Abstract
Background
Clostridium difficile infection (CDI) accounts for 20%-30% of cases of antibiotic-associated diarrhea and is the most commonly recognized cause of infectious diarrhea in healthcare settings. The incidence of CDI is rising, while the effectiveness of antibiotics for treatment decreases with recurrent episodes. The use of fecal microbiota transplantation (FMT) for cure of CDI has been reported since 1958, and the worldwide cure rate is reported to be 93%. We report our experience with FMT for the treatment of CDI.
Methods
We performed a retrospective chart review of patients undergoing FMT for CDI at Ochsner Clinic Foundation from August 2012 to November 2013. FMT was administered via colonoscopy for patients with recurrent or severe CDI. Stool donors were screened for infections in the majority of cases.
Results
FMT was performed in 20 CDI patients. The 16 female and 4 male patients ranged in age from 27 to 89 years (mean 62 years). The average duration of illness from diagnosis to treatment was 49.6 weeks, based on available data. Only 3 donors were unscreened for infectious pathogens. Nine donors were related to the recipients by blood; most of the other donors were spouses. The average length of follow-up after FMT was 3 months. No recurrences of CDI after treatment have been documented. Adverse events reported after treatment included abdominal cramping, bloating, flatulence, and nausea that resolved.
Conclusion
Although the US Food and Drug Administration currently considers FMT an experimental therapy, we demonstrate that FMT is safe, well tolerated, and effective for recurrent and severe CDI.
Keywords: Antibiotics, clostridium difficile, diarrhea, feces, microbiota
INTRODUCTION
Humans are inhabited by a vast array of microorganisms, including bacteria, viruses, fungi, and parasites that live in a state of symbiosis with us.1 The majority of our microbiota are anaerobic, and 2 main phyla (Bacteroidetes and Firmicutes) are predominant in our gut.2 Bacterial counts progressively increase distally in the gastrointestinal tract, with approximately 1 trillion bacteria in the colon. In fact, bacteria account for approximately 60% of the dry weight of feces. The colonization of the intestine accounts for subsequent immune reactions and our ability to mount responses (either appropriately or maladaptively) to various diseases.3
Leading voices in the scientific community, such as Dr Martin Blaser, have proposed that an alteration in the intestinal microbiome is associated with increasing numbers of gastrointestinal diseases.4 Alterations in the number and composition of bacteria in the gut may put the host at risk for colonization and/or proliferation of harmful pathogens or may alter the immune response even to ordinarily benign pathogens.5 Decreased colonic microbial diversity, possibly as a result of antibiotic therapy, appears to be associated with increased recurrence rates of Clostridium difficile infection (CDI), with a 10%-20% recurrence rate after initial antibiotic therapy and a 40%-65% recurrence rate after a second course of antibiotics.6 The concept is known as dysbiosis, and it likely explains why CDI persists in some individuals in spite of appropriate antimicrobial therapy.7 CDI accounts for 20%-30% of cases of antibiotic-associated diarrhea and is the most commonly recognized cause of infectious diarrhea in healthcare settings.1 The incidence of CDI is rising, while the effectiveness of antibiotics for treatment decreases with recurrent episodes.6
Fecal microbiota transplantation (FMT) has been documented in the medical literature since 1958, when 4 patients were given fecal enemas in addition to antimicrobial treatment.8 All 4 patients demonstrated resolution of symptoms within 48 hours. The efficacy of FMT for confirmed recurrent CDI was documented in 1983.9 Studies have described the administration of donor fecal material via physician-administered retention enemas,9 nasogastric duodenal tube,10 colonoscopy,11 and self-administered enemas.12 Worldwide cure rates of CDI with fecal transplantation are around 93%.13 In a 2013 randomized controlled trial, van Nood et al presented the results of a study in which patients were randomized to receive 1 of 3 therapies: an initial vancomycin regimen followed by bowel lavage and subsequent suspension of unrelated donor feces through a nasoduodenal tube, a standard vancomycin regimen, or a standard vancomycin regimen and bowel lavage.14 This trial was terminated early because of the overwhelming efficacy of FMT relative to the other treatment regimens.
