Abstract
Animals often exhibit consistent individual differences in behavior (i.e. animal personality) and correlations between behaviors (i.e. behavioral syndromes), yet the causes of those patterns of behavioral variation remain insufficiently understood. Many authors hypothesize that state-dependent behavior produces animal personality and behavioral syndromes. However, empirical studies assessing patterns of covariation among behavioral traits and state variables have produced mixed results. New statistical methods that partition correlations into between-individual and residual within-individual correlations offer an opportunity to more sufficiently quantify relationships among behaviors and state variables to assess hypotheses of animal personality and behavioral syndromes. In a population of wild Belding's ground squirrels (Urocitellus beldingi) we repeatedly measured activity, exploration, and response to restraint behaviors alongside glucocorticoids and nutritional condition. We used multivariate mixed models to determine whether between-individual or within-individual correlations drive phenotypic relationships among traits. Squirrels had consistent individual differences for all five traits. At the between-individual level, activity and exploration were positively correlated whereas both traits negatively correlated with response to restraint, demonstrating a behavioral syndrome. At the within-individual level, condition negatively correlated with cortisol, activity and exploration. Importantly, this indicates that although behavior is state-dependent, which may play a role in animal personality and behavioral syndromes, feedback mechanisms between condition and behavior appear not to produce consistent individual differences in behavior and correlations between them.
Keywords: Animal personality, asset protection, behavioral syndromes, condition, glucocorticoids, state-dependent safety
Introduction
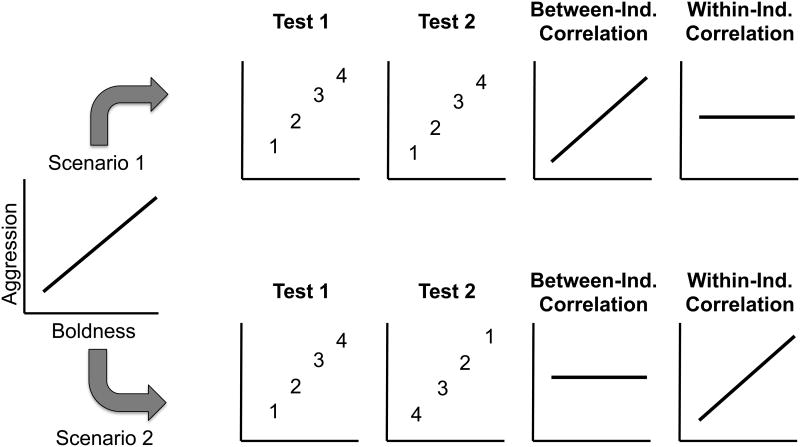
The study of behavioral syndromes and animal personality aims to understand the behavioral variation of individuals. Animal personality refers to consistent among-individual variation in behaviors that persist over time and across environments (Réale et al. 2007), whereas behavioral syndromes refer to correlations between those behaviors (Sih et al. 2004). Despite the widespread prevalence of consistent among-individual variation in behavior, within-individual variation contributes substantially to the overall phenotypic variance of behavior. In fact, a recent meta-analysis of behavioral repeatability (i.e. consistent among-individual variation) found that on average nearly two-thirds of overall behavioral variation was attributed to within-individual rather than among-individual differences (Bell et al. 2009). Therefore, phenotypic correlations between behavioral traits (i.e. Pearson's or Spearman's) could be due to a relationship at two levels. First, a phenotypic correlation could be due to a relationship between individuals' average levels of two behaviors, termed a between-individual correlation (Figure 1, Scenario 1). For example, in Ward's damselfish (Pomacentrus wardi), even though boldness and activity change with respect to temperature, there is a positive correlation between individuals' average level of the two behaviors (Biro et al. 2010). Second, a phenotypic correlation could be due to a relationship between individuals' change in each behavior, termed a within-individual correlation (Figure 1, Scenario 2; Dingemanse & Dochtermann 2013). Bell and Stamps (2004) documented a positive phenotypic correlation between boldness and aggression in three-spined sticklebacks (Gasterosteus aculeatus), but they noted that individuals with low boldness and aggression on one occasion were just as likely to have high boldness and aggression on the next. The overall phenotypic correlation appeared to be produced by a relationship between individuals' change in boldness and aggression rather than a relationship between individuals' average level of those behaviors. This demonstrates how statistically partitioning phenotypic correlations into between-individual correlations and residual correlations (which approximate within-individual correlations; Brommer 2013) will help understanding of the causes and consequences of behavioral variation in nature (Dingemanse & Dochtermann 2013).
Figure 1.
Hypothetical graphs demonstrating the difference between between-individual and within-individual correlations in a population of four numbered individuals (modified from Figure 4 in Dingemanse and Dochtermann 2013). The leftmost graph shows a positive phenotypic correlation between boldness and aggression. In Scenario 1, individuals maintain their place along the correlation across Test 1 and Test 2, meaning that the phenotypic correlation results from a relationship between individuals' average level of boldness and aggression (i.e. a between-individual correlation). In Scenario 2, individuals change their level of boldness and aggression across Test 1 and Test 2, meaning that the phenotypic correlation results from a relationship between individuals' change in boldness and aggression.
