Abstract
Background
The safety and efficacy of endovascular therapies for ascending aortic pseudoaneurysms (AAPs) are still controversial.
Methods
We report an endovascular correction of an AAP in a high-risk surgical patient and present the results of a literature review focusing on AAP treatment strategies. A multilingual search of AAP therapy was performed with limiting dates of January 1980 to May 2014. The studies were classified by intervention.
Results
A 79-year-old male with a 9 × 10 × 7 cm AAP in the anterior mediastinum was considered too high risk for surgery. An endovascular closure with a 12 mm Amplatzer septal occluder device (St. Jude Medical) was performed, and computed tomography angiography at 3-month follow-up exhibited a thrombosed AAP with minimal residual shunt. In our literature search, we identified 355 cases of AAPs, mostly case reports (91.5%) and a few patient series (8.5%). Surgical correction accounted for 73.8% of the cases, 5% of the patients were conservatively treated or considered too critically ill for any intervention, and 21.2% were treated with endovascular techniques. The most commonly reported endovascular techniques were stent grafts (9.8%) and septal occluder devices (9.8%).
Conclusion
Although endovascular closure of AAPs with off-label devices is a reliable option for controlling the expansion and symptoms in high-risk surgical patients, solid data on survival are lacking. Efforts to promote discussion within the heart team to expand the application of endovascular techniques can provide groundbreaking evidence to support the use of endovascular techniques as guideline therapy when facing these complicated cases.
Keywords: Aneurysm–false, aorta, endovascular procedures, septal occluder device
INTRODUCTION
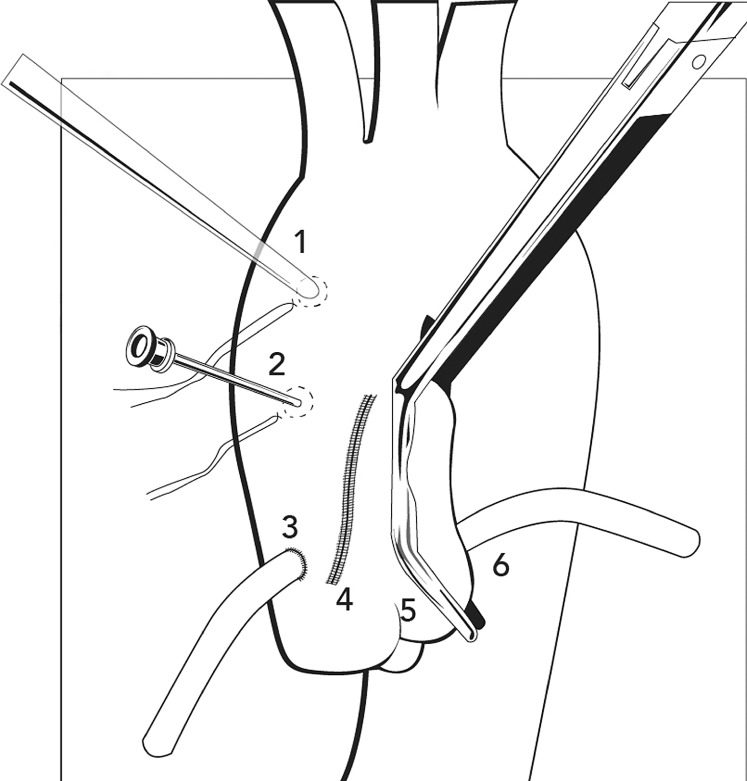
False aneurysms or ascending aortic pseudoaneurysms (AAPs) usually form in patients with a history of cardiac surgery and are associated with anastomosis or cannulation of the vessel, leading to a poor healing process and defects in the layers of the wall.1 Additionally, any traumatic, inflammatory, or infectious event can be associated with the development of an AAP.2 Spontaneous formation of AAPs, where no triggering factors were found, are also reported. The incidence of AAPs has been reported to be as low as 0.5%; however, higher incidence rates (up to 13%) were reported in a surveillance imaging series of patients after cardiac or aortic surgery.3 In up to 60% of aortic surgeries, AAPs occur at the level of the suture line after surgery (Figure 1).1,4,5 Aortic root procedures, such as the Bentall, are the most commonly associated procedures, accounting for 55% of cases.4-7 We present the case of a patient whose AAP, located in the anterior mediastinum compartment, was successfully treated via endovascular procedure. His age, history of repeat coronary artery bypass surgery, and multiple comorbidities deemed him to be a high-risk surgical candidate. After the case presentation, we discuss the diversity of approaches used to correct AAPs.
Figure 1.
Diagram of the locations for development of an ascending aortic pseudoaneurysm postsurgery. 1=Cannulation site, 2=Needle vent site, 3=Vein graft anastomosis, 4=Aortotomy suture line, 5=Clamp site, and 6=Vein graft. (Figure adapted with permission from Razzouk et al.1)
CASE REPORT
A 79-year-old Caucasian male with a history of repeat coronary artery bypass grafting (CABG), systolic dysfunction with a left ventricular ejection fraction (LVEF) of 40%, status post dual-chamber pacemaker implantation secondary to complete heart block, end-stage renal disease on hemodialysis, diabetes mellitus on insulin, hypertension, and a known 7 cm AAP was admitted to the emergency department with crescendo angina. A review of his medical records indicated the patient had had a cardiac evaluation 1 year prior to this hospitalization. During that evaluation, cardiothoracic surgery considered the patient a high-risk candidate for AAP repair and monitored him with conservative therapy. During the patient's current hospitalization, a computed tomography angiography (CTA) of the chest and abdomen revealed an expanding 9 × 10 × 7 cm AAP in proximity to the right coronary artery (RCA) vein graft (Figure 2A). A cardiothoracic surgery evaluation again deemed the patient as high risk, so he was considered for endovascular therapy.
