Abstract
Total knee replacement surgery begins with correct planning of both the incision and the exposure of the joint. Indeed, these are factors that are just as crucial to an optimal outcome as choosing the right implant, positioning the components, and balancing the ligaments. While it is true that the standard incision and arthrotomy (with which we are most familiar) will, in most primary implant cases, provide adequate joint exposure, it is also true that cases characterized by certain conditions, such as previous cutaneous incisions, a stiff knee or patella baja, present specific skin and exposure problems that need to be recognized, planned for and overcome.
Keywords: exposure, knee, osteoarthritis, skin incision, arthroplasty
Introduction
Total knee arthroplasty (TKA) is currently one of the most successful orthopedic surgical procedures, with rates of excellent or good long-term outcomes ranging from 90% to 98% (1–13). The success of the procedure, which undoubtedly demands compliance with certain technical parameters such as restoration of a correct leg axis, correct positioning of the components, and proper ligament balancing, also depends on correct exposure of the joint. This is crucial for reducing or avoiding errors of positioning and alignment of the prosthetic components, and thus early mechanical failure of the implant. While it is true that the standard incision and arthrotomy (with which we are most familiar) will, in most primary implant cases, provide adequate joint exposure, it is also true that certain cases, characterized, for example, by previous cutaneous incisions, a stiff knee or a patella baja, present specific skin and exposure problems that need to be recognized, planned for and overcome.
In order to provide an instructive and exhaustive overview of this topic, we have divided this article into the following sections: the standard cutaneous incision; at-risk incisions; standard arthrotomy; tissue-sparing arthrotomies; extensive arthrotomies; and the current role of mini-invasive surgery (MIS).
The standard cutaneous incision
The epidermis, which does not have its own vessels and is nourished by diffusion from the dermis, is the organ most at risk of necrosis. Proper respect for the skin is crucial when dealing with the knee, more so than with any other major joint. There are several reasons for this: the fact that skin of the knee lies directly over the joint, the presence of the bony prominences of the patella and anterior tibial tuberosity, and the joint’s considerable range of motion (ROM) in flexion.
There are several anatomical parameters that must be known and respected when performing the cutaneous incision:
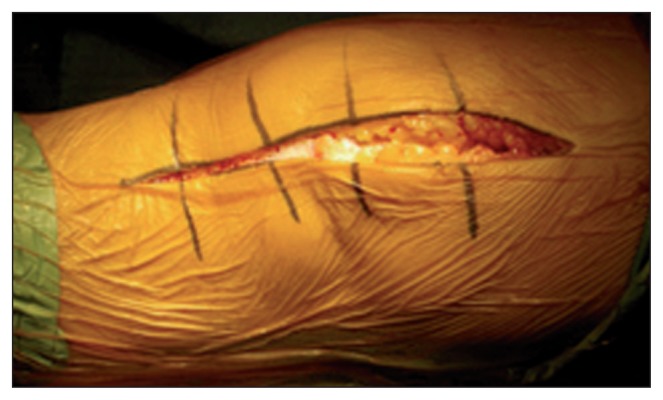
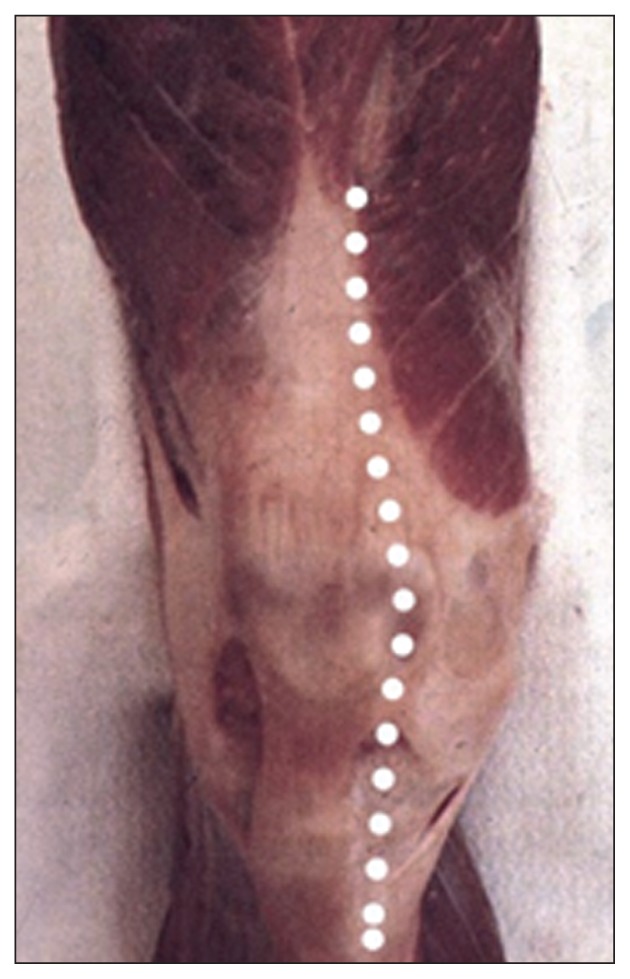
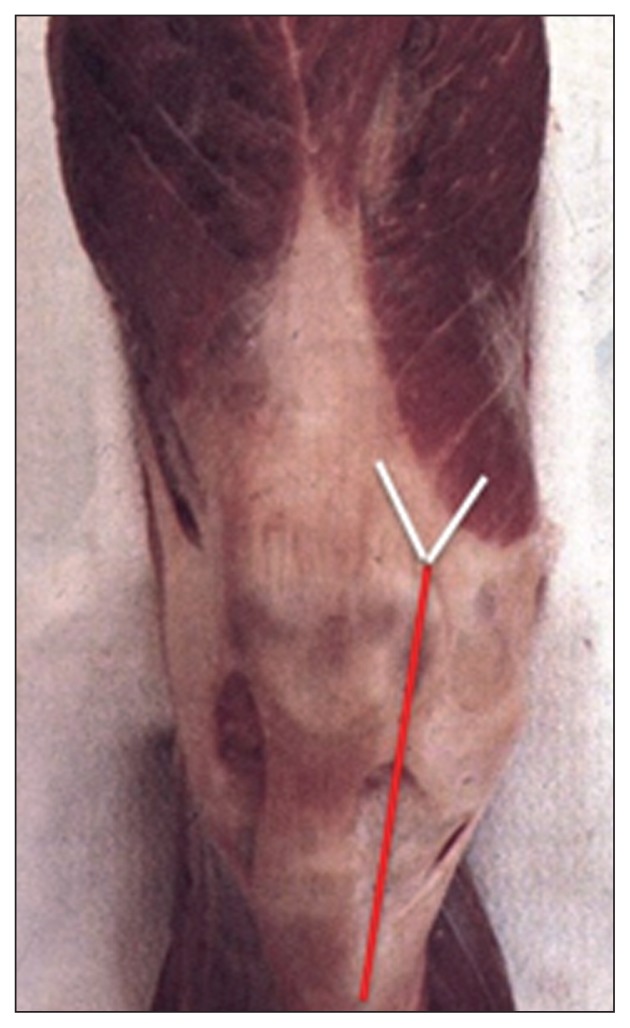
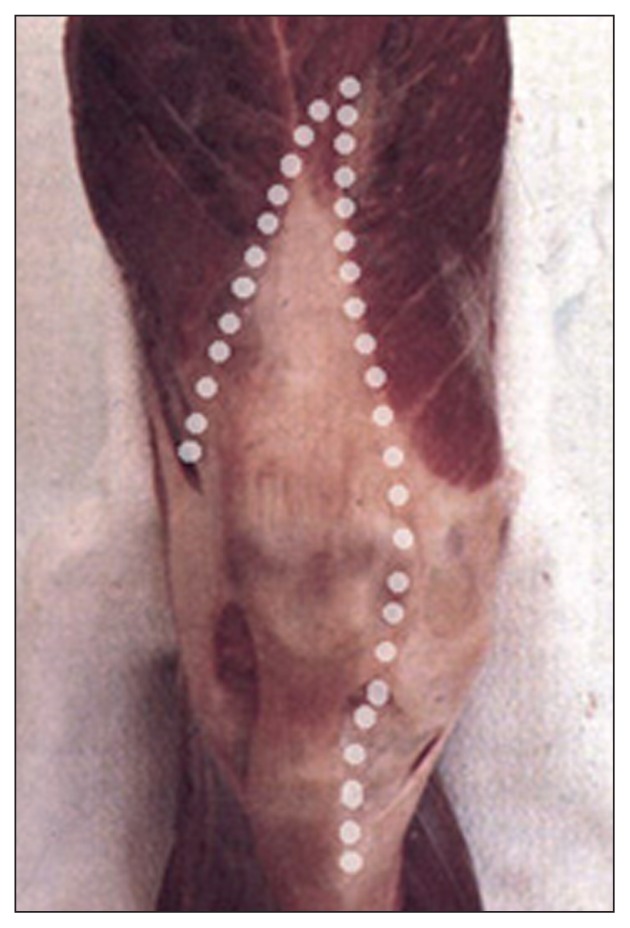
The incision from the cutaneous plane as far as the tendon fascia of the extensor mechanism must be vertical in order to avoid devascularization of the skin flap, which is fed by the arteries located at this level (Fig. 1).