Partitioning correlations is important for more than just behavioral traits. Many traits are expected to covary with behavior (Réale et al. 2010), and many of these non-behavioral traits are themselves labile. For example, consistent individual differences in behavior often associate with consistent individual differences in physiology (Koolhaas et al. 1999), but the patterns underlying phenotypic correlations between behavior and physiology are complex. In evaluating the relationships between behavioral traits and physiological traits in alpine marmots, Ferrari et al. (2013) found that cortisol was not related to activity or docility at the between-individual level but had a positive within-individual correlation with activity. In other words, as a marmot's cortisol level increases its activity also increases. However, an individual with relatively high average activity has no greater chance of having high average levels of cortisol than does an individual with low average activity. Given the prevalence of hypotheses that relationships between physiology and behavior explain animal personality and behavioral syndromes (Sih et al. 2004; Duckworth & Sockman 2012; Garamszegi et al. 2012), more data are needed to understand whether between-individual or within-individual correlations dictate patterns of phenotypic covariation among behavioral and physiological traits.
Some hypotheses of adaptive animal personality and behavioral syndromes propose that condition plays a primary role (Rands et al. 2003; McElreath & Strimling 2006; Luttbeg & Sih 2010). They propose different mechanisms by which condition may affect a single behavior to produce personality, whereas if condition affects multiple behaviors it may produce a behavioral syndrome. Condition is often defined as energy or nutritional reserves (Price et al. 1988; Rands et al. 2003; Schulte-Hostedde et al. 2005), but measures such as hematocrit (Kluen et al. 2014) and oxidative stress (von Schantz et al. 1999), among others (Hill 2011), have also been used. It is often found to be a flexible phenotypic trait, but environmental factors or feedbacks between condition and other traits could produce short or long term repeatability of condition (Rands et al. 2003; McElreath & Strimling 2006; Luttbeg & Sih 2010). Surprisingly, there has been a relative dearth of studies assessing condition and animal personality, and those that assess it often do not find the hypothesized relationships (Johnson & Sih 2005; David et al. 2011; Kurvers et al. 2011; Menzies et al. 2013), although some studies have found relationships between condition and behavior (Sinn et al. 2010; David & Giraldeau 2012; Seltmann et al. 2012). In an experiment exposing Iberian wall lizards (Podarcis hispanicus) to low-risk predation, Rodriguez-Prieto et al. (2010) found that an individual's consistent level of boldness did not impact its body condition. However, habituation to the predation threat (i.e. increase in boldness) did increase lizards' body condition. This experiment provided evidence that a phenotypic relationship between boldness and body condition resulted from a within-individual process rather than a between-individual process. The only study to partition between-individual and within-individual correlations of behaviors and condition found that activity, aggression, and hematocrit did not relate at either level (Kluen et al. 2014). More studies assessing the relationship between condition and behaviors are needed to evaluate predictions that condition-dependent behavior accounts for animal personality and behavioral syndromes, in particular studies that quantify between-individual and within-individual correlations.
If condition indeed accounts for animal personality and behavioral syndromes, then condition itself should show consistent individual differences and correlate with the behavior(s) at the between-individual level. Within-individual correlations are also expected, though the direction of the correlation will depend on the mechanism driving the link between behavior and condition. The potential relationship between condition and the behaviors will depend on whether a positive or negative feedback mechanism underlies the relationship. The asset protection principle is a negative feedback mechanism in which increases in an asset, like condition, cause decreases in a risky behavior, like activity or exploration, to avoid jeopardizing the asset (Clarke 1994). In the same fashion, starvation avoidance predicts that as an individual's condition decreases it will increase behaviors like activity and exploration to obtain resources and prevent starvation. This is also a negative feedback mechanism, and we will treat starvation avoidance as an aspect of asset protection (Luttbeg & Sih 2010). If asset protection and starvation avoidance are at work in a system, the negative relationship between change in an individual's condition and change in an individual's behavior would produce a negative within-individual correlation between condition and behavior, and potentially a positive between-individual correlation if other factors generate consistent differences in condition (McElreath et al. 2007). Alternatively, state-dependent safety is a positive feedback mechanism in which high condition enables an individual to better avoid predation (Luttbeg & Sih 2010). This allows the individual to become bolder and more active, which may in turn lead to a further increase in condition. Those individuals in low condition are more susceptible to predation, and therefore their condition decreases because they must be less bold. This relationship would produce both a positive within-individual correlation and a positive between-individual correlation between condition and a behavior. Both positive and negative feedback mechanisms are likely to coexist (Luttbeg & Sih 2010), but our data will evaluate which mechanism predominates and whether it contributes to animal personality and behavioral syndromes.
In this study, we repeatedly measured a suite of behavioral traits, fecal glucocorticoid metabolites (a proxy of cortisol), and body condition index in a wild population of Belding's ground squirrels (Urocitellus beldingi). We did this to test hypotheses regarding animal personality, behavioral syndromes, and the role of stress physiology and condition in those patterns of variation. U. beldingi are excellent for this study because fecal glucocorticoid metabolite (CORT) measures have been experimentally validated and shown to relate to antipredator behavior (Mateo & Cavigelli 2005; Mateo 2007), and body condition index (BCI) measures have been experimentally shown to reflect nutritional condition and relate to vigilance and foraging behavior (Bachman 1993). We focused on activity, exploration, and response to restraint (i.e. docility) behaviors because they are commonly measured personality traits (Réale et al. 2007) that could be repeatedly assayed in standardized tests. We predicted that the behaviors would show consistent individual differences, reflecting animal personality, and correlate at the between-individual level, constituting a behavioral syndrome (Dingemanse & Dochtermann 2013). We predicted that CORT and BCI would be negatively correlated at the within-individual level, because increases in an individual's cortisol cause release of energy stores (Sapolsky et al. 2000; Mateo 2007; Bonier et al. 2009). We predicted that CORT will have a positive within-individual correlation with activity, similar to Ferrari et al. (2013), because the energy stores mobilized by CORT increases could cause higher activity levels. All together, these data will test whether Belding's ground squirrels exhibit animal personality and a behavioral syndrome, as well as evaluate what process might account for that variation.
