Abstract
Background
Stroke is the fourth leading cause of death in the United States, leading to devastating disability. Most strokes are ischemic, and nearly one-third of these are caused by carotid disease. The primary mechanism of carotid-related stroke is an atheroembolic event from an unstable atherosclerotic plaque rupture. In the 1990s, randomized trials demonstrated the benefit of carotid endarterectomy (CEA) in reducing the risk of stroke in both symptomatic and asymptomatic carotid disease.
Methods
We review best medical therapy (BMT) for asymptomatic carotid disease and recent randomized trials comparing CEA and carotid angioplasty stenting (CAS), and we discuss the role of urgent carotid interventions in patients with acute neurologic symptoms.
Results
In 2010, 2 large trials demonstrated the efficacy of CAS in select patients, although CAS was associated with an increased procedural stroke risk compared to CEA. An age effect was observed; patients >75 years do worse with CAS compared to CEA. As BMT has evolved in the past decade, a future trial (CREST-2) will address whether BMT is equal to intervention (CEA or CAS) in asymptomatic carotid disease. In a subgroup of patients with asymptomatic carotid disease, CEA plus BMT will likely remain the mainstay therapy for carotid disease compared to BMT alone. CEA and CAS will continue to play complementary roles in the future, as CAS will be done in select patients in whom CEA cannot be undertaken because of high-risk anatomical or medical conditions. Finally, a role for urgent carotid interventions in a select group of patients who present with acute neurologic symptoms is developing as a way to prevent recurrent stroke after an initial carotid plaque rupture event.
Conclusion
CAS has an increasingly higher risk of stroke with advancing age. Patients treated with CAS have a 1.76-fold increased risk of stroke (95% CI, 1.35-2.31) with each 10-year increase in age. No such age effect is seen in patients treated with CEA. Age is a critical variable in making informed choices regarding treatment of severe carotid artery stenosis.
Keywords: Carotid angioplasty stenting, carotid disease, carotid endarterectomy, carotid stenosis
INTRODUCTION
An estimated 795,000 strokes will occur in 2014, making stroke the fourth leading cause of death and major disability in the United States. Not surprisingly, elderly patients have more difficulty recovering from stroke, and 26% percent of stroke patients >65 years will require long-term institutional care after their first stroke. The estimated yearly direct/indirect cost of stroke in the US is $36.5 billion.1 More than two-thirds of all strokes are ischemic, and 20%-30% of ischemic strokes are attributable to carotid atherosclerotic disease. The primary mechanism of stroke related to carotid disease is an embolic event from an unstable atherosclerotic plaque; however, although rare, acute thrombosis of the internal carotid artery can also occur.
Risk factors that lead to the development of atherosclerosis are numerous, some of which are not modifiable, such as age, race, sex, and genetics. As the US population ages, an estimated 72 million people will be over the age of 65 by the year 2030 (20%).1 Aggressive treatment of modifiable risk factors can have a dramatic impact on decreasing stroke and stroke risk, highlighting the importance of what is now referred to as best medical therapy (BMT) in carotid atherosclerotic disease.
BEST MEDICAL THERAPY
BMT has evolved since the landmark carotid endarterectomy (CEA) randomized trials were published in the 1990s.2,3 Modifiable risk factors including hypertension, diabetes, hypercholesterolemia, and tobacco use should be the focus of aggressive medical management when treating asymptomatic or symptomatic carotid stenosis.
Hypertension
Antihypertensive therapy can lower the incidence of stroke by 35%-44%.4 Blood pressure should be lowered to <140 mmHg in most patients and to <120 mmHg in patients >60 years with significant cardiovascular risk factors. Lifestyle modifications including a low-sodium and reduced saturated fat diet, exercise, smoking cessation, and limited alcohol consumption should augment pharmacologic therapy.5
Diabetes
Management of other modifiable risk factors is essential in the diabetic patient, as diabetes alone increases the relative risk of ischemic stroke 1.8 to 6 times that of nondiabetics. The use of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker has been shown to reduce the incidence of stroke by 21%-33%, as well as to reduce the incidence of diabetic complications, and should be considered first-line antihypertensive therapy.4,6 Remarkably, treatment with statins has resulted in a 24%-48% reduction in cardiovascular events and stroke.7
Hyperlipidemia
Elevated serum total cholesterol levels and low levels of high-density lipoprotein cholesterol are risk factors for ischemic stroke. Multiple clinical trials have shown a significant reduction in the risk of stroke in patients with coronary artery disease who are treated with a statin or other lipid-lowering medications.8 The secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial showed a reduced incidence of stroke and major coronary events in patients with recent transient ischemic attack (TIA) or stroke and no history of coronary artery disease.9 In a subset analysis of patients with carotid stenosis, the risk of stroke was reduced by 33% in patients treated with a statin versus placebo. Statins are now widely recognized as having pleiotropic cardiovascular effects after showing a significant benefit in the primary prevention of stroke and major cardiovascular events in healthy subjects,10 sometimes independent of the direct lipid-lowering effects.
