Abstract
Background
Breast cancer is the most frequently diagnosed cancer and the second leading cause of cancer death in American women. Post-surgery adjuvant radiotherapy (RT) significantly reduced the local recurrence rate. However, many patients develop early adverse skin reactions (EASRs) that impact quality of life and treatment outcomes.
Methods
We evaluated an inflammatory biomarker, C-reactive protein (CRP) in predicting RT-induced EASRs in 159 breast cancer patients undergoing RT. In each patient, we measured pre- and post-RT plasma CRP levels using a highly-sensitive ELISA CRP assay. RT-induced EASRs were assessed at weeks 3 and 6 using the National Cancer Institute Common Toxicity Criteria (v3.0). Association between EASRs and CRP levels were assessed using logistic regression models after adjusting for potential confounders.
Results
RT-induced grade 2+ EASRs were observed in 8 (5%) and 80 (50%) patients at weeks 3 and 6 (end of RT), respectively. At the end of RT, significantly higher proportion of African Americans developed grade 3 EASRs (13.8% vs. 2.3% in others); grade 2+ EASRs were significantly associated with: change of CRP>1 mg/L (OR=2.51; 95%CI=1.06, 5.95, p=0.04), obesity (OR=2.08; 95%CI=1.03, 4.21, p=0.04), or combined both factors (OR=5.21; 95%CI=1.77, 15.38, p=0.003).
Conclusion
This is the first study to demonstrate that an inflammatory biomarker CRP is associated with RT-induced EASRs, particularly combined with obesity.
Impact
Future larger studies are warranted to validate our findings and facilitate the discovery and development of anti-inflammatory agents to protect normal tissue from RT-induced adverse effects and improve quality of life in breast cancer patients undergoing RT.
INTRODUCTION
Breast cancer is the most frequently diagnosed cancer and the second leading cause of cancer death in American women (1). There are more than two million American breast cancer survivors and it is important to address cancer survivorship issues related to treatment adverse responses that significantly impact quality of life. Compared with breast conserving surgery alone, the addition of radiotherapy (RT) to breast cancer therapy reduced the rate of local cancer recurrence (2). However, it is not yet clear which patients can be successfully treated with lumpectomy alone. Although well tolerated by most patients, even with improved RT technology, breast cancer patients experience moist desquamation as early adverse skin reactions (EASRs) during or up to 6 weeks after RT; 31.2% with intensity-modulated RT and 47.8% with standard treatment, respectively (3). The breast remains tender to palpation and the skin remains hyperpigmented for 6 to 9 months after treatment. The most common permanent effects on normal tissue are minor changes in the aesthetic appearance of the breast resulting from volume loss, fibrosis, or retraction at the tumor-bed site (4, 5). Breast or chest wall pain, increased risk of rib fracture, increased risk of cardiac morbidity, and lymphedema are also known late side effects of radiation (6, 7). Increasing evidence has suggested that individual genetic variations may play a significant role in the development of adverse radiation responses (8–10).
Inflammation may play critical roles in RT-induced EASRs and previous studies showed that RT induces changes in pro-inflammatory (IL-1α, IL-4, IL-2, IL-6, IL-8, IL-10, TNF-α, IFN-γ), pro-fibrotic (TGF-β1), pro-angiogenic (VEGF) and stem cell mobilizing (GM-CSF) cytokines and growth factors that may contribute to normal tissue toxicities or tumor control (11, 12). In addition, an inflammatory biomarker, C-reactive protein (CRP) has been associated with elevated risk for vascular atherosclerosis, insulin resistance, type 2 diabetes mellitus, and cancer (13–15). CRP levels were associated with fatigue and sleep quality in breast cancer patients and RT-induced mucositis in head and neck cancer patients (16–18). Furthermore, CRP levels also have prognostic value in patients with: (1) breast cancer, (2) loco-regionally advanced laryngeal carcinoma, or (3) advanced esophageal cancer (14, 19, 20).
Using the plasma samples from the first 159 breast cancer patients undergoing adjuvant RT to the intact breast in an ongoing prospective study, we pilot tested the hypothesis that higher pre- and post-RT CRP levels are associated with RT-induced EASRs. To the best of our knowledge, this is the first study investigating CRP in RT-induced EASRs of breast cancer patients.
Materials and Methods
Study Population
We used the plasma samples/data from the first 159 patients recruited during the period of December of 2008 and June of 2011 from an ongoing study to conduct this pilot study. Women diagnosed with breast carcinoma, Stage 0-III (American Joint Committee on Cancer) after breast conserving surgery were recruited from the Radiation Oncology Departments at the Sylvester Comprehensive Cancer Center and Jackson Memorial Hospital in Miami, FL. Each patient was asked to complete a self-administered questionnaire to collect information on: (a) demographic factors, (b) race/ethnicity, and (c) smoking history/status. Blood samples (20 ml) were collected from each individual before the initiation of RT and immediately after completion of RT. They were processed within 2 hours of phlebotomy and plasma was stored at −80°C until assay. This study was approved by the institution’s review board at the University of Miami Miller School of Medicine and the Jackson Memorial Hospital. After receiving a detailed description of the study protocol, signed informed consent was obtained from each participant.
RT and EASRs Assessment
Breast cancer patients usually begin RT about 4 to 6 weeks after surgery or completion of chemotherapy. RT to the whole breast was given using standard opposed tangential fields alone, or to the whole breast plus regional lymph nodes at the treating physician’s discretion. A regular dose of 45 to 50.4 Gy, in 1.8 or 2.0 Gy per fraction was delivered to the whole breast with or without regional nodes using 6 and/or 10 MV photons. In selected cases, a medial electron field was used for inclusion of the internal mammary nodes and/or cardiac sparing. A boost dose of 10–16 Gy was delivered to the lumpectomy cavity in the majority of case. Two radiation oncologists used the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Effects (CTCAE, version 3.0; http://ctep.cancer.gov) to evaluate EASRs grade and the presence or absence of moist desquamation at week 3 and at the completion of RT (week 6). The NCI CTCAE for radiation dermatitis contains 4 severity grades: Grade 1, faint erythema or dry desquamation; Grade 2, moderate erythema, noncontiguous patchy moist desquamation and moderate edema; Grade 3, contiguous moist desquamation and bleeding induced by minor trauma or abrasion; Grade 4, necrosis or ulceration and spontaneous bleeding from involved site. Within grade 2, patients may or may not have moist desquamation, a clinically relevant endpoint.
High Sensitivity CRP Assay
CRP levels in the pre- and post-RT plasma samples were measured using the high sensitivity C-reactive protein ELISA Kit (Calbiotech, Spring Valley, CA) according to the manufacturer’s protocol. The standard curve was generated with each batch of samples based on the CRP ranged from 0.2 to 10 mg/L. We always re-run subset of samples that are outside the detection range by adjusting the dilution ratio from the standard 1:100 to either more concentrate (e.g., 1:50) or less concentrate (e.g., 1:200, 1:500, or 1:1000) for repeated assay to ensure that the diluted samples are within the linear range of the standard curve of 0.2 and 10 mg/L. Briefly, frozen plasma sample was thawed and centrifuged at 10,000 rpm for 3 minutes. The clarified supernatant was diluted and 10 µl was added to duplicate CRP-coated wells. 100 µl of the enzyme conjugate was added and the plate was agitated briefly to mix. Following one hour incubation at room temperature, unbound mixture was removed and the wells washed three times with wash buffer. The plate was blotted on paper towels to remove residual wash solution. 100 µl of substrate was added and the plate was incubated for 15 minutes at room temperature. 50 µl of stop solution was then added and the plate was agitated to mix. The absorbance at 450 mm was determined using the Synergy HT microplate reader (BioTek Instruments, Winooski, VT). A standard curve using known concentrations of CRP was generated and levels of CRP were extrapolated based on the standard curve. The average coefficient of variation of duplicate samples was 8.3% and the inter-assay variation was <10%.