Methods
Study species and field site
Belding's ground squirrels are diurnal rodents that live in the Sierra Nevada and southern Cascade mountains. They are active between April and August and hibernate the remainder of each year. Females live an average of 3.4 ± 0.3 years and males live an average of 2.1 ± 0.4 years. To address whether squirrels show consistent individual differences and/or correlations of traits, we measured traits in a free-ranging population of U. beldingi at Lower Horse Corral in Rock Creek Canyon, CA (37.4656°N, 118.7249°W). We trapped squirrels at their burrow and conducted the battery of behavioral tests described below, before releasing squirrels at their site of capture. We took 334 measurements of all traits on 157 squirrels (90 females, 67 males) for an average ± SD of 2.1 ± 1.2 measurements per individual, with a range of 1-7. Details regarding that study population can be found in Mateo (2007) and we collected data from 2009 to 2011. Sixty-one individuals were measured for the first time in 2009, 71 in 2010, and 25 in 2011, with 16% of the individuals measured across years. Institutional Animal Care and Use Committees of The University of Chicago (protocol no. 71255) and University of California at Santa Barbara (protocol no. 5-03-532) approved this study, and we had permits from California Fish & Game and the U.S. Forest Service.
Exploration
We measured exploration behavior of squirrels in a holeboard test (Martin & Réale, 2008), which is a 122cm × 122cm modified open-field apparatus. The arena had 61 cm tall walls and a wire mesh top to prevent escape. The testing apparatus contained four false burrows evenly spaced 40 cm apart, which provided species-relevant spaces to investigate. We released squirrels into the arena from a Tomahawk trap (Tomahawk Live Trap, Hazelhurst, WI, USA) via a door in the arena wall, and we counted the number of head dips into the false burrows during a five-minute trial as our dependent variable measuring exploration (File & Wardill 1976). To prevent the odors of previously tested squirrels from affecting results we lined the arena with acrylic and cleaned it with ethanol following every trial. We videotaped each holeboard test and scored it later, blind to squirrel identity.
Activity
We measured locomotor activity of squirrels in the holeboard apparatus during the same trial in which we measured exploration. We marked the holeboard into 16 quadrants and counted the number of lines crossed during the five-minute trial as our dependent variable measuring activity.
Behavioral response to restraint stress
Our last behavioral measure consisted of restraining squirrels in a small canvas bag (28cm × 22cm) to measure the proportion of time they spent immobile during one minute of restraint. This test was not videotaped. One observer (AD) pulled squirrels from a Tomahawk trap by hand, placed them into the bag and then suspended the bag in the air for one minute. We measured the amount of time spent immobile with a stopwatch. We interpret this test as a measure of docility, defined as the response to human handling (Martin & Réale, 2008).
Cortisol
We measured fecal glucocorticoid metabolites, a proxy of CORT, following Mateo & Cavigelli (2005). After collection during trapping, samples were placed on ice and brought to a freezer for short-term storage (< 2 months) before shipping to The University of Chicago for analysis. We dried fecal samples, weighed 0.2g, and extracted CORT from the samples with 1.5 mL 80% ethanol. We centrifuged the feces-ethanol mixture at 2500 g for 20 minutes and collected the supernatant for a 125I-cortisol Corticote® radioimmunoassay (MP Biomedicals, Irvine, CA, USA). For the radioimmunoassay, we ran duplicates of samples and reassayed any sample with a coefficient of variation over 20%. Two control samples, created by pooling samples from five individuals with high binding and five individuals with low binding, were analyzed at the beginning and end of each assay. The mean intra- and interassay coefficients of variation were 11.2% and 16.0%, respectively for the low control and 12.7% and 15.8% for the high control.
Body condition index
We first weighed squirrels on an OHAUS balance (OHAUS CORPORATION, Parsippany, NJ, USA) to the nearest gram. We also measured condylo-basal length (tip of the nose to the occipital condyle) to the nearest hundredth mm with Mitutoyo digital calipers (Mitutoyo America Corporation, Aurora, IL, USA). Using these measurements we calculated body condition index (BCI) from the regression of log weight on log skull length (Schulte-Hostedde et al. 2005). Bachman (1993) experimentally showed BCI reflects energy reserves in U. beldingi. We chose condyle-basal length as a measure of structural size because skull length had lower measurement error than skull zygomatic arch breadth or hind foot length in a pilot study. Only one observer (AD) measured skull length to eliminate inter-observer variation.
Sampling sequence
We sampled an individual during a single trapping event. We trapped individuals with Tomahawk Live Traps (Tomahawk Live Trap, Hazelhurst, WI, USA) at their burrow. Squirrels usually defecate upon capture, and we gathered fecal samples from under the trap in 2.0mL polypropylene tubes for later analysis of fecal glucocorticoid metabolite levels. We then weighed squirrels to the nearest gram, and released them into the test arena for the holeboard test of activity and exploration. After the five-minute test, we shepherded the squirrel back into the Tomahawk trap, before pulling the squirrel out by hand to place into the response to restraint test. Finally, we handled the squirrel to measure its condyle-basal length before releasing it at the site of capture.