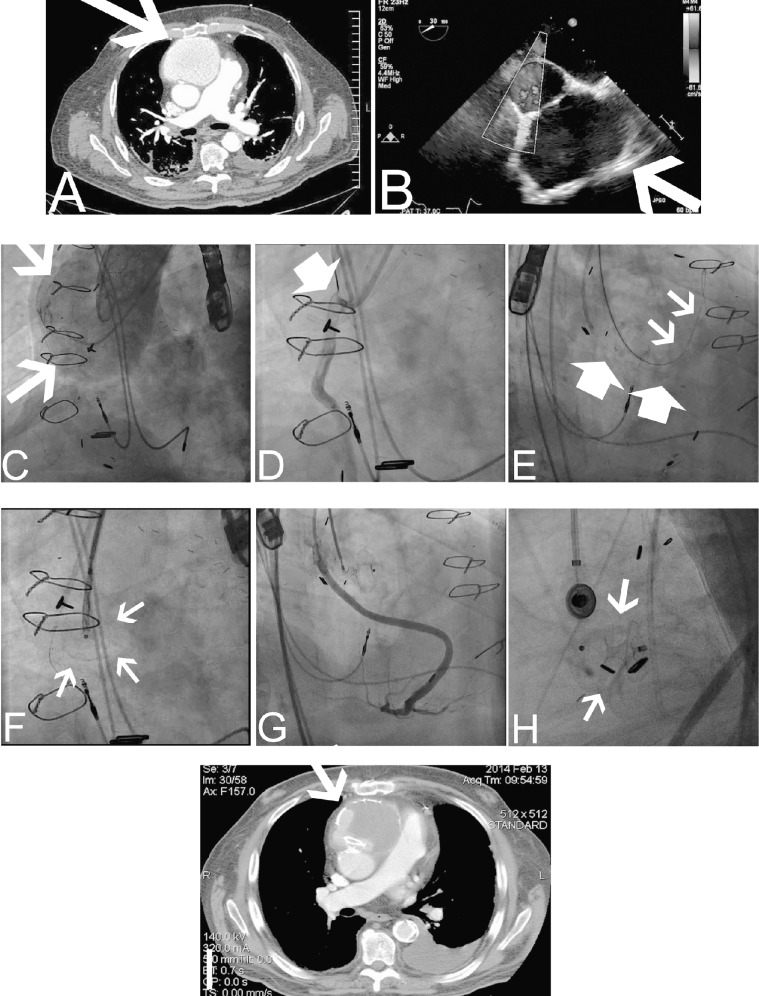
Figure 2.
Endovascular approach to repair an ascending aortic pseudoaneurysm (AAP).
A: Computed tomography angiography (CTA) of the chest exhibits an AAP (arrow) in proximity to the sternum. B: Transesophageal echocardiography (TEE) depicts the shunt between the ascending aorta (AA) and the pseudoaneurysm (arrow). C: Simultaneous contrast injection was performed from a JR4 catheter and pigtail catheter located in the AAP and AA, respectively. Arrows depict the AAP. D: Selective angiography of the venous graft was also performed to assess the distance of the vein graft to the AAP. E: An Amplatz Super Stiff guidewire (small arrows) was advanced via the JR4 catheter into the AAP. A multipurpose catheter was used to engage the nearby vein graft and a 0.014-inch workhorse guidewire was advanced into the graft for protection during the Amplatzer septal occluder device deployment (large arrows). F: The position of the Amplatzer septal occluder delivery system was confirmed in left anterior oblique and right anterior oblique angiographic views and TEE views (arrows). G: Prior to deployment of the device, a vein graft contrast injection was performed to evaluate its patency. H: Final position of the septal occluder device after deployment (arrows). I: Three-month CTA documented stable location of the Amplatzer septal occluder device with evidence of thrombosis in the lumen of the AAP (arrow).
The interventional cardiology team (interventionalist staff and 2 interventional fellows) performed the endovascular procedure with the patient under general anesthesia. A cardiothoracic surgeon was on standby. An iE33 transesophageal echocardiogram (TEE) (Philips Medical Systems) was performed throughout the procedure for assistance (Figure 2B). Arterial accesses were obtained in the left and right groin areas, and preclosing technique with a ProGlide closure device (Abbott Vascular Devices) was employed. A 6-French pigtail diagnostic catheter was advanced to the ascending aorta (AA) for root angiography. A JR4 diagnostic catheter was used to engage the lumen of the AAP. Simultaneous contrast injections through both catheters were then performed (Figure 2C). The pigtail catheter was exchanged over a guidewire for a 6-French multipurpose guide catheter. This catheter was used to engage the vein graft to the RCA. Selective contrast injection exhibited separation of the vein graft from the neck of the AAP (Figure 2D). The vein graft was wired for protection during the intervention (Figure 2E, large arrows). Through the JR4 catheter, the AAP was wired with an Amplatz Super Stiff guidewire (Boston Scientific) (Figure 2E, small arrows). The shaft sheath of a 12 mm Amplatzer septal occluder device (St. Jude Medical) was advanced through the neck of the AAP (Figure 2F, arrows). Graft angiography ensured patency of the graft prior to deployment of the Amplatzer septal occluder device (Figures 2G and 2H). Under TEE guidance, the distal disk of the septal occluder device was deployed into the AAP sac, the body of the occluder was placed in the neck of the AAP, and the proximal disk was deployed at the aortic side. A final aortogram was performed after deployment, confirming the location of the septal occluder device (Figure 2H). The patient was discharged 7 days after the procedure with no further chest pain. Clinical follow-up confirmed resolution of the symptoms. A 3-month follow-up CTA of the chest exhibited thrombosis of the AAP with minimal residual shunt (Figure 2I).
AAPs IN THE LITERATURE
We performed a literature search in English, Spanish, and Portuguese, limited by the dates January 1980 to May 2014. We searched the databases of the National Library of Medicine, PubMed, EMBASE, and SciELO for the following medical terms: ascending aorta, false aneurysm, and pseudoaneurysm. Our search included all age groups, and we applied no specific filters. We screened studies according to the critical appraisal tool STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) and summarized them by the number of participants, methodology, technical intervention, and outcomes.8 Discrepancies in the selection of the relevant literature were resolved by consensus. We classified the studies by treatment strategy—surgical vs endovascular repair—and summarized them in tables highlighting demographic characteristics, prior surgical history, intervention, and outcomes.
Our literature search screened 3,047 citations that included 355 cases of AAPs. Of the citations, 91.5% were single case reports and 8.5% were case series. All the studies were retrospective. Twenty-two percent of the publications reported either clinical or imaging follow-up, ranging from 1 month to 6 years, with the latter in the surgical series. No studies compared surgical treatment vs endovascular repair.
