Abstract
Purpose of Review
Much confusion has surrounded the purpose of the psychological assessment in the context of chronic pain. For many clinicians the psychological assessment is used to rule out psychiatric illness and/or to identify non- medical causes for pain and disability. In essence it is used to identify causes of pain that fall outside of the biomedical model. Supported by over 30 years of evidence, the bio-psycho-social model acknowledges that psychosocial factors are inherent in chronic pain and require assessment if meaningful diagnostics and treatments are to occur.
Recent Findings
Five broad categories of psychosocial assessment are relevant to chronic pain. These categories have been shown to enhance the diagnosis of underlying forms of pain, predict the transition from acute to chronic status, and help to phenotype individuals for the discovery of underlying mechanisms responsible for pain.
Summary
Informed assessment of chronic pain needs to include relevant biological, psychological, and social domains. This paper describes those domains and offers suggestions of specific instruments to use in clinical or research settings.
Keywords: Affect, Cognition, pain perception and modulation
Introduction
The field of pain medicine has been quite successful in developing and delivering interventions for the relief of acute pain. Pain medicine has been less successful treating chronic pain however achieving only modest success. (1) Excellence in treating one type of pain but not another suggests that chronic pain is more than prolonged acute pain; it is a separate clinical entity involving different underlying mechanisms and requiring different approaches to treatment. (2)
Pain is a perceptual experience created by the brain to warn of threat to specific body regions. Like other perceptual experiences (e.g., vision, taste, hunger, beauty etc.), input from peripheral sensory receptors gets combined cortically along with ongoing affect, cognition, and memories of previous threatening events. The integration of each of these factors is required for the conscious experience of pain. (3-5) When pain becomes chronic, sensory input plays a diminished role while affective and cognitive pathways play a more prominent role in the creation of painful perceptions. (6)
The assessment of pain has historically focused almost exclusively upon location and intensity (e.g., 0-10 rating scales). While such measures provide insights into how strong the sensory experience might feel for acute pain, little is revealed about the affective or cognitive processes influencing chronic pain over time. For example, if you are listening to music, knowing only the volume setting tells you little about instrumentation, quality, key or tempo of the piece that is being played. This manuscript reviews the various domains of relevance for a bio-psycho-social assessment of pain, provides examples of assessment instruments for each domain, and offers practical approaches to implementing psychological assessments into practice or research.
The Bio-Psycho-Social Perspective on Pain
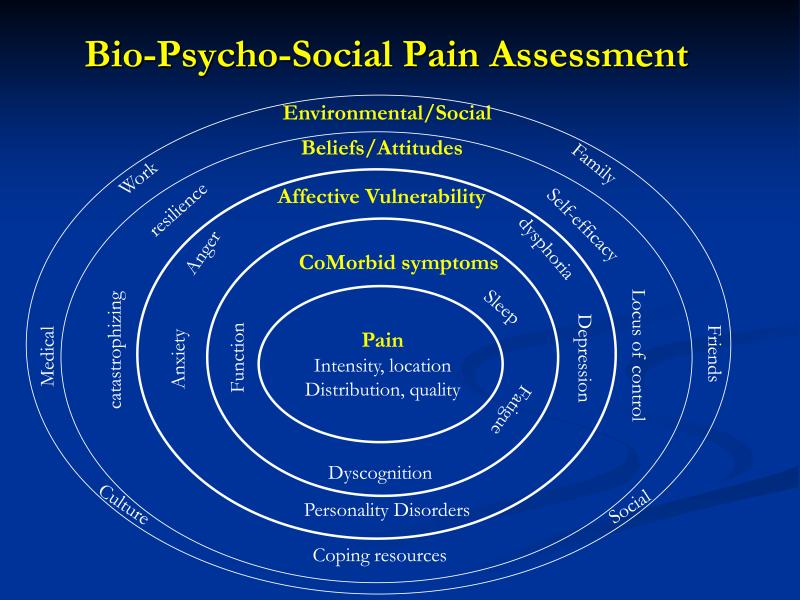
Psychological factors heavily influence the perception of chronic pain; as such there has been some confusion in knowing when and why it might be relevant to assess for them. Some clinicians confuse psychological factors with psychiatric illnesses (e.g. depression, anxiety etc.). While psychiatric illnesses can accompany chronic pain or result from persistent pain, there is little evidence that psychiatric illnesses are the root cause of most chronic pain conditions. (7) On- the- other-hand, non-psychiatric levels of affect and cognition play an essential role in developing the perception of pain and its unpleasantness. (8) Psychosocial factors also play an important role in determining who develops chronic pain and how a given individual responds to pain in terms of functional status, adaptation, and development of disability. (9) Thus to gain the best understanding of how chronic pain develops, how it's maintained, and the functional consequences of having chronic pain, information about pathophysiology (Bio), psychological (psycho) and social factors (social) need to be assessed and integrated for diagnostic purposes and treatment planning. Figure 1 provides an overview of the many potentially relevant domains for psychosocial assessment and will serve as a guide to the domains to be described. Table 1 provides examples of published instruments for assessing the various bio-psycho-social domains of relevance discussed in this paper.