Statistics
We aimed to quantify repeatability of activity, exploration, response to restraint, CORT, and BCI, and partition their phenotypic correlations into between-individual and within-individual correlations. To do so, we ran multivariate Bayesian linear mixed-effect models using the MCMCglmm package (Hadfield 2010) in R 3.0.2 (R Development Core Team 2013), specifying a random intercept for squirrel identity. Technically, this partitions correlations into between-individual and residual components (Brommer 2013), but we maintain the use of the term within-individual correlations for conceptual clarity. We followed the methodology of Brommer et al. (2014). We log-transformed CORT so that it better approximated a normal distribution, and we standardized all traits to mean zero and unit variance. We specified slightly informative priors using the raw phenotypic variation of traits for the within-individual matrix and the raw phenotypic variation multiplied by 0.1 for the between-individual matrix, with a degree of belief set at the number of variances to be estimated. However, models with both uninformative priors and low degrees of belief had results that were qualitatively the same. We inspected trace and density plots for the estimated parameters to ensure models were properly specified. Initial models showed parameter autocorrelation, so we ran the models for a larger number of iterations (nitt = 520000) discarding the first 20000 and specifying a thinning interval of 250, which corrected the model diagnostic issue. We included sex, age, test repetition, and year as fixed effects. We decomposed the variance into between-individual (VB) and within-individual (VW) components, calculating repeatability as r = VB/(VB + VW). We calculated the correlation at a given level by dividing the covariance between two traits by the square root of the product of the two variances. For phenotypic correlations, we added the between-individual and within-individual variances and covariances together to calculate the correlation. We used the mode of the posterior distribution as a point estimate for a given statistic and used the 95% Highest Posterior Density as a measure of the 95% Credibility Interval. Credibility Intervals that do not include zero indicate statistical significance. Following Mutzel et al. (2014), we evaluated CIs that only slightly overlapped zero by calculating how often the estimate was positive or negative, which is analogous to a p-value.
Results
Squirrels had an average (± SD) exploration of 1.4 ± 1.9 head dips and activity of 52.9 ± 44.0 lines crossed per test occasion. The average proportion of time squirrels spent static in the response to restraint test was 0.4 ± 0.3. Average fecal glucocorticoid levels were 235.3 ± 71.4 ng/ul. Because individuals' body condition index values were standardized mass-length residuals, we do not provide summary statistics.
Squirrels had consistent individual differences for each of the five traits, determined by repeatability estimates with 95% Credibility Intervals (CRI) that did not include zero. The repeatability of response to restraint behavior and activity were the highest (Table 1). Exploration was also significantly repeatable, but VB of exploration made up a lower proportion of the overall variance. CORT and BCI were significantly repeatable, but at low levels.
Table 1.
Repeatabilites of exploration, response to restraint (RR), activity, fecal glucocorticoid metabolites (CORT), and condition. For all traits, the sample size was 334 measurements on 157 squirrels, ranging between 1-7 measurements per individual. We calculated repeatability as r = VBetween-ind./(VBetween-ind.+ VWithin-ind.), using modes of the posterior distribution as estimates of variance components.
| Trait | Repeatability | Lower 95% CRI | Upper 95% CRI |
|---|---|---|---|
| Exploration | 0.13 | 0.07 | 0.28 |
| RR | 0.52 | 0.42 | 0.65 |
| Activity | 0.45 | 0.28 | 0.58 |
| CORT | 0.09 | 0.03 | 0.18 |
| Condition | 0.05 | 0.03 | 0.11 |
Bold values denote correlations whose 95% Credibility Intervals do not include zero.
The phenotypic, between-individual, and within-individual correlations between traits were largely concordant, but not entirely. At the phenotypic level, three correlations were significant. Activity was positively correlated with exploration and negatively correlated with response to restraint, whereas exploration had a negative phenotypic correlation with BCI (Table 2a). The positive phenotypic relationship between activity and exploration was driven by significantly positive between-individual (Table 2b) and within-individual correlations (Table 2c). However, the phenotypic relationship between activity and response to restraint was primarily driven by a between-individual correlation. Response to restraint had a phenotypic correlation with exploration at the trend level, but this reflected opposing between-individual and within-individual correlations. The negative phenotypic relationship between exploration and BCI primarily reflects a negative within-individual correlation between the traits. BCI also had a significant negative within-individual correlation with activity and a trend for the predicted negative relationship with CORT that drove negative phenotypic correlations that both trended toward significance.
Table 2.
