Abstract
Skeletal muscle injuries are common causes of severe long-term pain and physical disability, accounting for up to 55% of all sports injuries. The phases of the healing process after direct or indirect muscle injury are complex but clearly defined processes comprising well-coordinated steps: degeneration, inflammation, regeneration, and fibrosis.
Despite this frequent occurrence and the presence of a body of data on the pathophysiology of muscle injuries, none of the treatment strategies adopted to date have been shown to be really effective in strictly controlled trials. Most current muscle injury treatments are based on limited experimental and clinical data and/or were only empirically tested.
Platelet-rich plasma (PRP) is a promising alternative approach based on the ability of autologous growth factors (GFs) to accelerate tissue healing, improve muscular regeneration, increase neovascularization and reduce fibrosis, allowing rapid recovery after muscle lesions.
Thus, further experimental studies that include the quantification of specific GFs released by PRP, as well as additional data on angiogenesis, myogenesis and functional recovery are needed to ultimately validate the hypothesis of PRP efficacy in the treatment of muscle lesions and open the way for its wide clinical application.
Keywords: muscle injuries, growth factors, platelet-rich plasma, skeletal muscle
Introduction
Skeletal muscle injuries are common causes of severe long-term pain and physical disability, accounting for up to 55% of all sports injuries. Contusions and strains are the most frequent muscle lesions.
Skeletal muscle injuries account for 31% of all injuries in élite football (soccer), and their high prevalence in both football and other sports is well documented in the international literature. 92% of lesions occurring in football (soccer) affect the four major muscle groups of the lower limbs: hamstrings 37%, adductors 23%, quadriceps 19% and calf muscles 13%. As many as 96% of all muscle injuries in football (soccer) occur in non-contact situations, whereas contusions are more frequently encountered in contact sports, such as rugby, American football and ice hockey (1, 2) (Fig. 1). 16% of muscle injuries in élite football (soccer) are re-injuries and they are associated with a longer absence from competition than was necessary following the original injury.
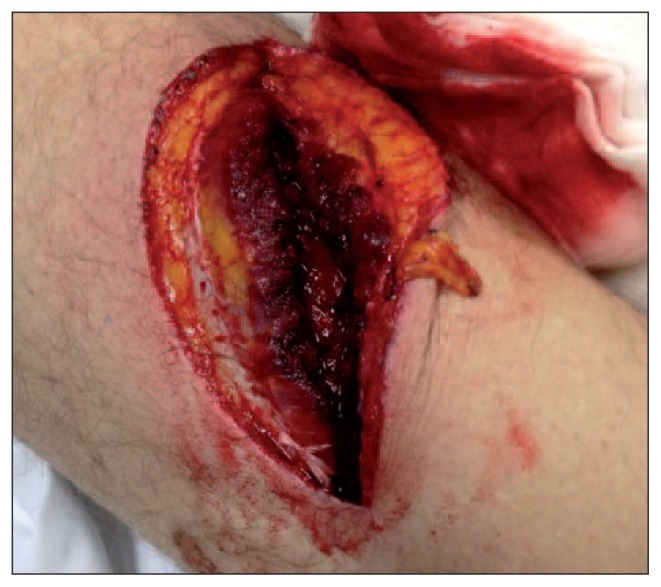
Fig. 1.
Male, 22 years old. An example of direct muscle injury caused by a sharp knife trauma (complete laceration of the muscle fibers of the right quadriceps).
Thigh muscle injuries often occur in track and field athletes (16%), but have also been documented in team sports like rugby (10.4%), basketball (17.7%) and American football (46%/22% practice/games) (3). In sport, the aim of muscle lesion treatment is to allow the athlete to resume training and competing as soon as possible, without any complications. Prognostic information is crucial to medical staff required (by coaches, managers, media, agents and players themselves) to indicate the length of an athlete’s expected lay off (4).
Muscle injury is a challenging problem in traumatology, as injured muscles heal very slowly and often with incomplete functional recovery; hence the critical importance of correct evaluation, diagnosis and therapy of these disorders.
Muscle injuries
The variety of criteria that can be taken into consideration makes it difficult to develop a single classification of muscle injuries. The severity of muscle injury is defined by the amount of muscle tissue involved and by the extent and the location of the effusion.