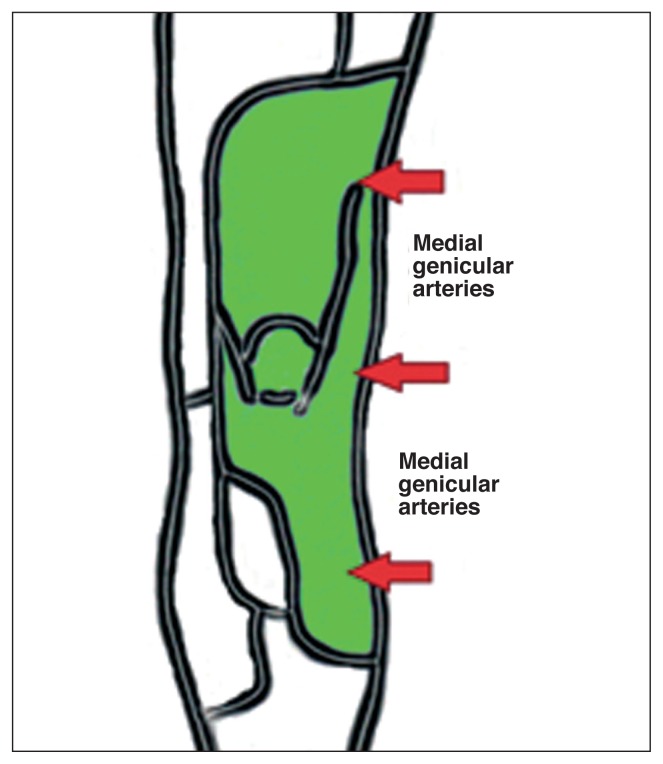
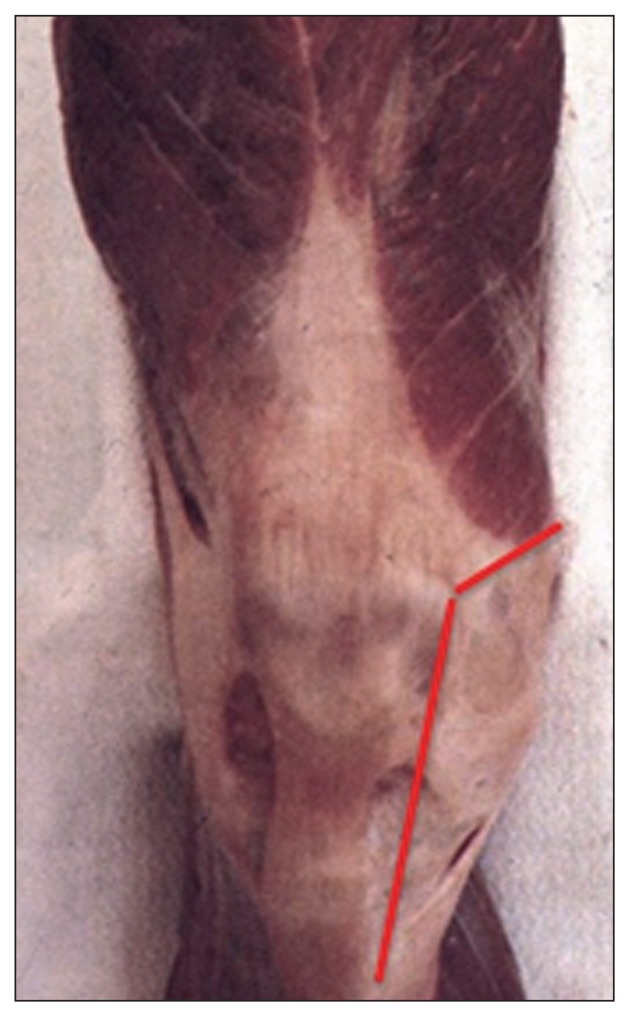
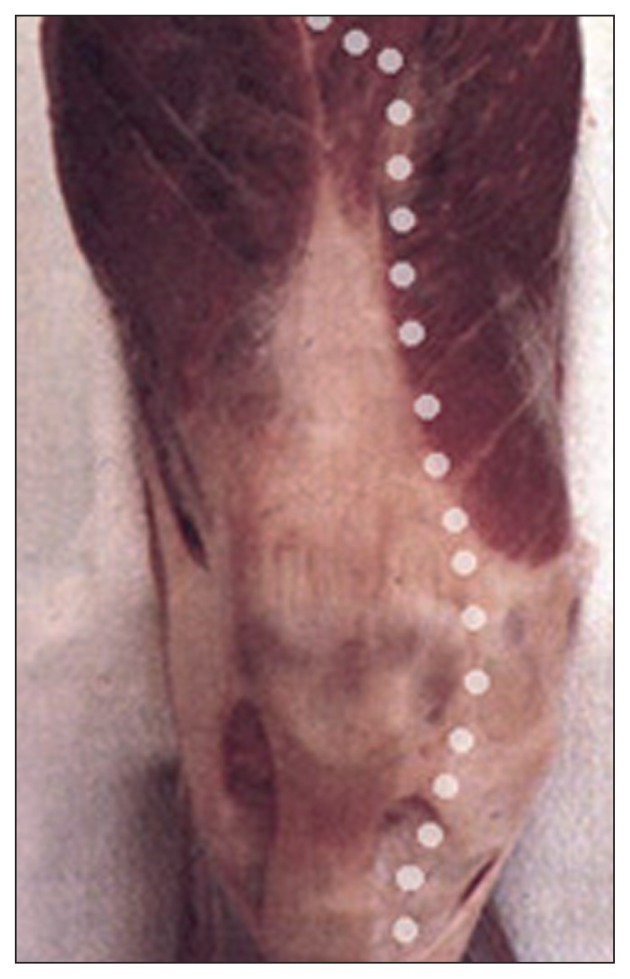
Since most of the cutaneous vasculature (14, 15) arises from the medial genicular arteries, the skin flap at greatest risk of necrosis is the lateral one (Fig. 2).