Based on the available data, we have identified FMT as a reasonable therapeutic option in the treatment of recurrent CDI. The US Food and Drug Administration initially considered FMT an experimental therapy and required institutional review board approval for usage but later amended this decision and allowed FMT to be performed under enforcement discretion as long as suggested screening guidelines are followed. The following data represent our experience in treating 20 patients with FMT.
METHODS
We retrospectively reviewed the electronic hospital record for patients who were treated with FMT for CDI from August 2012 to November 2013 at the Ochsner Clinic Foundation hospital and outpatient endoscopy center. All procedures were performed by a board-certified gastroenterologist with the occasional assistance of a gastroenterology fellow. The variables recorded included patient demographics, clinical diagnosis, duration of illness, number of antibiotics used to treat CDI prior to FMT, length of follow-up, and any adverse events reported in follow-up.
Patients were identified as candidates for FMT if they had 2 recurrences of CDI, defined by continued diarrhea (>3 loose stools a day) or positive C difficile stool sample after finishing appropriate courses of antibiotics (metronidazole, vancomycin, or fidaxomicin), or if they had life-threatening illness from CDI requiring hospitalization and/or admission to an intensive care unit (ICU). We did not treat patients with positive stool samples if they did not have continued diarrhea. Once a potential candidate for treatment was identified, we discussed identifying a stool donor with the patient.
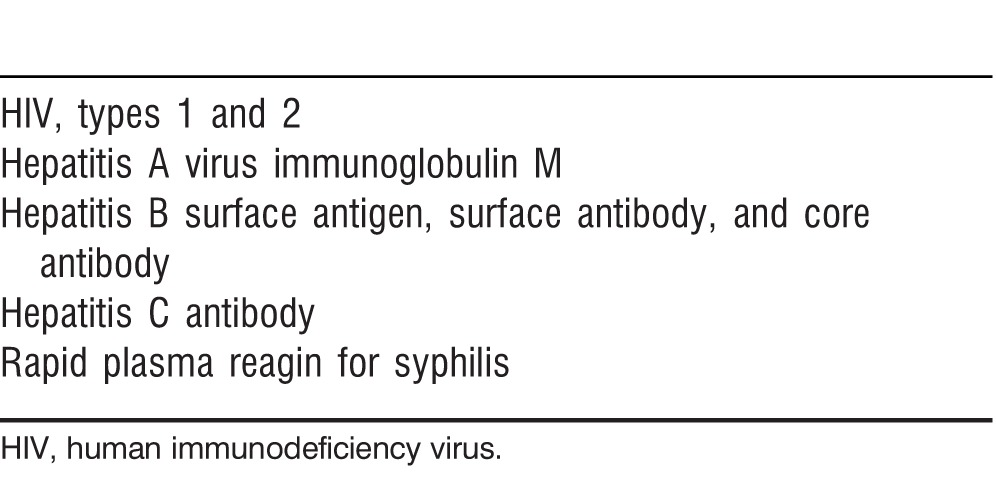
Most stool donors were screened for potential infectious pathogens and completed a questionnaire to determine their risk factors for potential infections as detailed in Table 1. Patients were also screened serologically for hepatitis (A, B, and C), syphilis, and HIV 1 and 2 at baseline to ensure that they were negative prior to FMT (Table 2). Once screening and testing were complete, the patient was scheduled for a colonoscopy with the usual bowel preparation the night before. Two days prior to FMT, all antibiotics were stopped. On the day of the treatment, patients were given the option to take 2 Imodium tablets 2 hours prior to the procedure and 1 tablet afterwards if they felt they needed help retaining the FMT infusion. The patient was also requested to bring a blender to the procedure to process the donor fecal material. The patient signed a consent form acknowledging that FMT is considered an experimental treatment and that unknown and unscreened infectious pathogens may possibly be transmitted via FMT. At the time of the FMT, the donated fecal material was brought into a separate room and blended with sterile water. The resulting slurry was drawn up into 60 cc catheter-tipped syringes. A colonoscopy was performed with anesthesia assistance, and the fecal material was infused through the biopsy port of the colonoscope into either the terminal ileum or cecum. The colonoscope was then withdrawn and random cold forceps biopsies of the colon were obtained to rule out other coexistent etiologies for diarrhea. The patient was awoken from anesthesia, monitored under standard nursing protocols, and discharged or returned to his or her hospital room. The blender was discarded in a biohazard bag.