Statistical Analysis
We first determined the distributions of demographics (age, race, and ethnicity) and other patient characteristics (body mass index [BMI], smoking status, and breast cancer stage), as well as EASR grade at weeks 3 and 6 respectively overall and by race. Test of symmetry was used to compare the marginal distributions of matching–pairs data on EASR grade at weeks 3 and 6. Chi-square or Fisher’s exact test was used to test the association between patient demographics and other characteristics and EASRs grade at weeks 3 and 6. A paired t-test was used to compare CRP pre- and post-RT, using CRP log-base-2 transformed data for better fit of normal distribution. Analysis of variance (ANOVA) was conducted for comparisons of CRP log-base-2 transformed data, either at pre-RT or post-RT, by categories of demographic and other patient characteristic variables (not shown). Alternatively, we used nonparametric methods (Wilcoxon-Mann-Whitney test, or Kruskal-Wallis test for ANOVA, Wilcoxon sign test for paired sample test) for corresponding analysis of CRP raw data when distributions were significantly right skewed.
Logistic regressions were used to test whether pre-RT CRP (≥2 vs. <2 mg/L), post-RT CRP (≥2 vs. <2 mg/L), or the CRP change (>1 vs. ≤1 mg/L, post- minus pre-RT) were significantly associated with grade 2/3 vs. 0/1 RT-induced EASRs at week 3 and 6, respectively. Optimal cut-off values, 2 for CRP and 1 for CRP change, were selected based on estimates of sensitivity, specificity, and accuracy from receiver operating characteristic (ROC) univariate logistic regression analyses including CRP variables as continuous. High sensitivity was prioritized to select common cut-off value for pre/post CRP, and cut-off for CRP change. Multiple logistic regression models were used to evaluate the association between EASR grade and CRP or CRP change, after adjustment for BMI (≥30 vs. <30), ethnicity (Hispanic vs. Non-Hispanic), race (African Americans vs. non-African Americans), and age (in years). Odds ratios (OR) and 95% confidence intervals (95% CI) are reported. All statistical analysis was carried out in SAS v 9.3 (SAS Institute, Cary, NC), and test results were considered significant at the two-sided 5% level.
RESULTS
Study Population Characteristics
As summarize in Table 1, the study population consists of 29 African Americans (18.2%), 96 Hispanic Whites (60.4%), 29 non-Hispanic Whites (18.2%), and 5 other (3.1%). There were significant racial/ethnic differences in BMI distributions; about 62.1% African Americans were obese compared to 36.5% and 27.6% in Hispanic Whites and non-Hispanic Whites, respectively. No significant racial/ethnic differences were observed for clinical stage, smoking history, and smoking status. There was no significant racial/ethnic difference in skin toxicity grade at week 3 or 6. At week 3, lower proportion of Hispanic Whites had a grade 2 skin toxicity (2.1%) compared to African Americans (10.7%) or non-Hispanic Whites (10.3%). At week 6, higher proportion of African Americans had a grade 3 skin toxicity (13.8%) compared to Hispanic Whites (2.1%) or non-Hispanic Whites (3.4%). There was no significant racial/ethnic difference in the distribution of total number of 11 comorbidity conditions or total RT doses. However, there was a significant racial/ethnic difference (p<0.0001) in breast volume; African American patients have the highest breast volume (mean±SD=1358±647) compared to that in Hispanic Whites (905±437), non-Hispanic Whites (808±408), or other (587±251).
Table 1.
Study Variables by Race/Ethnicity
| Variable | Patients | AA1 | HW1 | NHW1 | Other | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | P2 | ||
| Study Population | 159 | 100 | 29 | 18.2 | 96 | 60.4 | 29 | 18.2 | 5 | 3.1 | ||
| Age: Mean (SD) | 56.3 | (9.3) | 55.1 | (7.4) | 57.2 | (9.4) | 55.5 | (10.5) | 50.2 | (9.2) | 0.30 | |
| BMI (kg/m2) | ||||||||||||
| <25 | 47 | 29.6 | 4 | 13.8 | 24 | 25.0 | 15 | 51.7 | 4 | 80.0 | 0.003 | |
| 25 – 29.99 | 51 | 32.1 | 7 | 24.1 | 37 | 38.5 | 6 | 20.7 | 1 | 20.0 | ||
| ≥30 | 61 | 38.4 | 18 | 62.1 | 35 | 36.5 | 8 | 27.6 | 0 | 0 | ||
| Clinical stage | ||||||||||||
| 0 | 33 | 20.8 | 5 | 17.2 | 22 | 22.9 | 4 | 13.8 | 2 | 40.0 | 0.23 | |
| IA–B | 79 | 49.7 | 11 | 37.9 | 51 | 53.1 | 16 | 55.2 | 1 | 20.0 | ||
| IIA–IIIC | 47 | 29.6 | 13 | 44.8 | 23 | 24.0 | 9 | 31.0 | 2 | 40.0 | ||
| Smoking History | ||||||||||||
| Never | 108 | 67.9 | 18 | 62.1 | 8 | 70.8 | 18 | 62.1 | 4 | 80.0 | 0.53 | |
| Ever | 51 | 32.1 | 11 | 37.9 | 28 | 29.2 | 11 | 37.9 | 1 | 20.0 | ||
| Smoking Status | ||||||||||||
| Never | 108 | 67.9 | 18 | 62.1 | 68 | 70.8 | 18 | 62.1 | 4 | 80.0 | 0.30 | |
| Former | 45 | 28.3 | 8 | 27.6 | 26 | 27.1 | 10 | 34.5 | 1 | 20.0 | ||
