Abstract
Purpose
the aim of this study was to describe the clinical results obtained after intra-articular injection of a leukocyte-poor platelet-rich plasma (PRP) preparation for the treatment of knee osteoarthritis (OA).
Methods
forty-five patients (mean age: 59 years, mean BMI: 27) were included and treated with a cycle of three weekly injections of autologous conditioned plasma. Six patients were affected by bilateral symptomatic OA, therefore 51 knees in total were treated. The patients were divided into two groups: those affected by early/moderate OA and those affected by severe OA. The patients were submitted to baseline evaluation and evaluation after a mean follow-up of 14.5 months (range: 6–24 months), performed using the following outcome measures: IKDC-subjective, EQ-VAS, Tegner, and KOOS scores. Adverse events and patient satisfaction were also recorded. The results in the two groups of patients (“early/moderate” vs “severe OA”) were analyzed separately.
Results
the overall clinical outcome was positive and the treatment proved to be safe. In the “early/moderate OA” group, the IKDC-subjective score increased from 36.4 at the baseline evaluation to 57.3 at the follow-up (p<0.0005) and a similar trend was shown by the EQ-VAS, Tegner, and KOOS scores. Although an improvement was also recorded in the “severe OA” group, the clinical outcome of the patients in this group was significantly poorer and they reported less benefit. In the “early/moderate OA” group, BMI and longer symptom duration before treatment were found to be correlated with clinical outcome.
Conclusions
PRP injections are capable of reducing pain and improving knee functional status at short-term follow-up. The patients with a lower degree of joint degeneration were the best responders, whereas in severe osteoarthritic knees this biological treatment, used as a “salvage procedure”, produced a less favorable outcome.
Level of evidence
level IV; therapeutic case series.
Keywords: PRP, osteoarthritis, growth factors, injections, knee
Introduction
The application of biological treatments to favor tissue healing and regeneration is currently one of the most attractive and intense areas of research in orthopaedic practice. Cartilage is one of the tissues most targeted (1), due to its peculiar features: namely, its relative isolation from systemic regulation (due to the lack of nerves and vessels) and its complex histological architecture, consisting of chondrocytes surrounded by a specialized extra-cellular matrix, which determines an intrinsic vulnerability that allows small and focal lesions to develop into an accelerated degenerative process leading to osteoarthritis (OA) (2, 3). OA is a painful chronic condition that is very challenging to treat by conservative means and often requires more invasive surgical approaches such as joint replacement. Several treatments, both conservative and surgical, have been tested to address cartilage pathology, but their results were found to be time-dependent and recovery of full functional status was always difficult (4, 5). The constant search for innovative solutions has led to the development and testing of novel biological approaches for treating different stages of cartilage pathology, from chondropathy to early and severe OA.
In this particular field, the role played by blood derivatives, and in particular platelet-rich plasma (PRP), is preeminent. The injection of PRP preparations containing platelet-derived growth factors (GFs) is the most exploited mean of administering a biological stimulus to several different types of damaged tissue, such as cartilage, tendons and muscle, which might benefit from this particular approach (6).
The biological rationale behind this treatment has been extensively investigated in several studies: the topical administration of molecules normally involved in joint homeostasis has been hypothesized to contribute to the mechanisms of healing and tissue regeneration. Platelet-derived GFs are a group of polypeptides that play important roles in regulating the growth and development of several tissues, including cartilage. Platelets contain storage pools of GFs (1, 7–9), such as: platelet-derived growth factor (PDGF), transforming growth factor (TGF-β), platelet-derived epidermal growth factor (PDEGF), vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), fibroblastic growth factor (FGF), epidermal growth factor (EGF), and so on. Furthermore, platelet alpha granules also release cytokines, chemokines and many other proteins (7, 9), which play a crucial role in chemotaxis, cell proliferation and maturation, and inflammatory response modulation (1, 7, 9). Dense granules instead store molecules such as ADP, ATP, calcium ions, histamine, serotonin and dopamine, which are involved in tissue modulation and regeneration (1, 7, 9). Last but not least, lysosomal granules are involved in secreting acid hydrolases, cathepsin D and E, elastases and lysozyme (7), and probably other molecules, not yet well characterized, whose function is still under investigation by basic researchers.