Etiology of AAPs
Seventy-six percent of the cases involved prior aortic or cardiac surgery, including replacement or prior plasty of the AA, CABG, valve replacement, and heart transplant. Five percent of AAP cases were attributed to complications of blunt chest trauma. Up to 4.4% of AAP cases were related to inflammatory or autoimmune disease, mycotic pseudoaneurysms, tuberculosis, and foreign bodies in the digestive tract causing a fistula. Finally, 10.6% of the cases did not have a known triggering risk factor related to the AAP event.
In regard to the treatment strategy, 73.8% of the cases reported surgical repair with Dacron graft, Hemashield graft, or pericardial patch; interrupted stitches; or a hybrid procedure in combination with stent grafts. In contrast, 21.2% of AAPs were treated with endovascular techniques only, either with stent grafts (9.8%), coil embolization (1.1%), thrombin injection (0.5%), or occluder devices (9.8%). Specifically related to our patient, either the Amplatzer septal occluder device or vascular plugs were implanted in 36 patients. Of those cases, 75% had successful immediate periprocedural results, but follow-up was inconsistent. Finally, 5% of patients with AAPs were treated conservatively or considered too critically ill for any intervention, resulting in eventual palliative care.
Natural History of AAPs
Similar to other aneurysms, AAPs have the potential to create life-threatening conditions as a result of progressive expansion. AAPs can lead to compression or erosion of the surrounding structures, rupture, bleeding, or fistula development, or they can serve as a source of persistent infection or embolism.9,10 No definitive factors have been identified to predict the rate of AAP expansion or progression into a major complication. Most of the patients who declined surgical treatment survived up to 23 months.4,11 A small percentage of the patients with inflammatory disease (Behçet disease) or inherited conditions (Marfan syndrome) developed asymptomatic AAPs. These patients are usually monitored until they develop symptoms and eventually undergo successful surgical correction. One of the case series reported a mean period of 2 years for symptom development in patients with Behçet disease.12 Conversely, CTA imaging of aortic replacement cases involving postsurgical mediastinitis and AAP development exhibited regression of the AAP at 6-month postinfection control.13 In these cases, the authors hypothesized that a periprosthetic hematoma initially occurred and subsequently regressed without evolving into a definitive AAP or causing any clinical problems.3
Surgical AAP Treatments
Repeat sternotomy entails the risk of rupture of the AAP and/or cerebral embolism if the oscillating saw used for the procedure enters the thoracic cavity. Consequently, patients with an anteriorly located AAP situated <2 cm from the sternum are considered high-risk patients for chest reentry.10 Strategies to avoid the rupture of the AAP during surgical repair include the following: (1) extramediastinal cardiopulmonary bypass (femorofemoral, carotid, or axillary bypass), (2) moderate or deep hypothermia with partial or full circulatory arrest, (3) left ventricular venting (if the ventricle appears distended on TEE), (4) insertion and inflation of an endoclamp balloon in the AA, (5) balloon occlusion catheter positioned at the level of the disrupted aortic anastomosis, and (6) securing the proximal aortic branches.11,14
Most published surgical series are retrospective analyses of repeat cardiac surgery, specifically resternotomy for repeat AA repair or for bypass surgery. Hybrid procedures were performed in patients in whom the proximal lesion was treated with conventional open-heart procedure, whereas the distal communication was repaired with a stent graft deployment,15,16 or in whom the stent graft was delivered through a minimal thoracotomy access via transapical access.17