| Current | 6 | 3.8 | 3 | 10.3 | 2 | 2.1 | 1 | 3.4 | 0 | 0 | ||
| Week 3 EASR grade | ||||||||||||
| 0 | 25 | 15.8 | 5 | 17.9 | 16 | 16.7 | 2 | 6.9 | 2 | 40.0 | 0.10 | |
| 1 | 125 | 79.1 | 20 | 71.4 | 78 | 81.3 | 24 | 82.8 | 3 | 60.0 | ||
| 2 | 8 | 5.1 | 3 | 10.7 | 2 | 2.1 | 3 | 10.3 | 0 | 0 | ||
| Week 6 EASR grade | ||||||||||||
| 0 | 2 | 1.3 | 1 | 3.4 | 1 | 1.0 | 0 | 0 | 0 | 0 | 0.173 | |
| 1 | 77 | 48.4 | 12 | 41.4 | 46 | 47.9 | 15 | 51.7 | 4 | 80.0 | ||
| 2 | 73 | 45.9 | 12 | 41.4 | 47 | 49.0 | 13 | 44.8 | 1 | 20.0 | ||
| 3 | 7 | 4.4 | 4 | 13.8 | 2 | 2.1 | 1 | 3.4 | 0 | 0 | ||
| Pre-RT CRP (mg/L) | ||||||||||||
| < 10 | 145 | 91.2 | 24 | 82.8 | 90 | 93.8 | 27 | 93.1 | 4 | 80.0 | 0.25 | |
| ≥ 10 | 14 | 8.8 | 5 | 17.2 | 6 | 6.3 | 2 | 6.9 | 1 | 20.0 | ||
| < 2 | 58 | 36.5 | 8 | 27.6 | 36 | 37.5 | 13 | 44.8 | 1 | 20.0 | 0.39 | |
| ≥ 2 | 101 | 63.5 | 21 | 72.4 | 60 | 62.5 | 16 | 55.2 | 4 | 80.0 | ||
| Post-RT CRP (mg/L) | ||||||||||||
| < 10 | 141 | 88.7 | 23 | 79.3 | 87 | 90.6 | 27 | 93.1 | 4 | 80.0 | 0.20 | |
| ≥ 10 | 18 | 11.3 | 6 | 20.7 | 9 | 9.4 | 2 | 6.9 | 1 | 20.0 | ||
| < 2 | 56 | 35.2 | 7 | 24.1 | 34 | 35.4 | 13 | 44.8 | 2 | 40.0 | 0.25 | |
| ≥ 2 | 103 | 64.8 | 22 | 75.9 | 62 | 64.6 | 16 | 55.2 | 3 | 60.0 | ||
| Post-RT- pre-RT CRP(mg/L) | ||||||||||||
| <0 | 71 | 44.7 | 12 | 41.4 | 41 | 42.7 | 14 | 48.3 | 4 | 80.0 | 0.36 | |
| 0 | 10 | 6.3 | 0 | 0 | 8 | 8.3 | 2 | 6.9 | 0 | 0 | ||
| >0 and <1 | 44 | 27.7 | 7 | 24.1 | 30 | 31.3 | 6 | 20.7 | 1 | 20.0 | ||
| ≥1 | 34 | 21.4 | 10 | 34.5 | 17 | 17.7 | 7 | 24.1 | 0 | 0 | ||
| # of Comorbidities4 | ||||||||||||
| 0 | 65 | 40.9 | 6 | 20.7 | 42 | 43.8 | 14 | 48.3 | 3 | 60.0 | 0.13 | |
| 1 | 56 | 35.2 | 12 | 41.4 | 35 | 36.5 | 9 | 31.0 | 0 | 0 | ||
| 2 | 28 | 17.6 | 8 | 27.6 | 15 | 15.6 | 3 | 10.3 | 2 | 40.0 | ||
| ≥3 | 10 | 6.3 | 3 | 10.3 | 4 | 4.2 | 3 | 10.3 | 0 | 0 | ||
| Total RT dose (Gy)5 | ||||||||||||
| <60 | 33 | 20.8 | 5 | 17.2 | 18 | 18.8 | 9 | 31.0 | 1 | 20.0 | 0.31 | |
| 60 | 108 | 67.9 | 20 | 69.0 | 69 | 71.9 | 15 | 51.7 | 4 | 80.0 | ||
| >60 | 18 | 11.3 | 4 | 13.8 | 9 | 9.4 | 5 | 17.2 | 0 | 0 | ||
| Breast volume (CC) | ||||||||||||
| Mean (SD) | 958 | (509) | 1358 | (647) | 905 | (437) | 808 | (408) | 587 | (251) | <0.0001 | |
| Median | 864 | 1357 | 831 | 774 | 593 | |||||||
| Below Median (<864) | 77 | 49.7 | 8 | 28.6 | 49 | 52.7 | 16 | 55.2 | 4 | 80.0 | 0.06 | |
| Above median (≥ 864) | 78 | 50.3 | 20 | 71.4 | 44 | 47.3 | 13 | 44.8 | 1 | 20.0 | ||
AA, Black or African American; HW, Hispanic Whites; NHW, Non-Hispanic Whites.
Chi-square or Fisher’s exact tests excluding the other race category and missing.
Combined early adverse skin reaction (EASR) grades 0 and 1.
Sum of 11 comorbidity conditions: diabetes, hypertension, heart disease, lung disease, thyroid condition, cirrhosis liver, stroke, chronic bronchitis, hepatitis, tuberculosis, and other.
Sum of total breast and boost radiation doses; the ranged was between 50 to 66.4 Gy except for one patient at 38.5 Gy.
Progression of EASRs from Week 3 to Week 6 (End of RT)
In Table 2, we demonstrate a significant RT dosage-dependent progression of EASRs from week 3 to week 6 (p<0.001). There was only one patient who had grade 0 at both weeks 3 and 6. At week 3, 15.8%, 79.1%, and 5.1% patients had grades 0, 1, and 2 EASRs, respectively. At week 6, there was a significant increase in the severity of RT-induced EASRs: 1.3%, 48.1%, 46.2%, and 4.4% patients had grade 0, 1, 2, and 3 EASRs, respectively.
Table 2.
Progression of EASR1 from Week 3 to Week 6 (End of RT)
| Week 3 Grade | Week 6 Grade | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | P2 | |
| 0 | 1 | 14 | 9 | 1 | 25 (15.8%) | <0.001 |
| 1 | 1 | 62 | 57 | 5 | 125 (79.1%) | |
| 2 | 0 | 0 | 7 | 1 | 8 (5.1%) | |
| Total | 2 (1.3%) | 76 (48.1%) | 73(46.2%) | 7(4.4%) | 158 | |
EASR: early adverse skin reaction.
Wilcoxon signed-rank test.
EASR Grade by Patient Characteristics
In Table 3, we summarize the results of EASR grade by patient characteristics. At week 3, there was a marginally significant ethnic different in EASR grade (p=0.07); Hispanic patients have less grade 2 toxicity (2.0% vs. 10.3% in non-Hispanic). At week 6, a higher proportion of African Americans developed grade 3 EASRs (13.8% vs. 2.3% in others). A significantly higher proportion of obese (BMI ≥30) women experienced grade 3 EASRs at week 6 compared to those with BMI<30 (8.2% vs. 2.0%; p=0.02). A significantly higher proportion of current smoker experienced grade 3 EASRs at week 6 compared to non-smoker (33.3% vs. 4.7%; p=0.01). In addition, patients with above-median breast volume have a higher risk of developing grade 3 EASRs at week 6 compared to patients with below-median breast volume (9.0% vs. 0%; p=0.007).
Table 3.