Tobacco Use
Cigarette smoking alone leads to a nearly 2-fold increase in the risk of ischemic stroke.11 Smoking increases heart rate and blood pressure and decreases arterial distensibility; chronic use leads to increased atherosclerosis. It is important to stress to patients that smoking cessation can reduce their risk of stroke to a level that is near but not equivalent to the risk level of those who have never smoked.12
Antiplatelet Drugs
Antiplatelet drugs are considered a mainstay of therapy in patients with cardiovascular disease and in the secondary prevention of ischemic stroke. Aspirin has been recommended as prophylaxis against myocardial infarction (MI) for individuals with a 3%-10% risk of cardiac events over 5-10 years.13,14 A 2006 metaanalysis reviewed 6 major trials evaluating aspirin as primary prevention and found no benefit over placebo in reducing the incidence of stroke, although there was a benefit in overall cardiovascular events. However, the Women's Health Study demonstrated a 24% reduction in the incidence of ischemic stroke, a difference not born out in other studies that were composed mostly of men.15 In current practice, indirect data from trials such as the Women's Health Study and results from the metaanalysis mentioned above have resulted in the use of some form of antiplatelet treatment in this patient population.
LEVEL I EVIDENCE: CEA FOR SYMPTOMATIC AND ASYMPTOMATIC CAROTID STENOSIS
Carotid surgery for stroke prevention was introduced as a carotid segment excision and graft reconstruction in 1954 by Eastcott, Pickering, and Rob.16 During this time, DeBakey began to perform the standard CEA as it is still being done today, although he did not publish his series until 2 decades later.17 Surgical intervention for symptomatic carotid disease gained favor in the early 1990s with the publication of the North American Symptomatic Carotid Endarterectomy Trial (NASCET) in 1991.2 This landmark study demonstrated for the first time a significant reduction in risk of recurrent strokes for patients with symptomatic carotid disease: 2 years after an initial TIA or mild stroke, the risk of a recurrent stroke was reduced from 26% in patients treated with medical management alone to 9% in patients treated with CEA, a highly significant difference.
The management of asymptomatic carotid disease was addressed by a landmark randomized trial published in 1995 that looked at patients with ≥60% stenosis randomized to CEA vs medical management, the Asymptomatic Carotid Atherosclerosis Study (ACAS).3 The risk over 5 years for ipsilateral stroke and any perioperative stroke or death was 5.1% for surgical patients and 11% for patients treated medically, also highly significant. Despite differences in methods, these findings were replicated in another large European trial on asymptomatic carotid disease more than a decade later in the Asymptomatic Carotid Surgery Trial (ACST).18 This multicenter randomized trial allocated patients to immediate CEA vs deferral of CEA and medical therapy. The ACST trial, which is the largest body of evidence supporting a prophylactic CEA for asymptomatic high-grade carotid stenosis >80%, recently reported the benefit of CEA compared to medical therapy out to 10 years.19
CEA VS CAROTID ANGIOPLASTY STENTING
The Carotid Revascularization Endarterectomy Stenting Trial (CREST) was a multicenter, randomized, controlled trial funded by the National Institutes of Health (NIH) to evaluate the safety and effectiveness of carotid angioplasty stenting (CAS) in patients in the United States and Canada who were not at high risk for CEA.20 CREST was originally designed to evaluate only symptomatic patients; however, the inclusion criteria were expanded to include asymptomatic patients in 2005, likely because of low recruitment. The results of CREST, involving more than 1,200 patients, were published in 2010 and failed to show a significant difference between CEA and CAS in the primary endpoint, defined as the combined periprocedural incidence of MI, stroke, and death through a follow-up of 4 years. In comparing the 2 groups, the incidence of the periprocedural endpoints occurred in 5.2% ± 0.6% of CAS patients vs 4.5% ± 0.6% of CEA patients, a difference that did not reach statistical significance (P=0.38). When examining the individual endpoints, however, a statistically significant higher incidence of stroke occurred in the CAS group (4.1% ± 0.6% vs 2.3% ± 0.4%, P=0.01). Conversely, a statistically significant higher rate of MI occurred in the CEA group (1.1% ± 0.3% vs 2.3% ± 0.4%, P=0.03). No difference was seen in the 30-day mortality rate between the CAS and CEA patients (0.7% ± 0.2% vs 0.3% ± 0.2%, P=0.18). The equivalence between the 2 groups was maintained at estimated 4-year follow-up (CEA 6.8%, CAS 7.2%, P=0.51).