Different classification systems are published in the literature (5–12) (Tab. 1), but there is little consistency between studies and in daily practice (13).
Table 1.
Overview of muscle injury classification systems.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| O’Donoghue 1962 (5) | No appreciable tissue tearing No loss of function or strength Only a low-grade inflammatory response |
Tissue damage Strength of the musculotendinous unit reduced Some residual function |
Complete tear of musculotendinous unit Complete loss of function |
- |
| Ryan 1969 (6) (initially for quadriceps) | Tear of a few muscle fibers Fascia remaining intact |
Tear of a moderate number of fibers Fascia remaining intact |
Tear of many fibers Partial tearing of the fascia |
Complete tear of the muscle and fascia of the musculotendinous unit |
| Craig 1973 (7) | Tissue damage Strength of the musculotendinous unit |
No complete tear of musculotendinous unit | Complete tear of musculotendinous unit Complete loss of function |
- |
| Kouvalchouk 1992 (8) | Contracture | Elongation | Breakdown or tearing: grade I-IV From strength of the musculotendinous unit to complete tear of the musculotendinous unit |
- |
| Reid 1992 (9) | Minimal structural damage Small hemorrhage Rapid recovery |
Tissue damage Some residual function |
Complete tear of musculotendinous unit Complete loss of function. Hematoma aspiration helpful |
- |
|
Takebayashi 1995 (10) Peetrons 2002 (11) (Ultrasound-based) |
No abnormalities or diffuse bleeding with/without focal fiber rupture of less than 5% of the muscle involved | MRI-negative=0% structural damage. Hyperintense edema with or without hemorrhage |
Complete muscle rupture with retraction Fascial injury |
- |
| Stoller 2007 (12) (MRI-based) | Partial rupture: focal fiber rupture of more than 5% of the muscle involved with/without fascial injury | MRI-positive with tearing up to 50% of the muscle fibers Possible hyperintense focal defect and partial retraction of muscle fibers |
Muscle rupture=100% structural damage Complete tearing with/without muscle retraction |
- |
In imaging, the radiological classification system of muscle injuries introduced by Peetrons (11) is frequently used; Ekstrand et al. (4) recently showed that MRI can be helpful in verifying the diagnosis of hamstring injuries and that radiological grading is associated with lay-off times after injury.
Recently, the “Munich muscle injury classification system” was introduced providing terminology and a new classification system of muscle injuries (3). This clinical classification (Tab. 2) classifies muscle injuries into functional and structural types. Functional disorders are fatigue-induced or neurogenic injuries causing muscle dysfunction, while structural injuries consist of muscle fiber tears (3).
Table 2.
Classification of acute muscle disorders and injuries according to Mueller-Wohlfahrt et al. (3).
| A. Indirect muscle disorder/injury | Functional muscle disorder | Type 1: Overexertion-related muscle disorder |
Type 1A: Fatigue-induced muscle disorder Type 1B: Delayed-onset muscle soreness (DOMS) |
| Type 2: Neuromuscular muscle disorder |
Type 2A: Spine-related neuromuscular Muscle disorder Type 2B: Muscle-related neuromuscular Muscle disorder |
||
| B. Direct muscle injury | Structural muscle injury | Type 3: Partial muscle tear |
Type 3A: Minor partial muscle tear Type 3B: Moderate partial muscle tear |
| Type 4: (Sub)total tear Contusion Laceration | Subtotal or complete muscle tear Tendinous avulsion |
Jarvinen et al. (14) and Askling et al. (15) recommended that treatment strategy should start with a precise history of the injury, investigating the circumstances of its occurrence, the symptoms, and any previous problems, followed by a careful clinical examination with inspection, palpation of the injured area, comparison with the contralateral side and muscle function testing. Palpation serves to detect (more superficial and larger) tears, perimuscular edema and increased muscle tone. An early post-injury ultrasound (US) between 2 and 48 hours after the muscle trauma can provide helpful information about any existing muscle structure problem, particularly in the presence of hematoma or when the clinical examination indicates a functional disorder without evidence of structural damage (11). MRI is recommended for every situation in which structural muscle injury is suspected. MRI is helpful in determining the possible presence and pattern of edema and also in detecting a structural lesion and estimating its size. Furthermore, MRI is helpful in confirming the site of injury and the presence of any tendon involvement (15). However, it must be pointed out that MRI alone is not sensitive enough to measure the extent of muscle tissue damage accurately. For example, it is not possible to identify on MRI scans areas where edema/ hemorrhage (seen as a high signal) is obscuring structurally intact muscle tissue (3).