EASR Grade by Patient Characteristics
| Variable | Week 3 grade | Week 6 grade | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 0 | 1 | 2 | 3 | ||||||||||
| N | % | N | % | N | % | P1 | N | % | N | % | N | % | N | % | P1 | |
| Total Patient | 25 | 15.8 | 125 | 79.1 | 8 | 5.0 | 2 | 1.3 | 77 | 48.4 | 73 | 45.9 | 7 | 4.4 | ||
| Age (years) | ||||||||||||||||
| <50 | 6 | 13.3 | 34 | 75.6 | 5 | 11.4 | 0.35 | - | - | 19 | 42.2 | 25 | 55.6 | 1 | 2.2 | 0.13 |
| 50–59 | 10 | 18.5 | 43 | 79.6 | 1 | 1.9 | 1 | 1.9 | 29 | 53.7 | 19 | 35.2 | 5 | 9.3 | ||
| ≥60 | 9 | 15.3 | 48 | 81.4 | 2 | 3.2 | 1 | 1.7 | 29 | 48.3 | 29 | 48.3 | 1 | 1.7 | ||
| Ethnicity | ||||||||||||||||
| Non-Hispanic | 9 | 15.5 | 43 | 74.1 | 6 | 10.3 | 0.07 | 1 | 1.7 | 29 | 50.0 | 24 | 41.4 | 4 | 6.9 | 0.24 |
| Hispanic | 16 | 16.0 | 82 | 82.0 | 2 | 2.0 | 1 | 1.0 | 48 | 47.5 | 49 | 48.5 | 3 | 3.0 | ||
| Race/Ethnicity | ||||||||||||||||
| AA | 5 | 17.9 | 20 | 71.4 | 3 | 10.3 | 0.10 | 1 | 3.4 | 12 | 41.4 | 12 | 41.4 | 4 | 13.8 | 0.22 |
| HW | 16 | 16.7 | 78 | 81.3 | 2 | 2.1 | 1 | 1.0 | 46 | 47.9 | 47 | 49.0 | 2 | 2.1 | ||
| NHW | 2 | 6.9 | 24 | 82.8 | 3 | 10.3 | 0 | 0 | 15 | 51.7 | 13 | 44.8 | 1 | 3.4 | ||
| Other | 2 | 40.0 | 3 | 60.0 | 0 | 0 | 0 | 0 | 4 | 80.0 | 1 | 20.0 | 0 | 0 | ||
| AA | 5 | 17.9 | 20 | 71.4 | 3 | 10.7 | 0.22 | 1 | 3.4 | 12 | 41.4 | 12 | 41.4 | 4 | 13.8 | 0.02 |
| Non-AA | 21 | 16.2 | 104 | 80.0 | 5 | 3.8 | 1 | 0.8 | 65 | 50.0 | 61 | 46.9 | 3 | 2.3 | ||
| BMI(kg/m2) | ||||||||||||||||
| <25 | 9 | 19.1 | 37 | 78.7 | 1 | 2.1 | 0.83 | - | - | 30 | 63.8 | 16 | 34.0 | 1 | 2.1 | 0.06 |
| 25 – 29.99 | 7 | 14.0 | 40 | 80.0 | 3 | 5.9 | 1 | 2.0 | 25 | 49.0 | 24 | 47.1 | 1 | 2.0 | ||
| ≥30 | 9 | 14.8 | 48 | 78.7 | 4 | 6.6 | 1 | 1.6 | 22 | 36.1 | 33 | 54.1 | 5 | 8.2 | ||
| <30 | 16 | 16.5 | 77 | 79.4 | 4 | 4.1 | 0.78 | 1 | 1.0 | 55 | 56.1 | 40 | 40.8 | 2 | 2.0 | 0.02 |
| ≥30 | 9 | 14.8 | 48 | 78.7 | 4 | 6.6 | 1 | 1.6 | 22 | 36.1 | 33 | 54.1 | 5 | 8.2 | ||
| Clinical stage | ||||||||||||||||
| 0 | 7 | 21.2 | 25 | 75.8 | 1 | 3.0 | 0.60 | 2 | 6.1 | 20 | 60.6 | 10 | 30.3 | 1 | 3.0 | 0.29 |
| IA–IB | 9 | 11.5 | 65 | 83.3 | 4 | 5.1 | - | - | 36 | 45.6 | 39 | 49.4 | 4 | 5.1 | ||
| IIA–IIIC | 9 | 19.1 | 35 | 74.5 | 3 | 6.4 | - | - | 21 | 44.7 | 24 | 51.1 | 2 | 4.3 | ||
| Smoking History | ||||||||||||||||
| Never | 19 | 17.8 | 83 | 77.6 | 5 | 4.7 | 0.61 | 1 | 0.9 | 53 | 49.1 | 49 | 45.4 | 5 | 4.7 | 1.00 |
| Ever | 6 | 11.8 | 42 | 82.4 | 3 | 5.9 | 1 | 2.0 | 24 | 47.1 | 24 | 47.1 | 2 | 3.9 | ||
| Smoking Status | ||||||||||||||||
| Never | 19 | 17.8 | 83 | 77.6 | 5 | 4.7 | 0.78 | 1 | 0.9 | 53 | 49.1 | 49 | 45.4 | 5 | 4.7 | 0.01 |
| Former | 5 | 11.1 | 37 | 82.2 | 3 | 6.7 | 1 | 2.2 | 20 | 44.4 | 24 | 53.3 | 0 | 0 | ||
| Current | 1 | 16.7 | 5 | 83.3 | 0 | 0 | 0 | 0 | 4 | 66.7 | 0 | 0- | 2 | 33.3 | ||
| # Comorbidities | ||||||||||||||||
| 0 | 7 | 10.9 | 55 | 85.9 | 2 | 3.1 | 0.25 | 0 | 0 | 37 | 56.9 | 26 | 40.0 | 2 | 3.1 | 0.15 |
| 1 | 10 | 17.9 | 40 | 71.4 | 6 | 10.7 | 0 | 0 | 25 | 44.6 | 30 | 53.6 | 1 | 1.8 | ||
| 2 | 6 | 21.4 | 22 | 78.6 | 0 | 0 | 2 | 7.1 | 12 | 42.9 | 12 | 42.9 | 2 | 7.1 | ||
| ≥3 | 2 | 20.0 | 8 | 80.0 | 0 | 0 | 0 | 0 | 3 | 30.0 | 5 | 50.0 | 2 | 20.0 | ||
| RT Dose (Gy) | ||||||||||||||||
| <60 | 2 | 6.3 | 28 | 87.5 | 2 | 6.3 | 0.30 | 0 | 0 | 17 | 51.5 | 14 | 42.4 | 2 | 6.1 | 0.90 |
| 60 | 18 | 16.7 | 84 | 77.8 | 6 | 5.6 | 1 | 0.9 | 52 | 48.1 | 51 | 47.2 | 4 | 3.7 | ||
| >60 | 5 | 27.8 | 13 | 72.2 | 0 | 0 | 1 | 5.6 | 8 | 44.4 | 8 | 44.4 | 1 | 5.6 | ||
| Breast Volume (cc) | ||||||||||||||||
| <864 | 13 | 16.9 | 62 | 80.5 | 2 | 2.6 | 0.65 | 2 | 2.6 | 42 | 54.5 | 33 | 42.9 | 0 | 0 | 0.007 |
| ≥864 | 12 | 15.6 | 60 | 77.9 | 5 | 6.5 | 0 | 0 | 33 | 42.3 | 38 | 48.7 | 7 | 9.0 | ||
P-value of chi-square test or Fisher’s exact test excluding missing; for week 6, grade 0/1 vs. 2 vs. 3.