Figure 1.
Bio-Psycho-Social Pain Assessment
Table 1.
Examples of Assessment Instruments by Domain for Chronic Pain
| Domain | Purpose | Instrument |
|---|---|---|
| Pain Symptom | Pain Intensity | VAS (10), NRS, VRS |
| Pain Quality | MPQ (11) | |
| Pain Distribution | WPI (12) | |
| Combination: intensity/distribution/quality | PainDetect (13) | |
| Co-Morbid Symptoms | Fatigue | MFI (14) |
| Sleep problems | PSQI (15) | |
| Perceived cognitive problems | MASQ (16) | |
| Functional status | SF36 (17) | |
| Combination: Intensity/functional interference | BPI (18) | |
| Combination: Functional Symptom checklist | PILL (19) | |
| Combination: Functional Symptom checklist | CMSI (20) | |
| Combination: Functional Symptom checklist | SSI (12) | |
| Affective Vulnerability | Depressed mood | CESD (21) |
| Anxious mood | STAI (22) | |
| Anger | STAXI (23) | |
| Combination: Depression/Anxiety | HADS (24) | |
| Combination: Negative/Positive Affect | PANAS (25) | |
| Beliefs and Attitudes | Locus of Pain Control | BPCQ (26) |
| Coping Strategies | CSQ (27) | |
| Self-Efficacy to Manage Pain | SEQ (28) | |
| Catastrophizing | PCS (29) | |
| Environmental/Social | Couple marital satisfaction | DAS (30) |
| Combination: work, family, social | WHYMPI (31) |
Note. Visual Analogue Scale (VAS), Numeric Rating Scale (NRS), Verbal Rating Scale (VRS); McGill Pain Questionnaire (MPQ); Wide-spread Pain Index (WPI); Multidimensional Fatigue Inventory (MFI); Pittsburgh Sleep Quality Index (PSQI); Multiple Abilities Symptom Questionnaire (MASQ); Short Form 36 (SF36); Brief Pain Inventory (BPI); Pennebaker Inventory of Limbic Languidness (PILL); Complex Medical Symptom Inventory (CMSI); Symptom Severity Index (SSI); Center for Epidemiologic Studies Depression Scale (CESD); State Trait Anxiety Inventory (STAI); State Trait Anger Expression Inventory (STAXI); Hospital Anxiety and Depression Scale (HADS); Positive and Negative Affect Scale (PANAS); Beliefs in Pain Control Questionnaire (BPCQ); Coping Strategies Questionnaire (CSQ); Self-Efficacy Questionnaire (SEQ); Pain Catastrophizing Scale (PCS); Dyadic Adjustment Scale (DAS); West Haven Yale Multidimensional Pain Inventory
Traditional Symptoms of Pain
Depicted at the center of Figure 1, are traditional scales of pain intensity such as the visual analogue scale (VAS), numeric pain rating (NRS) or verbal rating scale (VRS). Such measures have been shown to be reliable and valid indices of pain intensity and are the most commonly used measures in clinical practice. (32) Aside from intensity, clinicians may need to understand the quality of the pain (e.g., burning, stinging, aching etc.) for differentiating musculoskeletal from neuropathic pain or to understand whether pain is confined to a single region or has wide-spread characteristics. Body Maps can be useful in documenting the distribution of pain.
