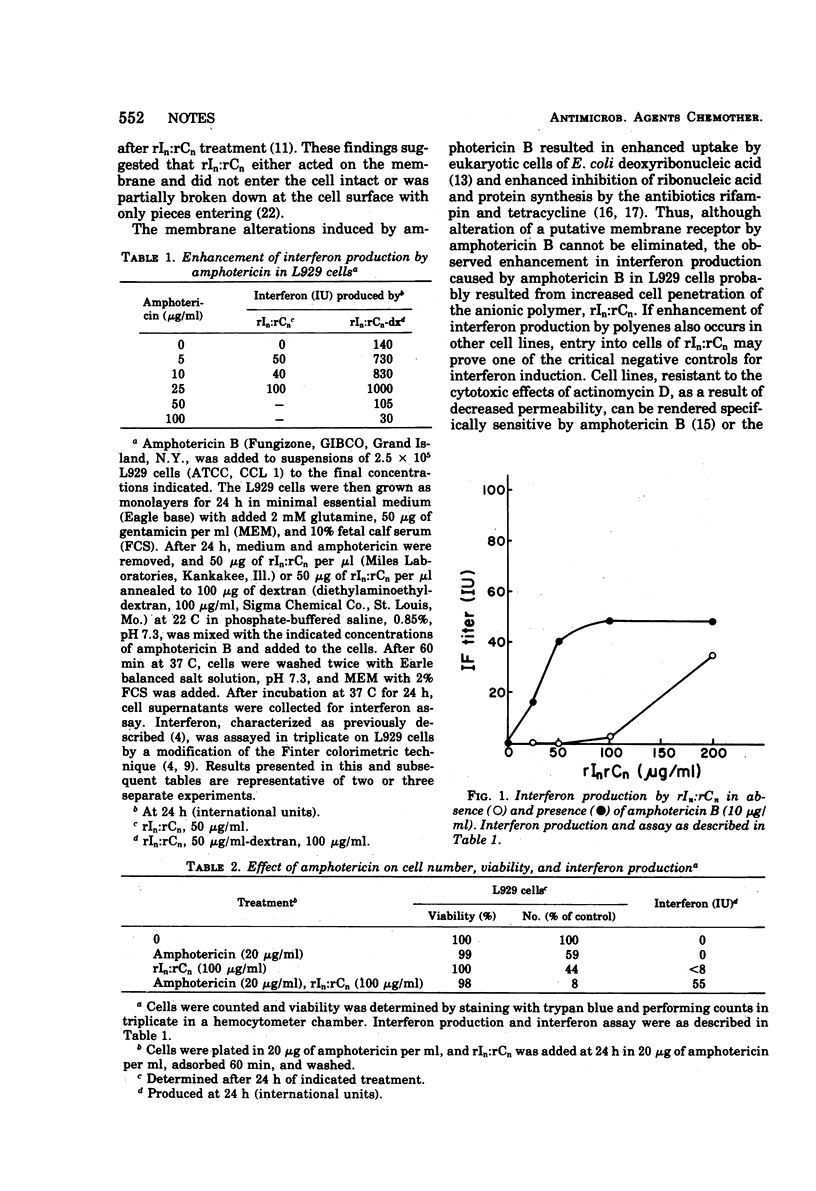
Abstract
When mouse L929 cells are treated with amphotericin B before exposure to polyriboinosinic-polyribocytidylic acid or polyriboinosinic-polyribocytidylic acid-dextran, they make significantly more interferon than do cells not receiving amphotericin. This effect may be due to enhanced cell membrane penetration by the polynucleotide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati P., Lago C. Sensitivity to amphotericin B of in vitro established cell lines. Nature. 1974 Feb 15;247(5441):466–469. doi: 10.1038/247466a0. [DOI] [PubMed] [Google Scholar]
- Andreoli T. E. On the anatomy of amphotericin B-cholesterol pores in lipid bilayer membranes. Kidney Int. 1973 Nov;4(5):337–345. doi: 10.1038/ki.1973.126. [DOI] [PubMed] [Google Scholar]
- Bausek G. H., Merigan T. C. Cell interaction with a synthetic polynucleotide and interferon production in vitro. Virology. 1969 Nov;39(3):491–498. doi: 10.1016/0042-6822(69)90097-x. [DOI] [PubMed] [Google Scholar]
- Borden E. C., Prochownik E. V., Carter W. A. The interferon refractory state. II. Biological characterization of a refractoriness-inducing protein. J Immunol. 1975 Feb;114(2 Pt 2):752–756. [PubMed] [Google Scholar]
- Colby C., Chamberlin M. J. The specificity of interferon induction in chick embryo cells by helical RNA. Proc Natl Acad Sci U S A. 1969 May;63(1):160–167. doi: 10.1073/pnas.63.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Stewart W. E., 2nd Integrity of cell-bound poly(I).poly(C). J Gen Virol. 1974 Jul;24(1):201–209. doi: 10.1099/0022-1317-24-1-201. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Wells R. D., Merigan T. C. Studies on the antiviral activity and cell interaction of synthetic double-stranded polyribo- and polydeoxyribonucleotides. Virology. 1972 Feb;47(2):405–415. doi: 10.1016/0042-6822(72)90276-0. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Holz R. Aqueous pores created in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. Membranes. 1973;2:377–408. [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Fungal sterols and the mode of action of the polyene antibiotics. Adv Appl Microbiol. 1974;17(0):109–134. doi: 10.1016/s0065-2164(08)70556-2. [DOI] [PubMed] [Google Scholar]
- Kelly T. A., Levy H. B. Lack of uptake of intact polyriboinosinic-polyribocytidylic acid by cells. Proc Soc Exp Biol Med. 1973 Nov;144(2):534–537. doi: 10.3181/00379727-144-37630. [DOI] [PubMed] [Google Scholar]
- Kinsky S. C. Antibiotic interaction with model membranes. Annu Rev Pharmacol. 1970;10:119–142. doi: 10.1146/annurev.pa.10.040170.001003. [DOI] [PubMed] [Google Scholar]