At the same time CREST was published in 2010, the European International Carotid Stenting Study (ICSS) found that the incidence of stroke, death, or periprocedural MI was 8.5% in the stenting group vs 5.2% in the endarterectomy group, which was highly significant.21 ICSS concluded that the risk of stroke and all causes of death was higher in the stenting group. Alarmingly, a follow-up study of a select ICSS cohort found that CAS patients had an increased incidence of ischemic lesions detected by magnetic resonance imaging (MRI) diffusion-weighted imaging studies compared to CEA patients (50% vs 17%).22 These microemboli could be a source of future cognitive decline that future studies will have to address.
The perioperative surgical results for CEA have improved since these studies, as evidenced in CREST (2.6%) compared to NASCET (7.5%), likely highlighting a partial effect of BMT.2,20
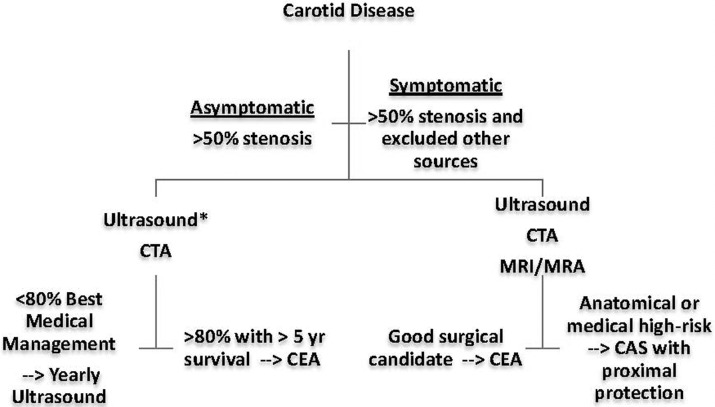
Given all these level I data, we propose an algorithm to assist in decision-making when treating our patients with carotid stenosis (Figure 1).
Figure 1.
Algorithm for the management of >50% carotid stenosis. Carotid endarterectomy (CEA) may be undertaken safely in the majority of asymptomatic and symptomatic patients. In symptomatic disease, if stenosis is <50% and all other sources of cerebral ischemia have been ruled out, best medical therapy should be utilized. However, if the symptoms recur (such as in cases with an ulcerated carotid plaque), a CEA may be undertaken.
*An ultrasound is the preferred way to image asymptomatic carotid and symptomatic carotid stenosis, and computed tomography angiography (CTA) is not recommended routinely for imaging the patient with asymptomatic disease unless an intervention is planned; in these cases, an ultrasound may suffice prior to CEA. CAS, carotid angioplasty stenting; MRI/MRA, magnetic resonance imaging/magnetic resonance angiography (of the head and neck).
AGE EFFECT FAVORS CEA OVER CAS
Interestingly, an effect of age on outcomes was also observed in CREST, demonstrating that age is an important factor in choosing the best therapy for a given patient. In patients undergoing CAS, those ≥75 years had an incidence of MI, stroke, or death of 12.7% compared with 6.3% for those 65-74 years old and 3.9% for the youngest patients (P<0.0001).23 No such age effect was seen in patients treated with CEA, however. Patients undergoing CEA had similar risks in the 3 age categories (6.1% at <64 years, 6.3% at 65-74 years, 7.4% at ≥75 years, P=0.5). When comparing CEA and CAS, no difference in the incidence of the primary endpoint was seen among patients in the 2 lower age strata. However, in patients ≥75 years, the incidence was significantly higher (12.7% CAS vs 7.4% CEA, P=0.05) with a significant treatment-by-age interaction (P=0.02). The increased incidence of the primary endpoint in the CAS cohort is primarily driven by the increase in the rate of stroke as the treatment age increases. This trend was not seen in patients treated with CEA.