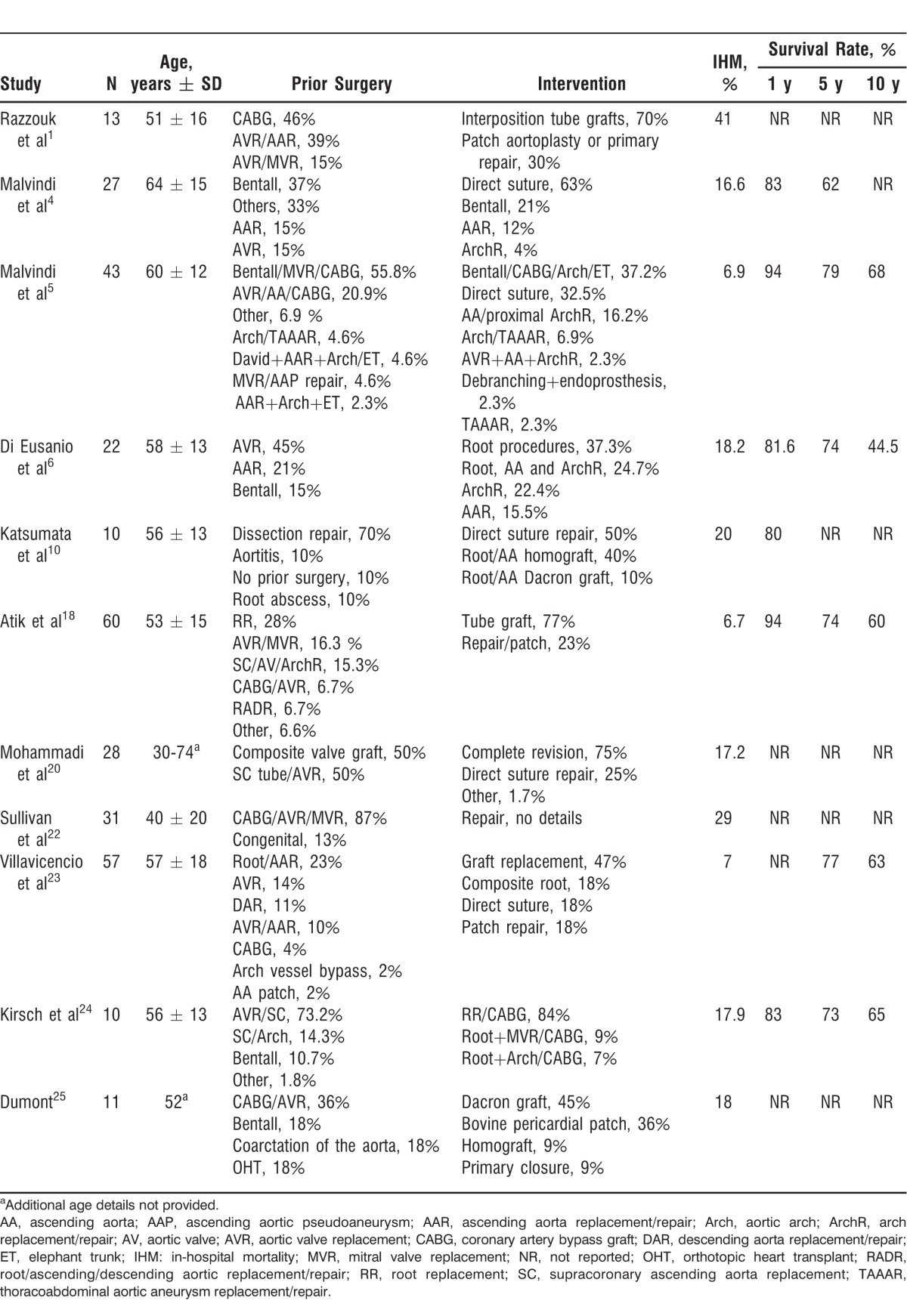
Table 1 summarizes surgical series that include 10 or more patients with AAP in their cohort. In-hospital mortality of surgical AAP correction ranged between 6.7% and 41%,10,18-25 whereas survival rates reached 94%, 79%, and 68% at 1, 5, and 10 years postprocedure, respectively. Predictors for complicated surgery include active endocarditis (odds ratio [OR] 5.1), New York Heart Association class III-IV (OR 3.8), urgent procedure, an AAP >55 mm in diameter, age >65 years, and the duration of cardiopulmonary bypass.6,23 Additionally, predictors for operative mortality include severe systolic dysfunction (LVEF <35%) and obesity (body mass index >30 kg/m2).23 A recurrence rate up to 12% at 10 years has been reported, but no risk factors have been strongly associated with recurrence.23
Table 1.
Case Series of Surgical Treatments for Pseudoaneurysm of the Ascending Aorta
Endovascular AAP Repair
Stent Grafts
Stent grafts are currently designed for the treatment of the distal arch, descending and abdominal aortic conditions, but they are not manufactured for the AA. The rationale lies in the tortuous trajectory of the AA and of the arch, as well as the presence of the aortic branches in which a potential exclusion of the coronary and aortic branches by the stent grafts could be harmful. For this reason, fenestrations in the stent may be required. The stiffness of the endoprosthesis may also be a factor to consider for the further development of aortic endoleaks.26 In this context, the treatment plan should ensure a safe landing zone (at least a 2 cm safety margin of healthy aorta), avoid the compromise of important aortic branches, and match the size of the shunt. The suggested diameter size of the stent is approximately 10%-15% larger than the diameter of the aorta measured on computed tomography (CT) scan.27 Other investigators suggest that intravascular ultrasound (IVUS) can overcome the limitations of the routine static cross-sectional imaging provided by CTA.28,29
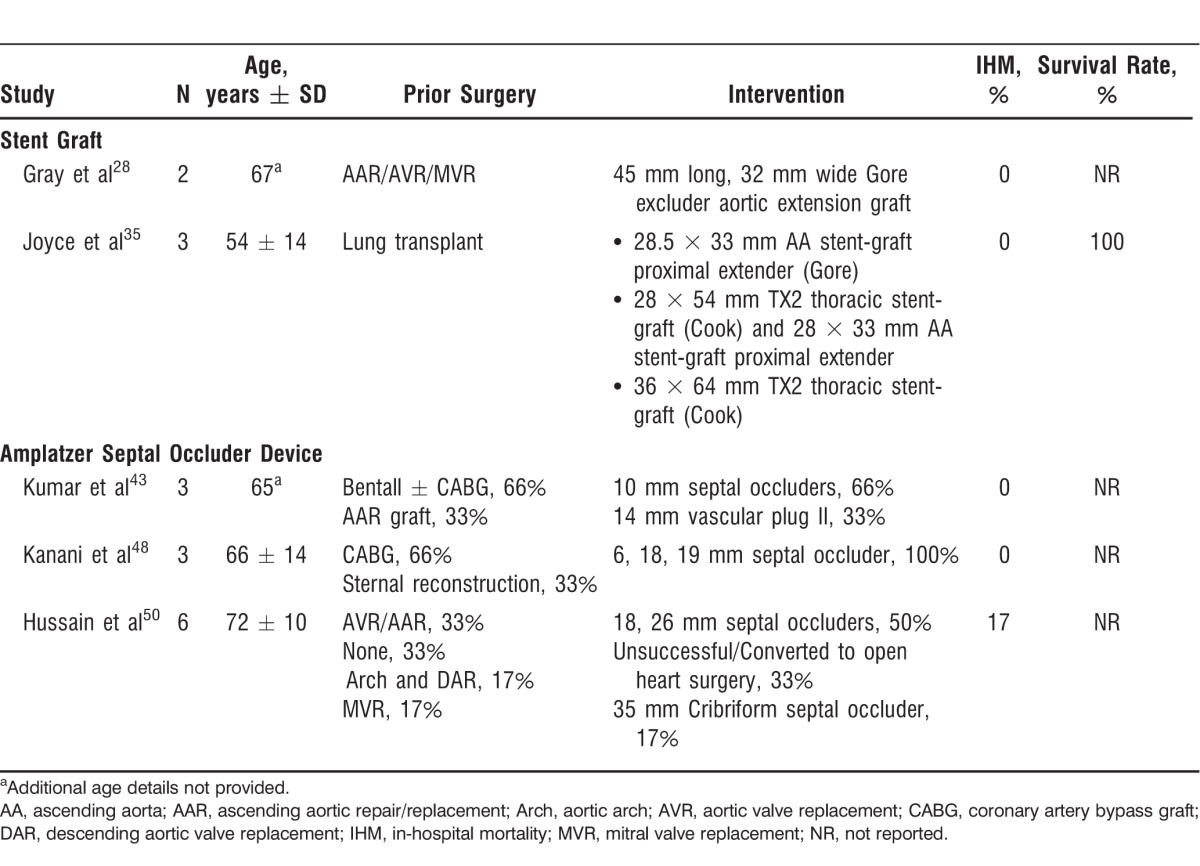
Interventions to assist in accurate stent deployment include decreasing the cardiac output, rapid pacing, inflation of a right atrial occlusion balloon, and administration of adenosine or beta blockers.28 These interventions can decrease the likelihood of stent migration and endoleaks.30 A small number of cases report treating AAPs and the arch with stent grafts such as the Gore Excluder Aortic Extender, Gore-Tex TAG (W. L. Gore and Associates), Talent thoracic endograft (Medtronic), self-expandable SEAL thoracic flex stent graft (S&G Biotech), Zenith TX aortoaortic endograft (Cook Medical), or Z-type stents customized to the patient's anatomy.28,31-34 Successful cases of patients with prior thoracic surgeries and stent graft exclusion of AAPs have been documented with CTA up to 4-month follow-up (Table 2).28,35 Other adjunctive procedures used to avoid complications with stent grafts placed in the AA and in the aortic arch include prophylactic transposition or bypass graft of the neck vessels to support brain perfusion prior to stent deployment and stent grafts with side branches. The latter approach has been associated with increased risk of stroke.36-38 The technical challenges faced in the treatment of AAPs with stent grafts include the large size of the sheath navigating through the tortuous diseased iliac vessels; the short-length delivery system currently in use with access from the femoral artery unable to reach the AA; the significant hemodynamic forces in the AA leading to device migration; retrograde dissection from oversizing of the graft; and overzealous postdeployment dilatation.28
Table 2.
