Abstract
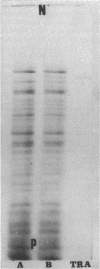
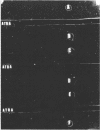
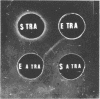
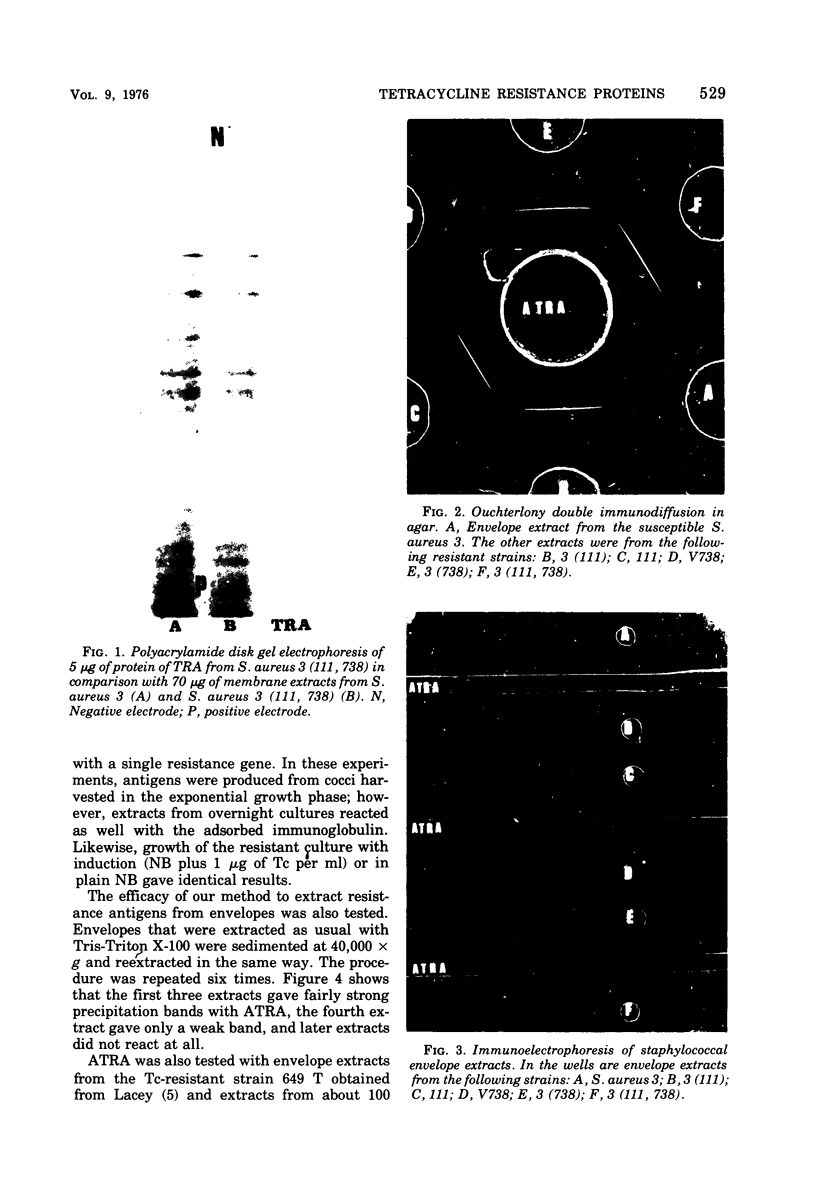
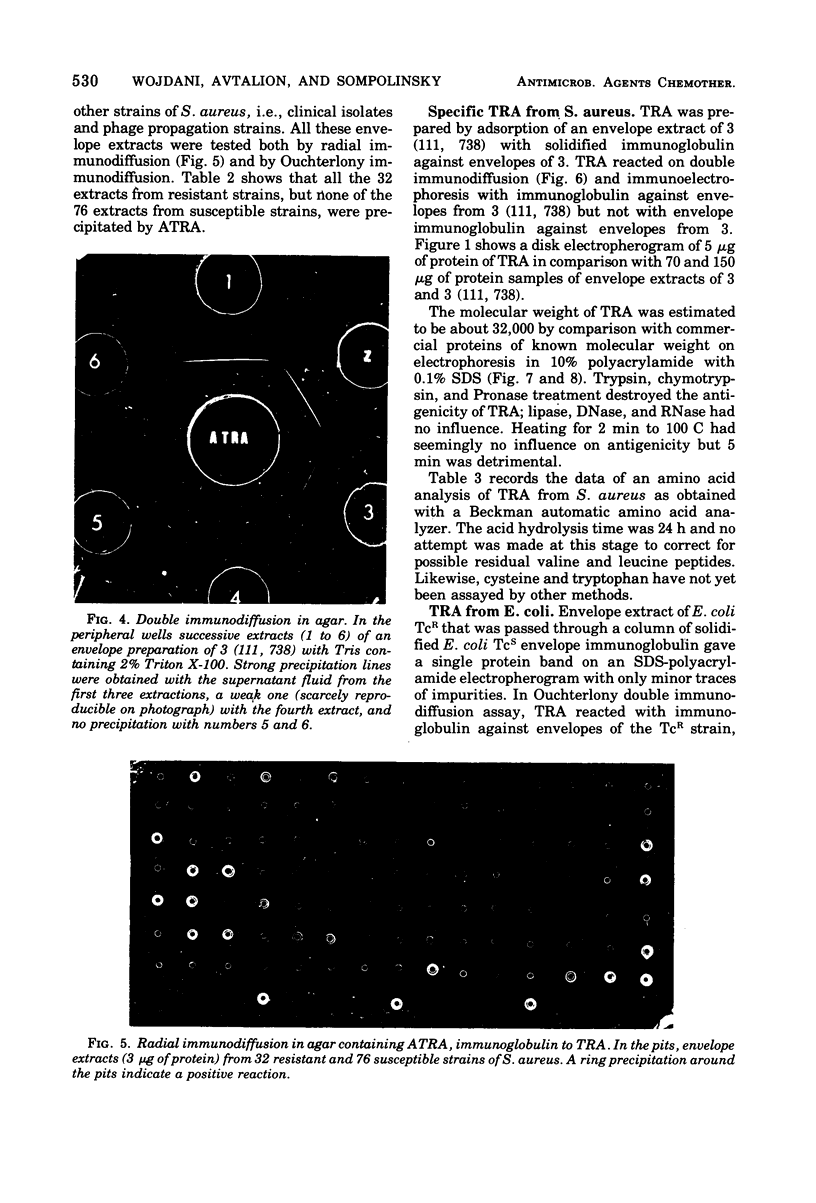
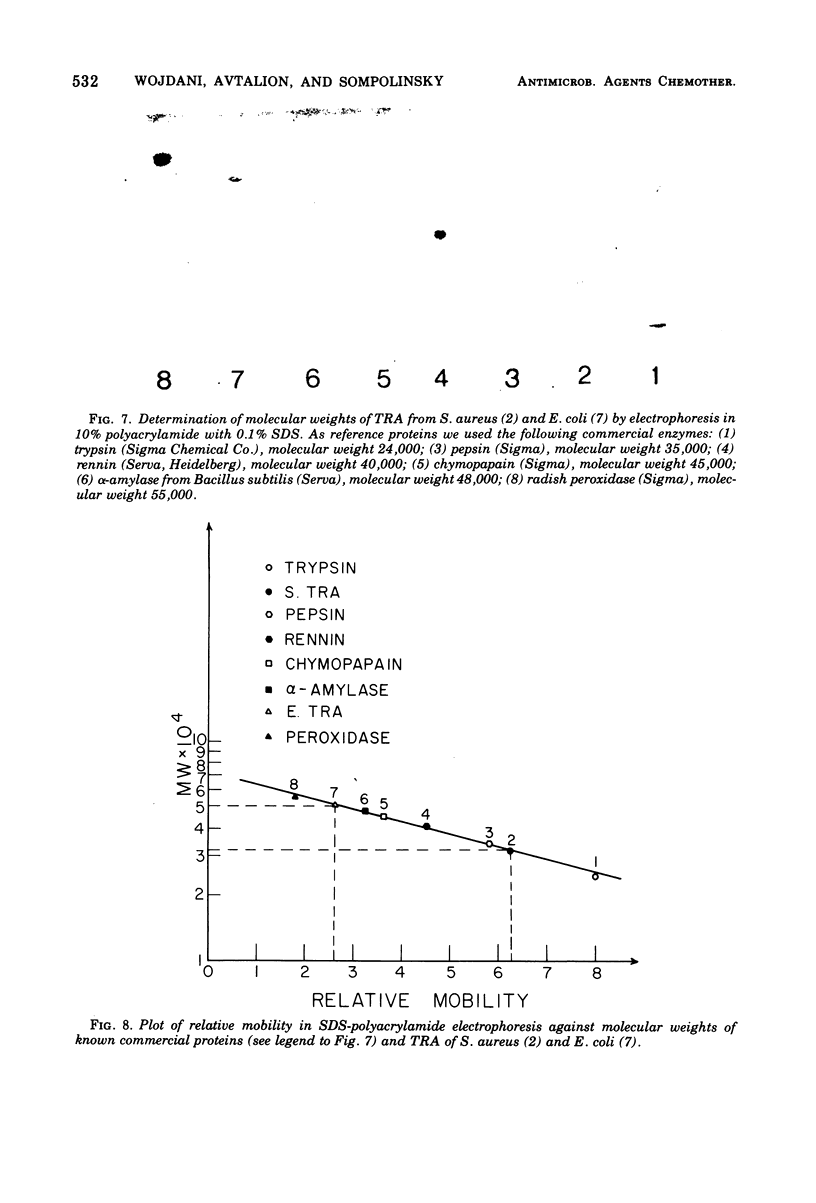
Immunoglobulin (adsorbed resistance antiserum) reacting specifically with antigens from tetracycline (Tc)-resistant Staphylcoccus aureus or Escherichia coli was produced by adsorbing immunoglobulin against cell envelopes of resistant strains with envelope extracts from the respective isogenic susceptible strains. Adsorbed resistance antiserum against S. aureus reacted with envelope extracts from 32 Tc-resistant strains and failed to react with similar extracts from 76 Tc-susceptible strains of S. aureus. An antigen (Tc resistance antigen [TRA]) found only in Tc-resistant strains was produced by adsorbing envelope extracts from these strains with immunoglobulin against envelopes from isogenic Tc-susceptible strains. On immunodiffusion no cross-reactivity between TRAs from S. aureus and E. coli was observed. The TRAs behaved as proteins. The molecular weight of TRA from S. aureus was determined to be 32,000 and from E. coli to be 50,000. Data obtained by preliminary amino acid analysis of the TRAs are presented.