CRP Levels by BMI and other Patient Characteristics
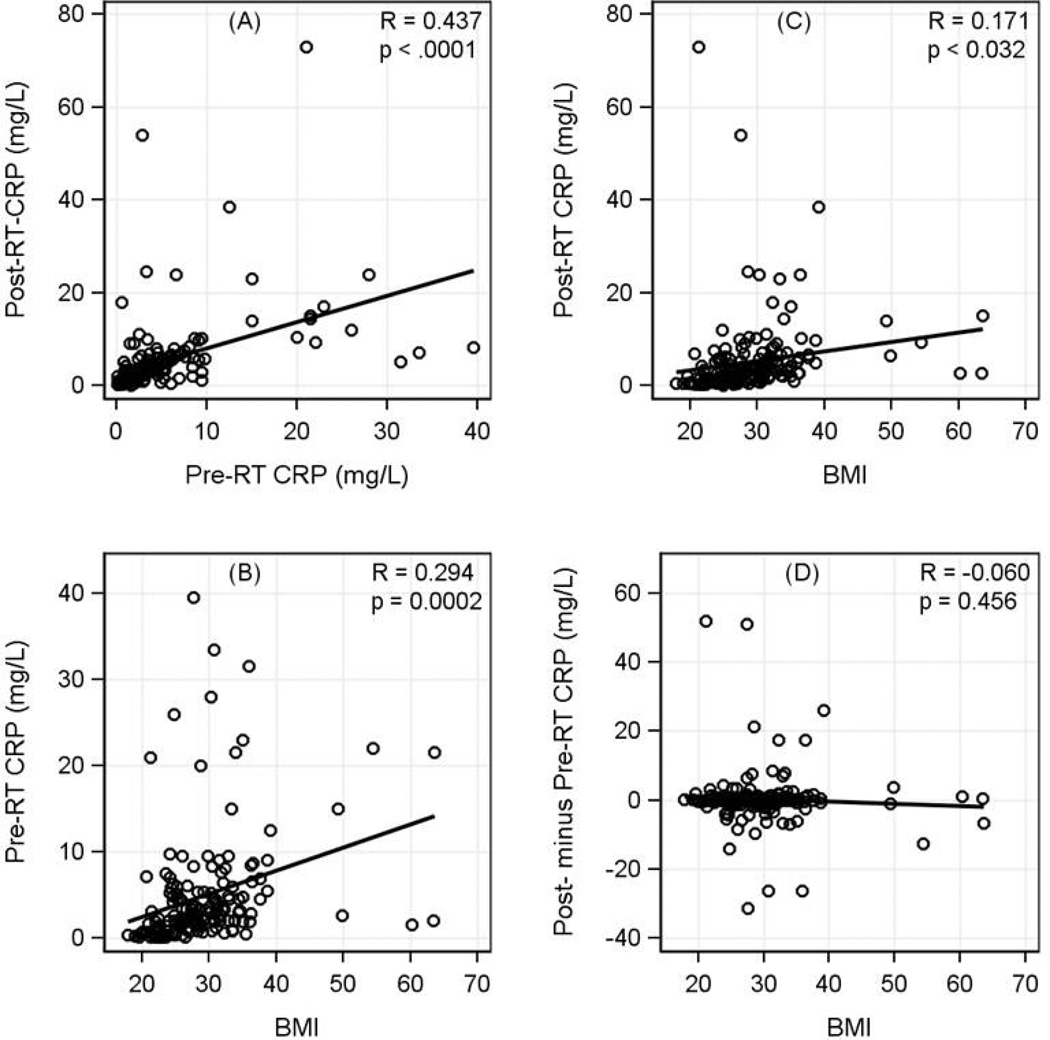
As shown in Fig. 1, there were significant correlations among individual’s pre- and post-RT CRP levels (R=0.437, p<0.0001) and BMI (pre-RT: R=0.294, p=0.0002; post-RT: R=0.171, p=0.032). The pre- and post-RT CRP levels by patient characteristics were summarized in Table 4. The mean±SD of pre- and post-RT CRP levels were 4.93±6.65 and 5.26±8.59 mg/L, respectively. For the pre-RT CRP levels, obese patients with BMI≥30 have a significantly higher pre-RT CRP level vs. patients with BMI<30 (p<0.0001). For post-RT CRP, in addition to the differences by obesity, significantly higher levels were observed in African Americans compared to other racial/ethnic groups (6.44±6.11 vs 5.00±9.05, p=0.02). Patients with multiple comorbidity conditions have significantly higher pre- and post-RT CRP values. When we evaluated the change of CRP between pre- and post-RT, patients with at least 3 comorbidity conditions have a significant increase (6.50±20.51; p=0.02) compared to other groups. Patients with above-median breast volume have significantly higher pre- and post-RT CRP values.
Figure 1.
Correlations among Pre-RT CRP, Post-RT CRP, and BMI. The mean of pre-RT CRP was 4.93 mg/L (SD=6.65, median=2.8, and range=0.1 to 39.5); the mean of post-RT CRP was 5.26 mg/L (SD=8.59, median=3.1, and range=0.1 to 73). CRP data was substantially skew. Comparison of log-2 transformed data indicated no statistically significant difference between pre- vs. post-RT CRP (mean of log2CRP: 1.33 vs. 1.42, p=0.34). Correlations were 0.437 (p<0.0001) between pre- and post-RT CRP, 0.171 (p=0.03) for post-RT CRP and BMI, and 0.294 (p<0.001) for pre-RT CRP and BMI. After excluding the two largest outliers for post-RT CRP (values 54 and 73), the correlations were 0.529 for pre- and post-RT, and 0.373 for post-RT CRP vs. BMI.
Table 4.
CRP Levels (mg/L) by Demographic Factors and Other Patient Characteristics
| Variable | N | Pre-RT CRP | Post-RT CRP | Post-pre RT CRP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | SD | P1 | Median | Mean | SD | P1 | Median | Mean | SD | P1 | ||
| Total | 159 | 2.80 | 4.93 | 6.65 | 3.10 | 5.26 | 8.59 | 0.00 | 0.33 | 8.25 | |||
| Age (yrs) | |||||||||||||
| <50 | 45 | 2.10 | 4.39 | 6.32 | 0.12 | 2.70 | 5.45 | 11.32 | 0.04 | 0.10 | 1.06 | 9.42 | 0.29 |
| 50–59 | 54 | 2.40 | 4.46 | 5.95 | 2.25 | 3.83 | 4.35 | −0.20 | −0.63 | 3.66 | |||
| ≥60 | 60 | 3.35 | 5.75 | 7.47 | 4.20 | 6.41 | 9.04 | 0.05 | 0.66 | 10.13 | |||
| Ethnicity | |||||||||||||
| Non-Hispanic | 58 | 2.65 | 5.54 | 7.70 | 0.98 | 2.75 | 6.06 | 11.92 | 0.66 | −0.05 | 0.52 | 11.64 | 0.52 |
| Hispanic | 101 | 3.00 | 4.58 | 5.98 | 3.20 | 4.80 | 5.92 | 0.10 | 0.23 | 5.50 | |||
| Race/ethnicity | |||||||||||||
| AA | 29 | 4.50 | 6.67 | 7.79 | 0.16 | 4.70 | 6.44 | 6.11 | 0.04 | 0.40 | −0.23 | 7.43 | 0.27 |
| HW | 96 | 2.90 | 4.54 | 6.02 | 3.15 | 4.62 | 5.74 | 0.00 | 0.08 | 5.54 | |||
| NHW | 29 | 2.20 | 4.02 | 6.66 | 2.20 | 6.50 | 16.09 | 0.00 | 2.48 | 14.53 | |||
| Other | 5 | 4.20 | 7.50 | 10.51 | 2.00 | 3.64 | 4.84 | −0.80 | −3.86 | 5.93 | |||
| AA | 29 | 4.50 | 6.67 | 7.79 | 0.08 | 4.70 | 6.44 | 6.11 | 0.02 | 0.40 | −0.23 | 7.43 | 0.24 |
| Non-AA | 130 | 2.65 | 4.54 | 6.34 | 2.80 | 5.00 | 9.05 | 0.00 | 0.46 | 8.44 | |||
| BMI(kg/m2) | |||||||||||||
| <25 | 47 | 0.90 | 2.93 | 5.02 | <0.0001 | 1.20 | 3.55 | 10.63 | <0.0001 | 0.00 | 0.61 | 8.09 | 0.91 |
| 25 − 29.99 | 51 | 2.50 | 4.14 | 5.96 | 3.20 | 4.84 | 8.07 | 0.10 | 0.69 | 9.37 | |||
| ≥30 | 61 | 4.60 | 7.12 | 7.68 | 4.70 | 6.94 | 6.93 | 0.10 | −0.18 | 7.45 | |||
| <30 | 98 | 2.00 | 3.56 | 5.54 | <0.0001 | 1.75 | 4.22 | 9.36 | <0.0001 | 0.00 | 0.66 | 8.73 | 0.46 |
| ≥30 | 61 | 4.60 | 7.12 | 7.68 | 4.70 | 6.94 | 6.93 | 0.10 | −0.18 | 7.45 | |||