Table 1.
Donor Screening and Testing
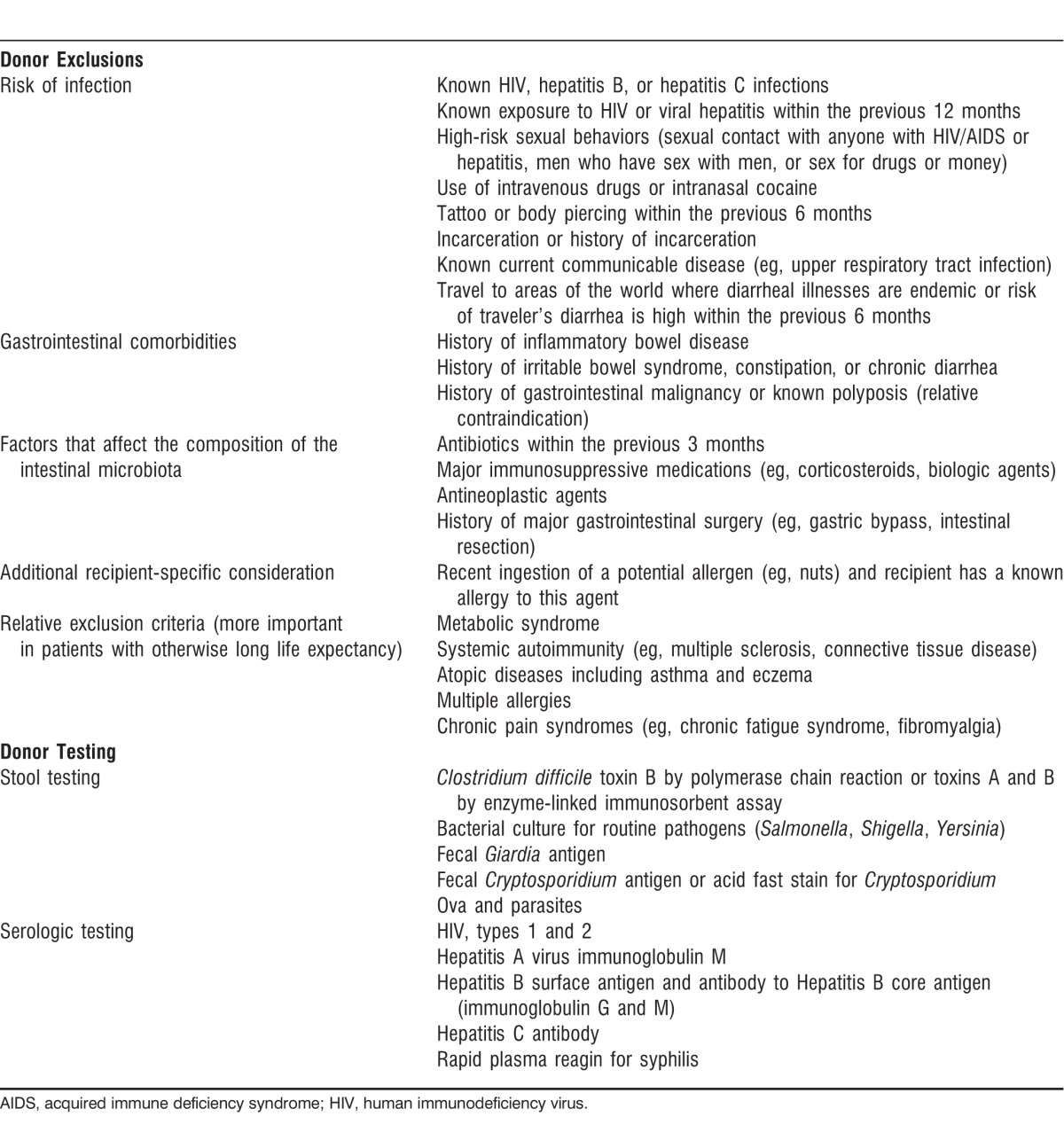
Table 2.