Injecting PRP is therefore an easy way of modulating the joint environment and promoting tissue regeneration; however, current scientific evidence fails to allow a precise definition of PRP (10, 11). Indeed, the blood-derived products applied in clinical practice vary considerably and there exist several different PRP formulations. All this makes it very difficult to compare clinical results and to gain a full understanding of the potential and limits of each formulation. In this context, there are several aspects to be taken into account:
- the preparation methods, which can influence the final platelet concentration (and therefore the amount of GFs administered), but also the cell content of PRP (in this particular regard, the role played by leukocytes is one of the aspects most discussed among scientists) (12, 13);
- the storage procedures: some authors prefer a single blood harvesting to prepare PRP, and therefore they store PRP and thaw it for subsequent injections (12);
- the activation method: some authors do not activate PRP prior to injection, whereas others use different substances for this purpose, such as thrombin or calcium chloride (12).
Finally, the particular therapeutic protocol applied by different authors (i.e. the number of PRP injections and the time interval between them) should also be considered as a peculiar factor that could lead to a different clinical outcome.
The present authors have published a number of studies on the use of a particular laboratory-made leukocyte-rich PRP preparation, which also features a high platelet concentration (at least five times the basal level). Interesting clinical results were reported (5, 14–20), especially in young patients affected by low grades of knee joint degeneration. The purpose of this study was to describe the clinical results obtained after intra-articular injection of a leukocyte-poor PRP preparation for the treatment of knee OA. The study hypothesis was that this particular treatment can improve the clinical outcome of these patients.
Methods
Patient selection and outcome measures
The following diagnostic criteria were used for patient selection: a history of chronic (at least 4 months) pain or swelling of the knee and imaging findings of degenerative changes of the knee joint (Kellgren Lawrence score of 0 to IV on X-ray evaluation or MRI findings of degenerative changes in patients without X-ray evidence of OA). Exclusion criteria were: systemic disorders such as diabetes, rheumatoid arthritis, major axial deviation (varus or valgus deformity > 5°), hematological diseases (coagulopathy), severe cardiovascular diseases, infections, immunodepression, treatment with anticoagulants or antiaggregants, use of non-steroidal anti-inflammatory drugs (NSAIDs) in the five days before blood harvesting, hemoglobin value < 11 g/dl and platelet count < 150,000/mmc.
For this study, 45 patients (21 men and 24 women; mean age 59 years, range: 20–87 years) received three weekly intra-articular injections of PRP. Thirty-nine patients were affected by unilateral lesions while the other six presented bilateral lesions, thus making a total of 51 knees treated. The mean BMI was 27 (range: 21–39); 22 patients had undergone previous knee surgery and 34 patients had undergone previous intra-articular knee injections with hyaluronic acid (HA) or cortison. The mean symptom duration was four years (range: 1.5–10 years).
The standard radiographs of the knees treated were evaluated according to the Kellgren-Lawrence grading scale: 41 knees were classified as affected by early/moderate OA (Kellgren-Lawrence score ranging from 0 to III), while 10 were considered affected by severe OA (Kellgren Lawrence score of IV). The results in these two groups of patients (“early/moderate” vs “severe OA”) were analyzed separately.
The patients were evaluated basally and at a mean follow-up of 14.5 months (range: 6–24 months) using the following evaluation tools: the IKDC Subjective Knee Form, the Knee Injury and Osteoarthritis Outcome Score (KOOS), EQ-VAS for general health status, and the Tegner activity scale. Patient satisfaction and adverse events were also reported.