- Kumar B. V., Medoff G., Kobayashi G., Schlessinger D. Uptake of Escherichia coli DNA into HeLa cells enhanced by amphotericin B. Nature. 1974 Jul 26;250(464):323–325. doi: 10.1038/250323a0. [DOI] [PubMed] [Google Scholar]
- Kwan C. N., Medoff G., Kobayashi G. S., Schlessinger D., Raskas H. J. Potentiation of the antifungal effects of antibiotics by amphotericin B. Antimicrob Agents Chemother. 1972 Aug;2(2):61–65. doi: 10.1128/aac.2.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Kwan C. N., Schlessinger D., Kobayashi G. S. Permeability control in animal cells by polyenes: a possibility. Antimicrob Agents Chemother. 1973 Mar;3(3):441–443. doi: 10.1128/aac.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Kwan C. N., Schlessinger D., Kobayashi G. S. Potentiation of rifampicin, rifampicin analogs, and tetracycline against animal cells by amphotericin B and polymyxin B. Cancer Res. 1973 Jun;33(6):1146–1149. [PubMed] [Google Scholar]
- Pitha P. M., Harper H. D., Pitha J. Dependence of interferon induction on cell membrane integrity. Virology. 1974 May;59(1):40–50. doi: 10.1016/0042-6822(74)90204-9. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Marshall L. W., Carter W. A. Interferon induction: rate of cellular attachment of poly IC. J Gen Virol. 1972 Apr;15(1):89–92. doi: 10.1099/0022-1317-15-1-89. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Pitha J. Interferon induction site: poly IC on solid substrate carriers. J Gen Virol. 1973 Oct;21:31–37. doi: 10.1099/0022-1317-21-1-31. [DOI] [PubMed] [Google Scholar]
- Riehm H., Biedler J. L. Potentiation of drug effect by Tween 80 in Chinese hamster cells resistant to actinomycin D and daunomycin. Cancer Res. 1972 Jun;32(6):1195–1200. [PubMed] [Google Scholar]
- Schell P. L. Uptake of polynucleotides by intact mammalian cells. 8. Synthetic homoribopolynucleotides. Biochim Biophys Acta. 1971 Jul 29;240(4):472–484. doi: 10.1016/0005-2787(71)90704-0. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Kallos J. Induction of interferon by "Sepharose"-bound poly(I)-poly(C). Nat New Biol. 1973 Oct 3;245(144):143–144. doi: 10.1038/newbio245143a0. [DOI] [PubMed] [Google Scholar]
- Tytell A. A., Field A. K. Interferons and host resistance: with particular emphasis on induction by complexed polynucleotides. CRC Crit Rev Biochem. 1972 Feb;1(1):1–32. doi: 10.3109/10409237209102542. [DOI] [PubMed] [Google Scholar]
- Wagner A. F., Bugianesi R. L., Shen T. Y. Preparation of sepharose-bound poly (rI:rC). Biochem Biophys Res Commun. 1971 Oct 1;45(1):184–189. doi: 10.1016/0006-291x(71)90067-2. [DOI] [PubMed] [Google Scholar]