Case Series of Endovascular Treatment for Pseudoaneurysm of the Ascending Aorta
Coils
Closure of AAPs with coils as stand-alone therapy has been reported, but coils are more commonly used as adjunctive therapy along with stent grafts, occluder devices, or vascular plugs.39 The indications for using coils include small AAPs with narrow necks and saccular aneurysms with a sac diameter greater than the neck that will enable intraaneurysmal coil packing without risk of coil displacement into the arterial lumen. Each coil is introduced into the aneurysmal cavity via a microcatheter and is deployed by pushing the device. If the coil is unstable or too large, it can still be retrieved.40 As such, coils can be used for any anatomic location and their mechanical effects are not an issue.41-43
Thrombin Injections
An initial experience of treating an AAP with thrombin injection was complicated by a stroke that was rescued with abciximab infusion.44 Another exceptional case of CT-guided thrombin injection of an AAP was performed as a bridge to reduce its size prior to surgical repair.45 Currently, no recommendations favor the use of thrombin in AAP cases.
Septal Occluder Devices and Vascular Plugs
A variety of devices have been employed for off-label closure of AAPs: Amplatzer septal occluder device (or its multifenestrated variant Cribriform with a shorter connecting waist); Amplatzer persistent foramen ovale (PFO) occluder (the connecting waist consists of a narrow pin with less efficiency in a high pressure flow environment); Amplatzer muscular ventricular septal defect (mVSD) occluder with symmetrical disk sizes and a wider midconnecting portion that provides better anchoring; Amplatzer vascular plugs (named I-IV) available in single or multilayered mesh lobes; and Amplatzer duct occluder (all St. Jude Medical). In deciding the type and size of the occluder device, the disk located in the aorta should not interfere with any of the coronary ostia.46 In complex adult or congenital cases, a combination of more than one type of device has been successfully used.47
The off-label practice of using occluder devices for AAP closure has been restricted to patients with histories of multiple cardiac surgeries and are thus considered unsuitable for other thoracotomy procedures (Table 2).43,48-50 Of the 36 cases reported employing Amplatzer septal occluder devices or vascular plugs, 75% were successfully deployed with minor residual flow postdeployment. In most of the cases (52.7%), Amplatzer septal occluder devices were used, including Cribriform septal occluders (5.5%). Vascular plugs were employed in 27.7% of the cases, mVSDs were used in 11.3% of the cases, and only 1 case utilized a PFO occluder (2.7%). In the largest case series, comprised of 6 patients, an Amplatzer septal occluder device was successfully deployed in 4 patients.50 No follow-up was reported in this series. In a 2012 case series of 3 patients, a follow-up of 6 months after successful deployment of the device was reported.43
DISCUSSION
The important findings of this systematic review are as follows: (1) AAPs are almost always related to history of aortic surgery or history of a procedure that needed cannulation of the AA, (2) surgical repair of AAPs remains the standard treatment, and (3) endovascular therapies for AAPs are limited to high-risk surgical patients; consequently, limited experience has been reported.
We described the endovascular treatment of a highly symptomatic patient with an expanding AAP who was considered a nonoperative candidate. The present case was not suitable for stent grafts given the proximity of the AAP to the saphenous vein graft to the RCA. The size of the AAP neck was also too large to employ coils. The fact that the 3-month imaging follow-up exhibited a thrombosed AAP was an encouraging indication of progression toward definitive AAP closure.