The results of CREST refuted the notion that a less-invasive procedure, CAS, would be more beneficial in older patients compared to CEA. The increase in the rate of stroke with advancing age associated with CAS neutralized the modest reduction in MI when compared to CEA. Surprisingly, CAS was most beneficial in the youngest patient cohort, a group that may not have the same comorbidities and risk factors needed to be considered high risk for CEA. Multiple explanations have been given for this increased procedural risk of stroke with CAS, including the finding in older patients of increased aortic arch calcification and the decreased ability to tolerate the nearly universal microemboli that occur with CAS compared to CEA.
Several anatomic risk factors have been described that place elderly patients at higher risk for complications after CAS than younger patients. A single-center series of 133 patients treated with CAS demonstrated a significant increase in the risk of adverse outcomes among patients ≥80 years old.24 The patients within the older cohort had a statistically significant increase in the incidence of arch elongation, arch calcification, vessel origin stenosis, common carotid tortuosity, and severity of the target lesion stenosis compared to patients <80 years old. These factors, when present, can increase the difficulty of successful wire and catheter manipulation, leading to cerebral embolization of vulnerable atherosclerotic plaques from the arch, associated vessel, or target carotid lesion. We have previously demonstrated the impact of aortic arch calcification and elongation on the outcomes of CAS. In our series, the aortic calcium content was calculated using the Agatston coronary calcium score from thoracic computed tomography (CT) scans.25 The results showed a correlation between increased aortic calcification and age ≥75 years. Older patients in this series were also more likely to have a type II arch. Another series showed an increase in the number of embolic lesions seen on post-CAS diffusion-weighted MRI among patients with severe aortic arch calcifications and ulceration of the target carotid lesion.26 Octogenarians had a significantly higher number of new embolic lesions than younger patients. A third study showed that aortic arch type was the only variable independently associated with adverse neurologic outcomes following CAS,27 again highlighting the prevalence of arch calcifications and ulcerated carotid plaque phenotype among older patients. Last, in addition to unfavorable anatomic factors that occur in elderly patients, the cerebral reserve may also be decreased, making them more susceptible to ischemic events after CAS. A review of 916 cerebral blood flow studies revealed an abnormal response to intracranial vasodilator administration, indicating decreased cerebral vascular reserve in patients >70 years old with significant carotid stenosis or peripheral vascular disease.28 This finding suggests that elderly patients are less tolerant of the cerebral embolization that occurs during CAS, making them more likely to have a symptomatic ischemic event.
URGENT CAROTID INTERVENTIONS
The basis for undertaking an urgent CEA during the index hospitalization is based on natural history data that suggest the risk of stroke after a TIA is near 10% at 7 days.29 Pooled analysis from CEA trials has demonstrated the greatest benefit within 14 days from the ischemic event, and the benefit falls rapidly with delay of surgery.30 Debate remains as to the timing of surgical intervention in the acutely symptomatic patient. It has been shown that if a patient has a TIA, the likelihood of another event is highest within 1 week of the event, and a model exists based on large natural history data that quantifies the risk of stroke after TIA.29 A total of 7 points are calculated based on the assignment of single points for each of the following: age >60 years (A), systolic blood pressure >140 mmHg (B), unilateral weakness (C), duration of TIA >60 minutes (D), and presence of diabetes (D). Two days after a low ABCD2 TIA score (0-3), the risk of stroke is low: 1%. However, 2 days after a high score (6-7) the risk of stroke is 8.1%. Additionally, the aggressive natural history is highlighted by a risk of stroke of 11.7% 7 days after presentation with an ABCD2 TIA score of 6-7.
These data have prompted us and others to be aggressive in employing CEA during the index hospitalization in select patients with nondisabling stroke.30,31 The absence of level I evidence is because of the lack of randomized trials addressing urgent carotid interventions. A multidisciplinary recommendation from the American Heart/American Stroke associations deemed a class IIa, level of evidence B for undertaking CEA within 2 weeks after a TIA or stroke rather than delaying surgery if there are no contraindications to the early revascularization.32 We previously demonstrated that in patients with few medical comorbidities urgent CEA could be undertaken safely with outcomes similar to electively performed CEAs.33
We have also recently reported the importance of close collaboration with a stroke/vascular neurology team to be able to treat more patients who present with acute neurologic symptoms because of carotid disease.31 The periprocedural outcomes for urgent CEAs in patients with stable and unstable neurologic symptoms presenting with a National Institutes of Health Stroke Scale (NIHSS) score <10 were safely tolerated. There is a paucity of data in the neurovascular surgery literature regarding the NIHSS scores under which urgent CEAs are well tolerated, which we defined as NIHSS <10. Interestingly, the proportion of asymptomatic patients undergoing CEAs per year fell from 69% to 56% during 4 years. This interesting observation likely reflects an increasingly conservative stance on asymptomatic carotid occlusive disease, consistent with the increasing trend of utilizing BMT alone in select patients with asymptomatic carotid disease.