Despite the frequent occurrence of skeletal muscle injuries and the availability of a body of data on their pathophysiology, none of the treatment strategies adopted to date have been shown to be really effective in strictly controlled trials. One possible explanation for this apparently paradoxical situation is the broad heterogeneity of injuries of this type and their widely varying severity. In addition, most current muscle injury treatments are based on limited experimental data and/or were only empirically tested.
The aims of correct treatment of muscle injuries are: 1) to limit the consequences of the damage on the tissues involved in the trauma, 2) to prevent future damage, and 3) to ensure the athlete’s prompt return to competitive activity while nevertheless respecting the necessary biological healing times.
These three points are closely related to each other and dependent on the treatment carried out in the initial phase (24–48 hours).
For less severe muscle injuries, non-surgical treatment usually results in good functional outcomes. As a rule, the treatment protocol comprises simply RICE (i.e. Rest, Ice, Compression and Elevation), non-steroidal anti-inflammatory drugs (NSAIDs), early mobilization, and physical therapy (active and passive modalities). An alternative to non-surgical treatment of muscle injury is surgical re-approximation of muscle tears. However, in other circumstances, especially in the presence of extensive muscle lesions and in athletes with a high functional demand, these therapeutic approaches are often all unsatisfactory. The phases of the healing process after direct or indirect muscle injury are well defined. The initial degeneration/ necrosis phase is principally characterized by the formation of hematoma. The subsequent inflammatory/cell response phase and the repair/fibrosis phase are interrelated and time-dependent. Local swelling and hematoma formation occur rapidly after injury with clot formation and associated platelet degranulation. The latter leads to local release of growth factors (GFs) and cytokines that determine the chemotactic migration of neutrophils and macrophages.
Subsequently, myogenic precursor cells (also known as satellite cells) undergo activation and proliferation, also facilitated by GFs (16).
These satellite cells, which are located between the basal lamina and the plasma membrane of each individual myofiber, are quiescent in the uninjured state; once activated, however, they proliferate and differentiate into multinucleated myotubes and, eventually, myofibers. Many of these cells are able to fuse with existing necrotic myofibers and may prevent the muscle fibers from completely degenerating. Regenerating cells are centrally nucleated and are easily identifiable histologically. In the majority of cases, this healing process results in the formation of regenerated muscle characterized by an area of fibrotic scar tissue (differing in size depending on the size of the primary lesion) and by incomplete restoration of functional capacity.
Thus, the reparative capacity of muscle lesions varies widely and depends on the severity of the trauma (17). The chance of a complete restitutio ad integrum following a muscle injury is proportional to the extent of the lesion and dependent on the pathophysiological processes that characterize the early post-injury phase (0–72 hours).
Growth factors, platelet-rich plasma and muscle injuries
It is important to emphasize the critical role played by GFs in the process of muscle regeneration and satellite cell activation. Scarring and fibrosis are both obstacles to complete muscle recovery following injury. For this reason, regulation of fibrosis is one of the goals of the use of GFs in the management of muscle lesions.
Platelet-rich plasma (PRP) is an autologous concentration of human platelets to supra-physiologic levels (18). At baseline levels, platelets function as a natural reservoir for GFs including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), transforming growth factor-beta 1 (TGF-β1), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), and insulin-like growth factor type 1 (IGF-1). PRP is commonly used in orthopaedic practice to enhance healing in sports-related skeletal muscle, tendon, and ligament injuries (14, 19).
However, the use of PRP in the treatment of skeletal muscle lesions is based on limited experimental data and no meta-analysis studies or randomized controlled trials have been conducted to allow the safe and effective use of these therapies (19, 20).
Only a few in vivo studies have shown that GFs are able to improve muscle regeneration and increase muscle strength after a trauma. In experimental studies of animal models, it has been shown that IGF-1, bFGF and nerve growth factor (NGF) are potent stimulators of myoblast proliferation and fusion. However, injured muscles need to be treated with high concentrations of GFs, due to the rapid clearance of these molecules and their short half-life.