Correlation matrices between exploration, response to restraint (RR), activity, fecal glucocorticoid metabolites (CORT) and condition at the a) phenotypic level, b) between-individual level, and c) within-individual level. For all traits, the sample size was 334 measurements on 157 squirrels, ranging between 1-7 measurements per individual.
| a) Phenotypic correlations | ||||
| Exploration | RR | Activity | CORT | |
| Exploration | --- | |||
| RR | -0.057 (-0.194, 0.030)† | --- | ||
| Activity | 0.567 (0.495, 0.647) | -0.202 (-0.345, -0.116) | --- | |
| CORT | -0.046 (-0.175, 0.036) | 0.016 (-0.077, 0.139) | -0.007 (-0.123, 0.095) | --- |
| Condition | -0.116 (-0.226, -0.022) | 0.003 (-0.111, 0.100) | -0.090 (-0.195, 0.020)† | -0.087 (-0.183, 0.027)† |
|
| ||||
| b) Between-individual correlations | ||||
| Exploration | RR | Activity | CORT | |
| Exploration | --- | |||
| RR | -0.545 (-0.823, -0.185) | --- | ||
| Activity | 0.694 (0.327, 0.868) | -0.639 (-0.823, -0.378) | --- | |
| CORT | -0.207 (-0.569, 0.450) | 0.095 (-0.388, 0.554) | -0.028 (-0.421, 0.608) | --- |
| Condition | -0.101 (-0.447, 0.561) | -0.219 (-0.585, 0.376) | 0.071 (-0.360, 0.638) | -0.012 (-0.509, 0.474) |
|
| ||||
| c) Within-individual correlations | ||||
| Exploration | RR | Activity | CORT | |
| Exploration | --- | |||
| RR | 0.128 (-0.014, 0.288)* | --- | ||
| Activity | 0.594 (0.482, 0.681) | 0.142 (-0.057, 0.253) | --- | |
| CORT | -0.065 (-0.185, 0.063) | -0.046 (-0.146, 0.159) | -0.035 (-0.191, 0.104) | --- |
| Condition | -0.128 (-0.249, -0.022) | 0.037 (-0.113, 0.144) | -0.148 (-0.286, -0.025) | -0.073 (-0.192, 0.028)† |
Bold values denote correlations whose 95% Credibility Intervals (in parentheses) do not include zero. The asterisk (*) and dagger (†) indicate p < 0.05 and p < 0.10, with p values determined by calculating how often a given estimate was positive or negative (see text for details).
Discussion
Understanding the causes and consequences of animal personality and behavioral syndromes requires quantifying complex patterns of variation, so methods that partition behavioral correlations into between-individual and within-individual correlations are a substantial step forward (Brommer 2013; Dingemanse & Dochtermann 2013). In wild Belding's ground squirrels we found that activity, exploration, response to restraint behavior, fecal glucocorticoids and body condition index were all significantly repeatable, supporting our prediction that squirrels would show animal personality. Also, there were a number of significant correlations at both the between-individual and within-individual level, supporting our prediction of a behavioral syndrome influenced by other phenotypic traits. To date, researchers have documented significant repeatability and phenotypic correlations of behaviors in many animal taxa, as well as phenotypic correlations between behaviors and non-behavioral traits (Koolhaas et al. 1999; Sih et al. 2004; Réale et al. 2010). However, phenotypic correlations may be comprised of conflicting relationships at the between-individual and within-individual level (Ferrari et al. 2013; Brommer et al. 2014), which we found to be the case. We still know little about whether phenotypic relationships among behaviors and other traits reflect processes operating at the between-individual or within-individual level because few studies have applied the statistical tools necessary to partition variance between those levels (Brommer et al. 2014; Fresneau et al. 2014), especially when considering behavior alongside physiology or condition (Ferrari et al. 2013; Kluen et al. 2014). Our results supplement this growing area of research and demonstrate unique relationships among behaviors and condition that provide evidence to evaluate hypotheses explaining animal personality and behavioral syndromes.
Animal personality – Consistent individual differences in traits
All traits measured in wild Belding's ground squirrels were significantly repeatable (Table 1). The repeatabilities of the behavioral traits are within range of the average 0.37 repeatability found in behavioral traits across taxa (Bell et al. 2009). Activity, exploration, and response to restraint were all significantly repeatable, similar to results found in other sciurid species (Martin & Réale 2008; Boyer et al. 2010; Ferrari et al. 2013). CORT and BCI had significant repeatabilities, but were substantially lower than those of the behaviors. The relatively low repeatability of BCI in squirrels contrasts with other studies, such as Wilcoxon et al. (2010), who found that BCI in Florida scrub-jays (Aphelocoma coerulescens) was highly repeatable over roughly six months (r = 0.64), and Chappell et al. (2011) who found that BCI of wild satin bowerbirds (Ptilonorhynchus violaceus) was repeatable from year to year (r = 0.26). The repeatability of CORT in squirrels was similar to the repeatability of fecal corticosterone metabolites (r = 0.12) in yellow-bellied marmots recorded across multiple years (Marmota flaviventrus: Smith et al. 2012) but substantially lower than the repeatability of plasma cortisol in alpine marmots (r = 0.76) (Ferrari et al. 2013). The high positive correlation between plasma and fecal cortisol levels in Belding's ground squirrels suggests that the methodological difference does not account for the discrepancy in repeatabilities (Mateo & Cavigelli 2005). In a recent review on repeatabilities of glucocorticoids in birds, Ouyang et al. (2011) found a mix of significant and non-significant repeatabilities, and reported data showing that glucocorticoids were repeatable within the breeding season but not across seasons or years in great tits (Parus major) and tree swallows (Tachycineta bicolor). Our data are primarily made up of repeated measures taken within a single year, and so the time frame issue suggested by the data of Ouyang et al. (2011) is unlikely to explain the low repeatabilities. However, the life-history of Belding's ground squirrels raises the possibility that within-year weight gain for hibernation may explain the relatively lower repeatabilities of CORT and BCI even with short-term data. Hypotheses proposing that state-dependence of behavior explain animal personality predict that the state itself should be repeatable. As predicted by hypotheses of state-dependent behavior, we found that CORT and BCI were repeatable, but the fact that their repeatabilities are lower than the repeatabilities of the behaviors suggests that state-dependence of behavior can only partially account for animal personality and behavioral syndromes.