Given the absence of randomized controlled trials supporting the notion of an aggressive approach for a patient with severe carotid-related acute neurologic ischemic symptoms such as stroke in evolution, better protocols need to be developed in the future to determine which patients will benefit from emergent compared to urgent CEA. Increasing evidence supports the notion of aggressive management for patients presenting with stroke in evolution34 as we undertook in 5 of 49 patients in our urgent CEA cohort, leading to revascularization within 4 hours of initiation of symptoms.
CASE STUDY
A 75-year-old man with a history of hypertension and hyperlipidemia experienced a sudden onset of left-sided weakness and poor coordination while having lunch. He complained of headache over his right eye, describing it as sharp and nonradiating, with no known aggravating or relieving factors. He denied slurred speech, double vision, nausea, vomiting, and/or numbness. He presented to the emergency department where the stroke service found him to have an NIHSS of 4, representing a minor stroke. The patient also had a history of recurrent deep venous thrombosis in his lower extremities and was on warfarin but not on any antiplatelet medication. An MRI of his head demonstrated small foci of restricted diffusion in the right occipital, parietal, and frontal lobes compatible with small areas of embolic infarction (Figure 2). A CT perfusion scan showed increased mean transit time and decreased blood flow with maintained blood volume involving the majority of the right cerebral hemisphere, suggesting an ischemic penumbra from slow vascular blood flow (Figure 3). The CT angiography portion of the study demonstrated a high-grade left carotid stenosis with large amounts of soft atheroma (Figure 4). He was outside the window for thrombolysis, and his anticoagulation status put him at high bleeding risk. Given his constellation of signs and symptoms combined with the large penumbra found on CT perfusion, he was taken to the operating room for an urgent CEA within 2 hours of his presentation. Intraoperatively, a large friable and soft atherosclerotic plaque with areas of ulceration and subplaque hemorrhage was found. The surgery was completed without incident. The patient recovered well with no neurologic deficits and was discharged home 3 days postoperatively.
Figure 2.
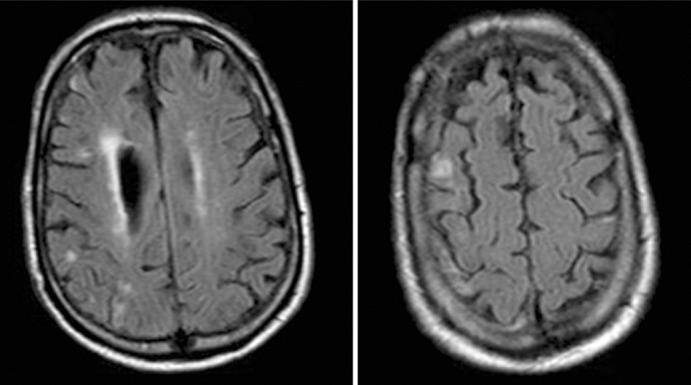
Magnetic resonance imaging demonstrates several small foci of restricted diffusion within the right occipital, parietal, and frontal lobes compatible with small areas of embolic infarction.
Figure 3.