As far as possible, it is necessary to avoid cutting the skin overlying the bony prominences, where the tissue is thinner and subject to greater strain.
Fig. 1.
To preserve the vascularization of the dermis, it is necessary to make a vertical incision from the cutaneous plane as far as the tendon fascia.
Fig. 2.
Most of the cutaneous vasculature arises from the medial genicular arteries.
The two standard incisions most widely used in total knee replacement surgery are the midline incision and the medial parapatellar incision.
Midline incision
The standard midline incision, running from a point 2 cm proximal to the base of the patella to the anterior tibial tuberosity (ATT), is made with the knee flexed, following the midline of the knee.
To avoid cutting the skin overlying the bony prominence of the ATT, it is advisable to keep the incision 0.5–1 cm medially to it.
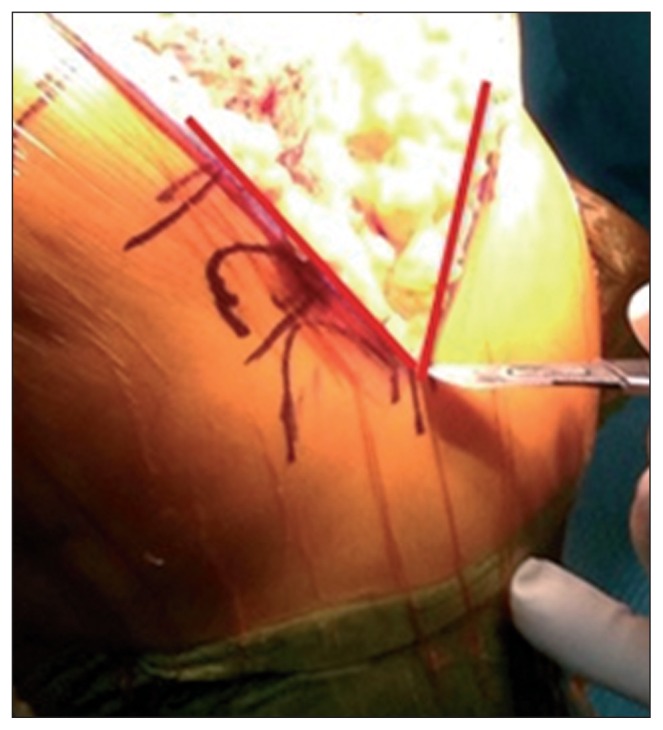
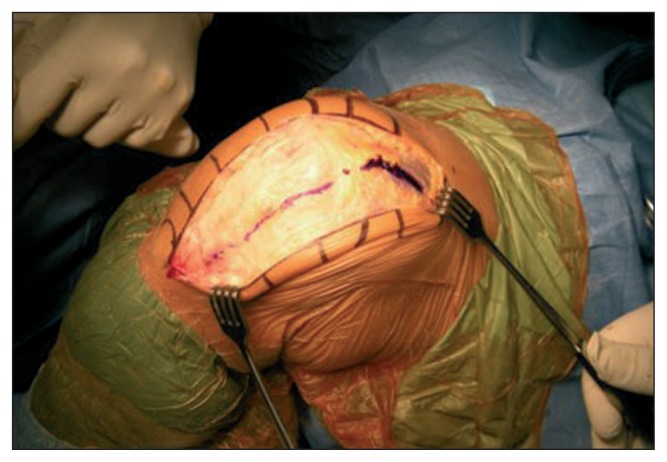
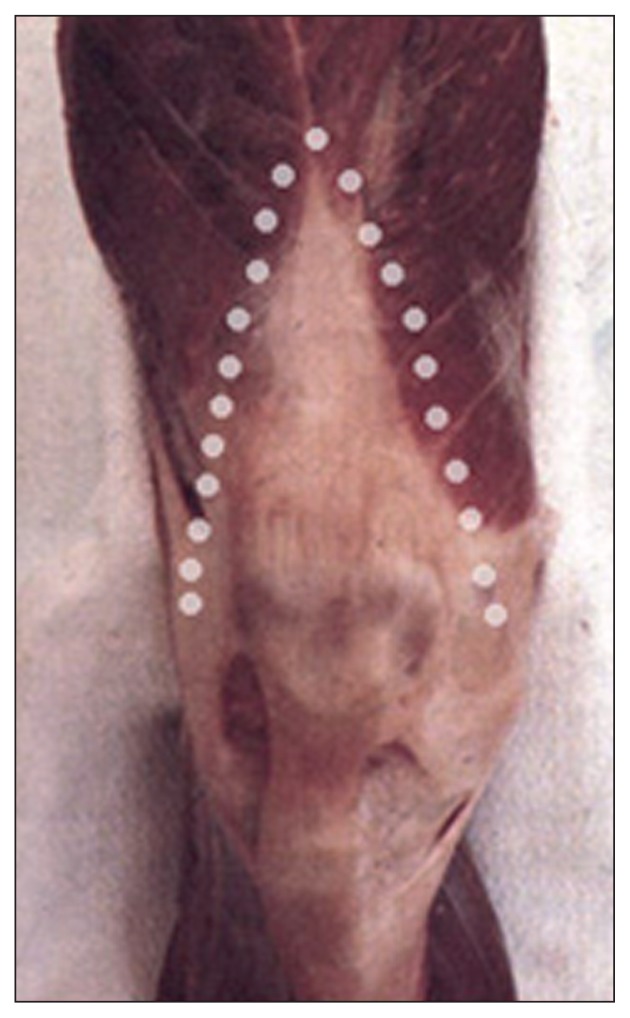
The length of the incision will depend on the size of the knee and on the patient’s body mass index (BMI). Since the literature provides no reliable parameter for determining the minimum length needed to obtain sufficient exposure of the joint, it is good practice to adhere to the empirical concept that the incision should be extended when its ends are under tension, i.e. when the V-shape of the incision is more similar to a “U” shape (Fig. 3).
Fig. 3.
The incision must be V-shaped.
Vertically, the incision extends vertically and deep to the fascia of the extensor mechanism, allowing its exposure.
Medial parapatellar incision
The medial parapatellar incision is slightly curvilinear incision with a lateral concavity that, from a point 2 cm proximal to the base of the patella, extends as far as the ATT.
Johnson et al. (16, 17), comparing the O2 tension of the lateral and medial skin flaps following different incisions – anterior midline, medial parapatellar and curved medial –, found a reduction from 55.8±15 mm di Hg to 18.4±13.8 mm di Hg in the mean O2 tension of the lateral flap on the first day after the operation. This reduction was smaller for the midline incision (mean O2 tension: 23.6±16.7) and greater for the medial parapatellar and curved medial incisions (mean O2 tension: 16.6±9.7 and 15.7±15.2, respectively).