Co Morbid Symptoms
While acute pain typically occurs in response to an injury and is limited to the injury site, chronic pain is often accompanied by co-morbid symptoms that can add to the overall suffering and discomfort of the individual. The most commonly reported co-morbid symptoms of chronic pain include diminished physical functioning, sleep problems, fatigue, and cognitive problems such as difficulties with concentration and memory. The emergence of these additional symptoms has been associated with the transition from acute to chronic pain status and a worsening clinical course across many pain conditions (e.g., osteoarthritis, fibromyalgia, tempromandibular joint disorder, and cancer pain). (33-36) A wide variety of assessment instruments can be used to validly assess each symptom individually or comprehensive symptom checklists such as the Pennebaker Inventory of Limbic Languidness (PILL) (19) or the Complex Medical Symptom Index (CMSI) (20) can assess global functional symptom burden. Pain conditions with aberrantly strong central nervous system involvement can be identified using a combination of pain distribution measures (e.g., Wide-spread Pain Index) along with assessment of the above co-morbid symptoms (e.g., Symptom Severity Index). (12)
Affective Vulnerability
Psychiatric illnesses such as major depression, anxiety disorders, and personality disorders commonly occur in individuals with chronic pain. For example, in individuals with chronic pain, co-morbid depression has been reported to be as high as 52% in pain clinics, 27% in primary care practices, and 18% in population-based studies of pain. (37) In individuals without pain, depression occurs at a base rate between 5-10%. (38) Anxiety disorders occurring along with depression are also more common in individuals with chronic pain (i.e., 23%), (39) while anxiety in individuals without pain is as low as 12%. (38) Personality disorders, which are relatively uncommon in the general population (e.g., 14%) (40) can occur with rates as high as 51-58% in individuals with chronic pain. (41, 42) The high rate of psychiatric illness cooccurring along with chronic pain does not mean that one causes the other or that they can be viewed as the same condition. Each condition requires its own assessment and often involves separate interventions. Psychiatric illnesses are best diagnosed based upon Diagnostic and Statistical Manual criteria. (43)
The perception of chronic pain requires the presence of non-psychiatric emotional factors (6) which may in part be subject to genetic influences. (44) The three most commonly assessed moods in chronic pain studies have been depressed mood, anxiety, and anger. In most studies, higher levels these three negative moods have been associated with reduced pain thresholds, reduced pain tolerances, and increased reported pain. (45-47) By first assessing mood and then intervening on mood through medications or behavioral lifestyle interventions, clinicians can modify how pain is processed and modulated via the affective pathways. (48)
Beliefs and Attitudes about Pain
People form beliefs about the pain that they experience. Such pain-specific beliefs can include topics such as whether the pain is curable, whether it is understood by clinicians, what treatments would be relevant, and who caused the pain and/or who is responsible for fixing it. Beliefs are powerful predictors of health care utilization (49, 50) and can influence not only pain perception but treatment adherence, and treatment responsively. (51, 52) For example, 40% of the variance in physical functioning and 30% of the variance in affective symptoms of pain are attributable simply to what patients think about their pain. (53)
One cognitive process known to influence the experience of pain is “locus of control.” An “internal” locus of control for pain refers to a patient's belief that he/she has the ability to influence pain by what they do or think. Such a belief has been associated with lowered symptoms and better treatment outcomes. (54, 55) Related to the concept of locus of control, is the assessment of coping resources, efficacy to use coping resources, and characteristics of resilience. Assessment of these topics gives the clinician a sense of which patients already have viable self-management resources and those who may need additional training or assistance in obtaining self-management skills.