Behavioral syndrome and patterns of correlations between traits
Squirrels' behaviors were correlated with one another primarily at the between-individual level. Activity and exploration were positively correlated at the between-individual and within-individual level, although they both negatively correlated with response to restraint at the between-individual level (Table 2b, c). This indicates that the relationships among the behaviors were primarily due to an association between individuals' behavioral averages rather than change in one behavior being matched by change in another. The patterns of covariation between activity and response to restraint mirror those of alpine marmots, with a significant negative between-individual correlation but a nonsignificant positive within-individual correlation (Ferrari et al. 2013). The significant between-individual correlations between behaviors shows the traits comprise a behavioral syndrome on which selection may operate (Dingemanse & Dochtermann 2013).
We found that CORT and the behaviors were not correlated. This was contrary to expectations and results in other sciurids (Montiglio et al. 2012; Ferrari et al. 2013) but similar to results in collared flycatchers (Fidecula albicollis; Garamszegi et al. 2012). An alternative explanation for the lack of relationships between CORT and the behaviors is that the effect of CORT on an individual's behavior may depend on its condition. For example, in breeding female eiders (Somateria mollissima) corticosterone responses and body condition have an interactive effect on boldness (Seltmann et al. 2012), with corticosterone affecting boldness less when body condition is high. However, the multivariate mixed models that we used to partition between and within-individual correlations cannot provide insight about the possibility of between or within-individual interaction effects among response variables.
Squirrel BCI and CORT did not significantly correlate at the between-individual level, but did correlate at the within-individual level. The relationship was only significant at the trend level, but was in the predicted negative direction (Sapolsky et al. 2000; Bonier et al. 2009), suggesting that as an individual's CORT increased, its BCI decreased over repeated measures, and vice versa. This suggests that CORT is an element of condition in squirrels, particularly under the definition that condition is the capacity to withstand environmental challenges (Hill 2011).
The asset protection principle and state-dependent safety hypothesis propose different mechanisms by which condition may produce consistent individual differences in behaviors and correlations between those behaviors. The asset protection principle predicts that individuals will have negative feedback between condition and risk-associated behaviors. We found that activity and exploration negatively correlated with BCI at the within-individual level (Table 2c), as predicted by the asset protection principle rather than the state-dependent safety hypothesis, which predicts a positive within-individual correlation between condition and behavior (Clark 1994; Luttbeg & Sih 2010). In other words, when a squirrel's condition increased, it was likely to decrease its level of activity and exploration, and vice versa. However, two additional observations are necessary to support the hypothesis that asset protection explains consistent individual differences in a behavior, and consistent correlations among a set of behaviors. First, condition should be a repeatable trait. We found this to be the case in U. beldingi. Second, individuals' average levels of condition and behavior should be related. In other words, there should be a between-individual correlation. We found a lack of significant between-individual BCI/exploration or BCI/activity correlations, suggesting that BCI does not produce or reinforce animal personality or behavioral syndromes by the asset protection principle. However, this does not preclude condition playing a role in the maintenance of genetic variance for personality and behavioral syndromes over evolutionary time (Price et al. 1988; Rowe & Houle 1996; Luttbeg & Sih 2010), nor the possibility that interactive relationships among traits produce personality and behavioral syndromes (Seltmann et al. 2012). Additionally, we should qualify this interpretation by mentioning that our sample size (334 observations on 157 individuals) does not provide sufficient statistical power to detect between-individual correlations of small effect. Simulations run by Dingemanse & Doctermann (2013; see their Figure 1b) indicate that for our sample size the likelihood of failing to detect a true relationship is over 30% for between-individual correlations 0.3 or lower (Power < 0.7 for RB < 0.3) and roughly 50 more individuals measured twice would be needed to increase statistical power to recommended levels for a between-individual correlation of 0.3 (Power = 0.8 for RB = 0.3). However, we have more information to estimate the within-individual correlations than the between-individual correlations, which is an inherent aspect of the models (Brommer et al. 2014). Therefore, the power to detect asset protection is greater than our power to test whether asset protection produces between-individual correlations among condition and behaviors. This may account for our results showing evidence that Belding's ground squirrel activity and exploration are governed by the asset protection principle, but that asset protection appears to not produce the animal personality of those traits or the behavioral syndrome they constitute. This calls for studies of larger size to more rigorously test the hypothesis that condition differences explain animal personality and behavioral syndromes through asset protection or state-dependent safety. Nonetheless, given that only one other study, to our knowledge, has partitioned correlations of behavioral and condition traits (Kluen et al. 2014), these data provide novel evidence of relationships between condition and behaviors and worthwhile insights on the role condition may play in animal personality and behavioral syndromes in nature.
Conclusion
Wild Belding's ground squirrels exhibited consistent individual differences in activity, exploration, response to restraint behavior, CORT, and BCI. Activity, exploration and response to restraint formed a behavioral syndrome, as all traits correlated at the between-individual level. Within-individual correlations indicate that as the condition of individuals change, CORT, activity and exploration move in the opposite direction. However, despite the within-individual covariation between BCI and behaviors, we conclude that the patterns of variation in Belding's ground squirrels do not support hypotheses that feedback between condition and behaviors, either positive or negative, produce animal personality or behavioral syndromes. In general, more studies that partition correlations between behaviors and state variables are needed to evaluate hypotheses explaining animal personality and behavioral syndromes. Additionally, it will be useful to assess whether condition may be involved in evolutionary processes that produce consistent individual differences in behaviors and correlations between them.