Patient Serologic Testing
RESULTS
Demographics
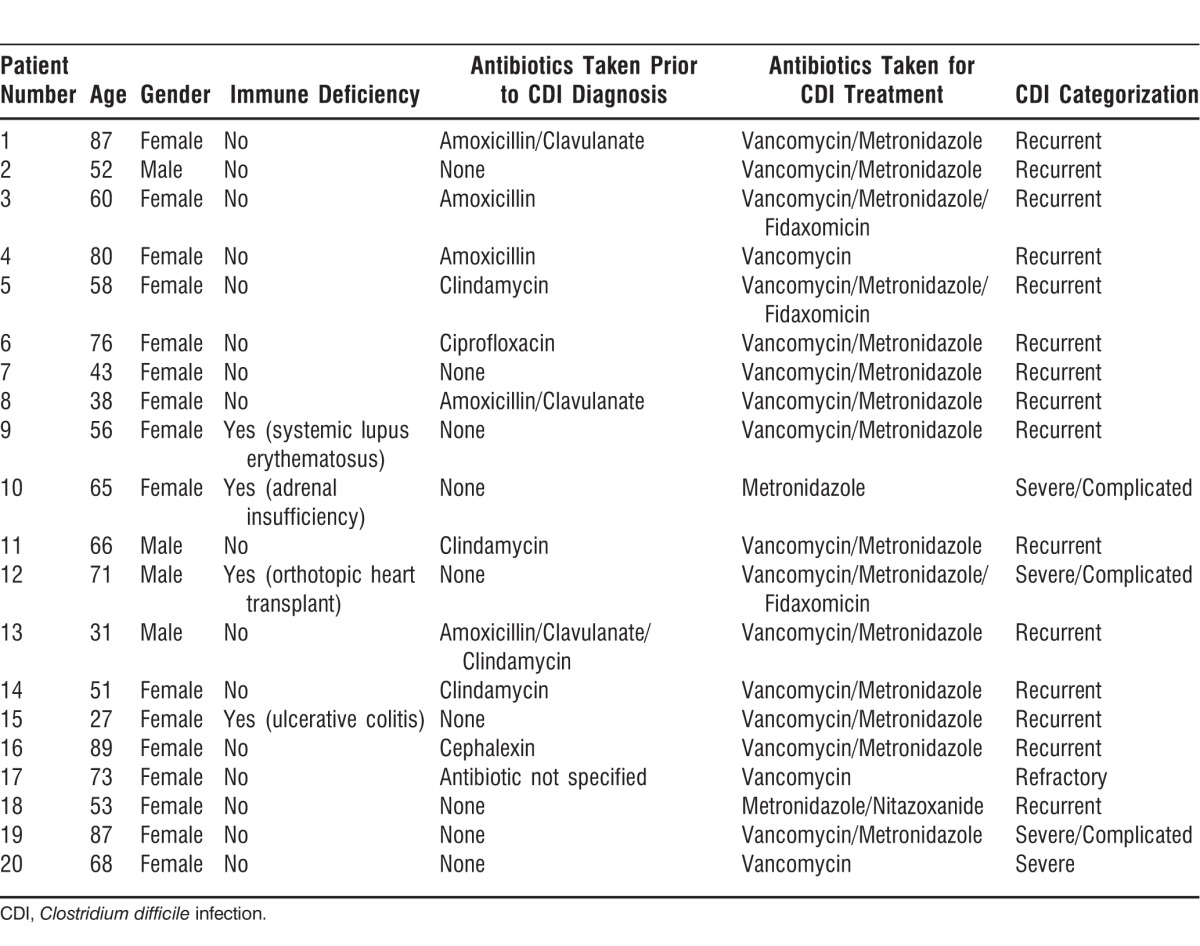
Of the 20 patients included in this study, 90% were white (n=18), 1 was Hispanic, and 1 was African American, with an average age of 62 years (range, 27-89) for the group. All patients had documented positive CDI prior to FMT. Sixteen women (80%) and 4 men (20%) participated. Sixteen patients were not considered immunosuppressed (80%). Of the 4 patients (20%) who were considered immunosuppressed, 1 had inflammatory bowel disease on infliximab, 1 had had a heart transplant, 1 had lupus, and 1 had adrenal insufficiency requiring chronic corticosteroid use. Fifteen patients (75%) underwent FMT in the outpatient setting, and 5 patients (25%) underwent the procedure as inpatients. Eleven (55%) of the 20 patients surveyed had a history of antibiotic use prior to their first episode of CDI. All the patients received antibiotic treatment for CDI prior to FMT. Table 3 lists demographic information for each patient, including their antibiotic regimens.
Table 3.
Patient Demographics
Donor Status
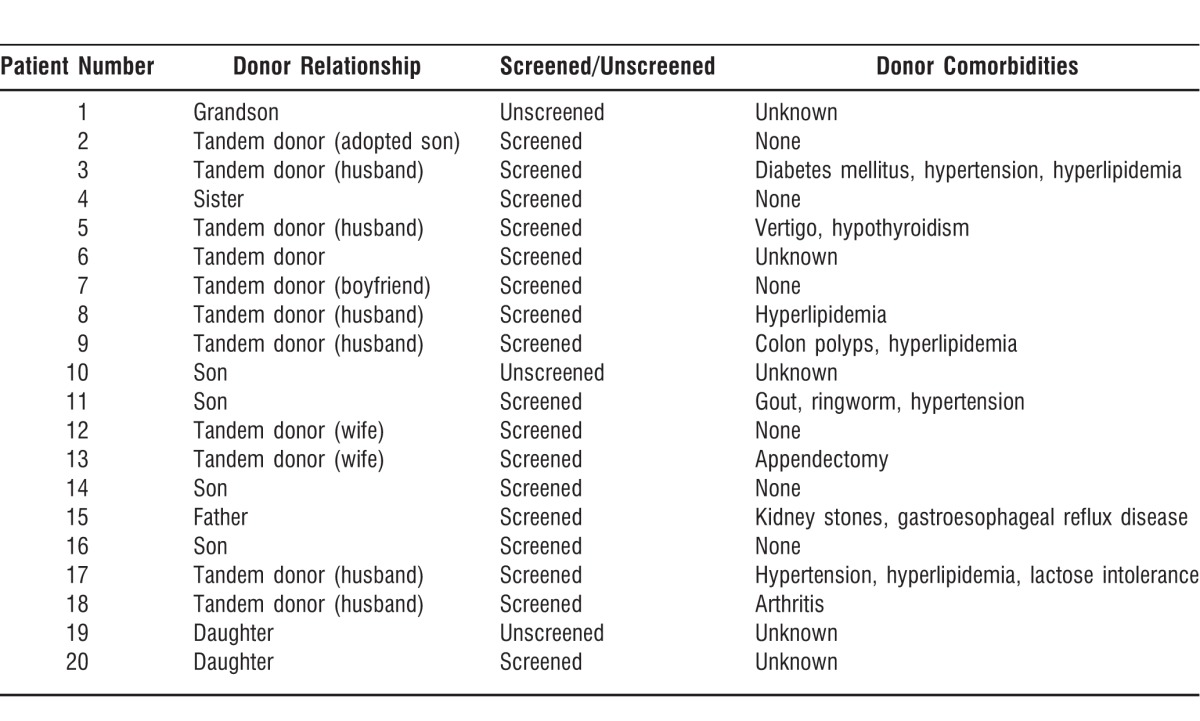
Only 3 donors were unscreened. All 3 of the unscreened donors were relatives who had no comorbid conditions or gastrointestinal complaints. One patient exempted her donor; the other 2 donors were unscreened because the patients were critically ill, and waiting for the screening results could have been potentially life threatening.