In our view, therefore, the midline incision should be preferred as its exposure capacity is similar to that of the medial parapatellar one, but it involves less vascular sacrifice of the lateral skin flap and, in theory, carries a smaller risk of necrosis.
At-risk incisions
At-risk incisions are incisions at increased risk of necrosis, due to causes that may be local (the presence of previous incisions), or general.
Local causes
As far as the presence of previous incisions is concerned, the following practical rules, easy to apply, can be very helpful in choosing the most suitable incision site:
In the presence of a single previous incision, the old scar, providing it is suitable, can be used.
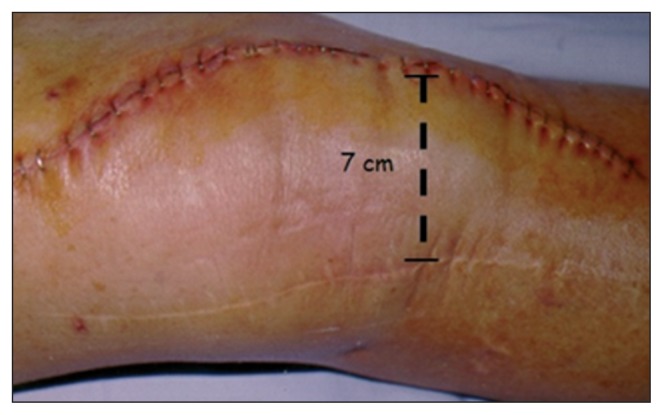
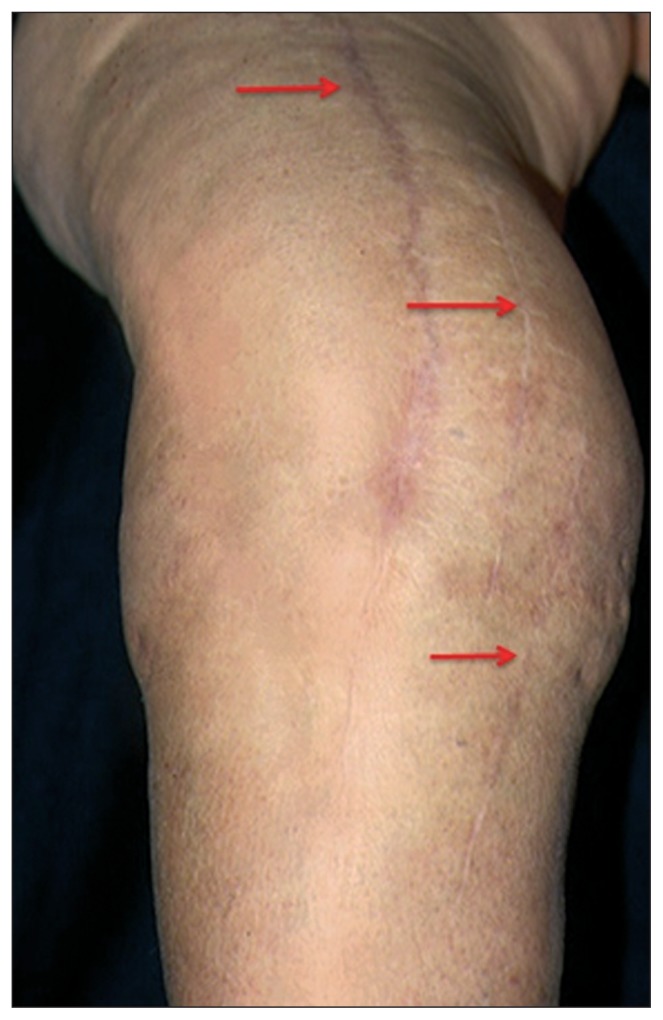
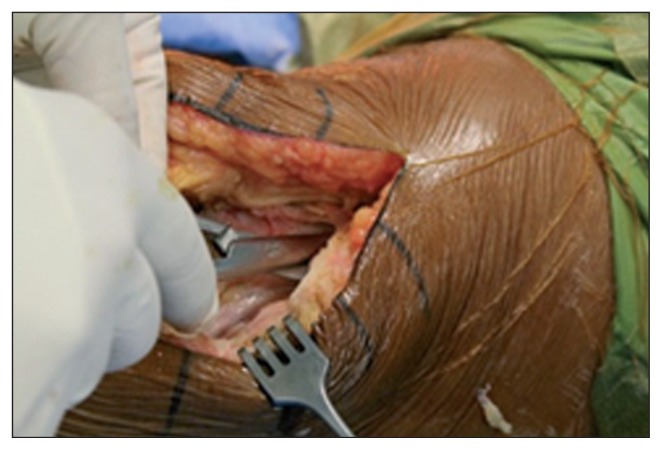
If it is not possible to incorporate the previous scar, the distance between the old and the new incisions should be no less than 2/3 of the length of the existing scar and there must be an at least 5-cm bridge of skin between the two incisions (Fig. 4).
A horizontal previous incision must be crossed perpendicularly.
In the presence of multiple previous incisions, use the most lateral one. In order to avoid excessive prefascial separation, the medial one can be used, but only if the bridge of skin between the two incisions is greater than 5 cm (Fig. 5).
Fig. 4.
Cutaneous incision with a 7-cm bridge of skin between it and the previous one.
Fig. 5.
In the presence of multiple previous incisions, it is preferable to use the most lateral one.
General causes
Of the various general risk factors (18–20), the following are the most frequent:
prolonged use of corticosteroids
rheumatoid arthritis (RA)
diabetes mellitus (DM)
obesity
smoking
use of non-steroidal anti-inflammatory drugs (NSAIDs).
Prolonged use of corticosteroids (20–23), as seen in patients affected by RA (24), seems to be related to reduced proliferation of fibroblasts and to inhibition of the action of collagenases, which, in turn, leads to accumulation of collagen and delayed wound healing. Obesity is associated with an increased risk of wound complications due to the need for greater retraction of the skin flaps and to more precarious vascularization of the dermis (20, 25, 26). The involvement of cigarette smoking seems to be linked to the vasoconstrictor effect of nicotine (27, 28). The relationship between DM and wound complications is not yet entirely clear (20, 25), although altered collagen metabolism or reduced neoangiogenesis are hypotheses that have been advanced. Prolonged use of NSAIDs, through the mechanism of inflammatory response inhibition, can also alter the wound healing process (18–20).
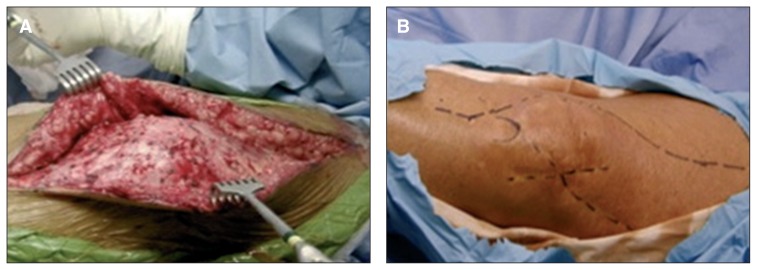
In the case of patients presenting both general and local risk factors, it may be useful, before permanently implanting the prosthesis, to perform a simulation of the operation (sham procedure). An incision (the most appropriate) is made in the skin, which is then separated as far as the fascial layer. The wound is then sutured and observed for two weeks. If the skin flaps remain viable, the definitive procedure can be performed using the same incision site (Fig. 6).