PRP preparation method and injection technique
The Autologous Conditioned Plasma (ACP) Preparation Kit (Arthrex Inc.; Naples, Florida, USA) was used for PRP preparation. A 15-ml sample of peripheral venous blood was collected slowly in a special double syringe, included in the preparation kit provided by the manufacturer. Then, under sterile conditions, the double syringe was placed in one bucket of the centrifuge and a counterweight was inserted into the opposite bucket. At this point a single centrifugation was performed (1500 rpm for five minutes). At the end, the supernatant PRP was transferred from the larger outer syringe into the small inner syringe, carefully avoiding mixing. The small inner syringe was unscrewed and fitted with a needle, so that the PRP was ready for use. The injection was performed in sterile conditions through a classic lateral approach with a 22-gauge needle. At the end of the procedure, the patient was encouraged to bend and extend the knee a few times to allow the PRP to distribute over the entire joint before becoming a gel. After the injection, patients were sent home with instructions to restrict the use of the leg for at least 24 hours and to use cold therapy/ice on the affected area to relieve pain. During this period, the use of non-steroidal medication was forbidden. During the treatment period, rest or mild activities (such as using an exercise bike or mild exercise in a pool) were permitted, and subsequently a gradual resumption of normal sport or recreational activities was allowed, as tolerated.
Data Analysis
All continuous data are expressed as mean and standard deviation of the mean. One-way ANOVA was performed to assess differences between groups when the Levene test for homogeneity of variances was not significant (p<0.05); otherwise, the Mann-Whitney U-test (two groups) or the Kruskal-Wallis test (more than two groups) was used. Paired t-test was performed to test differences in the scores at different follow-up times. The non-parametric Pearson’s chi-square test was performed to investigate the relationships between discrete variables; Fisher’s exact test was performed to investigate relationships between dichotomous variables. Spearman’s rank correlation was used to assess the correlation between continuous variables. For all tests, p<0.05 was considered significant.