Nine donors were related to the recipients by blood, and the other donors were tandem donors: spouses, a partner, an adopted child, and an unrelated donor. Comorbid conditions present in the donor population are listed in Table 4.
Table 4.
Donor Information
Duration of Illness and Efficacy of Treatment
The average length of time from initial diagnosis and problems with CDI to FMT was 49.6 weeks (2-192 weeks) based on available data (Table 5). The average number of stools positive for C difficile per patient in the time period prior to FMT was 3.15 (1-8 positive stools). All patients surveyed received only 1 round of treatment with FMT, and stools positive for CDI were documented in this group during follow-up.
Table 5.
Patient Outcome Information
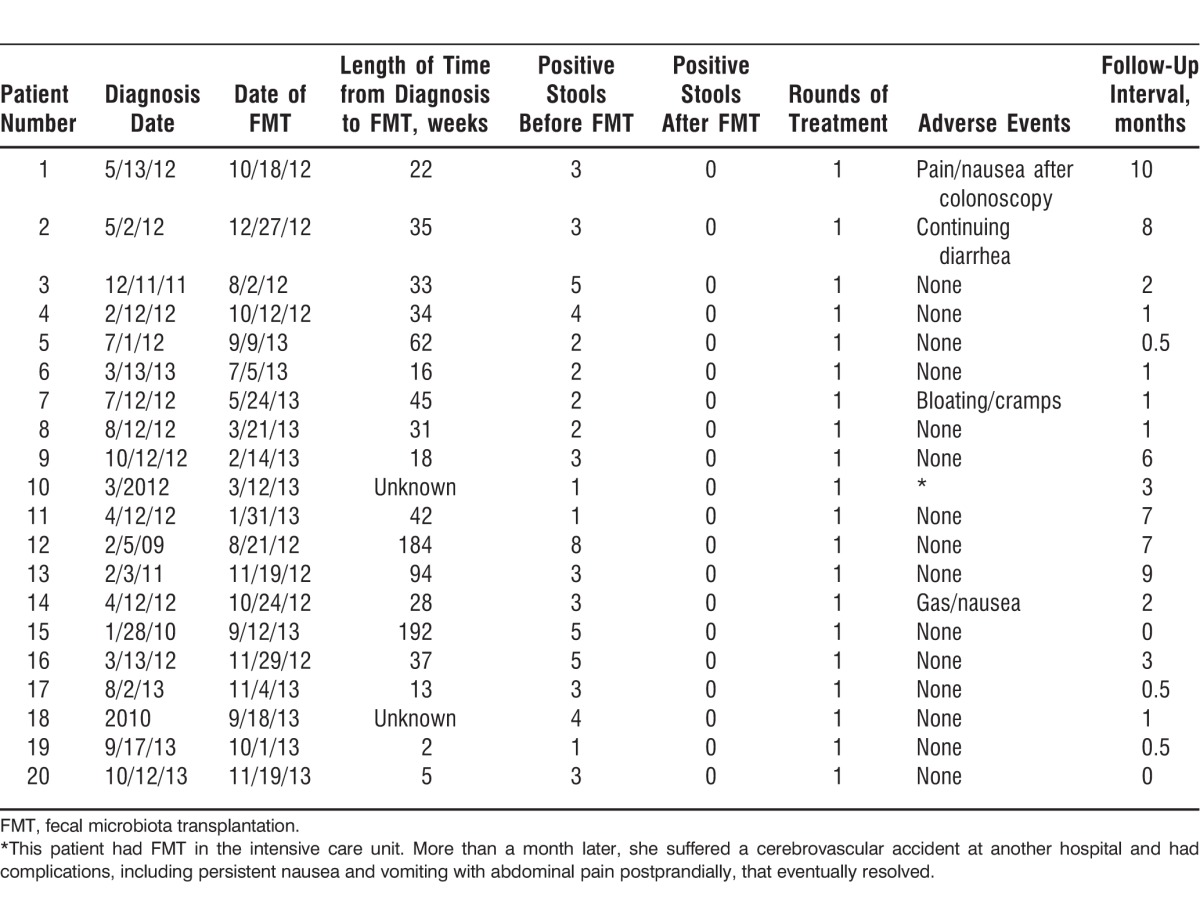
The average length of follow-up after FMT was 3.175 months (0-10 months). Five patients reported adverse events/complaints after FMT. Patient 1 described abdominal cramps and nausea after the colonoscopy but had substantial improvement in diarrhea frequency with only 1-2 semisolid consistency stools per day 6 months after FMT. These initial symptoms resolved fairly quickly after colonoscopy. Patient 2 did not have resolution of his diarrhea after the FMT procedure but remained negative for C difficile during the 8 months of follow-up. The patient reported having 60%-70% fewer loose stools per day. Patient 2 had had a cholecystectomy prior to having diarrheal symptoms, so the etiology of his diarrhea is somewhat unclear. Patient 7 stated that her diarrhea improved after the procedure but that she continued to experience bloating and cramps daily, consistent with her preexistent inflammatory bowel disease symptoms. The patient stated that the symptoms did not worsen after the procedure. Patient 14 experienced an increase in flatulence and nausea for a few weeks after the procedure but noted improvement in symptoms of diarrhea. Patient 10 had FMT performed urgently while in the ICU for severe CDI. She was initially much improved after FMT and discharged from the ICU but suffered a stroke more than 1 month later. She subsequently experienced significant weight loss, nausea, vomiting, and diffuse abdominal pains. Because this patient had multiple comorbid conditions, the etiology of her symptoms was not directly evident but seemed to be more related to her other underlying disease processes than to FMT. One patient had a minor mucosal tear during the colonoscopy that was successfully clipped without any complication.