Fig. 6.
“Sham procedure”. A: The most appropriate incision is made in the skin. The wound is sutured and observed for two weeks. B: If the skin flaps remain viable, the definitive procedure can be performed using the same incision site.
Standard arthrotomy
Standard arthrotomy is a medial incision. With the patient’s knee flexed, the surgeon begins by cutting the quadriceps tendon longitudinally up to a point 1 cm from the vastus medialis obliquus (VMO), before proceeding distally, cutting the medial patellofemoral ligament and medial capsule 5 mm from the medial border of the patella, to reach the medial borders of the patellar tendon and ATT (Fig. 7). With the patient’s knee extended, the deep medial collateral ligament is detached to expose the proximal tibia. The cleavage plane between the patellar tendon and the fat pad is identified after which, remaining strictly parallel to the course of the patellar tendon, the fat pad is removed. The patella is laterally subluxated and the knee is exposed in flexion. It is actually possible to use either patellar eversion or patellar subluxation, and there seem to be no significant differences between the two maneuvers in terms of bleeding, recovery of quadriceps strength, pain and long-term outcome (29).
Fig. 7.
Standard medial arthrotomy.
The Insall version
The version of the procedure described by Insall in 1971 (30) is a more lateral parapatellar arthrotomy, which facilitates the lateral subluxation or eversion of the patella.
In this case, after cutting the quadriceps tendon, the incision is continued over the medial 1/3 of the patella, medially detaching the medial patellofemoral ligament, and over the medial 1/3 of the patellar tendon as far as the ATT.
Tissue-sparing arthrotomies
Some authors have recently been led to use less invasive surgical approaches on the basis of an intuitive theoretical assumption, namely that a reduction of the surgical incision and of soft tissue damage will mean a better outcome, in esthetic and surgical terms, as well as in terms of pain and functional recovery (31). In particular, these approaches are proposed in order to avoid violation of the extensor mechanism. The most commonly used are the subvastus approach, the midvastus approach and the trivector approach.
The subvastus approach
First described in the literature in 1929 (32), this approach was taken up again by Bechtol (33) in 1976 and by Hofmann in 1991 (34).
Once the extensor mechanism and VMO have been isolated, the medial border of the VMO is identified and, by means of blunt dissection, detached from the intermuscular septum for approximately 10 cm proximally to the adductor tubercle. The perforating vessels in the intermuscular septum are coagulated. The descending genicular artery and the saphenous nerve are not at risk when using blunt dissection (Fig. 8).
Fig. 8.
Subvastus approach.
The incision is continued in the usual way, distally to the medial joint capsule, and the extensor mechanism is laterally displaced.
Hofmann (34) advised against using this approach in obese individuals weighing over 90 kg, especially if they also have a short femur or patella baja, on account of the difficulties that, in these conditions, may be encountered in displacing the extensor mechanism. The procedure is also relatively contraindicated in subjects requiring revision surgery and in the presence of a stiff knee with a less than 50° ROM.
The subvastus approach, as well as allowing exposure comparable to that obtained through the standard parapatellar approach, seems to be associated with less need to perform a lateral release, less blood loss, less postoperative pain and faster recovery of quadriceps strength, compared with standard arthrotomy (35, 36). Potential drawbacks include the formation of a hematoma below the VMO, excessive stretching of the fibers of the VMO during displacement of the patella (making the procedure unsuitable for obese or muscular patients), and difficulty increasing the exposure in situations in which this is necessary.
The midvastus approach
This approach was first described by Engh et al. in 1997 (37) as a compromise between the standard parapatellar and the subvastus approaches.
The surgical technique involves a midline skin incision and deep dissection exposing the VMO. With the knee flexed, the direction of the muscle fibers of the VMO is identified and, following this course, an approximately 2–4 cm incision is made in the VMO fascia and parenchyma (Fig. 9).
Fig. 9.
Midvastus approach.
The arthrotomy proceeds distally, in the usual way, through cutting of the medial patellofemoral ligament and capsule to reach the ATT.
Most of the VMO, including the entire portion attached to the quadriceps tendon, is preserved.
The superior-medial genicular artery is cut, but the preservation of the descending genicular artery should guarantee sufficient blood supply to the patella (38).
The potential advantages of this approach, like the subvastus one, are linked to sparing of the extensor mechanism, faster recovery of quadriceps muscle strength, greater patellofemoral stability, and a reduction of the need to perform lateral releases, compared with the standard medial parapatellar approach (39).
Engh et al. (40), in a prospective study of two approaches, reported a 3% prevalence of lateral release in the midvastus group versus a 50% prevalence in the standard group, however subsequent studies failed to confirm this finding (41).
The main drawback of this approach is potential denervation of the VMO.
Parentis et al. (42), in a prospective randomized study of 51 knees, compared the median parapatellar approach with the midvastus approach. No significant differences we re found in strength, ROM, knee score, tourniquet time and proprioception, whereas bleeding and the prevalence of lateral release were both found to be significantly lower in the midvastus group.
The most important finding emerging from this study was the presence of abnormal VMO electromyograms in 43% of the patients treated with the midvastus approach. Instead, the group treated with the parapatellar approach showed 100% normal electromyograms. Since the EMG abnormality was transient and the authors did not report a VMO recruitment deficit, further studies are needed to clarify this problem.
The trivector approach
In this case the arthrotomy starts at the medial margin of the tibial tuberosity and runs upward along the edge of the patellar tendon and patella, 1 cm medially to the latter. At the superior-medial pole of the patella, it divides into two “snips”: one following the direction of the VMO fibers for a distance of around 1.5 cm and the other following the quadriceps tendon (Fig. 10). Exploiting both snips it is possible to obtain better mobility of the patella, especially when it is stiff or thick, thus preventing extension of the snips during knee flexion.
Fig. 10.
Trivector approach.
Fisher et al. (43) reported the clinical results obtained in 10 patients submitted to simultaneous bilateral total knee arthroplasty. The right and left knees were randomized to treatment with the trivector approach or with the standard parapatellar approach. At six-week and six-month follow-ups no differences were found between the two groups in pain, ROM, knee score and pain score. However, the knees treated with the tri vector appoach showed faster recovery of straight-leg raising and, at six months, greater quadriceps strength in concentric contractions (15% more than the contralateral leg).
Extensive arthrotomies
Sometimes, as we have said, exposure of the joint can be difficult for various reasons, such as fibrosis, joint stiffness or patella baja, obesity, fractures and previous surgery.
In these cases, solutions such as the restoration of the suprapatellar pouch described by Tarabichi and Tarabichi (44) (Fig. 11) and extensive posteromedial release, are not sufficient to adequately expose the joint and it is necessary to have recourse to extensive arthrotomies. These can be proximal or distal and the procedures most frequently used are the quadriceps snip, the quadriceps turndown and osteotomy of the ATT.
Fig. 11.
Detachment of the extensor apparatus from the bone surface according to Tarabichi and Tarabichi.
The quadriceps snip
First described by Insall (45), this is an extensive medial parapatellar arthrotomy in which the incision begins at the origin of the quadriceps tendon and extends at a 45° angle along the fibers of the vastus lateralis.