Statistical analysis was carried out using the Statistical Package for the Social Sciences (IBM-SPSS) software, version 19.0.
Results
No major adverse events were described after the intra-articular injections. Only mild pain and/or slight swelling were reported which resolved spontaneously within 24–48 hours.
Both groups showed a statistically significant improvement of the clinical scores from preoperative evaluation to final follow-up. The results are discussed separately for each subgroup.
Early/moderate OA
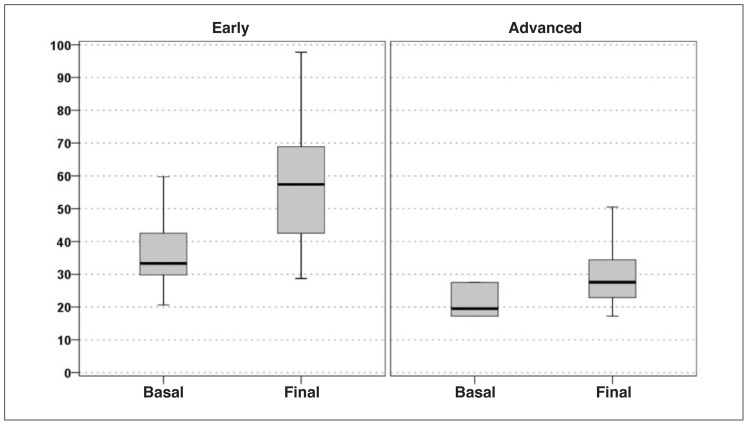
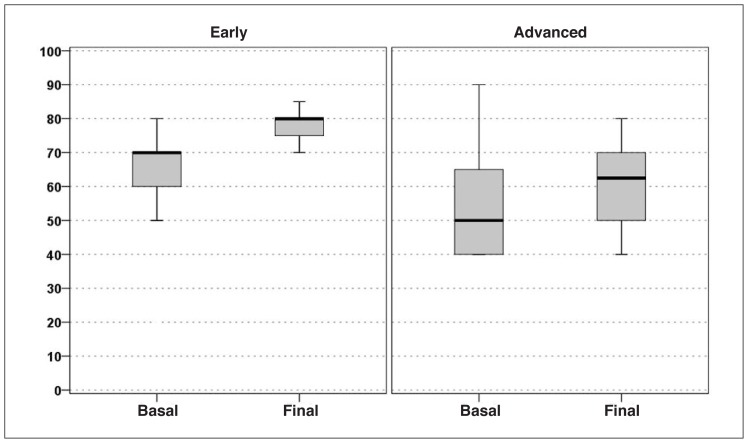
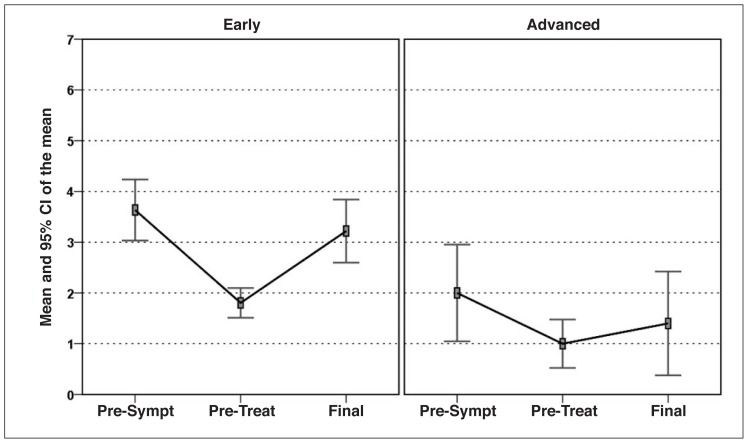
Statistically significant increases from baseline to follow-up was recorded in all the clinical scores considered. In particular, the IKDC-subjective score increased from 36.4 at the basal evaluation to 57.3 at the mean 14.5-month final evaluation (p<0.0005) (Fig. 1). The EQ-VAS score increased significantly from 64.3 to 76.2 (p<0.0005) (Fig. 2), and the Tegner score showed a similar trend (increasing from 1.8 to 3.2; p<0.0005) (Fig. 3).
Fig. 1.
IKDC-subjective score improvement in the two groups of patients considered: “early/moderate OA” vs “severe OA”.
Fig. 2.
EQ-VAS score improvement in the two groups of patients considered: “early/moderate OA” vs “severe OA”.
Fig. 3.
Tegner score improvement in the two groups of patients considered: “early/moderate OA” vs “severe OA”.
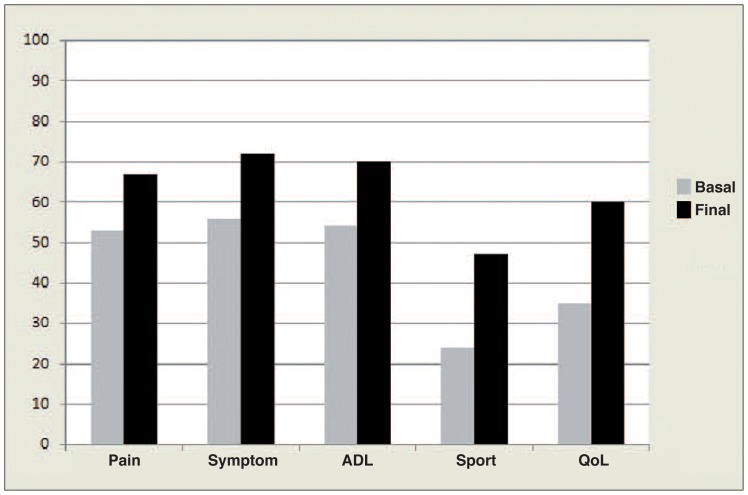
However, the patients were not able to recover their pre-symptom-onset level of activity. For what regards the KOOS results, significant improvement was obtained in all five subscale scores (“Pain” increased from 52.9 to 66.8, p=<0.0005; “other Symptoms” from 56.2 to 71.6, p=<0.0005; “ADL” from 53.8 to 69.9, p=<0.0005; “Function in sports and recreation (Sport/Rec)” from 24.2 to 47.2, p=<0.0005; “knee-related Quality of Life (QoL)” from 35.2 to 59.5, p=<0.0005) (Fig. 4).