A second cognitive process that has received wide study is Catastrophizing. Catastrophizing is a superlatively negative evaluation of pain and its impact on the sufferer. The presence of catastrophizing in patients appears to negatively influence pain perception and diminishes functional status. (56-58) The presence of catstrophizing is also thought to be an important factor in predicting the transition of an acute pain state to one that is chronic; predicting up to 47% of the variance in making such a transition. (59)
Environmental / Social Factors
Pain cannot be seen by others. In fact, it is only through pain behaviors such as non-verbal postures and gestures, paraverbal groans, or verbal communication that others can learn of ones’ discomfort. (60) When patients feel as though others are inaccurately hearing their message about pain, they are likely to communicate more frequently and with greater intensity in hopes of being “heard” and acknowledged. Such escalation of pain communication in medical settings is common given there is often limited time available for physicians and patients to communicate about pain and its impact.
Similarly, well-meaning but overly solicitous partners may unwittingly encourage patients to increase pain behaviors by offering more social support in the presence of pain behaviors, while engaging in an overly sedentary lifestyle, or when patients display dependence upon family members. (61) For these reasons, it is often useful to include partners or significant family members/caregivers in the assessment of individuals with pain as these individuals not only shed light on factors that can worsen pain but can serve as active participants in reinforcing more adaptive behavior under the guidance of clinical staff. (62)
As pain behaviors are likely to increase or decrease in accordance with the need to communicate about pain, it is also important to understand the nature (e.g., supportive, solicitous, adversarial etc.), of other important social relationships in patients’ lives such as relationships at work, friends, religious relationships, and any relationships involving litigation or financial matters. For example, if the work environment is unsupportive or there is low job satisfaction (i.e., a psychosocial factor), the likelihood of pain and functional status improving to the point of returning to work is highly unlikely. (63) The assessment of work place relationships is often accomplished using standardized job satisfaction or occupational stress questionnaires that are non-specific to pain.
Practical Application
Compared to no pain assessment at all, which was common before the 5th vital sign guidelines were enacted,(64) assessment of pain intensity could at least alert health care providers to a persons’ suffering. Assessing other aspects of pain however greatly broadens the treatment options that can be offered to patients. What domains actually get assessed depends upon the purpose of the assessment as some purposes require more comprehensive assessment than others: diagnostics (least comprehensive), outcomes measurement for clinical trials (more comprehensive), and phenotyping individuals with different forms of chronic pain for research (most comprehensive).
When considering diagnostics it is tempting to simply assess intensity and location; but a measure of central augmentation or centralization of pain (e.g., WPI+SSI), can offer further guidance regarding the likelihood of transitioning to chronic pain, alternative treatment options, and prognosis. (65) When assessing outcomes of a clinical trial for pain, a panel of experts from academia, industry, and government recommended that at least six areas be considered: (1) pain intensity, (2) physical functioning, (3) emotional functioning, (4) patient global rating of improvement, (5) co-morbid symptoms / adverse events, and (6) the participants’ disposition (e.g., adherence and the reason for any early withdrawal). (66) Finally, phenotyping studies seek to identify distinct subgroups of individuals who each present with pain; but who likely have differing underlying pathophysiology. For such studies, the most comprehensive approach to biopsychosocial assessment can be helpful. (67)
Conclusion
The study of pain has largely occurred redundantly within medical sub-specialties; each publishing their own literatures and gaining an understanding of pain from the perspective of the organ systems relevant to each sub-specialty. Some specialties where pain is prominent have long considered the psychological assessment essential to dealing with chronic pain and have included it in standardized treatment guidelines. The domains reviewed in this manuscript are perhaps the most common or generic across chronic pain conditions. Some specific forms of pain might benefit from additional topics within each domain. For example, some forms of pain might benefit from the assessment of childhood abuse, fear avoidance, post-traumatic stress disorder, and personality factors. Historically, the field of Urology has not assessed psychological factors of chronic pain with the same regularity as other subspecialties focused on pain. This review provides a framework for enhancing the overall assessment of chronic pain which involves the inclusion of psychosocial constructs.
Key Points.
The experience of chronic pain inherently involves psychological factors thus the failure to include psychological assessment results in an incomplete understanding of the condition being treated or studied.
The influence of psychological factors in chronic pain is separate from and should not be confused with co-morbid psychiatric illnesses.
Psychosocial assessment should be included in diagnostics, clinical outcomes assessment and in phenotyping individuals with pain.
Psychological factors are salient predictors of the transition from acute to chronic pain status, underlying mechanisms of pain, disability, treatment adherence, and outcomes for various interventions.