DISCUSSION
As evidenced by the data represented above, our FMT program has had remarkable success in curing patients of their CDI symptoms. The vast majority of patients undergoing the procedure had almost immediate improvement of their presenting symptoms, primarily consisting of diarrhea, abdominal pain, and laboratory abnormalities such as leukocytosis. Additionally, the effect seems to be durable, with an average postprocedural follow-up period of 3 months. All of our patients required only 1 FMT; no patient has needed more than 1 treatment for durable relief of symptoms. The majority of our patients have not been retested for CDI after transplantation, consistent with recommendations of the Infectious Disease Society of America that state repeat testing is not necessary if symptoms have not recurred after intervention.15 One patient in our study population did not experience complete relief of diarrhea with FMT, but this patient had multiple potential reasons for ongoing increased stool frequency. We retested this patient's stool for CDI, and it was negative.
Our screening program for potential donors is rigorous. We require general health screening, as well as examination for potentially transmissible diseases and underlying gastrointestinal pathology. Thus far, we have had little difficulty identifying donors for transplantation; the majority have been family members. The use of family members is potentially concerning, as they likely have had exposure to a disease known to be communicable. However, given our success rate in eradicating symptoms of infection, using family members as donors does not seem to be a warranted concern at present. Other studies have demonstrated increased patient tolerability of the procedure with the knowledge that a family member would be the donor, making the patient more likely to undergo the procedure. Three of our patients used an unscreened donor, and these patients were apprised of the potential risks prior to undergoing the procedure. We do not recommend this practice; however, 2 patients were critically ill, and we believed that identifying a family member or close associate donor and screening him or her would have created an avoidable delay in care. Neither patient has subsequently developed a transmissible infection or other process.