| Clinical Stage | |||||||||||||
| 0 | 33 | 3.70 | 5.03 | 5.98 | 0.70 | 3.30 | 4.99 | 5.71 | 0.75 | 0.10 | −0.04 | 2.44 | 0.93 |
| IA–B | 79 | 2.80 | 4.56 | 5.82 | 2.60 | 6.04 | 11.30 | 0.00 | 1.48 | 10.20 | |||
| IIA–IIIC | 47 | 2.30 | 5.46 | 8.31 | 3.20 | 4.14 | 3.43 | 0.00 | −1.33 | 6.92 | |||
| Smoking history | |||||||||||||
| Never | 108 | 2.75 | 4.87 | 6.59 | 0.87 | 3.20 | 5.37 | 9.31 | 0.74 | 0.00 | 0.50 | 8.90 | 0.67 |
| Ever | 51 | 2.90 | 5.05 | 6.84 | 2.80 | 5.04 | 6.89 | 0.10 | −0.01 | 6.73 | |||
| Smoking Status | |||||||||||||
| Never | 108 | 2.75 | 4.87 | 6.59 | 0.84 | 3.20 | 5.37 | 9.31 | 0.96 | 0.00 | 0.50 | 8.90 | 0.87 |
| Former | 45 | 2.50 | 5.16 | 7.21 | 2.80 | 5.17 | 7.24 | 0.10 | 0.01 | 6.98 | |||
| Current | 6 | 3.55 | 4.22 | 2.92 | 2.05 | 4.07 | 3.57 | 0.40 | −0.15 | 4.95 | |||
| Week 3 EASR grade | |||||||||||||
| 0 | 25 | 3.60 | 4.80 | 5.19 | 0.27 | 5.20 | 5.61 | 4.93 | 0.08 | 0.20 | 0.82 | 6.06 | 0.29 |
| 1 | 125 | 2.30 | 4.68 | 6.48 | 2.60 | 5.24 | 9.37 | 0.00 | 0.56 | 8.54 | |||
| 2 | 8 | 3.65 | 9.15 | 11.89 | 3.25 | 4.71 | 4.85 | −0.60 | −4.44 | 9.49 | |||
| Week 6 EASR grade | |||||||||||||
| 0/1 | 79 | 3.00 | 4.45 | 5.60 | 0.22 | 2.20 | 5.21 | 10.03 | 0.13 | 0.00 | 0.76 | 7.65 | 0.07 |
| 2 | 73 | 2.90 | 5.73 | 7.82 | 3.90 | 5.39 | 7.17 | 0.10 | −0.34 | 9.15 | |||
| 3 | 7 | 2.00 | 1.91 | 0.94 | 2.70 | 4.49 | 4.21 | 0.60 | 2.57 | 3.56 | |||
| # comorbidities | |||||||||||||
| 0 | 65 | 2.10 | 3.66 | 4.16 | 0.02 | 1.70 | 4.60 | 10.25 | <0.001 | −0.20 | 0.93 | 7.91 | 0.02 |
| 1 | 56 | 2.75 | 4.77 | 6.81 | 3.20 | 4.36 | 4.86 | 0.20 | −0.42 | 4.20 | |||
| 2 | 28 | 5.35 | 7.80 | 7.99 | 4.65 | 6.05 | 4.96 | −0.25 | −1.76 | 7.29 | |||
| ≥3 | 10 | 2.70 | 5.94 | 11.84 | 8.60 | 12.44 | 16.21 | 3.95 | 6.50 | 20.51 | |||
| Total RT dose (Gy) | |||||||||||||
| <60 | 33 | 3.20 | 6.48 | 7.51 | 0.20 | 3.30 | 7.22 | 13.11 | 0.45 | −0.20 | 0.74 | 9.83 | 0.39 |
| 60 | 108 | 2.55 | 4.81 | 6.81 | 2.70 | 4.99 | 7.38 | 0.00 | 0.17 | 8.40 | |||
| ≥60 | 18 | 2.50 | 2.77 | 1.79 | 3.30 | 3.33 | 2.75 | 0.50 | 0.56 | 2.52 | |||
| Breast volume (cc) | |||||||||||||
| <864 | 77 | 2.00 | 3.74 | 5.95 | <0.001 | 2.00 | 4.46 | 10.41 | <0.001 | 0.00 | 0.72 | 9.31 | 0.98 |
| ≥864 | 78 | 3.40 | 5.99 | 7.02 | 3.85 | 6.05 | 6.43 | 0.05 | 0.06 | 7.30 | |||
P value, Wilcoxon-Mann-Whitney or Kruskal-Wallis test (except the firs line).
Wilcoxon sign test.
Association between EASRs and CRP and/or Obesity
In multivariable logistic regression models, we evaluated the associations between specific CRP variable and obesity (BMI≥30 vs. <30) and EASRs after adjustment for age (years), race (African Americans vs. others), ethnicity (Hispanic or non-Hispanic), and tumor stage (II-IV or I vs. 0). Because interactions between specific CRP variable with BMI were not significant, OR estimates for combination high CRP and obesity vs. low CRP and non-obesity were calculated by multiplying the individual ORs. As shown in Table 5, neither pre-RT CRP nor obesity alone was significantly associated with EASRs at week 3 or 6. However, there was a significant association between combined higher pre-RT CRP with obesity and grade 2+ EASRs at week 6 (OR=3.03, 95%CI=1.28, 7.17; p=0.01). Grades 2/3 EASRs at week 6 were significantly associated with a higher post-RT CRP combined with obesity (OR=2.70, 95%CI=1.18, 6.19; p=0.02). Grades 2/3 EASRs at week 6 were significantly associated with change (post-RT minus pre-RT) of CRP >1 mg/L (OR=2.51; 95%CI=1.06, 5.95; p=0.04), and obesity (OR=2.08; 95%CI=1.03, 4.21; p=0.04). Furthermore, grades 2/3 EASRs at week 6 was significantly associated with combined elevated CRP (>1 mg/L) and obesity (BMI≥30) vs. CRP≤1 and non-obesity (OR=5.21, 95%CI=1.77, 15.38; p=0.003).
Table 5.
