tractography and electrocorticography. Hum Brain Mapp 35:2333–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. (2001): Frequencies contributing to functional connectivity in the cerebral cortex in “resting‐state” data. AJNR Am J Neuroradiol 22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblaere K, Boon PA, Vandemaele P, Tieleman A, Vonck K, Vingerhoets G, et al. (2004): MRI language dominance assessment in epilepsy patients at 1.0 T: Region of interest analysis and comparison with intracarotid amytal testing. Neuroradiology 46:413–420. [DOI] [PubMed] [Google Scholar]
- Doucet G, Naveau M, Petit L, Delcroix N, Zago L, Crivello F, et al. (2011): Brain activity at rest: A multiscale hierarchical functional organization. J Neurophysiol 105:2753–2763. [DOI] [PubMed] [Google Scholar]
- Doucet G, Osipowicz K, Sharan A, Sperling M, Tracy J (2013a): Hippocampal functional connectivity patterns during spatial working memory differ in right versus left temporal lobe epilepsy. Brain Connect 3:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G, Osipowicz K, Sharan A, Sperling MR, Tracy JI (2013b): Extratemporal functional connectivity impairments at rest are related to memory performance in mesial temporal epilepsy. Hum Brain Mapp 34:2202–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G, Sharan A, Pustina D, Skidmore C, Sperling MR, Tracy JI (2014): Early and late age of seizure onset have a differential impact on brain resting‐state organization in temporal lobe epilepsy. doi: 10.1007/s10548-014-0366-6 [DOI] [PMC free article] [PubMed]
- Engel J Jr (2001): Mesial temporal lobe epilepsy: What have we learned? Neuroscientist 7:340–352. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF (2012): Epilepsy surgery trends in the United States, 1990–2008. Neurology 78:1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Raichle ME, Balota DA, Tallal P, Petersen SE (1996): PET activation of posterior temporal regions during auditory word presentation and verb generation. Cereb Cortex 6:1–10. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, et al. (2002): Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology 59:256–265. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Cole J (2011): Language organization and reorganization in epilepsy. Neuropsychol Rev 21:240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington GS, Buonocore MH, Farias ST (2006): Intrasubject reproducibility of functional MR imaging activation in language tasks. AJNR Am J Neuroradiol 27:938–944. [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C (2004): Neuropsychological aspects of epilepsy surgery. Epilepsy Behav 5:S45–S55. [DOI] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, et al. (2010): Altered functional connectivity and small‐world in mesial temporal lobe epilepsy. PLoS One 5:e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD (2006): Detecting awareness in the vegetative state. Science 313:1402. [DOI] [PubMed] [Google Scholar]
- Pang EW, Wang F, Malone M, Kadis DS, Donner EJ (2011): Localization of Broca's area using verb generation tasks in the MEG: Validation against fMRI. Neurosci Lett 490:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, et al. (2010): Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: Evidence from resting state fMRI. BMC Neurosci 11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravata E, Sestieri C, Mantini D, Briganti C, Colicchio G, Marra C, et al. (2011): Functional connectivity MR imaging of the language network in patients with drug‐resistant epilepsy. AJNR Am J Neuroradiol 32:532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch R, Walsh GO (1984): Right‐hemisphere language dominance in right‐handed epileptic patients. Arch Neurol 41:1077–1080. [DOI] [PubMed] [Google Scholar]
- Ruff IM, Petrovich Brennan NM, Peck KK, Hou BL, Tabar V, Brennan CW, Holodny AI (2008): Assessment of the language laterality index in patients with brain tumor using functional MR imaging: Effects of thresholding, task selection, and prior surgery. AJNR Am J Neuroradiol 29:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan A, Bustamante JC, Forn C, Ventura‐Campos N, Barros‐Loscertales A, Martinez JC, et al. (2010): Comparison of two fMRI tasks for the evaluation of the expressive language function. Neuroradiology 52:407–415. [DOI] [PubMed] [Google Scholar]
- Sharan A, Ooi YC, Langfitt J, Sperling MR (2011): Intracarotid amobarbital procedure for epilepsy surgery. Epilepsy Behav 20:209–213. [DOI] [PubMed] [Google Scholar]
- Sperling MR, O'Connor MJ, Saykin AJ, Phillips CA, Morrell MJ, Bridgman PA, et al. (1992): A noninvasive protocol for anterior temporal lobectomy. Neurology 42:416–422. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, et al. (1999): Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain 122:2033–2046. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M (2008): Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav 12:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivard L, Hombrouck J, du Montcel ST, Delmaire C, Cohen L, Samson S, et al. (2005): Productive and perceptive language reorganization in temporal lobe epilepsy. Neuroimage 24:841–851. [DOI] [PubMed] [Google Scholar]
- Tracy J, Johnson V, Sperling MR, Cho R, Glosser D (2007): The association of mood with quality of life ratings in epilepsy. Neurology 68:1101–1107. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Boswell S(2008): Modeling the Interaction between Language and Memory: The case of temporal lobe epilepsy In: Whitaker BSH, editor. Handbook of the Neuroscience of Language. San Diego, CA: Academic Press; pp 319–328. [Google Scholar]
- Tracy JI, Shah S (2008): Presurgical functional brain mapping and neurocognitive testing in epilepsy In: Morgan JRJ, editor. Textbook of Clinical Neuropsychology. New York: Taylor and Francis. [Google Scholar]
- Tracy JI, Waldron B, Glosser D, Sharan A, Mintzer S, Zangaladze A, et al. (2009): Hemispheric lateralization and language skill coherence in temporal lobe epilepsy. Cortex 45:1178–1189. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. (2006): Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage 30:1414–1432. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Jobard G, Petit L, Crivello F, et al. (2011): What is right‐hemisphere contribution to phonological, lexico‐semantic, and sentence processing? Insights from a meta‐analysis. Neuroimage 54:577–593. [DOI] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD (2006): Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol 59:335–343. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ (2006): A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage 33:522–530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