Hammond et al. (21), in an experimental study investigating the biomechanical and biochemical effects of PRP in muscle injury in rats, showed that PRP can promote and accelerate myogenesis.
In 2012, some of the present authors conducted an experimental study of muscle injury in a rat model, analyzing histologically and immunohistochemically the effects of platelet-rich fibrin matrix (PRFM) in the regeneration of damaged muscle tissue (22). Bilateral lesions were created on the longissimus dorsi muscle of Wistar rats (Fig. 2). In each rat, one lesion was filled with a PRFM while the contralateral lesion was left untreated, as control. Animals were sacrificed at five, 10, 40 and 60 days from surgery. Histological, immunohistochemical and histomorphometric analyses were performed to evaluate muscle regeneration, neovascularization, fibrosis and inflammation (Fig. 3). We also assessed the presence of metaplasia zones, calcifications and heterotopic ossification. The PRFM-treated muscles showed better muscle regeneration and more neovascularization. Immunohistochemical data further strengthened our hypothesis of PRP efficacy in the treatment of muscle lesions: both MyoD and myogenin play a key role during embryonic and neonatal myogenesis and have a crucial regulatory function in the processes of plasticity, adaptation and regeneration in adult muscle. MyoD- and myogenin-positive cells were located both inside the basal lamina of the fiber and in the interstitial spaces in the muscle sacrificed at five days. No staining was detected in 10 day-sacrificed animals, nor in those sacrificed at 40 and 60 days. These findings are therefore consistent with a significant enhancement of early myogenesis and subsequent neovascularization in the presence of PRFM compared to the untreated control condition. The levels of fibrosis and inflammation were similar to those found in the controls; metaplasia, heterotopic calcification and ossification were absent both in PRFM-treated and control lesions, suggesting that there are no side effects related to the use of PRFM in the treatment of muscle injury (22).
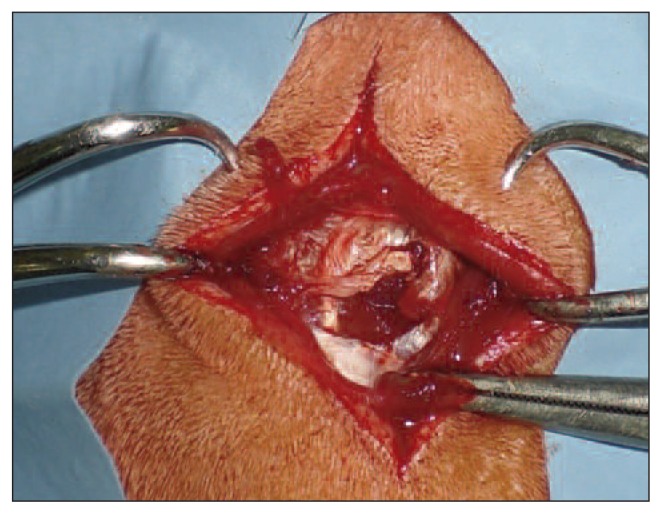
Fig. 2.
Male Wister rat: dorsal incision in the paravertebral region (3 cm in length) and muscle lesion on the longissimus dorsi.
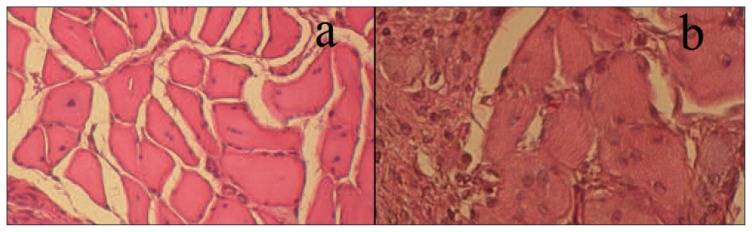
Fig. 3.
Histological sections of a longissimus dorsi muscle lesion treated with PRFM (a) and of an untreated muscle lesion (b) at 10 days after injury. The presence of fibers with central nuclei is suggestive of muscle regeneration in the PRFM-treated lesion (a); these features are less evident in the control lesion (b).
This morphological experimental study showed that the use of PRFM could improve muscle regeneration and long-term vascularization. Since autologous blood products are safe, PRFM may be a useful and convenient product in the clinical treatment of muscle injuries.