Association between EASRs and CRP and/or Obesity
| Variable | Level | Week 3 Grade (n=158) | Week 6 Grade (n=159) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 0/1 | 2 | Multivariable | Total | 0/1 | 2/3 | Multivariable | ||||||||||
| N | % | N | % | N | % | OR (95%CI)1 | P | N | % | N | % | N | % | OR(95%CI)1 | P | ||
| Pre-RT CRP (mg/L) | < 2 | 58 | 36.7 | 57 | 38.0 | 1 | 12.5 | Reference | 58 | 36.5 | 34 | 43.0 | 24 | 30.0 | Reference | ||
| ≥ 2 | 100 | 63.3 | 93 | 62.0 | 7 | 87.5 | 4.90 (0.50, 48.32) | 0.17 | 101 | 63.5 | 45 | 57.0 | 56 | 70.0 | 1.56 (0.76, 3.21) | 0.22 | |
| BMI | <30 | 97 | 61.4 | 93 | 62.0 | 4 | 50.0 | Reference | 98 | 61.6 | 56 | 70.9 | 42 | 52.5 | Reference | ||
| ≥30 | 61 | 38.6 | 57 | 38.0 | 4 | 50.0 | 1.18(0.23, 6.21) | 0.85 | 61 | 38.4 | 23 | 29.1 | 38 | 47.5 | 1.94 (0.94, 4.02) | 0.08 | |
| Pre-RT CRP≥2 and BMI≥30 vs. CRP<2 and BMI<30 | 5.81 (0.49, 69.23)2 | 0.16 | 3.03 (1.28, 7.17)2 | 0.01 | |||||||||||||
| Post-RT CRP (mg/L) | < 2 | 56 | 35.4 | 53 | 35.3 | 3 | 37.5 | Reference | 56 | 35.2 | 33 | 41.8 | 23 | 28.8 | Reference | ||
| ≥ 2 | 102 | 64.6 | 97 | 64.7 | 5 | 62.5 | 0.82 (0.13, 5.15) | 0.84 | 103 | 64.8 | 46 | 58.2 | 57 | 71.3 | 1.38 (0.65, 2.94) | 0.40 | |
| BMI | <30 | 97 | 61.4 | 93 | 62.0 | 4 | 50.0 | Reference | 98 | 61.6 | 56 | 70.9 | 42 | 52.5 | Reference | ||
| ≥30 | 61 | 38.6 | 57 | 38.0 | 4 | 50.0 | 2.02 (0.30, 13.63) | 0.47 | 61 | 38.4 | 23 | 29.1 | 38 | 47.5 | 1.96 (0.92, 4.17) | 0.08 | |
| Post-RT CRP≥2 and BMI≥30 vs. CRP<2 and BMI<30 | 1.66 (0.27, 10.32)2 | 0.59 | 2.70 (1.18, 6.19)2 | 0.02 | |||||||||||||
| Post- minus pre-RT CRP | ≤ 1 | 125 | 79.1 | 118 | 78.7 | 7 | 87.5 | Reference | 126 | 79.2 | 69 | 87.3 | 57 | 71.3 | Reference | ||
| > 1 | 33 | 20.9 | 32 | 21.3 | 1 | 12.5 | 0.47 (0.05, 4.38) | 0.51 | 33 | 20.8 | 10 | 12.7 | 23 | 28.8 | 2.51 (1.06, 5.95) | 0.04 | |
| BMI | <30 | 97 | 61.4 | 94 | 62.0 | 4 | 50.0 | Reference | 98 | 61.6 | 56 | 70.9 | 42 | 52.5 | Reference | ||
| ≥30 | 61 | 38.6 | 57 | 38.0 | 4 | 50.0 | 2.09 (0.40,11.14) | 0.39 | 61 | 38.4 | 23 | 29.1 | 38 | 47.5 | 2.08 (1.03, 4.21) | 0.04 | |
| CRP change>1 and BMI≥30 vs. change≤1 and BMI<30 | 0.97(0.08, 11.97)2 | 0.98 | 5.21 (1.77, 15.38)2 | 0.003 | |||||||||||||
OR estimates of CRP variables (high vs. low category, mg/L), BMI (≥30 vs. <30 kg/m2), or combination, obtained from logistic regression models including the specific CRP variable, BMI, age (years), ethnicity (Hispanic vs. non-Hispanic), race (AA vs. others), and tumor stage (II–IV or I, vs. 0).
Interactions between specific CRP variable with BMI were not significant, OR estimates for combination high CRP and obesity vs. low CRP and non-obesity were calculated by multiplying the individual ORs.
DISCUSSION
After breast-conserving surgery, adjuvant RT to the breast contributes to improved local regional recurrence and survival in breast cancer patients. However, whole breast RT is associated with EASRs, increased cardiovascular mortality, and lung cancer development. In general, African Americans have worse treatment-related side effects and survival. Therefore, we designed this tri-racial/ethnic study to evaluate whether inflammatory biomarker CRP is related to RT-induced EASRs. To the best of our knowledge, this is the first study to date investigating the association between CRP and RT-induced EASRs in breast cancer. We reported significant association between elevated risk of RT-induced EASRs and higher levels of CRP, particularly the change between pre- and post-RT, combined with obesity. We also observed that African Americans have higher CRP and are more susceptible to RT-induced EASRs compared to Whites.
Our data showed that African Americans are more susceptible to RT-induced EASRs, which is consistent with the data from a previous study using a self-administered questionnaire to assess skin reactions (21). In our study, two radiation oncologists used the NCI CTC v3.0, a well-established tool for assessing RT-induced EASRs, which may present a more consistent and objective evaluation of EASRs. Patients with pre-RT CRP or post-RT CRP levels greater than 2 mg/L or patients experienced an increase in CRP levels greater than 1 mg/L have a higher risk for grade 2+ EASRs at week 6. This is supported by a previous study that expression of human CRP in mice was correlated with up-regulation of the TGF-β/Smad3 signaling pathway, which has been associated with RT-induced fibrosis or moist desquamation of the skin (22). Other risk factors, such as obesity and breast volume, have been related to late effects (23). This could be due to dosimetric variation across the breast related to skin folding in patients with higher BMI and/or breast volume. Similarly, we reported an association between obesity and RT-induced EASRs, although larger breast volume was not significantly associated with EASRs.
The CRP level in normal human serum ranges from 0.2 to 10 mg/L, where 90% of apparently healthy individuals have CRP levels <3 mg/L and only 1% have levels >10 mg/L. As shown in our study, 14 (8.8%) and 18 (11.3%) patients have pre-RT and post-RT CRP ≥10 mg/L, respectively. We also observed that higher proportion of African Americans have CRP ≥10 mg/L at both pre-RT (17.2%) and post-RT (20.7%). This is consistent with the previous finding that higher CPR levels were reported in African Americans compared to Whites, Chinese or Japanese in a multi-ethnic study in non-cardiovascular disease women (24, 25). Furthermore, racial/ethnic differences have been documented in multiple inflammatory cytokine polymorphisms indicating that groups of the African or black Americans have higher frequency of cytokine variants responsible for the regulation of immune/inflammatory responses (26, 27). Similarly, we report that African-American patients had higher pre-RT CRP levels compared to Whites. Although our data suggest that higher pre-RT CRP may be associated with extremely early onset of grade 2+ EASRs at week 3 (OR=4.90, 95%CI=0.50, 48.32); the association was not significant probably due to small sample size of patients with grade 2+ EASRs at week 3 (n=8). Another consideration is that racial/ethnic differences in EASRs may be attributed to multiple genetic risk factors. For example, previous studies have shown that racial/ethnic differences in RT-induced skin reactions in ATM sequence variant carriers: 17% Hispanics, 23% African Americans and 8% Whites (9, 28). Therefore, future large genetic studies are warranted to elucidate contribution of genetic variants in racial/ethnic differences of RT-induced EASRs and late effects.
Previous studies suggest that RT-induced changes in pro-inflammatory cytokines and growth factors may contribute to normal tissue toxicities (11, 12). Our current data provide new evidence that the inflammatory biomarker, plasma CRP levels are associated with RT-induced EASRs in breast cancer patients undergoing RT. Our current findings will have several clinical implications. First, although CRP produced by tumor cells may act as an opsonin, mediating tumor cell lysis, or it may also stimulate production of prostaglandins, which would facilitate tumor progression; although tumor CRP synthesis may be too low to influence circulating CRP levels. Second, elevated circulating CRP has been associated with cancer prognosis, vascular atherosclerosis, insulin resistance, and type 2 diabetes mellitus that may also impact overall survival. Therefore, patients with CRP levels ≥ 10 mg/L (8.8% pre-RT and 10.3% post-RT) in our study population will need to be monitored for cancer recurrence and other clinical conditions. Third, considering the involvement of CRP in fatigue and prognosis of breast cancer patients, our future follow-up study will focus on monitoring CRP levels, quality of life, and clinical outcomes, including fatigue, late effects, and recurrence/metastasis.