Acknowledgements
Funding: U01 DK82345, Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network.
Footnotes
The author has no conflicts of interest associated with this manuscript.
Reference List
- 1.IOM . Relieving Pain in America: A Blueprint for Transforming Prevention, Care Education, and Reserach. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 2.Bonica JJ, Loeser JD, Chapman CR, Fordyce WE. The Management of Pain. Lea & Febiger; Philadelphia: 1990. History of pain concepts and theories. pp. 2–17. [Google Scholar]
- 3.Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150(699):971–9. doi: 10.1126/science.150.3699.971. PubMed PMID: 6501. [DOI] [PubMed] [Google Scholar]
- 4.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–91. doi: 10.1016/j.neuron.2007.07.012. PubMed PMID: 13502. [DOI] [PubMed] [Google Scholar]
- 5*.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013 Apr 11;368(15):1388–97. doi: 10.1056/NEJMoa1204471. PubMed PMID: 23574118. [Use of fMRI imaging can help identify differences in types of pain based upon brain regions being used to process pain in humans.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. EurJPain. 2005;9(4):463–84. doi: 10.1016/j.ejpain.2004.11.001. PubMed PMID: 12701. [DOI] [PubMed] [Google Scholar]
- 7.Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003 Aug 1;54(3):399–409. doi: 10.1016/s0006-3223(03)00545-6. PubMed PMID: 12893114. Epub 2003/08/02. eng. [DOI] [PubMed] [Google Scholar]
- 8.Loggia ML, Mogil JS, Bushnell MC. Experimentally induced mood changes preferentially affect pain unpleasantness. The journal of pain : official journal of the American Pain Society. 2008 Sep;9(9):784–91. doi: 10.1016/j.jpain.2008.03.014. PubMed PMID: 18538637. [DOI] [PubMed] [Google Scholar]
- 9.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine. 2002 Mar 1;27(5):E109–20. doi: 10.1097/00007632-200203010-00017. PubMed PMID: 11880847. [DOI] [PubMed] [Google Scholar]
- 10*.Jensen MP, Karoly P. Self-Report Scales and Procedures for Assing Pain in Adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. the Guilford Press; New York, NY: 2011. pp. 19–44. [Review of self-report scales for pain assessment. Covers pros and cons of most commonly used scales used in clinical and research for pain.] [Google Scholar]
- 11.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–7. doi: 10.1016/0304-3959(87)91074-8. PubMed PMID: 1087. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. The Journal of rheumatology. 2011 Jun;38(6):1113–22. doi: 10.3899/jrheum.100594. PubMed PMID: 21285161. Epub 2011/02/03. eng. [DOI] [PubMed] [Google Scholar]
- 13.Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Current medical research and opinion. 2006 Oct;22(10):1911–20. doi: 10.1185/030079906X132488. PubMed PMID: 17022849. Epub 2006/10/07. eng. [DOI] [PubMed] [Google Scholar]
- 14.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. Journal of psychosomatic research. 1995;39(3):315–25. doi: 10.1016/0022-3999(94)00125-o. PubMed PMID: 8287. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. PubMed PMID: 12889. [DOI] [PubMed] [Google Scholar]
- 16.Seidenberg M, Taylor MA, Haltiner A. Personality and self report of cognitive functioning. Archives of Clinical Neuropsychology. 2000;9(4):353–61. PubMed PMID: 1425. [PubMed] [Google Scholar]
- 17.Ware JE, Kosinski M, Dewey J. How to Score Version Two of the SF-36r Health Survey. QualityMetric, Inc.; Lincoln, RI: 2000. [Google Scholar]
- 18.Cleeland C. The Brief Pain Inventory: User Guide. MD Anderson Cancer Center; Houston , TX: 2009. [Google Scholar]
- 19.Pennebaker JW. The psychology of physical symptoms. Springer-Verlag; New York, New York: 1982. [Google Scholar]