With reference to fibrosis, Visser et al. (23) demonstrated that PRFM, in vitro, contains significantly higher concentrations of TGF-β1, which has the capacity to significantly increase connective cell proliferation over time compared with whole blood concentrate of similar volume. However in our in vivo study, we did not observe an increase in fibrotic tissue formation during PRFM treatment in comparison with controls; therefore, we hypothesize that the amount of TGF-β released by PRFM in vivo is not sufficient for this to occur (22).
In 2013, Huard et al. (24) performed an experimental study of PRP and losartan combinatorial therapy, which was found to improve overall skeletal muscle healing after muscle contusion injury by enhancing angiogenesis and follistatin expression and by reducing the expression of phosphorylated Smad 2/3 and the development of fibrosis. These results suggested that blocking the expression of TGF-β1 with losartan improves the effect of PRP therapy on muscle healing after a contusion injury (24).
In an experimental Wistar rat model (data currently unpublished), we used different concentrations of PRP and studied their different effects after a skeletal muscle injury. Unilateral muscle lesions were created on the longissimus dorsi muscle of Wistar rats. The lesion was filled with PRP (injected intramuscularly at different concentrations) 24 hours after the surgical trauma. A group of rats was left untreated (controls). Animals were sacrificed at 3, 15 and 60 days from surgery. Histological, immunohistochemical (M-caderin, CD-34, V-CAM-1, Myo-D and myogenin) and histomorphometric analyses were performed to evaluate muscle regeneration, neovascularization, fibrosis and inflammation. We also assessed the presence of metaplasia, calcifications and heterotopic ossification.
The PRP-treated muscles showed better muscle regeneration, more neovascularization and a slight reduction of fibrosis compared with the control muscles. The preliminary results of this study suggest that myogenesis induced by PRP could be a dose-dependent process.
These experimental results on muscle healing after PRP administration are, however, an incomplete representation of the clinical situation; data on pain and functional recovery are lacking.
In fact, there exist few clinical data on the use of PRP in the treatment of muscle injuries. However, some clinical pilot studies on the use of PRP under US guidance after muscle injury demonstrated the efficacy of this treatment, showing it to be associated with a higher level of pain relief, better physical recovery, less fibrosis and faster regeneration compared with conventional conservative treatment of acute muscle trauma.