The overall tolerability of the procedure seems to be good, with the majority of patients demonstrating no adverse events related to the procedure. A reported postprocedural symptom was abdominal cramping that was self-limited. A serious adverse event, a cerebrovascular accident, was noted in 1 patient, but we do not believe that the event is directly attributable to the FMT procedure because it has not been reported in the medical literature to date and did not occur in the immediate postprocedural period. The patient who experienced the cerebrovascular accident had a known history of renal insufficiency requiring hemodialysis, which is a more likely risk factor for vascular disease. Still, while patients are counseled regarding the potential risks of FMT, our knowledge of the associated consequences is evolving.
FMT appears to be effective in high-risk populations, including patients with severe or complicated disease, immunosuppressed patients, and elderly patients. As mentioned previously, our cure rate so far is 100% in terms of eradication of symptoms of CDI, and the aforementioned patient populations are represented in that number.
The efficacy of manipulating a patient's gastrointestinal microbiota in the treatment of disease raises the question of whether this particular therapy would be beneficial in other circumstances. Because chronic inflammatory processes such as CDI originate in part from gut dysbiosis, restoring symbiosis may be beneficial. Metronidazole and vancomycin are recommended as initial therapy for CDI, depending upon disease severity.15 However, recurrent episodes are less responsive to antimicrobial therapy, and one wonders whether it would be advantageous to manipulate a patient's microbiota earlier in the course of the disease. Currently, we are only using FMT for cases of recurrent CDI that have failed multiple antibiotic regimens, but it may be prudent to expand the use of FMT to even first recurrences, potentially obviating further antibiotic therapy. Additionally, it is conceivable that other disease entities, such as ulcerative colitis, may result from a similar dysbiosis; indeed, case reports have documented the efficacy of FMT in this disease state.16 This treatment modality opens the door to exciting potential areas of study. We are currently participating in research trials to assess the safety and efficacy of standardized stool banks, and eventually we hope to be able to offer a fecal pill.
CONCLUSION
Based on our experience, FMT represents an effective therapeutic option for the treatment of recurrent CDI. It is generally safe and well tolerated by most patients. It has applicability for patients with mild to complicated disease and appears to be useful even in high-risk populations.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012 Jun 13;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005 Jun 10;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huurre A, Kalliomäki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of delivery–effects on gut microbiota and humoral immunity. Neonatology. 2008;93(4):236–240. doi: 10.1159/000111102. [DOI] [PubMed] [Google Scholar]
- 4.Blaser MJ. Missing Microbes: How The Overuse of Antibiotics Is Fueling Our Modern Plagues. New York, NY: Henry Holt and Company;; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004 Jan;5(1):104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 6.McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999 Jan;20(1):43–50. doi: 10.1086/501553. [DOI] [PubMed] [Google Scholar]
- 7.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8(10):e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958 Nov;44(5):854–859. [PubMed] [Google Scholar]
- 9.Schwan A, Sjölin S, Trottestam U, Aronsson B. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983 Oct 8;2(8354):845. doi: 10.1016/s0140-6736(83)90753-5. [DOI] [PubMed] [Google Scholar]
- 10.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003 Mar 1;36(5):580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 11.Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol. 2000 Nov;95(11):3283–3285. doi: 10.1111/j.1572-0241.2000.03302.x. [DOI] [PubMed] [Google Scholar]
- 12.Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010 May;8(5):471–473. doi: 10.1016/j.cgh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridum difficile infection. Clin Infect Dis. 2011 Nov;53(10):994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 14.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013 Jan 31;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010 May;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 16.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003 Jul;37(1):42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]