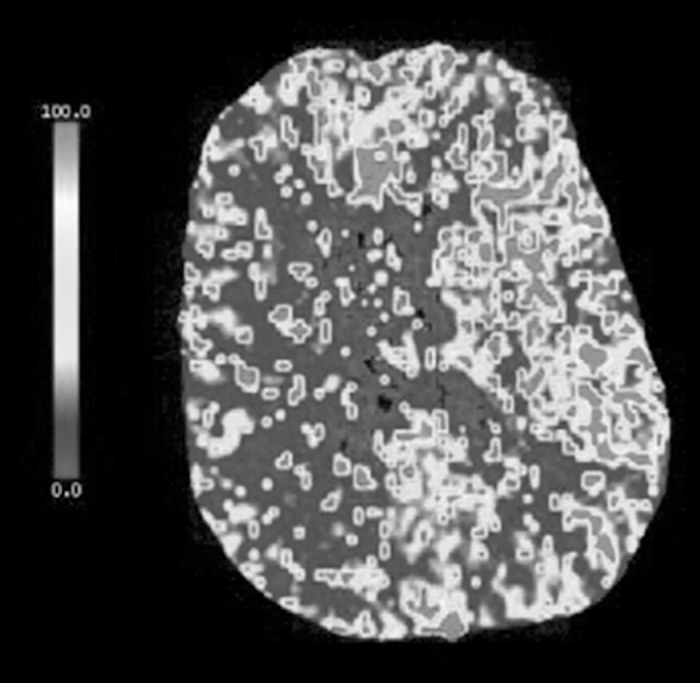
A computed tomography perfusion scan shows increased mean transit time and decreased blood flow with maintained blood volume involving the majority of the right cerebral hemisphere, suggesting possible ischemic penumbra from slow vascular blood flow. No evidence suggests significant core infarction. There is relative sparing of the right anterior cerebral artery territory likely via collateral flow from the anterior communicating artery.
Figure 4.
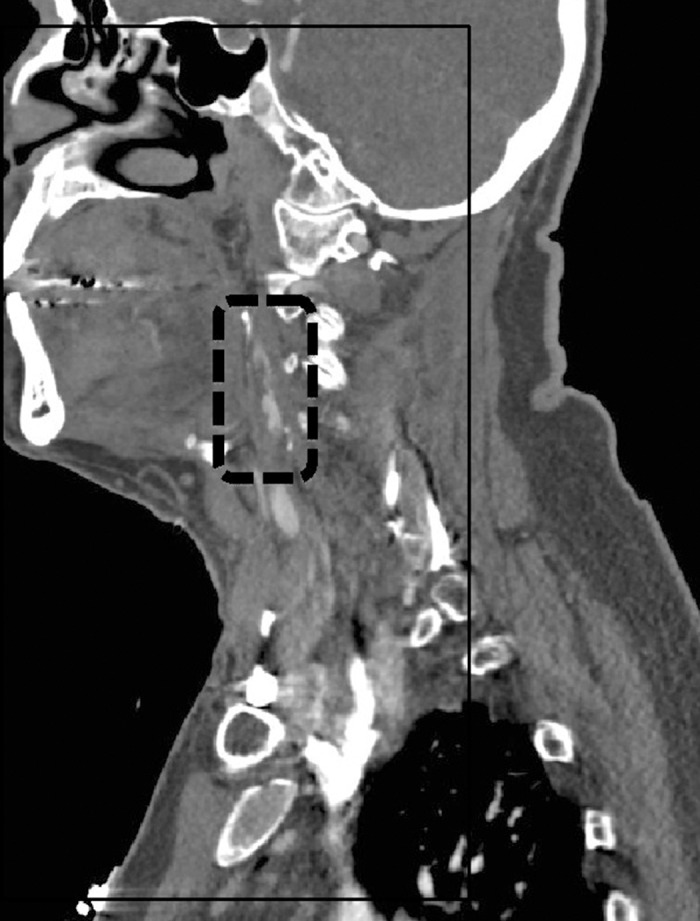
A sagittal representative computed tomography angiography image of the left internal carotid artery shows an atherosclerotic plaque resulting in high-grade 90% stenosis approximately 1.5 cm distal to the bifurcation and spanning a distance of approximately 3.5 cm along the vessel course. Superimposed thrombus is also noted in this region.
FUTURE DIRECTIONS
Current research efforts are reviewing imaging modalities and serum and plaque markers to help assess plaque vulnerability and instability. Early data from this work have revealed that patients who have undergone CEA for symptomatic stenosis were more likely to have soft plaque than patients undergoing CEA for asymptotic lesions. Also, a biobank of carotid plaques is being studied to identify biomarkers that may assist in the identification of vulnerable and unstable plaques.