However, some cases report failure of the device to maintain its position over time. In these cases, the occluder device either prolapsed into the AAP cavity allowing expansion,51 migrated from the initial deployment location, or embolized to other organs after a year. Consequently, the Amplatzer septal occluder device was captured with a goose snare and followed by open surgical repair of the AAP. The authors hypothesized that the device was not properly oversized at deployment or an appropriate firm rim of tissue to anchor the device was absent.16,46,49,51,52 In other cases with evidence of persistent shunt or recurrence of a partially thrombosed AAP, an additional septal occluder device was placed a year after the index procedure.50 In successful cases, the devices were oversized by 2-4 mm at deployment.46
Imaging guidance in sizing the neck of the AAP is crucial. Hence, IVUS has been used to assist in the deployment of occluders to precisely define the diameter of the AAP neck and appropriately select the device size.50,53 In cases of device malposition, concerning features include persistence of residual shunting into the AAP more than 6 weeks after deployment or continued expansion of the AAP for more than 2 weeks postprocedure.54
Multimodality imaging for surveillance follow-up of asymptomatic patients after aortic repair is currently done in the postoperative period within 3 months and yearly thereafter.3 Specifically, with surgeries after Dacron graft (Gelseal) replacement, a patient series reported a 3.2% diameter expansion per year.55 In cases of endovascular repair of the aorta with stent grafts, some case series recommend imaging follow-up at 30 days, 6 months, and yearly after the repair. However, repeated imaging follow-up remains controversial because of the amount of radiation exposure, the use of intravenous contrast that can worsen kidney function, and the cost of imaging studies.56
In summary, catheter-based procedures for correcting AAPs have been regarded as palliative interventions in high-risk surgical patients. Surgical techniques remain the gold standard given the longer period of experience. Despite the opinions of some authors that transcatheter therapies should be relegated to very high-risk patients, we consider endovascular therapies for the treatment of AAPs to be an evolving field that will find a niche in the contemporary practice with the training of experienced operators and the compilation of solid survival data.57
CONCLUSION
Because of the paucity of long-term survival data, percutaneous therapies to correct AAPs are not generally recommended as a primary therapy. Surgical patch aortotomy or graft interpositions have significant data on survival benefit and continue to represent the data-driven recommendations. Future long-term multicenter data involving transcatheter procedures will provide the essential outcomes to compare with the current gold standard therapy.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Razzouk A, Gundry S, Wang N, et al. Pseudoaneurysms of the aorta after cardiac surgery or chest trauma. Am Surg. 1993 Dec;59(12):818–823. [PubMed] [Google Scholar]
- 2.Deshpande A, Mossop P, Gurry J, et al. Treatment of traumatic false aneurysm of the thoracic aorta with endoluminal grafts. J Endovasc Surg. 1998 May;5(2):120–125. doi: 10.1583/1074-6218(1998)005<0120:TOTFAO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Mesana TG, Caus T, Gaubert J, et al. Late complications after prosthetic replacement of the ascending aorta: what did we learn from routine magnetic resonance imaging follow-up? Eur J Cardiothorac Surg. 2000 Sep;18(3):313–320. doi: 10.1016/s1010-7940(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 4.Malvindi PG, Cappai A, Raffa GM, et al. Analysis of postsurgical aortic false aneurysm in 27 patients. Tex Heart Inst J. 2013;40(3):274–280. [PMC free article] [PubMed] [Google Scholar]
- 5.Malvindi PG, van Putte BP, Heijmen RH, Schepens MA, Morshuis WJ. Reoperations for aortic false aneurysms after cardiac surgery. Ann Thorac Surg. 2010 Nov;90(5):1437–1443. doi: 10.1016/j.athoracsur.2010.06.103. [DOI] [PubMed] [Google Scholar]
- 6.Di Eusanio M, Berretta P, Bissoni L, Petridis FD, Di Marco L, Di Bartolomeo R. Re-operations on the proximal thoracic aorta: results and predictors of short- and long-term mortality in a series of 174 patients. Eur J Cardiothorac Surg. 2011 Nov;40(5):1072–1076. doi: 10.1016/j.ejcts.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 7.Miyahara S, Okita Y. Reoperative aortic root replacement [in Japanese] Kyobu Geka. 2013 Jul;66(8 Suppl):649–654. [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct 20;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 9.Mulder EJ, van Bockel JH, Maas J, van den Akker PJ, Hermans J. Morbidity and mortality of reconstructive surgery of noninfected false aneurysms detected long after aortic prosthetic reconstruction. Arch Surg. 1998 Jan;133(1):45–49. doi: 10.1001/archsurg.133.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Katsumata T, Moorjani N, Vaccari G, Westaby S. Mediastinal false aneurysm after thoracic aortic surgery. Ann Thorac Surg. 2000 Aug;70(2):547–552. doi: 10.1016/s0003-4975(00)01300-x. [DOI] [PubMed] [Google Scholar]
- 11.Tang GH, Pinney SP, Broumand SR, Adams DH, Anyanwu AC. Excellent outcomes with use of synthetic vascular grafts for treatment of mycotic aortic pseudoaneurysms after heart transplantation. Ann Thorac Surg. 2011 Dec;92(6):2112–2116. doi: 10.1016/j.athoracsur.2011.07.080. [DOI] [PubMed] [Google Scholar]
- 12.Navaravong L, Saab F, Cook JR, Peterman M, Flack J. Ascending aortic pseudoaneurysm, a ticking bomb after cardiac surgery. Cardiovasc Revasc Med. 2011 May-Jun;12(3):177–180. doi: 10.1016/j.carrev.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Agrifoglio M, Pontone G, Andreini D, Biglioli P, Cheema FH, Barili F. Conservative management of the pseudoaneurysms of ascending aortic graft: a case of spontaneous regression at follow-up. J Cardiovasc Med (Hagerstown) 2011 Aug;12(8):586–588. doi: 10.2459/JCM.0b013e3283474e71. [DOI] [PubMed] [Google Scholar]