- 20.Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am. 2009 May;35(2):339–57. doi: 10.1016/j.rdc.2009.05.007. PubMed PMID: 19647147. Pubmed Central PMCID: 2721827. Epub 2009/08/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. PubMed PMID: 1312. [Google Scholar]
- 22.Spielberger CD, Rickman RL, Eisenberg P, Kielholz P, Pancheri P, Racagni G. Assessment of state and trait anxiety. Anxiety: psychobiological and clinical perspectives. Hemisphere/Taylor and Francis; Washington: 1991. pp. 69–83. [Google Scholar]
- 23.Spielberger CD. STAXI-2: State-Trait Anger Expression Inventory - 2. Professional Manual. Psychological Assessment Resources (PAR),Inc.; Odessa, FL: 1999. p. 94. [Google Scholar]
- 24.Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. PubMed PMID: 12914662. Pubmed Central PMCID: 183845. Epub 2003/08/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality & Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. PubMed PMID: 1700. [DOI] [PubMed] [Google Scholar]
- 26.Skevington SM. A standardized scale to measure beliefs about controlling pain (BPCQ): A preliminary study. Psychology and Health. 1990;4:221–32. PubMed PMID: 8255. [Google Scholar]
- 27.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain. 1983;17(1):33–44. doi: 10.1016/0304-3959(83)90125-2. PubMed PMID: 7692. [DOI] [PubMed] [Google Scholar]
- 28.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis & Rheumatism. 1989;32(1):37–44. doi: 10.1002/anr.1780320107. PubMed PMID: 1009. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan M, Bishop S, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessments. 1995;7:524–32. [Google Scholar]
- 30.Spanier GB. The measurement of marital quality. J Sex Marital Ther. 1979;5(3):288–300. doi: 10.1080/00926237908403734. Fall PubMed PMID: 513146. [DOI] [PubMed] [Google Scholar]
- 31.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain. 1985;23(4):345–56. doi: 10.1016/0304-3959(85)90004-1. PubMed PMID: 855. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain. 1993;55(2):195–203. doi: 10.1016/0304-3959(93)90148-I. PubMed PMID: 5187. [DOI] [PubMed] [Google Scholar]
- 33.Henry NL, Clauw DJ. Thinking beyond the tumor to better understand chronic symptoms in breast cancer survivors. Breast cancer research and treatment. 2012 Jun;133(2):413–6. doi: 10.1007/s10549-011-1804-8. PubMed PMID: 21987035. [DOI] [PubMed] [Google Scholar]
- 34.Murphy SL, Lyden AK, Phillips K, Clauw DJ, Williams DA. Subgroups of older adults with osteoarthritis based upon differing comorbid symptom presentations and potential underlying pain mechanisms. Arthritis Res Ther. 2011;13(4):R135. doi: 10.1186/ar3449. PubMed PMID: 21864381. Pubmed Central PMCID: 3239378. Epub 2011/08/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisser ME, Strader Donnell C, Petzke F, Gracely RH, Clauw DJ, Williams DA. Comorbid somatic symptoms and functional status in patients with fibromyalgia and chronic fatigue syndrome: sensory amplification as a common mechanism. Psychosomatics. 2008 May-Jun;49(3):235–42. doi: 10.1176/appi.psy.49.3.235. PubMed PMID: 18448779. Epub 2008/05/02. eng. [DOI] [PubMed] [Google Scholar]
- 36.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, et al. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. The journal of pain : official journal of the American Pain Society. 2011 Nov;12(11 Suppl):T46–60. doi: 10.1016/j.jpain.2011.08.007. PubMed PMID: 22074752. Pubmed Central PMCID: 3233685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 38.Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys' estimates. ArchGenPsychiatry. 2002;59(2):115–23. doi: 10.1001/archpsyc.59.2.115. PubMed PMID: 13775. [DOI] [PubMed] [Google Scholar]