The patella is then dislocated or everted and the tibia is externally rotated so as to expose the joint.
A quadriceps snip does not necessitate modification of the postoperative course (Fig. 12).
Fig. 12.
Quadriceps snip.
Inverted V-shaped qua driceps incision
Originally described by Coonse and Adams (46) (Fig. 13), this arthrotomy starts at the center of the quadriceps tendon, approximately 1 cm from the base of the patella, divides and continues medially and laterally along the margin of the patella and of the patellar tendon, thereby allowing distal turning of the patella and complete exposure of the joint. A modified version (45) is a medial parapatellar arthrotomy that continues proximally with a further incision, angled at 45°, that, from the apex of the quadriceps tendon, extends laterally along the vastus lateralis and the most proximal portion of the iliotibial tract (Fig. 14).
Fig. 13.
Inverted V-shaped quadriceps incision.
Fig. 14.
A modified version of the inverted V-shaped quadriceps incision.
This approach, on the other hand, does demand modification of the postoperative course, as the functional recovery needs to be more gradual during the first four weeks (45).
Osteotomy of the anterior tibial tuberosity
Preferred and used routinely by some authors in knee joints that are difficult to expose, this is an approach that offers excellent exposure.
A midline skin incision is made that extends 8–10 cm below the ATT. The medial parapatellar arthrotomy begins at a point about 6 cm proximal to the patella and ends distally on the anterior tibial crest.
A trapezoidal osteotomy is performed (7–8 cm long and 2 cm in width and depth), leaving the periosteum and lateral muscular attachments intact to the bone fragment (47). Once the ATT has been overturned, the extensor mechanism can be raised proximally allowing complete exposure of the joint. However, this approach is technically demanding, carries a high risk of complications and should be reserved for selected cases.
The rate of complications occurring with this approach and reported in the literature, such as loss of reduction, impaired synthesis and fracture of the tibia, ranges from 7% to 22% (48, 49). Wolff (50) reported a risk of loss of reduction if the bone block of the ATT is too short (3 cm). Whiteside (51) highlighted a risk of fracture of the tibia if the tibial bone block is too long (10 cm).
Engh and Rorabeck (52), in 185 revision knee arthroplasties, used a standard approach in 54% of the cases, a quadriceps snip in 29%, a “V–Y” approach in 13%, and ATT osteotomy in only 4%.
The current role of mini-invasive surgery (MIS)
The concept of mini-invasive prosthetic surgery was introduced in the late 1990s by Repicci (53), and it was based on the principles of a small incision (3 inches) and maximum preservation of the extensor mechanism on implantation of a unicompartmental prosthesis. Since then, many other authors have felt it necessary to extend this concept to total knee and hip replacement (54–57). Today, however, more than a decade since its advent and around five years since its application to total joint replacement surgery, even the very meaning of the term mini-invasive prosthetic surgery remains to be clarified. Bonutti et al. (55) maintain that the skin incision does not define MIS per se, but then state that it should not exceed 10 cm. DiGioia et al. (58) said that incisions should be between 8 and 10 cm.
Furthermore, consideration of the length of the incision, as an absolute value, without reference to other parameters related to the patient’s size, is confusing. Is it right to state in absolute terms that MIS is defined by a 10-cm incision, when 10 cm may not be enough for an individual weighing 130 kg, and too much for one weighing 50 kg? In the literature, there is no consensus even on the definition of the parameters useful for establishing what constitutes an adequate minimum incision. Bonutti et al. suggest that the incision should be twice the length of the patella (55), yet no correlation has been shown between patella diameter and patient weight, height and BMI (i.e. the ratio of weight to height). Despite this, the authors are adamant that MIS is applicable even in subjects weighing over 150 kg (55). Tria considered BMI inadequate for this purpose, and instead suggested using an index relating incision length to thigh circumference (31).
Another aspect that the literature fails to clarify is whether the concept of MIS should mean only less surgical soft tissue trauma, or less surgical trauma to both soft tissue and bones. Of course, this question is more pertinent to the hip joint (for which resurfacing is only one of the possible options) than it is to the knee (for which this is the only available option). Therefore, the question that has to be asked is whether it makes sense to strive to reduce soft tissue trauma to the minimum, only then to implant an invasive prosthesis that demands sacrifice of bone tissue. In fact, if mini-invasive prosthetic surgery is meant to mean surgery geared at maximum preservation of the soft tissues, through progressively smaller incisions and capsulotomies (an evolution that goes hand in hand with improvements to instruments and increasing surgeon experience), then it would probably be more appropriate to call it “reduced tissue trauma surgery” (RTTS), as suggested by Ranawat and Ranawat (59), given that this is the term that best describes – and not in an ambiguous or forced way –, the natural evolution of surgical techniques generally. Ranawat and Ranawat (59) maintain that in hip arthroplasty (although the concept can also be extended to the knee joint), the difference between RTTS and MIS is that the latter approach involves less splitting of the gluteus maximus (reducing it by 30 to 50%), does not involve partial detachment of the distal insertion of the gluteus maximus, and requires that the quadriceps femoris be left in situ.
However, they argue that even internal rotation stress can cause quadriceps femoris laceration and that gluteus maximus tensing can cause sciatic nerve compression. Applying these data to knee arthroplasty, one is led to think that the VMO splitting in the minimally invasive midvastus approach proposed by Bonutti (54) should entail progressively extending the snip through the quadriceps far beyond the recommended 2 cm, during knee flexion, and retraction of the soft tissues using retractors. If, on the other hand, the aim is to promote a new surgical concept based on a small incision just large enough to allow the passage of the prosthetic components (no longer than a few centimeters), maximum preservation of the soft tissues, even at the expense of an adequate overview of the whole joint, and the use of a new bone cutting technique, then it is necessary to establish clearly that this new technique really does give the expected benefits (60), without increasing the incidence of complications (61) or changing the survivorship of the prosthetic implants. Furthermore, if MIS really does constitute a new technique, then it must be pointed out that, to date, it has not been disseminated globally in the correct manner, i.e., according to the algorithm proposed by Malchau (62), and that it is rather, an example of what Dunbar (63) called the “chaotic innovation of new technology”. The theoretical principles of less invasive surgery are sound, and certainly desirable. A smaller incision with less esthetic impact, greater patient satisfaction, greater respect for the capsule, tendon and muscle structures, faster post-operative recovery, less blood loss and an earlier discharge are undoubtedly parameters of interest to all orthopedic surgeons, but it remains to be demonstrated that these objectives can be achieved by going down the MIS route. Ultimately, a “less invasive” approach based on maximum safeguarding of the anatomical structures through reduction of surgical exposure (in accordance with the surgeon’s skill and experience) is desirable, but only if it is not at the expense of the surgical precision that is crucial for a good long-term outcome.
Conclusions
The planning of the surgical approach is crucially important in knee replacement surgery and even more so in complex and difficult and cases.
In the case of a standard primary implant, it is advisable to use a classical midline incision, while less invasive approaches, such as the subvastus or the midvastus ones, should be chosen only if the surgeon has a high level of expertise and has performed many implant procedures. In difficult cases, we recommend starting with release of the suprapatellar pouch, followed by a quadriceps snip, reserving ATT osteotomy only for the most difficult knees, which, even in revision cases, amount to no more than 5% of the total.