Fig. 4.
KOOS subscale score improvements evaluated in the “early/moderate OA” group.
Among the factors analyzed for possible correlations with the clinical results, BMI was found to significantly influence outcome. In particular, BMI correlated with the IKDC-subjective, EQ-VAS, and Tegner scores, and also with the KOOS “Symptoms” and “Sport/Rec” scores. Interestingly, higher BMI showed a trend to reduce the duration of the beneficial effects of PRP treatment (p=0.06), which was, overall, estimated to be about 9 months.
Finally, a correlation was found between symptom onset and the EQ-VAS score: a longer pain duration determined a lower perception of general health status after PRP treatment.
Severe OA
The IKDC-subjective score showed a slight but significant improvement from 26.1 at the basal evaluation to 34.7 at the mean 14.5-month final evaluation (p=0.023) (Fig. 1); the EQ-VAS score showed a similar trend, rising from 55.0 to 62.0 at the final evaluation (p=0.013) (Fig. 2). The Tegner score was the only parameter whose increase, from 1.0 to 1.4, did not reach statistical significance (p=0.223) (Fig. 3). In this group, too, full recovery of the pre-injury level of activity was not possible. For what regards the KOOS, significant improvement was reported in four of the five subscale scores (“Pain” increased from 41.7 to 46.5, p=0.195; “Symptoms” from 47.2 to 52.1, p=0.048; “ADL” from 31.5 to 41, p=0.010; and “Sport/Rec” from 14 to 23.5, p=0.035); the only one not reaching statistical significance was “QoL”.
Due to the small size of this group, it was not possible to study the correlation between patients’ characteristics and clinical outcome.
Group comparison
Although, overall, the clinical results were positive in both groups, the patients affected by early/moderate OA showed a better clinical outcome than those affected by severe OA, as testified by the significantly greater improvement in the IKDC-subjective score in the cases with less degeneration (p=0.014). In fact, the patients with severe knee OA recorded lower clinical scores at the basal evaluation and were found to have only a marginal chance of deriving benefit from this biological approach.
This trend was confirmed by the analysis of the EQ-VAS and Tegner scores, which documented a modest increase in the “severe OA” with respect to the “early/moderate OA” group (p=0.016 and p=0.008, respectively). The KOOS subscale scores confirmed this different performance in favor of the “early/moderate OA” group (“Pain”: p=0.005; “Symptoms”: p=0.004; “ADL”: p=<0.0005; “Sport/Rec”: p=0.013; “QoL”: p=<0.0005).
Discussion
The main finding of the present study is that leukocyte-poor low-concentrate PRP can provide symptomatic relief and improve functional status in cases of knee OA.
In spite of the large number of reviews available, current clinical evidence on intra-articular injections of PRP is based mainly on low quality studies (20); some randomized trials were published only recently.
Clinical experience with PRP dates back to 2008. Sanchez et al. (21) published a retrospective observational study on the effectiveness of intra-articular injections of a platelet concentrate in 60 patients, half treated with intra-articular injections of PRGF and half with injections of HA. The results at short-term evaluation were encouraging. Similar findings were reported by Sampson et al. (22) and Wang-Saegusa et al. (23) in their subsequent case series.