Musculoskeletal US and ultrasonic elastography (USE, sonoelastography) are able to provide clinicians with adequate data about muscle injuries. Also, US and USE are the most effective methods for guiding the puncture of soft tissues for hematoma aspiration or local drug administration (Fig. 4). USE measures tissue deformation as a response to the application of an external force, the assumption being that the deformation will be lower in rigid tissues, compared with elastic, soft tissues.
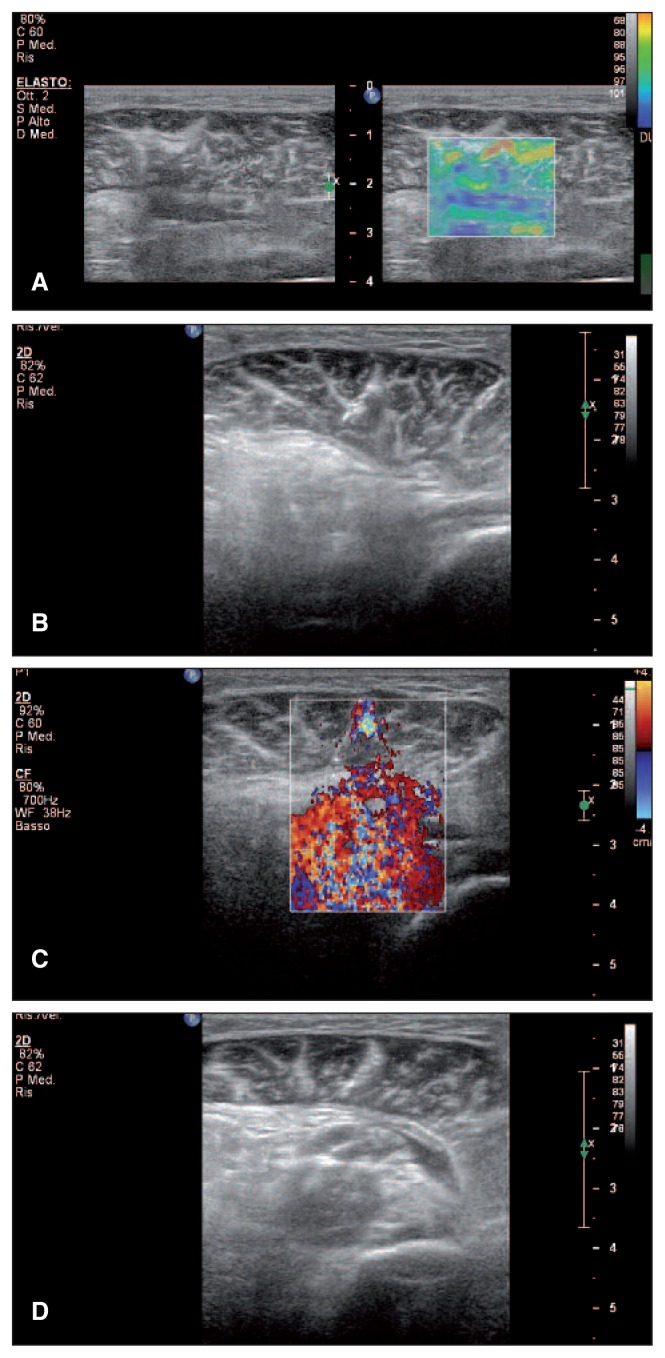
Fig. 4.
Male, 41 years old. Post-trauma hematoma of the left gastrocnemius. A: Ultrasonic elastography of the muscle injury. B: Hematoma aspiration under ultrasound guidance, longitudinal scan. C: Sonoelastography of PRP administration in the region of interest. D: Sonoelastography after PRP administration in the region of interest.
Evaluation of musculoskeletal pathology in vivo is one of the main applications of USE, which provides information about soft tissue quality and evolution of muscle injury over time, by measuring the stiffness/ elasticity of muscle. This method is based on comparison of the radiofrequency of ultrasonic waves obtained before and after compression, which is easily applied with a conventional transducer, using a freehand technique (25). A transducer can be used to obtain specific information in USE imaging: by exerting low pressure with the transducer in the region of interest (ROI) it is possible to determine a proportional correlation between pressure and deformation. The size of the ROI determined by the examiner must exceed 5 mm all around the explored lesion. In this way, lesions detected on two-dimensional image and also invisible damage can by analyzed by gray scale examination (Fig. 4A,B,D). Tissue elasticity is represented by color coding. Every pixel in the ROI is assigned one of 256 specific colors depending on the amplitude of deformation. The color scale ranges from red (soft components - areas with significant deformation) to blue (rigid elements - areas with low distortion). Green indicates the average deformation of the ROI. This use of these three basic colors is known as RGB encoding (red-green-blue) (25).
Further multicenter studies are needed in order to establish the clinical utility of USE in the development of muscle injury treatment strategies.
Conclusions
A number of questions related to PRP remain unanswered, such as the optimal concentration of platelets in PRP, the ideal PRP formulations, the types of cell that should be present, the ideal frequency of application, and the rehabilitation regime setting best able to optimize tissue repair and return to full function.
Thus, further experimental studies that include the quantification of specific GFs released by PRP, additional details on angiogenesis and myogenesis as well as functional recovery are required to ultimately validate our hypothesis and before PRP can be used in a wide clinical application. It will also be important to improve the accuracy of instrumental investigations, to improve the identification of types and extents of injury and to define new and specific rehabilitation protocols.