- 14.D'Attellis N, Diemont FF, Julia PL, Cardon C, Fabiani JN. Management of pseudoaneurysm of the ascending aorta performed under circulatory arrest by port-access. Ann Thorac Surg. 2001 Mar;71(3):1010–1011. doi: 10.1016/s0003-4975(00)02265-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhu P, Yang Q, Qiu F, Liao C. Post traumatic large pseudoaneurysms of the aortic arch and descending aorta. Eur J Cardiothorac Surg. 2009 Mar;35(3):535. doi: 10.1016/j.ejcts.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Ruggieri VG, Malezieux R, Bina N, Favre JP. Hybrid treatment of an ascending aortic pseudoaneurysm following multiple sternotomies. J Vasc Surg. 2010 Mar;51(3):729–731. doi: 10.1016/j.jvs.2009.08.092. [DOI] [PubMed] [Google Scholar]
- 17.Szeto WY, Moser WG, Desai ND, et al. Transapical deployment of endovascular thoracic aortic stent graft for an ascending aortic pseudoaneurysm. Ann Thorac Surg. 2010 Feb;89(2):616–618. doi: 10.1016/j.athoracsur.2009.06.090. [DOI] [PubMed] [Google Scholar]
- 18.Atik FA, Navia JL, Svensson LG, et al. Surgical treatment of pseudoaneurysm of the thoracic aorta. J Thorac Cardiovasc Surg. 2006 Aug;132(2):379–385. doi: 10.1016/j.jtcvs.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 19.Settepani F, Muretti M, Barbone A, et al. Reoperation for aortic false aneurysms: our experience and strategy for safe resternotomy. J Card Surg. 2008 May-Jun;23(3):216–220. doi: 10.1111/j.1540-8191.2008.00597.x. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadi S, Bonnet N, Leprince P, et al. Reoperation for false aneurysm of the ascending aorta after its prosthetic replacement: surgical strategy. Ann Thorac Surg. 2005 Jan;79(1):147–152. doi: 10.1016/j.athoracsur.2004.06.032. discussion 152. [DOI] [PubMed] [Google Scholar]
- 21.Schepens MA, Dossche KM, Morshuis WJ. Reoperations on the ascending aorta and aortic root: pitfalls and results in 134 patients. Ann Thorac Surg. 1999 Nov;68(5):1676–1680. doi: 10.1016/s0003-4975(99)00760-2. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan KL, Steiner RM, Smullens SN, Griska L, Meister SG. Pseudoaneurysm of the ascending aorta following cardiac surgery. Chest. 1988 Jan;93(1):138–143. doi: 10.1378/chest.93.1.138. [DOI] [PubMed] [Google Scholar]
- 23.Villavicencio MA, Orszulak TA, Sundt TM, 3rd, et al. Thoracic aorta false aneurysm: what surgical strategy should be recommended? Ann Thorac Surg. 2006 Jul;82(1):81–89. doi: 10.1016/j.athoracsur.2006.02.081. discussion 89. [DOI] [PubMed] [Google Scholar]
- 24.Kirsch EW, Radu NC, Mekontso-Dessap A, Hillion ML, Loisance D. Aortic root replacement after previous surgical intervention on the aortic valve, aortic root, or ascending aorta. J Thorac Cardiovasc Surg. 2006 Mar;131(3):601–608. doi: 10.1016/j.jtcvs.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Dumont E, Carrier M, Cartier R, et al. Repair of aortic false aneurysm using deep hypothermia and circulatory arrest. Ann Thorac Surg. 2004 Jul;78(1):117–120. doi: 10.1016/j.athoracsur.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Yuri K, Yamaguchi A, Hori D, et al. A fenestrated stent graft for endovascular repair of an ascending aortic pseudoaneurysm. Ann Vasc Dis. 2010;3(3):228–231. doi: 10.3400/avd.cr00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SW, Lee do Y, Kim MD, et al. Outcomes of endovascular treatment for aortic pseudoaneurysm in Behcet's disease. J Vasc Surg. 2014 Mar;59(3):608–614. doi: 10.1016/j.jvs.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 28.Gray BH, Langan EM, 3rd, Manos G, Bair L, Lysak SZ. Technical strategy for the endovascular management of ascending aortic pseudoaneurysm. Ann Vasc Surg. 2012 Jul;26(5):734–738. doi: 10.1016/j.avsg.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 29.van Prehn J, Vincken KL, Muhs BE, et al. Toward endografting of the ascending aorta: insight into dynamics using dynamic cine-CTA. J Endovasc Ther. 2007 Aug;14(4):551–560. doi: 10.1177/152660280701400418. [DOI] [PubMed] [Google Scholar]
- 30.Zago AC, Saadi EK, Zago AJ. Endovascular approach to treat ascending aortic pseudoaneurysm in a patient with previous CABG and very high surgical risk. Catheter Cardiovasc Interv. 2011 Oct 1;78(4):551–557. doi: 10.1002/ccd.23005. [DOI] [PubMed] [Google Scholar]
- 31.Ono T, Midorikawa H, Morishima S, Takano T, Nakazawa M, Kudo Y. Open aortic stent grafting and prosthetic bypass in a child. Ann Thorac Surg. 2011 Oct;92(4):1518–1520. doi: 10.1016/j.athoracsur.2011.03.142. [DOI] [PubMed] [Google Scholar]
- 32.Vaughan-Huxley E, Hamady MS, Metcalfe MJ, et al. Endovascular repair of an acute, mycotic, ascending aortic pseudoaneurysm. Eur J Vasc Endovasc Surg. 2011 Apr;41(4):488–491. doi: 10.1016/j.ejvs.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Heye S, Daenens K, Maleux G, Nevelsteen A. Stent-graft repair of a mycotic ascending aortic pseudoaneurysm. J Vasc Interv Radiol. 2006 Nov;17(11 Pt 1):1821–1825. doi: 10.1097/01.RVI.0000244834.71601.65. [DOI] [PubMed] [Google Scholar]
- 34.Rayan SS, Vega JD, Shanewise JS, Kong LS, Chaikof EL, Milner R. Repair of mycotic aortic pseudoaneurysm with a stent graft using transesophageal echocardiography. J Vasc Surg. 2004 Sep;40(3):567–570. doi: 10.1016/j.jvs.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Joyce DL, Singh SK, Mallidi HR, Dake MD. Endovascular management of pseudoaneurysm formation in the ascending aorta following lung transplantation. J Endovasc Ther. 2012 Feb;19(1):52–57. doi: 10.1583/11-3601.1. [DOI] [PubMed] [Google Scholar]