The optimal treatment for asymptomatic, high-grade carotid stenosis has become increasingly controversial during the past 5 years. Prior randomized controlled studies demonstrated that CEA provided significantly better reduction in stroke compared to medical therapy.3 The annual risk of stroke in patients with asymptomatic >60% carotid stenosis was demonstrated to be 2.2%-2.4% in the medical arms of the ACAS3 and ACST18 trials. However, medical therapy for carotid artery disease has improved since these trials were performed, making it less clear whether there continues to be a net benefit for carotid intervention (CEA or CAS) in this patient cohort. Future studies will hopefully shed light on which asymptomatic patients BMT is adequate for and identify the patients for whom CEA (or CAS) is the best mode of therapy. There is likely a subpopulation of patients, such as those with a tight carotid stenosis (>90%) or with rapidly progressing carotid stenosis, whose stroke risk cannot completely be abolished by medical treatment alone.
As healthcare costs become increasingly scrutinized, the cost effectiveness of these 2 interventions has also been investigated. Most of the studies have come to a similar conclusion, which we recently highlighted, that CEA is more cost effective than CAS.35
Given these increasing uncertainties about the optimal management of asymptomatic carotid artery disease, the NIH has funded CREST-2. This multicenter randomized trial has 2 arms—CEA vs BMT only and CAS vs BMT only—and a 1:1 randomization between intervention and BMT. The patient will have the choice of enrolling in either the CEA arm or the CAS arm of the study. Thus, the randomization assigns intervention or BMT, not the type of intervention (CEA or CAS). Patients assigned to CEA or CAS will also receive the same BMT as those randomized to medical treatment. This trial will likely take nearly a decade to enroll and produce results with at least a 4-year follow-up.
In the future, patients presenting with acute neurologic ischemic events should primarily be routed toward CEA because of the lower periprocedural stroke risk with CEA compared to CAS. In conjunction with a dedicated stroke/vascular neurology service, a clinical pathway could be implemented so all patients with acutely symptomatic carotids are referred by the vascular neurology service to vascular surgery for evaluation and revascularization management. Although CEA has been the preferred primary mode of treatment at our institution by our stroke/vascular neurology team, in select medically high-risk acute symptomatic patients, such as those with chronic obstructive pulmonary disease with home oxygen dependency or debilitated cardiac disease with ejection fraction <20%, CAS with proximal protection or embolic protection device has been undertaken. If an intracranial intervention is necessary for neurorescue, a neurointerventional team is available for endoluminal therapy. As significant stroke reduction could be achieved by undertaking an aggressive approach toward patients presenting with an acutely symptomatic carotid lesion, future research will address new ideas about establishing further novel stroke service lines, rapid access TIA/stroke clinics, and collaborations between vascular surgery and neurology, particularly a stroke/vascular neurology team, in the care of select patients with stable and unstable neurologic symptoms.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014 Jan 21;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Eng J Med. 1991 Aug 15;325(7):445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 3.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995 May 10;273(18):1421–1428. [PubMed] [Google Scholar]
- 4.Neal B, MacMahon S, Chapman N. Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet. 2000 Dec 9;356(9246):1955–1964. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006 Jun;37(6):1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. Erratum in: Stroke. 2007 Jan;38(1):207. [DOI] [PubMed] [Google Scholar]
- 6.Lindholm LH, Ibsen H, Dahlöf B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002 Mar 23;359(9311):1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 7.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004 Aug 21-27;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 8.Paciaroni M, Hennerici M, Agnelli G, Bogousslavsky J. Statins and stroke prevention. Cerebrovasc Dis. 2007;24(2-3):170–182. doi: 10.1159/000104474. [DOI] [PubMed] [Google Scholar]
- 9.Sillesen H, Amarenco P, Hennerici MG, et al. Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2008 Dec;39(12):3297–3302. doi: 10.1161/STROKEAHA.108.516450. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez BL, D'Agostino R, Abbott RD, et al. Risk of hospitalized stroke in men enrolled in the Honolulu Heart Program and the Framingham Study: A comparison of incidence and risk factor effects. Stroke. 2002 Jan;33(1):230–236. doi: 10.1161/hs0102.101081. [DOI] [PubMed] [Google Scholar]