Acknowledgments
We would like to thank Kevin Bender, Nichole Cudworth, Elizabeth Hiroyasu, Page Kannor, Jennifer Lieb, Charlie Norton, Tamara Rocabado, Loni Silver, and Krista Stewart for assistance with data collection, and Adam Hannon-Hatfield for helping score videotaped behavioral tests. Erin Loeding assisted with hormone analyses. We would also like to acknowledge grant support from The American Museum of Natural History's Theodore Roosevelt Memorial Fund, The American Society of Mammalogists, The University of Chicago's Hinds Fund, and Valentine Eastern Sierra Reserve to both AD and KCB, as well as grants to JMM by NSF (IOB 05-17137) and NIH (R01 MH63921-01A1).
Contributor Information
Andy J. Dosmann, Email: dosmann1@gmail.com, Stanford University, Thinking Matters, Sweet Hall 219B, 590 Escondido Mall, Stanford, CA 94305.
Katherine C. Brooks, Email: kcraig@uchicago.edu, The University of Chicago, Committee on Evolutionary Biology, 940 E. 57th St. Chicago, IL 60637.
Jill. M. Mateo, Email: jmateo@uchicago.edu, The University of Chicago, Committee on Evolutionary Biology, 940 E. 57th St. Chicago, IL 60637.
References
- Bachman GC. The effect of body condition on the trade-off between vigilance and foraging in Belding's ground squirrels. Animal Behaviour. 1993;46:233–244. [Google Scholar]
- Bell AM, Stamps JA. Development of behavioural differences between individuals and populations of sticklebacks, Gasterosteus aculeatus. Animal Behaviour. 2004;68:1339–1348. [Google Scholar]
- Bell AM, Hankison SJ, Laskowski KL. The repeatability of behavior: a meta-analysis. Animal Behaviour. 2009;77:771–783. doi: 10.1016/j.anbehav.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro PA, Beckmann C, Stamps JA. Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proceedings of the Royal Society B. 2010;277:71–77. doi: 10.1098/rspb.2009.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonier F, Martin PR, Moore IT, Wingfield JC. Do baseline glucocorticoids predict fitness? Trends in Ecology and Evolution. 2009;21:634–642. doi: 10.1016/j.tree.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Boyer N, Réale D, Marmet J, Pisanu B, Chapuis JL. Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus. Journal of Animal Ecology. 2010;79:538–547. doi: 10.1111/j.1365-2656.2010.01659.x. [DOI] [PubMed] [Google Scholar]
- Brommer JE. On between-individual and residual covariances in the study of animal personality: are you willing to take the “individual gambit”? Behavioral Ecology and Sociobiology. 2013;67:1027–1032. [Google Scholar]
- Brommer JE, Karell P, Ahola A, Karstinen T. Residual correlations, and not individual properties, determine a nest defense boldness syndrome. Behavioral Ecology. 2014 doi: 10.1093/beheco/aru057. [DOI] [Google Scholar]
- Chappell MA, Savard JF, Siani J, Coleman SW, Keagy J, Borgia G. Aerobic capacity in wild satin bowerbirds: repeatability and effects of age, sex and condition. Journal of Experimental Biology. 2011;214:3186–3196. doi: 10.1242/jeb.055046. [DOI] [PubMed] [Google Scholar]
- Clarke CW. Antipredator behavior and the asset-protection principle. Behavioral Ecology. 1994;5:159–170. [Google Scholar]
- David M, Auclair Y, Cézilly F. Personality predicts social dominance in female zebra finches, Taeniopygia guttata, in a feeding context. Animal Behaviour. 2011;81:219–224. [Google Scholar]
- David M, Giraldeau LA. Zebra finches in poor condition produce more and consume more in a producer-scrounger game. Behavioral Ecology. 2012;23:174–180. [Google Scholar]
- Dingemanse NJ, Dochtermann NA. Quantifying individual variation in behavior: mixed-effect modeling approaches. Journal of Animal Ecology. 2013;82:39–54. doi: 10.1111/1365-2656.12013. [DOI] [PubMed] [Google Scholar]
- Duckworth RA, Sockman KW. Proximate mechanisms of behavioral inflexibility: implications for the evolution of personality traits. Functional Ecology. 2012;26:559–566. [Google Scholar]
- Ferrari C, Pasquaretta C, Carere C, Cavallone E, von Hardenberg A, Réale D. Testing for the presence of coping styles in a wild mammal. Animal Behaviour. 2013;85:1385–1396. [Google Scholar]
- File SE, Wardill AG. Validity of head-dipping as a measure of exploration in a modified hole-board. Psychopharmacologia. 1975;44:53–59. doi: 10.1007/BF00421184. [DOI] [PubMed] [Google Scholar]
- Fresneau N, Kluen E, Brommer JE. A sex-specific behavioral syndrome in a wild passerine. Behavioral Ecology. 2014;25:359–367. [Google Scholar]
- Garamszegi LZ, Rosivall B, Rettenbacher S, Markó G, Zsebők S, Szöllősi E, Eens M, Potti J, Török J. Corticosterone, Avoidance of Novelty, Risk-Taking and Aggression in a Wild Bird: No Evidence for Pleiotropic Effects. Ethology. 2012;118:621–635. [Google Scholar]
- Hadfield JD. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. Journal of Statistical Software. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Hill GE. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecology Letters. 2011;14:625–634. doi: 10.1111/j.1461-0248.2011.01622.x. [DOI] [PubMed] [Google Scholar]
- Johnson JC, Sih A. Precopulatory sexual cannibalism in fishing spiders (Dolomedes triton): a role for behavioral syndromes. Behavioral Ecology and Sociobiology. 2005;58:390–396. [Google Scholar]