- 39.Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of Depression and Anxiety Alone and in Combination With Chronic Musculoskeletal Pain in Primary Care Patients. PsychosomMed. 2008 doi: 10.1097/PSY.0b013e318185c510. PubMed PMID: 13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant BF, Hasin DS, Stinson FS, Dawson DA, Chou SP, Ruan WJ, et al. Prevalence, correlates, and disability of personality disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J ClinPsychiatry. 2004;65(7):948–58. doi: 10.4088/jcp.v65n0711. PubMed PMID: 13777. [DOI] [PubMed] [Google Scholar]
- 41.Fishbain DA, Goldberg M, Meagher BR, Steele R, Rosomoff H. Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain. 1986;26(2):181–97. doi: 10.1016/0304-3959(86)90074-6. PubMed PMID: 4608. [DOI] [PubMed] [Google Scholar]
- 42.Polatin PB, Kinney RK, Gatchel RJ, Lillo E, Mayer T. Psychiatric illness and chronic low-back pain. The mind and the spine--which goes first? Spine. 1993;18(1):66–71. doi: 10.1097/00007632-199301000-00011. PubMed PMID: 1283. [DOI] [PubMed] [Google Scholar]
- 43.Association AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association; Washongton, DC: 2012. [Google Scholar]
- 44*.Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nature reviews Rheumatology. 2013 Jun;9(6):340–50. doi: 10.1038/nrrheum.2013.43. PubMed PMID: 23545734. Reviews approaches to penotyping chronic pain based upon genetics and self-report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang NK, Salkovskis PM, Hodges A, Wright KJ, Hanna M, Hester J. Effects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patients. Pain. 2008 Aug 31;138(2):392–401. doi: 10.1016/j.pain.2008.01.018. PubMed PMID: 18325674. [DOI] [PubMed] [Google Scholar]
- 46.Wagner G, Koschke M, Leuf T, Schlosser R, Bar KJ. Reduced heat pain thresholds after sad-mood induction are associated with changes in thalamic activity. Neuropsychologia. 2009 Mar;47(4):980–7. doi: 10.1016/j.neuropsychologia.2008.10.021. PubMed PMID: WOS:000264519200004. English. [DOI] [PubMed] [Google Scholar]
- 47.van Middendorp H, Lumley MA, Jacobs JW, Bijlsma JW, Geenen R. The effects of anger and sadness on clinical pain reports and experimentally-induced pain thresholds in women with and without fibromyalgia. Arthritis Care Res (Hoboken) 2010 Oct;62(10):1370–6. doi: 10.1002/acr.20230. PubMed PMID: 20506177. [DOI] [PubMed] [Google Scholar]
- 48.Hassett AL, Williams DA. Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best practice & research Clinical rheumatology. 2011 Apr;25(2):299–309. doi: 10.1016/j.berh.2011.01.005. PubMed PMID: 22094203. Epub 2011/11/19. eng. [DOI] [PubMed] [Google Scholar]
- 49.Brown M, Dean S, Hay-Smith EJ, Taylor W, Baxter GD. Musculoskeletal pain and treatment choice: an exploration of illness perceptions and choices of conventional or complementary therapies. Disabil Rehabil. 2010;32(20):1645–57. doi: 10.3109/09638281003649896. PubMed PMID: 20170384. [DOI] [PubMed] [Google Scholar]
- 50.Gross DP, Deshpande S, Werner EL, Reneman MF, Miciak MA, Buchbinder R. Fostering change in back pain beliefs and behaviors: when public education is not enough. Spine J. 2012 Nov;12(11):979–88. doi: 10.1016/j.spinee.2012.09.001. PubMed PMID: 23073211. [DOI] [PubMed] [Google Scholar]
- 51.Jensen MP, Turner JA, Romano JM. Changes after multidisciplinary pain treatment in patient pain beliefs and coping are associated with concurrent changes in patient functioning. Pain. 2007;131(1-2):38–47. doi: 10.1016/j.pain.2006.12.007. PubMed PMID: 13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romano JM, Jensen MP, Turner JA, Good AB. Changes in Pain-Related Beliefs Predict Outcome 6 Months After Pain Treatment. University of Washington Multidisciplinary Pain Center; Seattle WA: 1996. [Google Scholar]