- 36.Saito N, Kimura T, Odashiro K, et al. Feasibility of the Inoue single-branched stent-graft implantation for thoracic aortic aneurysm or dissection involving the left subclavian artery: short- to medium-term results in 17 patients. J Vasc Surg. 2005 Feb;41(2):206–212. doi: 10.1016/j.jvs.2004.11.030. discussion 212. [DOI] [PubMed] [Google Scholar]
- 37.Kpodonu J, Wheatley GH, 3rd, Williams JP, Rodriguez-Lopez JA, Ramaiah VG, Diethrich EB. A novel approach for the endovascular repair of the small thoracic aorta: customizing off-the-shelf endoluminal grafts to treat a post-coarctation pseudoaneurysm. Ann Thorac Surg. 2008 Mar;85(3):1115–1117. doi: 10.1016/j.athoracsur.2007.04.120. [DOI] [PubMed] [Google Scholar]
- 38.Taurino M, Fantozzi C, Stella N, Rizzo L. Hybrid treatment of anastomotic pseudoaneurysm of the isthmus portion of the thoracic aorta. J Vasc Surg. 2013 Oct;58(4):1088. doi: 10.1016/j.jvs.2012.10.100. [DOI] [PubMed] [Google Scholar]
- 39.Barbetakis N, Xenikakis T, Efstathiou A, Fessatidis I. Percutaneous coil embolisation of a false aortic aneurysm following coronary surgery and mediastinitis. Hellenic J Cardiol. 2007 Jul-Aug;48(4):246–248. [PubMed] [Google Scholar]
- 40.Chapot R, Aymard A, Saint-Maurice JP, Bel A, Merland JJ, Houdart E. Coil embolization of an aortic arch false aneurysm. J Endovasc Ther. 2002 Dec;9(6):922–925. doi: 10.1177/152660280200900630. [DOI] [PubMed] [Google Scholar]
- 41.Al-Husami WF, Piemonte T. Percutaneous repair of a pseudoaneurysm associated with coarctation of the aorta. J Invasive Cardiol. 2008 Oct;20(10):E293–E295. [PubMed] [Google Scholar]
- 42.Faganello G, Hamilton M, Wilde P, Turner MS. Percutaneous closure of false aneurysms of the aorta in Wiskott Aldrich syndrome. Eur Heart J. 2008 Jan;29(1):6. doi: 10.1093/eurheartj/ehm349. [DOI] [PubMed] [Google Scholar]
- 43.Kumar PV, Alli O, Bjarnason H, Hagler DJ, Sundt TM, Rihal CS. Percutaneous therapeutic approaches to closure of cardiac pseudoaneurysms. Catheter Cardiovasc Interv. 2012 Oct 1;80(4):687–699. doi: 10.1002/ccd.24300. [DOI] [PubMed] [Google Scholar]
- 44.Lin PH, Bush RL, Tong FC, Chaikof E, Martin LG, Lumsden AB. Intra-arterial thrombin injection of an ascending aortic pseudoaneurysm complicated by transient ischemic attack and rescued with systemic abciximab. J Vasc Surg. 2001 Nov;34(5):939–942. doi: 10.1067/mva.2001.116968. [DOI] [PubMed] [Google Scholar]
- 45.Perek B, Urbanowicz T, Zabicki B, Puślecki M, Juszkat R, Jemielity M. CT-guided thrombin injection to control rapid expansion of ascending aortic false aneurysm 15 months after Bentall-Bono operation. Cardiovasc Intervent Radiol. 2011 Feb;34(Suppl 2):S83–S85. doi: 10.1007/s00270-010-9808-z. [DOI] [PubMed] [Google Scholar]
- 46.Noble S, Ibrahim R. Embolization of an Amplatzer mVSD occluder device used for percutaneous closure of an ascending aortic pseudoaneurysm: case report and literature review. Catheter Cardiovasc Interv. 2012 Feb 1;79(2):334–338. doi: 10.1002/ccd.23094. [DOI] [PubMed] [Google Scholar]
- 47.Hashizume K, Shimizu H, Koizumi K, Inoue S. Endovascular aneurysm repair using the periscope graft technique for thoracic aortic anastomotic pseudoaneurysm. Interact Cardiovasc Thorac Surg. 2013 Apr;16(4):553–555. doi: 10.1093/icvts/ivs519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanani RS, Neilan TG, Palacios IF, Garasic JM. Novel use of the Amplatzer septal occluder device in the percutaneous closure of ascending aortic pseudoaneurysms: a case series. Catheter Cardiovasc Interv. 2007 Jan;69(1):146–153. doi: 10.1002/ccd.20794. [DOI] [PubMed] [Google Scholar]
- 49.Bibiloni Lage I, Benussi S, Verzini A, Alfieri O. Amplatzer device migration through the sternum: a rare complication of percutaneous treatment for an aortic pseudoaneurysm solved by 2 length-adjustable bovine pericardium conduits. J Thorac Cardiovasc Surg. 2012 Aug;144(2):e27–e28. doi: 10.1016/j.jtcvs.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 50.Hussain J, Strumpf R, Wheatley G, Diethrich E. Percutaneous closure of aortic pseudoaneurysm by Amplatzer occluder device-case series of six patients. Catheter Cardiovasc Interv. 2009 Mar 1;73(4):521–529. doi: 10.1002/ccd.21833. [DOI] [PubMed] [Google Scholar]
- 51.Westaby S, Luthra S, Anthony S, Ormerod O, Wilson N. Amplatzer device deployment for saccular aortic arch aneurysm: a note of caution. Circulation. 2012 Mar 13;125(10):1318–1320. doi: 10.1161/CIRCULATIONAHA.111.048710. [DOI] [PubMed] [Google Scholar]
- 52.Attia R, Venugopal P, Whitaker D, Young C. Management of a pulsatile mass coming through the sternum. Pseudoaneurysm of ascending aorta 35 years after repair of tetralogy of Fallot. Interact Cardiovasc Thorac Surg. 2010 May;10(5):820–822. doi: 10.1510/icvts.2009.227900. [DOI] [PubMed] [Google Scholar]
- 53.Garg N, Bacharach JM, Reynolds TR. Endovascular repair of ascending aortic pseudoaneurysm. Ann Vasc Surg. 2011 Jul;25(5):696.e1–696.e5. doi: 10.1016/j.avsg.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Patel AV, Gupta S, Laffin LJ, Retzer EM, Dill KE, Shah AP. One size does not fit all: case report of two percutaneous closures of aortic pseudoaneurysm and review of the literature. Cardiovasc Revasc Med. 2014 Apr;15(3):160–164. doi: 10.1016/j.carrev.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Takami Y, Tajima K, Kato W, et al. Long-term size follow-up of knitted Dacron grafts (Gelseal) used in the ascending aorta. Interact Cardiovasc Thorac Surg. 2012 May;14(5):529–531. doi: 10.1093/icvts/ivr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katsargyris A, Verhoeven EL. Part Two: Against the motion. All TEVAR patients do not require lifelong follow-up by annual CTA/MRA. [Con] Eur J Vasc Endovasc Surg. 2012 Dec;44(6):538–541. doi: 10.1016/j.ejvs.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Zicho D, Cartwright N, Bizzarri F, et al. Endovascular stent graft repair of suture-line pseudoaneurysm following ascending aorta replacement. Vasc Endovascular Surg. 2014 Apr;48(3):251–255. doi: 10.1177/1538574413513847. [DOI] [PubMed] [Google Scholar]