In 2010 Kon et al. (13) published a prospective study on 91 patients (115 knees) treated with three 5-mL injections of PRP, one every three weeks. The patients underwent clinical follow-up evaluation for up to 12 months: 80% of the patients were satisfied with the treatment received. At two and six months of follow-up there was a statistically significant improvement in all the scores considered, whereas at 12 months there emerged a worsening trend. A significant difference was detected when comparing patients affected by chondropathy alone, who presented better and more lasting results, with those affected by early or severe OA. A subsequent evaluation at 24 months of follow-up showed a further and marked deterioration in the clinical outcome, thus confirming this trend and the time dependence of intra-articular therapy with platelet-derived GFs (17). Napolitano et al. (24) treated 27 patients, affected by either simple chondropathy or initial OA. Significant results were obtained after treatment without the occurrence of adverse events. Within last two years a case report and four case series have been published (25–29): these studies confirmed the safety of the procedure and the encouraging clinical results obtained by previous authors.
Looking at comparative or randomized trials, the first one was published by Kon et al. (16) in 2011. PRP injections were tested against both low molecular weight HA (LW-HA) and high molecular weight HA (HW-HA) injections in three homogeneous groups of patients. The authors found a better performance of the PRP treatment at six months of follow-up. In particular, in the early cartilage degeneration group the biological approach gave results superior to those obtained with HA. Conversely, in the early OA group there was no significant difference versus HA, and in the severe OA group no difference in clinical outcome was observed. The same authors, in a multicenter study, were the first to compare two different PRP preparations: high-concentrate leukocyte-rich PRP versus low-concentrate leukocyte-free PRP. In the sample of 144 patients, treated and evaluated for up to six months, similar positive results were reported for both treatments even though the PRP-leukocyte group suffered from more swelling and pain reaction immediately after the injections (19).
Recently, Sanchez et al. (30) investigated the efficacy of single-spinning leukocyte-free PRP versus HA in 153 patients followed up for up to six months. The percentage of responders (patients with at least 50% pain reduction), which was found to be significantly higher in the PRP group, was the only parameter in which a clear superiority of PRP emerged. The same study confirmed that PRP is no more effective than HA in moderate/severe OA. Similar considerations were made by Filardo et al. (18) on the basis of the preliminary results (109 patients) of their randomized double blind trial comparing PRP and HA: no statistical inter-group difference was reported, only a trend towards better results for the PRP group at six and 12 months of follow-up in patients affected by low-grade cartilage degeneration (Kellgren Lawrence score up to II).
Cerza et al. (31), on the other hand, in their randomized trial, treated 120 patients with either autologous conditioned plasma (ACP) or HA and, surprisingly, the ACP group showed a significantly better performance than the HA group in all treatment categories, including patients affected by grade-III knee OA. Finally, Patel et al. (32) were the first to test PRP versus saline in a randomized trial. Seventy-eight patients affected by Kellgren-Lawrence grade I–III OA were included and treated bilaterally with one injection of PRP, two injections of PRP (three weeks apart) or one injection of saline. A significant difference was observed between PRP and saline solution in terms of clinical outcome. Interestingly, no difference was reported between patients who received one or two PRP injections, but the study was not designed to support this conclusion.
The present study confirms some of the findings emerging from the literature. First of all, it documented that this biological approach is not capable of determining substantial benefit in the category of patients affected by severe OA. These patients are candidates for more invasive approaches, such as metal resurfacing, and the most recent clinical trials on PRP injections considered end-stage OA as an exclusion criterion. In our series, 10 knees affected by severe OA were included because the patients refused surgical treatment. In these cases, PRP constituted a “salvage procedure”, performed in an attempt to provide temporary benefit and delay the prosthetic solution. Basically, the PRP was used as a “biological modulator” with the aim of providing a stimulus to normalize the articular environment, without any intention of promoting tissue regeneration in joints characterized by advanced degenerative changes and low cellular vitality and responsiveness to GFs. Although these patients’ overall satisfaction was lower than that of patients with less degeneration, a modest beneficial effect was obtained and their quality of life was found to be marginally improved. Results were, instead, clearly superior in the group of patients affected by early/moderate OA, as also shown by other authors (30). The mean duration of the effect of PRP therapy was about 9 months in our study, confirming the value recorded in a previous trial (17). Furthermore, we demonstrated the role of BMI in determining the clinical outcome: a higher BMI correlates with poorer results and is also associated with a trend towards a shorter effect duration, thus suggesting that mechanical overload could impair the beneficial effect of this treatment. Another interesting finding was that longer pain duration before treatment led to lower scores on general health status evaluation.