- Kluen E, Siitari H, Brommer JE. Testing for between-individual correlations of personality and physiological traits in a wild bird. Behavioral Ecology and Sociobiology. 2014;68:205–213. [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neuroscience and Biobehavioral Reviews. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Frontiers in Neuroendocrinology. 2010;31:307–321. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kurvers RHJM, Adamczyk VMAP, van Wieren SE, Prins HHT. The effect of boldness on decision-making in barnacle geese is group-size-dependent. Proceedings of the Royal Society B. 2011;278:2018–2024. doi: 10.1098/rspb.2010.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttbeg B, Sih A. Risk, resources and state-dependent adaptive behavioural syndromes. Philosophical Transactions of the Royal Society B. 2010;365:3977–3990. doi: 10.1098/rstb.2010.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JG, Réale D. Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus. Animal Behaviour. 2008;75:309–318. [Google Scholar]
- Mateo JM. Ecological and hormonal correlates of antipredator behaviour in adult Belding's ground squirrels (Spermophilus beldingi) Behavioral Ecology and Sociobiology. 2007;62:37–49. doi: 10.1007/s00265-007-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo JM, Cavigelli SA. A validation of extraction methods for noninvasive sampling of glucocorticoids in free-living ground squirrels. Physiological and Biochemical Zoology. 2005;78:1069–1084. doi: 10.1086/432855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElreath R, Strimling P. How noisy information and individual asymmetries can make ‘personality’ an adaptation: a simple model. Animal Behaviour. 2006;72:1135–1139. [Google Scholar]
- McElreath R, Luttbeg B, Fogarty SP, Brodin T, Sih A. Evolution of animal personalities. Nature. 2007;450:E5. doi: 10.1038/nature06326. [DOI] [PubMed] [Google Scholar]
- Menzies AK, Timonin ME, McGuire LP, Willis CKR. Personality Variation in Little Brown Bats. PLoS ONE. 2013;8:e80230. doi: 10.1371/journal.pone.0080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiglio PO, Garant D, Pelletier F, Réale D. Personality differences are related to long-term stress reactivity in a population of wild eastern chipmunks, Tamias striatus. Animal Behaviour. 2012;84:1071–1079. [Google Scholar]
- Mutzel A, Dingemanse NJ, Aray-Ajoy YG, Kempenaers B. Parental provisioning behavior plays a key role in linking personality with reproductive success. Proceedings of the Royal Society of London B. 2014;280:20131019. doi: 10.1098/rspb.2013.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang JQ, Hau M, Bonier F. Within seasons and among years: When are corticosterone levels repeatable? Hormones and Behavior. 2011;60:559–564. doi: 10.1016/j.yhbeh.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Price T, Kirkpatrick M, Arnold SJ. Directional selection and the evolution of breeding date in birds. Science. 1988;240:798–799. doi: 10.1126/science.3363360. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA. Spontaneous emergence of leaders and followers in foraging. Nature. 2003;423:432–434. doi: 10.1038/nature01630. [DOI] [PubMed] [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biological Reviews. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Prieto I, Martín J, Fernández-Juricic Habituation to low-risk predators improves body condition in lizards. Behavioral Ecology and Sociobiology. 2010;64:1937–1945. [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proceedings of the Royal Society of London B. 1996;263:1415–1421. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H. Good genes, oxidative stress and condition-dependent sexual signals. Proceedings of the Royal Society of London. Series B: Biological Sciences. 1999;266:1–12. doi: 10.1098/rspb.1999.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ. Restitution of Mass-Size Residuals: Validating Body Condition Indices. Ecology. 2005;86:155–163. [Google Scholar]
- Seltmann MW, Öst M, Jaatinen K, Atkinson S, Mashburn K, Hollmén T. Stress responsiveness, age and body condition interactively affect flight initiation distance in breeding female eiders. Animal Behaviour. 2012;84:889–896. [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioural syndromes: an ecological and evolutionary overview. Trends in Ecology and Evolution. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sinn DL, Moltschaniwskyj NA, Wapstra E, Dall SRX. Are behavioral syndromes invariant? Spatiotemporal variation in shy/bold behavior in squid. Behavioral Ecology and Sociobiology. 2010;64:693–702. [Google Scholar]
- Smith JE, Monclús R, Wantuck D, Florant GL, Blumstein DT. Fecal glucocorticoid metabolites in wild yellow-bellied marmots: Experimental validation, individual differences and ecological correlates. General and Comparative Endocrinology. 2012;178:417–426. doi: 10.1016/j.ygcen.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Wilcoxon TE, Boughton RK, Schoech SJ. Selection on innate immunity and body condition in Florida scrub-jays throughout an epidemic. Biology Letters. 2010;6:552–554. doi: 10.1098/rsbl.2009.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]