- 12.Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003 Jul-Aug;46(1):11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- 13.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002 Jan 15;136(2):161–172. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 14.Pearson TA, Blair SN, Daniels SR, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002 Jul 16;106(3):388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005 Mar 31;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 16.Eastcott HH, Pickering GW, Rob CG. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet. 1954 Nov 13;267(6846):994–996. doi: 10.1016/s0140-6736(54)90544-9. [DOI] [PubMed] [Google Scholar]
- 17.DeBakey ME. Successful carotid endarterectomy for cerebrovascular insufficiency. Nineteen-year follow-up. JAMA. 1975 Sep 8;233(10):1083–1085. [PubMed] [Google Scholar]
- 18.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004 May 8;363(9420):1491–1502. doi: 10.1016/S0140-6736(04)16146-1. Erratum in: Lancet. 2004 Jul 31;364(9432):416. [DOI] [PubMed] [Google Scholar]
- 19.Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010 Sep 25;376(9746):1074–1084. doi: 10.1016/S0140-6736(10)61197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010 Jul 1;363(1):11–23. doi: 10.1056/NEJMoa0912321. Errata in: N Engl J Med. 2010 Jul 8;363(2):198. N Engl J Med. 2010 Jul 29;363(5):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Carotid Stenting Study investigators. Ederle J, Dobson J, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010 Mar 20;375(9719):985–997. doi: 10.1016/S0140-6736(10)60239-5. Erratum in: Lancet. 2010 Jul 10;376(9735):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonati LH, Jongen LM, Haller S, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS) Lancet Neurol. 2010 Apr;9(4):353–362. doi: 10.1016/S1474-4422(10)70057-0. Erratum in: Lancet Neurol. 2010 Apr;9(4):345. [DOI] [PubMed] [Google Scholar]
- 23.Khatri R, Chaudhry SA, Vazquez G, et al. Age differential between outcomes of carotid angioplasty and stent placement and carotid endarterectomy in general practice. J Vasc Surg. 2012 Jan;55(1):72–78. doi: 10.1016/j.jvs.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent KC, Faries PL. The impact of increasing age on anatomic factors affecting carotid angioplasty and stenting. J Vasc Surg. 2007 May;45(5):875–880. doi: 10.1016/j.jvs.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 25.Bazan HA, Pradhan S, Mojibian H, Kyriakides T, Dardik A. Increased aortic arch calcification in patients older than 75 years: implications for carotid artery stenting in elderly patients. J Vasc Surg. 2007 Nov;46(5):841–845. doi: 10.1016/j.jvs.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 26.Kastrup A, Gröschel K, Schnaudigel S, Nägele T, Schmidt F, Ernemann U. Target lesion ulceration and arch calcification are associated with increased incidence of carotid stenting-associated ischemic lesions in octogenarians. J Vasc Surg. 2008 Jan;47(1):88–95. doi: 10.1016/j.jvs.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 27.Faggioli GL, Ferri M, Freyrie A, et al. Aortic arch anomalies are associated with increased risk of neurological events in carotid stent procedures. Eur J Vasc Endovasc Surg. 2007 Apr;33(4):436–441. doi: 10.1016/j.ejvs.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Chaer RA, Shen J, Rao A, Cho JS. Abu Hamad G, Makaroun MS. Cerebral reserve is decreased in elderly patients with carotid stenosis. J Vasc Surg. 2010 Sep;52(3):569–574. doi: 10.1016/j.jvs.2010.04.021. discussion 574-575. [DOI] [PubMed] [Google Scholar]
- 29.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2007 Dec;6(12):1063–1072. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- 30.Naylor AR. Occam's razor: Intervene early to prevent more strokes! J Vasc Surg. 2008 Oct;48(4):1053–1059. doi: 10.1016/j.jvs.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Bazan HA, Caton G, Talebinejad S, et al. A stroke/vascular neurology service increases the volume of urgent carotid endarterectomies performed in a tertiary referral center. Ann Vasc Surg. 2014 Jul;28(5):1172–1177. doi: 10.1016/j.avsg.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Jan;42(1):227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 33.Bazan HA, Pradhan S, Westvik TS, Sumpio BE, Gusberg RJ, Dardik A. Urgent carotid endarterectomy is safe in patients with few comorbid medical conditions. Ann Vasc Surg. 2008 Jul-Aug;22(4):505–512. doi: 10.1016/j.avsg.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 34.Capoccia L, Sbarigia E, Speziale F, et al. The need for emergency surgical treatment in carotid-related stroke in evolution and crescendo transient ischemic attack. J Vasc Surg. 2012 Jun;55(6):1611–1617. doi: 10.1016/j.jvs.2011.11.144. [DOI] [PubMed] [Google Scholar]
- 35.Sternbergh WC, 3rd, Crenshaw GD, Bazan HA, Smith TA. Carotid endarterectomy is more cost-effective than carotid artery stenting. J Vasc Surg. 2012 Jun;55(6):1623–1628. doi: 10.1016/j.jvs.2011.12.045. [DOI] [PubMed] [Google Scholar]