Given the good clinical outcome of our patients with early/moderate OA, leukocyte-poor low-concentrate PRP emerges as a valid therapeutic option and thus the debate about cell content in various PRP formulations remains open: the present trial lacks the strength necessary to answer this question but this is an aspect that should be properly investigated in future studies. Previous studies in animal models revealed that different platelet concentrations could have an influence on outcomes, even affecting histological features of the osteochondral unit (15, 33). Therefore, the need to define the best PRP formulation is strictly linked to the search for the optimal clinical response.
This study, despite its limitations (the small number of patients included and the lack of a control group), confirmed some of the most important findings of other trials and also showed that this particular PRP formulation, characterized by a reduced leukocyte count and low platelet concentration, is effective in early/moderate knee degenerative pathology and may provide short-term clinical benefit.
In conclusion, leukocyte-poor low-concentrate PRP injections are a safe conservative approach in patients affected by early/moderate OA, capable of reducing pain and improving knee functional status as shown by encouraging results at short-term evaluation. BMI negatively affects clinical outcome and duration of symptomatic relief. Conversely, this biological treatment used as a “salvage procedure” in severe OA knees produced a less favorable outcome and therefore presents a limited indication even for patients refusing more invasive solutions.
References
- 1.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 3.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–182. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Gomoll AH, Filardo G, de Girolamo L, Esprequeira-Mendes J, Marcacci M, Rodkey WG, Steadman RJ, Zaffagnini S, Kon E. Surgical treatment for early osteoarthritis. Part I: cartilage repair procedures. Knee Surg Sports Traumatol Arthrosc. 2012;20:450–466. doi: 10.1007/s00167-011-1780-x. [DOI] [PubMed] [Google Scholar]
- 5.Kon E, Filardo G, Drobnic M, Madry H, Jelic M, van Dijk N, Della Villa S. Non-surgical management of early knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20:436–449. doi: 10.1007/s00167-011-1713-8. [DOI] [PubMed] [Google Scholar]
- 6.Mishra A, Woodall J, Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28:113–125. doi: 10.1016/j.csm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 8.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 9.Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28:429–439. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 10.DohanEhrenfest DM, Rasmuson L, Albrektsson T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Arnoczky S, Delos D, Rodeo S. What is platelet-rich plasma? Oper Tech Sports Med. 2011;19:142–148. [Google Scholar]
- 12.Tschon M, Fini M, Giardino R, Filardo G, Dallari D, Torricelli P, Martini L, Giavaresi G, Kon E, Maltarello MC, Nicolini A, Carpi A. Lights and shadows concerning platelet products for musculoskeletal regeneration. Front Biosci (Elite Ed) 2011;3:96–107. doi: 10.2741/e224. [DOI] [PubMed] [Google Scholar]
- 13.Kon E, Buda R, Filardo G, Di Martino A, Timoncini A, Cenacchi A, Fornasari PM, Giannini S, Marcacci M. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010;18:472–479. doi: 10.1007/s00167-009-0940-8. [DOI] [PubMed] [Google Scholar]
- 14.Wasterlain A, Braun HJ, Dragoo JL. Contents and formulations of platelet-rich plasma. Oper Tech Orthop. 2012;22:33–42. [Google Scholar]