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Asheshov E. H. The genetics of tetracycline resistance in Staphylococcus aureus. J Gen Microbiol. 1975 May;88(1):132–140. doi: 10.1099/00221287-88-1-132. [DOI] [PubMed] [Google Scholar]
- Avtalion R. R., Ziegler-Schlomowitz R., Pearl M., Wojdani A., Sompolinsky D. Depressed resistance to tetracycline in Staphylococcus aureus. Microbios. 1971 Mar;3(10):165–180. [PubMed] [Google Scholar]
- Boldur I., Sompolinsky D. Antigen specific for bacteria resistant to tetracycline. Antimicrob Agents Chemother. 1974 Aug;6(2):117–120. doi: 10.1128/aac.6.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Bennett P. M., Lacey R. W. A variety of Staphylococcal plasmids present as multiple copies. J Gen Microbiol. 1973 Dec;79(2):343–345. doi: 10.1099/00221287-79-2-343. [DOI] [PubMed] [Google Scholar]
- Dunnick J. K., O'Leary W. M. Correlation of bacteria lipid composition with antibiotic resistance. J Bacteriol. 1970 Mar;101(3):892–900. doi: 10.1128/jb.101.3.892-900.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L., Palmer E. R factor proteins synthesized in Escherichia coli minicells: membrane-associated R factor proteins. J Bacteriol. 1974 Dec;120(3):1464–1471. doi: 10.1128/jb.120.3.1464-1471.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinski D., Ben-Yakov M., Aboud M., Boldur I. Transferable resistance factors with mutator effect in Salmonella typhi. Mutat Res. 1967 Mar-Apr;4(2):119–127. doi: 10.1016/0027-5107(67)90063-2. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Krausz J. Action of 12 tetracyclines on susceptible and resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1973 Sep;4(3):237–247. doi: 10.1128/aac.4.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Krawitz T., Zaidenzaig Y., Abramova N. Inducible resistance to tetracycline in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):341–349. doi: 10.1099/00221287-62-3-341. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Samra Z. Influence of magnesium and manganese on some biological and physical properties of tetracycline. J Bacteriol. 1972 May;110(2):468–476. doi: 10.1128/jb.110.2.468-476.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Zaidenzaig Y., Ziegler-Schlomowitz R., Abramova N. Mechanism of tetracycline resistance in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):351–362. doi: 10.1099/00221287-62-3-351. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]