References
- 1.Colizza WA, Insall JN, Scuderi GR. The posterior stabilized total knee prosthesis. Assessment of polyethylene damage and osteolysis after a ten year minimum follow-up. J Bone Joint Surg Am. 1995;77:1716–1720. doi: 10.2106/00004623-199511000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Diduch DR, Insall JN, Scott WN, et al. Total knee replacement in young, active patients. Long-term follow up and functional outcome. J Bone Joint Surg Am. 1997;79:575–582. doi: 10.2106/00004623-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Font-Rodriguez DE, Scuderi GR, Insall JN. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997;(345):79–86. [PubMed] [Google Scholar]
- 4.Gill GS, Chan KC, Mills DM. 5- to 18-year follow-up study of cemented total knee arthroplasty for patients 55 years old or younger. J Arthroplasty. 1997;12:49–54. doi: 10.1016/s0883-5403(97)90046-2. [DOI] [PubMed] [Google Scholar]
- 5.Insall JN, Ranawat CS, Scott WN, et al. Total condylar knee replacement. Preliminary report. Clin Orthop. 1976;120:149–54. [PubMed] [Google Scholar]
- 6.Malkani A, Rand JA, Bryan RS, et al. Total knee arthroplasty with the kinematic condylar prosthesis: a ten-year follow-up study. J Bone Join Surg Am. 1995;77:423–431. doi: 10.2106/00004623-199503000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Ranawat CS, Padgett DE, Ohashi Y. Total knee arthroplasty for patients younger than 55 years. Clin Orthop Relat Res. 1989;(248):27–33. [PubMed] [Google Scholar]
- 8.Ranawat CS, Flynn WF, Jr, Saddler S, et al. Long-term results of the total condylar knee arthroplasty: a fifteen-year survivorship study. Clin Orthop. 1993;286:96–102. [PubMed] [Google Scholar]
- 9.Ranawat CS, Flynn WF, Jr, Deshmukh RG. Impact of modern technique on long-term results of total condylar knee arthroplasty. Clin Orthop Relat Res. 1994;(309):131–135. [PubMed] [Google Scholar]
- 10.Ritter MA, Herbst SA, Keating EM, et al. Long-term survivorship analysis of a posterior cruciate-retaining total condylar total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):136–145. [PubMed] [Google Scholar]
- 11.Scott RD, Volatile TB. Twelve years’ experience with posterior cruciate-retaining total knee arthroplasty. Clin Orthop Relat Res. 1986;(205):100–107. [PubMed] [Google Scholar]
- 12.Stern SH, Wills RD, Gilbert JL. The effect of tibial stem design on component micromotion in knee arthroplasty. Clin Orthop Relat Res. 1997;(345):44–52. [PubMed] [Google Scholar]
- 13.Stern SH, Insall JN. Posterior stabilized prosthesis: results after follow-up of nine to twelve years. J Bone Joint Surg Am. 1992;74:980–986. [PubMed] [Google Scholar]
- 14.Clarke HD. Anatomy. In: Insall JN, Scott WN, editors. Insall & Scott surgery of the knee. Elsevier Churchill Livingstone; Philadelphia: 2011. pp. 40–42. [Google Scholar]
- 15.Haertsch P. The blood supply to the skin of the leg: a postmortem investigation. Br J Plast Surg. 1981;34:470–477. doi: 10.1016/0007-1226(81)90061-8. [DOI] [PubMed] [Google Scholar]
- 16.Johnson DP, Houghton TA, Radford P. Anterior midline or medial parapatellar incision for arthroplasty of the knee. A comparative study. J Bone Joint Surg Br. 1986;68:812–814. doi: 10.1302/0301-620X.68B5.3782252. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DP. Midline or parapatellar incision for knee arthroplasty. A comparative study of wound viability. J Bone Joint Surg Br. 1988;70:656–658. doi: 10.1302/0301-620X.70B4.3403619. [DOI] [PubMed] [Google Scholar]
- 18.Dennis DA. Wound complications in total knee arthroplasty. Instr Course Lect. 1997;46:165–169. [PubMed] [Google Scholar]
- 19.Garvin KL, Konigsberg BS. Infection following total knee arthroplasty: prevention and management. Instr Course Lect. 2012;61:411–419. [PubMed] [Google Scholar]
- 20.Holt GE, Dennis DA. Skin exposure issues. In: Bono JV, Scott RD, editors. Revision Total Knee Arthroplasty. Springer; New York: 2005. pp. 55–60. [Google Scholar]
- 21.Green JP. Steroid therapy and wound healing in surgical patients. Br J Surg. 1965;52:523–525. doi: 10.1002/bjs.1800520710. [DOI] [PubMed] [Google Scholar]
- 22.McNamara JJ, Lamborn PJ, Mills D, et al. Effect of shortterm pharmacologic doses of adrenocorticosteroid therapy on wound healing. Ann Surg. 1969;170:199–202. doi: 10.1097/00000658-196908000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werb Z. Biochemical actions of glucocorticoids on macrophages in culture. Specific inhibition of elastase, collagenase, and plasminogen activator secretion and effects on other metabolic functions. J Exp Med. 1978;147:1695–1712. doi: 10.1084/jem.147.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garner RW, Mowat AG, Hazleman BL. Wound healing after operations of patients with rheumatoid arthritis. J Bone Joint Surg Br. 1973;55:134–144. [PubMed] [Google Scholar]
- 25.Malinzak RA, Ritter MA, Berend ME, et al. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24( 6 Suppl):84–88. doi: 10.1016/j.arth.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Winiarsky R, Barth P, Lotke P. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80:1770–1774. doi: 10.2106/00004623-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Craig S, Rees TD. The effects of smoking on experimental skin flaps in hamsters. Plast Reconstr Surg. 1985;75:842–846. doi: 10.1097/00006534-198506000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Mosely LH, Finseth F, Goody M. Nicotine and its effect on wound healing. Plast Reconstr Surg. 1978;61:570–575. doi: 10.1097/00006534-197804000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Dalury DF, Mulliken BD, Adams MJ, et al. Early recovery after total knee arthroplasty performed with and without patellar eversion and tibial translation. A prospective randomized study. J Bone Joint Surg Am. 2009;91:1339–1343. doi: 10.2106/JBJS.H.00435. [DOI] [PubMed] [Google Scholar]
- 30.Insall JN. A midline approach to the knee. J Bone Joint Surg Am. 1971;53:1584–1586. [PubMed] [Google Scholar]
- 31.Tria AJ., Jr Minimally invasive total knee arthroplasty: the importance of instrumentation. Orthop Clin North Am. 2004;35:227–234. doi: 10.1016/S0030-5898(03)00118-4. [DOI] [PubMed] [Google Scholar]
- 32.Erkes F. Weitere Erfahrungen mit physiologischer Schnitt fuhrung zur Eroffnung des Kniegelenks. Bruns' Beitr. zur Win. Chir. 1929;147:221. [Google Scholar]