References
- 1.Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36:1597–1603. doi: 10.1177/0363546508316021. [DOI] [PubMed] [Google Scholar]
- 2.Lopez V, Jr, Galano GJ, Black CM, et al. Profile of an American amateur rugby union sevens series. Am J Sports Med. 2012;40:179–184. doi: 10.1177/0363546511427124. [DOI] [PubMed] [Google Scholar]
- 3.Mueller-Wohlfahrt HW, Haensel L, Mithoefer K, et al. Terminology and classification of muscle injuries in sport: the Munich consensus statement. Br J Sports Med. 2013;47:342–350. doi: 10.1136/bjsports-2012-091448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekstrand J, Healy JC, Waldén M, et al. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med. 2012;46:112–117. doi: 10.1136/bjsports-2011-090155. [DOI] [PubMed] [Google Scholar]
- 5.O’Donoghue DH. The Treatment of Injuries to Athletes. W.B. Saunders; Philadelphia: 1962. [Google Scholar]
- 6.Ryan AJ. Quadriceps strain, rupture and charlie horse. Med Sci Sports. 1969;1:106–111. [Google Scholar]
- 7.Craig TT. Comments in Sports Medicine. American Medical Association; Chicago: 1973. [Google Scholar]
- 8.Kouvalchouk JF, Durey A. Encycl Med Chir. Editions Tecniques; Paris: 1992. Pathologie traumatique de muscle striè chez le sportif. [Google Scholar]
- 9.Reid DC. Sports injury assessment and rehabilitation. Churchill Livingstone; New York: 1992. [Google Scholar]
- 10.Takebayashi S, Takasawa H, Banzai Y, et al. Sonographic findings in muscle strain injury: clinical and MR imaging correlation. J Ultrasound Med. 1995;14:899–905. doi: 10.7863/jum.1995.14.12.899. [DOI] [PubMed] [Google Scholar]
- 11.Peetrons P. Ultrasound of muscles. Eur Radiol. 2002;12:35–43. doi: 10.1007/s00330-001-1164-6. [DOI] [PubMed] [Google Scholar]
- 12.Stoller DW. MRI in orthopaedics and sports medicine. 3rd edn. Philadelphia: Wolters Kluwer/Lippincott; 2007. [Google Scholar]
- 13.Bryan Dixon J. Gastrocnemius vs. soleus strain: how to differentiate and deal with calf muscle injuries. Curr Rev Musculoskelet Med. 2009;2:74–77. doi: 10.1007/s12178-009-9045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Järvinen TA, Järvinen TL, Kääriäinen M, et al. Muscle injuries: optimising recovery. Best Pract Res Clin Rheumatol. 2007;21:317–331. doi: 10.1016/j.berh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Askling CM, Tengvar M, Saartok T, et al. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35:197–206. doi: 10.1177/0363546506294679. [DOI] [PubMed] [Google Scholar]
- 16.Kasemkijwattana C, Menetrey J, Bosch P, et al. Use of growth factors to improve muscle healing after strain injury. Clin Orthop Relat Res. 2000;370:272–285. doi: 10.1097/00003086-200001000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Dubowitz V. Muscle Biopsy: A Practical Approach. Baillière Tindall; London: 1985. pp. 129–181. [Google Scholar]
- 18.Castillo TN, Pouliot MA, Kim HJ, et al. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266–271. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 19.Menetrey J, Kasemkijwattana C, Day CS, et al. Growth factors improve muscle healing in vivo. J Bone Joint Surg Br. 2000;82:131–137. doi: 10.1302/0301-620x.82b1.8954. [DOI] [PubMed] [Google Scholar]
- 20.Benazzo F, Perticarini L. Use of “tissue bioengineering”: clinical applications on muscles. G.I.O.T. 2010;36:206–210. [Google Scholar]
- 21.Hammond JW, Hinton RY, Curl LA, et al. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am J Sports Med. 2009;37:1135–1142. doi: 10.1177/0363546508330974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gigante A, Del Torto M, Manzotti S, et al. Platelet rich fibrin matrix effects on skeletal muscle lesions: an experimental study. J Biol Regul Homeost Agents. 2012;26:475–484. [PubMed] [Google Scholar]
- 23.Visser LC, Arnoczky SP, Caballero O, et al. Platelet-rich fibrin constructs elute higher concentrations of transforming growth factor-β1 and increase tendon cell proliferation over time when compared to blood clots: a comparative in vitro analysis. Vet Surg. 2010;39:811–817. doi: 10.1111/j.1532-950X.2010.00739.x. [DOI] [PubMed] [Google Scholar]
- 24.Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84:822–832. [PubMed] [Google Scholar]
- 25.Botar Jid C, Vasilescu D, Damian L, et al. Musculoskeletal sonoelastography. Pictorial essay. Medical Ultrason. 2012;14:239–245. [PubMed] [Google Scholar]