- 53.Turner JA, Jensen MP, Romano JM. Do beliefs, coping, and catastrophizing independently predict functioning in patients with chronic pain? Pain. 2000;85(1-2):115–25. doi: 10.1016/s0304-3959(99)00259-6. PubMed PMID: 10018. [DOI] [PubMed] [Google Scholar]
- 54.Crisson JE, Keefe FJ. The relationship of locus of control to pain coping strategies and psychological distress in chronic pain patients. Pain. 1988;35(2):147–54. doi: 10.1016/0304-3959(88)90222-9. PubMed PMID: 4001. [DOI] [PubMed] [Google Scholar]
- 55.Lipchik GL, Milles K, Covington EC. The effects of multidisciplinary pain management treatment on locus of control and pain beliefs in chronic non-terminal pain. Clinical Journal of Pain. 1993;9(1):49–57. doi: 10.1097/00002508-199303000-00007. PubMed PMID: 994. [DOI] [PubMed] [Google Scholar]
- 56.Geisser ME, Robinson ME, Keefe FJ, Weiner ML. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain. 1994;59(1):79–83. doi: 10.1016/0304-3959(94)90050-7. PubMed PMID: 580. [DOI] [PubMed] [Google Scholar]
- 57.Turner JA, Jensen MP, Warms CA, Cardenas DD. Catastrophizing is associated with pain intensity, psychological distress, and pain-related disability among individuals with chronic pain after spinal cord injury. Pain. 2002;98(1-2):127–34. doi: 10.1016/s0304-3959(02)00045-3. PubMed PMID: 11492. [DOI] [PubMed] [Google Scholar]
- 58.Geisser ME, Roth RS. Knowledge of and agreement with chronic pain diagnosis: Relation to affective distress, pain beliefs and coping, pain intensity, and disability. Journal of Occupational Rehabilitation. 1998;8(1):73–88. PubMed PMID: 582. [Google Scholar]
- 59.Burton AK, Tillotson KM, Main CJ, Hollis S. Psychosocial predictors of outcome in acute and subchronic low back trouble. Spine. 1995;20(6):722–8. doi: 10.1097/00007632-199503150-00014. PubMed PMID: 2831. [DOI] [PubMed] [Google Scholar]
- 60.Fordyce WE. Behavioral Methods for Chronic Pain and Illness. Mosby; St. Louis: 1976. p. 236. [Google Scholar]
- 61.Keefe FJ, Somers TJ, Williams DA, Smith SJ. Assessment of Pain Behaviors. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. 3rd ed. The Guilford Press; New York, NY: 2011. pp. 134–50. [Google Scholar]
- 62.Keefe FJ, Caldwell DS, Baucom D, Salley A, Robinson E, Timmons K, et al. Spouse-assisted coping skills training in the management of knee pain in osteoarthritis: long-term followup results. Arthritis Care Res. 1999 Apr;12(2):101–11. doi: 10.1002/1529-0131(199904)12:2<101::aid-art5>3.0.co;2-9. PubMed PMID: 10513498. [DOI] [PubMed] [Google Scholar]
- 63.van Poppel MN, Koes BW, Deville W, Smid T, Bouter LM. Risk factors for back pain incidence in industry: a prospective study. Pain. 1998 Jul;77(1):81–6. doi: 10.1016/S0304-3959(98)00085-2. PubMed PMID: 9755022. [DOI] [PubMed] [Google Scholar]
- 64.Dahl JL, Saeger L, Stein W, Huss RD. The new JCAHO pain assessment standards: implications for the medical director. J Am Med Dir Assoc. 2000;1(6 Suppl):S24–31. Nov-Dec PubMed PMID: 12818012. [PubMed] [Google Scholar]
- 65**.Phillips K, Clauw DJ. Central pain mechanisms in the rheumatic diseases: future directions. Arthritis Rheum. 2013 Feb;65(2):291–302. doi: 10.1002/art.37739. PubMed PMID: 23045168. Pubmed Central PMCID: 3610409. [Introduces the concept of centralized pain and how this mechanism can influence pain perception across a variety of chronic pain conditions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–45. doi: 10.1016/j.pain.2003.08.001. PubMed PMID: 11380. [DOI] [PubMed] [Google Scholar]
- 67.Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, et al. Psychosocial phenotyping in women with interstitial cystitis/painful bladder syndrome: a case control study. The Journal of urology. 2010 Jan;183(1):167–72. doi: 10.1016/j.juro.2009.08.133. PubMed PMID: 19913812. [DOI] [PubMed] [Google Scholar]