- 33.Bechtol CO. Richards Patello-Femoral Replacement Surgical Technique Brochure. 1976;2:14, 26. [Google Scholar]
- 34.Hofmann AA, Plaster RL, Murdock LE. Subvastus (Southern) approach for primary total knee arthroplasty. Clin Orthop Relat Res. 1991;(269):70–77. [PubMed] [Google Scholar]
- 35.Cila E, Güzel V, Ozalay M, et al. Subvastus versus medial parapatellar approach in total knee arthroplasty. Arch Orthop Trauma Surg. 2002;122:65–68. doi: 10.1007/s004020100319. [DOI] [PubMed] [Google Scholar]
- 36.Roysam GS, Oakley MJ. Subvastus approach for total knee arthroplasty: a prospective randomized, and observer-blinded trial. J Arthroplasty. 2001;16:454–457. doi: 10.1054/arth.2001.22388. [DOI] [PubMed] [Google Scholar]
- 37.Engh GA, Holt BT, Parks NL. Midvastus muscle-splitting approach for total knee arthroplasty. J Arthroplasty. 1997;12:322–331. doi: 10.1016/s0883-5403(97)90030-9. [DOI] [PubMed] [Google Scholar]
- 38.Cooper RE, Jr, Trinidad G, Buck WR. Midvastus approach in total knee arthroplasty: a description and a cadaveric study determining the distance of the popliteal artery from the patellar margin of the incision. J Arthroplasty. 1999;14:505–508. doi: 10.1016/s0883-5403(99)90109-2. [DOI] [PubMed] [Google Scholar]
- 39.Engh GA, Parks NL. Surgical technique of the midvastus arthrotomy. Clin Orthop Relat Res. 1998;(351):270–274. doi: 10.1097/00003086-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 40.Engh GA, Parks NL, Ammen DJ. Influence of surgical approach on lateral retinacular releases in total knee arthroplasty. Clin Orthop Relat Res. 1996;(331):56–63. doi: 10.1097/00003086-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Keating EM, Faris PM, Meding JB, et al. Comparison of the mid-vastus muscle-splitting approach with the median parapatellar approach in total knee arthroplasty. J Arthroplasty. 1999;14:29–32. doi: 10.1016/s0883-5403(99)90198-5. [DOI] [PubMed] [Google Scholar]
- 42.Parentis MA, Rumi MN, Deol GS, et al. A comparison of the vastus splitting and median parapatellar approcheas in total knee arthroplasty. Clin Orthop Relat Res. 1999;(367):107–116. [PubMed] [Google Scholar]
- 43.Fisher DA, Trimble SM, Breedlove K. The medial trivector approach in total knee arthroplasty. Orthopedics. 1998;21:53–56. doi: 10.3928/0147-7447-19980101-13. [DOI] [PubMed] [Google Scholar]
- 44.Tarabichi S, Tarabichi Y. Can an anterior quadriceps release improve range of motion in the stiff arthritic knee? J Arthroplasty. 2010;25:571–575. doi: 10.1016/j.arth.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Insall JN. Surgery of the Knee. Churchill Livingstone; New York: 1984. p. 41. [Google Scholar]
- 46.Coonse KD, Adams JD. A new operative approach to the knee joint. Surg Gynecol Obstet. 1943;77:344. [Google Scholar]
- 47.Whiteside LA, Ohl MD. Tibial tubercle osteotomy for exposure of the difficult total knee arthroplasty. Clin Orthop Relat Res. 1990;(260):6–9. [PubMed] [Google Scholar]
- 48.Menderes A, Demirdover C, Yilmaz M, et al. Reconstruction of soft tissue defects following total knee arthroplasty. Knee. 2002;9:215–219. doi: 10.1016/s0968-0160(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 49.Ritter MA, Herbst SA, Keating EM, et al. Patellofemoral complications following total knee arthroplasty. Effect of a lateral release and sacrifice of the superior lateral geniculate artery. J Arthroplasty. 1996;11:368–372. doi: 10.1016/s0883-5403(96)80024-6. [DOI] [PubMed] [Google Scholar]
- 50.Wolff AM, Hungerford DS, Krackow KA, et al. Osteotomy of the tibial tubercle during total knee replacement. A report of twenty-six cases. J Bone Joint Surg Am. 1989;71:848–852. [PubMed] [Google Scholar]
- 51.Whiteside LA. Exposure in difficult total knee arthroplasty using tibial tubercle osteotomy. Clin Orthop Relat Res. 1995;(321):32–35. [PubMed] [Google Scholar]
- 52.Engh GA, Rorabeck CH. Revision Total Knee Arthroplasty. Williams & Wilkins; Baltimore: 1997. pp. 227–36. [Google Scholar]
- 53.Repicci JA, Eberle RW. Minimally invasive surgical technique for unicondylar knee arthroplasty. J South Orthop Assoc. 1999;8:20–27. [PubMed] [Google Scholar]
- 54.Bonutti PM. Minimally invasive total knee arthroplasty - midvastus approach. In: Hozack WJ, Krismer M, Nogler M, et al., editors. Minimally Invasive Total Joint Arthroplasty. Springer; Heidelberg: 2004. pp. 139–145. [Google Scholar]
- 55.Bonutti PM, Mont MA, Kester MA. Minimally invasive total knee arthroplasty: a 10-feature evolutionary approach. Orthop Clin North Am. 2004;35:217–226. doi: 10.1016/j.ocl.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Chauhan SK. Computer-assisted minimally invasive total knee arthroplasty. In: Hozack WJ, Krismer M, Nogler M, et al., editors. Minimally Invasive Total Joint Arthroplasty. Springer; Heidelberg: 2004. pp. 216–225. [Google Scholar]
- 57.Rosenberg AG. How should we teach MSI? In: Hozack WJ, Krismer M, Nogler M, et al., editors. Minimally Invasive Total Joint Arthroplasty. Springer; Heidelberg: 2004. pp. 329–332. [Google Scholar]
- 58.DiGioia AM, 3rd, Blendea S, Jaramaz B. Computer-assisted orthopaedic surgery: minimally invasive hip and knee reconstruction. Orthop Clin North Am. 2004;35:183–189. doi: 10.1016/S0030-5898(03)00133-0. [DOI] [PubMed] [Google Scholar]
- 59.Ranawat CS, Ranawat AS. MIS in total hip arthroplasty: present status and future direction. In: Hozack WJ, Krismer M, Nogler M, et al., editors. Minimally Invasive Total Joint Arthroplasty. Springer; Heidelberg: 2004. pp. 246–248. [Google Scholar]
- 60.Ogonda L, Wilson R, Archbold P, et al. A minimal-incision technique in total hip arthroplasty does not improve early postoperative outcomes. A prospective, randomized, controlled trial. J Bone Join Surg Am. 2005;87:701–710. doi: 10.2106/JBJS.D.02645. [DOI] [PubMed] [Google Scholar]
- 61.Fehring TK, Mason JB. Catastrophic complications of minimally invasive hip surgery. A series of three cases. J Bone Join Surg Am. 2005;87:711–714. doi: 10.2106/JBJS.D.02666. [DOI] [PubMed] [Google Scholar]
- 62.Malchau H. Introducing new technology: a stepwise algorithm. Spine 2000;(Phila Pa. 1976;25:285. doi: 10.1097/00007632-200002010-00004. [DOI] [PubMed] [Google Scholar]
- 63.Dunbar MJ. Introducing new surgical technologies. In: Hozack WJ, Krismer M, Nogler M, et al., editors. Minimally Invasive Total Joint Arthroplasty. Springer; Heidelberg: 2004. pp. 300–303. [Google Scholar]