- 15.Kon E, Filardo G, Delcogliano M, Fini M, Salamanna F, Giavaresi G, Martin I, Marcacci M. Platelet autologous growth factors decrease the osteochondral regeneration capability of a collagen-hydroxyapatite scaffold in a sheep model. BMC Musculoskelet Disord. 2010;27;11:220. doi: 10.1186/1471-2474-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, Timoncini A, Fornasari PM, Giannini S, Marcacci M. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27:1490–1501. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Filardo G, Kon E, Buda R, Timoncini A, Di Martino A, Cenacchi A, Fornasari PM, Giannini S, Marcacci M. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19:528–535. doi: 10.1007/s00167-010-1238-6. [DOI] [PubMed] [Google Scholar]
- 18.Filardo G, Kon E, Di Martino A, Di Matteo B, Merli ML, Cenacchi A, Fornasari PM, Marcacci M. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229. doi: 10.1186/1471-2474-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filardo G, Kon E, Pereira Ruiz MT, Vaccaro F, Guitaldi R, Di Martino A, Cenacchi A, Fornasari PM, Marcacci M. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single-versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20:2082–2091. doi: 10.1007/s00167-011-1837-x. [DOI] [PubMed] [Google Scholar]
- 20.Filardo G, Kon E. PRP: more words than facts... Knee Surg Sports Traumatol Arthrosc. 2012;20:1655–1656. doi: 10.1007/s00167-012-2136-x. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol. 2008;26:910–913. [PubMed] [Google Scholar]
- 22.Sampson S, Reed M, Silvers H, Meng M, Mandelbaum B. Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study. Am J Phys Med Rehabil. 2010;89:961–969. doi: 10.1097/PHM.0b013e3181fc7edf. [DOI] [PubMed] [Google Scholar]
- 23.Wang-Saegusa A, Cugat R, Ares O, Seijas R, Cuscó X, Garcia-Balletbó M. Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Arch Orthop Trauma Surg. 2011;131:311–317. doi: 10.1007/s00402-010-1167-3. [DOI] [PubMed] [Google Scholar]
- 24.Napolitano M, Matera S, Bossio M, Crescibene A, Costabile E, Almolla J, Almolla H, Togo F, Giannuzzi C, Guido G. Autologous platelet gel for tissue regeneration in degenerative disorders of the knee. Blood Transfus. 2012;10:72–77. doi: 10.2450/2011.0026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torrero JI, Aroles F, Ferrer D. Treatment of knee chondropathy with platelet rich plasma. Preliminary results at 6 months of follow-up with only one injection. J Biol Regul Homeost Agents. 2012;26( 2 Suppl 1):71S–78S. [PubMed] [Google Scholar]
- 26.Jang SJ, Kim JD, Cha SS. Platelet-rich plasma (PRP) injections as an effective treatment for early osteoarthritis. Eur J Orthop Surg Traumatol. 2012;23:573–580. doi: 10.1007/s00590-012-1037-5. [DOI] [PubMed] [Google Scholar]
- 27.Gobbi A, Karnatzikos G, Mahajan V, Malchira S. Platelet-rich plasma treatment in symptomatic patients with knee osteoarthritis: preliminary results in a group of active patients. Sports Health. 2012;4:162–172. doi: 10.1177/1941738111431801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freitag JB, Barnard A. To evaluate the effect of combining photo-activation therapy with platelet-rich plasma injections for the novel treatment of osteoarthritis. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-007463. pii: bcr2012007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halpern B, Chaudhury S, Rodeo SA, Hayter C, Bogner E, Potter HG, Nguyen J. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013;23:238–239. doi: 10.1097/JSM.0b013e31827c3846. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez M, Fiz N, Azofra J, Usabiaga J, Aduriz Recalde E, Garcia Gutierrez A, Albillos J, Gárate R, Aguirre JJ, Padilla S, Orive G, Anitua E. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28:1070–1078. doi: 10.1016/j.arthro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Cerza F, Carnì S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, De Biasi G, Ciuffreda M. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 32.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 33.Torricelli P, Fini M, Filardo G, Tschon M, Pischedda M, Pacorini A, Kon E, Giardino R. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35:1569–1576. doi: 10.1007/s00264-011-1237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]