Intriguingly, we observed a subset of breast cancer patients who experienced extreme hypersensitivity to RT and developed grade 2+ EASRs within the first 3 weeks and majority of patients developed RT-induced EASRs (98%) at the end of RT in a dose-dependent manner. In addition, we also observe that patients with at least 3 comorbidity conditions have significantly elevated CRP post-RT. Thus, the development of accurate prediction models in identifying high-risk patients and targets for personalized intervention to minimize EASRs in breast cancer patients may be essential. In summary, this is the first study to demonstrate racial/ethnic differences in CRP levels and disparities in RT-induced EASRs in breast cancer patients undergoing RT. Inflammatory biomarker CRP is associated with RT-induced EASRs, particularly when combined with obesity. With limited sample size, our results should be interpreted with caution. Future larger studies are warranted to confirm our findings. We are currently conducting two larger studies with a total sample size of 1,400 to validate our promising findings. If confirmed, our data will support and facilitate the application of anti-inflammatory agents in protecting normal tissue from RT-induced EASRs and improving quality of life in breast cancer patients.
Acknowledgements
We thank the participants who volunteered to be part of the study, the clinical staff at the radiation oncology clinics. We would also like to thank Martine Poitevien, Glenn O. Allen, Venetta Thomas, Marguerite Meitzler, Denise Larson, Dawn Watkins-Chow, Stacie Loftus Melissa Harris, William J. Pavan and The NIH Fellows Editorial Board for valuable scientific advice and discussion.
Grant Support
J. J. Hu was supported by NIH/NCI grant CA135288.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 4.Collette S, Collette L, Budiharto T, Horiot JC, Poortmans PM, Struikmans H, et al. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: a study based on the EORTC Trial 22881-10882 'boost versus no boost'. Eur J Cancer. 2008;44:2587–2599. doi: 10.1016/j.ejca.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Poortmans PM, Collette L, Horiot JC, Van den Bogaert WF, Fourquet A, Kuten A, et al. Impact of the boost dose of 10 Gy versus 26 Gy in patients with early stage breast cancer after a microscopically incomplete lumpectomy: 10-year results of the randomised EORTC boost trial. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009;90:80–85. doi: 10.1016/j.radonc.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Buchholz TA. Radiation therapy for early-stage breast cancer after breast-conserving surgery. N Engl J Med. 2009;360:63–70. doi: 10.1056/NEJMct0803525. [DOI] [PubMed] [Google Scholar]
- 7.Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. The lancet oncology. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 8.Raabe A, Derda K, Reuther S, Szymczak S, Borgmann K, Hoeller U, et al. Association of single nucleotide polymorphisms in the genes ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with risk of severe erythema after breast conserving radiotherapy. Radiation oncology (London, England) 2012;7:65. doi: 10.1186/1748-717X-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanteles GA, Murray RJ, Mills J, Barwell J, Chakraborti P, Chan S, et al. Variation in telangiectasia predisposing genes is associated with overall radiation toxicity. International journal of radiation oncology, biology, physics. 2012;84:1031–1036. doi: 10.1016/j.ijrobp.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Terrazzino S, La Mattina P, Gambaro G, Masini L, Franco P, Canonico PL, et al. Common variants of GSTP1, GSTA1, and TGFbeta1 are associated with the risk of radiation-induced fibrosis in breast cancer patients. International journal of radiation oncology, biology, physics. 2012;83:504–511. doi: 10.1016/j.ijrobp.2011.06.2012. [DOI] [PubMed] [Google Scholar]
- 11.Gallet P, Phulpin B, Merlin JL, Leroux A, Bravetti P, Mecellem H, et al. Long-term alterations of cytokines and growth factors expression in irradiated tissues and relation with histological severity scoring. PloS one. 2011;6:e29399. doi: 10.1371/journal.pone.0029399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Citrin DE, Hitchcock YJ, Chung EJ, Frandsen J, Urick ME, Shield W, et al. Determination of cytokine protein levels in oral secretions in patients undergoing radiotherapy for head and neck malignancies. Radiation oncology (London, England) 2012;7:64. doi: 10.1186/1748-717X-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfutzner A, Schondorf T, Hanefeld M, Forst T. High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: effects of insulin-sensitizing treatment with pioglitazone. J Diabetes Sci Technol. 2010;4:706–716. doi: 10.1177/193229681000400326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48:155–170. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26:706–713. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, Meeske K, et al. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1280–1287. doi: 10.1200/JCO.2011.36.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammed FF, Poon I, Zhang L, Elliott L, Hodson ID, Sagar SM, et al. Acute-phase response reactants as objective biomarkers of radiation-induced mucositis in head and neck cancer. Head Neck. 2012;34:985–993. doi: 10.1002/hed.21848. [DOI] [PubMed] [Google Scholar]
- 19.Zeng YC, Xue M, Chi F, Xu ZG, Fan GL, Wu R, et al. C-reactive protein level predicts prognosis in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Tumour Biol. 2012;33:891–895. doi: 10.1007/s13277-012-0330-6. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara H, Suchi K, Okamura S, Okamura H, Umehara S, Todo M, et al. Elevated serum CRP levels after induction chemoradiotherapy reflect poor treatment response in association with IL-6 in serum and local tumor site in patients with advanced esophageal cancer. Journal of surgical oncology. 2011;103:62–68. doi: 10.1002/jso.21751. [DOI] [PubMed] [Google Scholar]
- 21.Ryan JL, Bole C, Hickok JT, Figueroa-Moseley C, Colman L, Khanna RC, et al. Post-treatment skin reactions reported by cancer patients differ by race, not by treatment or expectations. Br J Cancer. 2007;97:14–21. doi: 10.1038/sj.bjc.6603842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamama S, Gilbert-Sirieix M, Vozenin MC, Delanian S. Radiation-induced enteropathy: molecular basis of pentoxifylline-vitamin E anti-fibrotic effect involved TGF-beta1 cascade inhibition. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;105:305–312. doi: 10.1016/j.radonc.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Mukesh M, Harris E, Jena R, Evans P, Coles C. Relationship between irradiated breast volume and late normal tissue complications: a systematic review. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;104:1–10. doi: 10.1016/j.radonc.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 26.Brown MD, Feairheller DL, Thakkar S, Veerabhadrappa P, Park JY. Racial differences in tumor necrosis factor-alpha-induced endothelial microparticles and interleukin-6 production. Vasc Health Risk Manag. 2011;7:541–550. doi: 10.2147/VHRM.S22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park NJ, Kang DH. Inflammatory cytokine levels and breast cancer risk factors: racial differences of healthy caucasian and african american women. Oncol Nurs Forum. 2013;40:490–500. doi: 10.1188/13.ONF.40-05AP. [DOI] [PubMed] [Google Scholar]
- 28.Ho AY, Fan G, Atencio DP, Green S, Formenti SC, Haffty BG, et al. Possession of ATM sequence variants as predictor for late normal tissue responses in breast cancer patients treated with radiotherapy. International journal of radiation oncology, biology, physics. 2007;69:677–684. doi: 10.1016/j.ijrobp.2007.04.012. [DOI] [PubMed] [Google Scholar]