Abstract
Background
Various factors must be taken into account when considering lung transplantation, including candidacy, contraindications, and outcomes.
Methods
This article presents a review of the data and literature on lung transplantation, tracking the evolution of the treatment as it applies to different conditions, as well as an examination of patient survival rates in relation to pathology and treatment.
Results
Timely referral and careful selection of candidates for lung transplantation maximize the outcomes of the procedure, resulting in a longer lifespan with improved physical health for patients.
Conclusion
Lung transplantation is a therapeutic option for patients with various lung diseases. Adapting treatment options and follow-up treatment to the individual patient's lifestyle and pathology optimizes patient survival rates after transplantation.
Keywords: Heart-lung transplantation, lung transplantation
INTRODUCTION
In 1963, Dr James Hardy attempted the first lung transplant in humans at the University of Mississippi. He performed a left lung transplant on a patient who had bronchogenic squamous cell cancer. This patient survived for 18 days.1 The 1980s saw the development of lung transplantation, starting in 1981 when Dr Bruce Reitz at Stanford University performed a heart-lung transplant on a patient with pulmonary arterial hypertension.2 Dr Joel Cooper from the Toronto Lung Group followed by performing the first successful single lung transplant in a patient with idiopathic pulmonary fibrosis in 1983. He continued to experiment with bilateral lung transplantation until in 1989 he developed the bilateral sequential lung transplant with Dr Michael Pasque at Washington University in St. Louis.3 Thanks to the breakthroughs of past decades, the field of lung transplantation continues to evolve as a therapeutic option for patients with various lung diseases.
In 2011, 3,640 lung transplants were performed and registered with the International Society for Heart and Lung Transplantation (ISHLT). This figure has continued to rise on an annual basis.4 Despite its evolution, lung transplantation is still unfamiliar territory for pulmonologists and nonpulmonologists. The goal of this review is to familiarize the reader with the different aspects of lung transplantation, including candidacy, contraindications, and outcomes.
INDICATIONS FOR EVALUATION AND TRANSPLANTATION
The general indication for lung transplantation is a progressive respiratory condition for which no proven therapy is available or maximal medical therapy is ineffective.
The decision to transplant varies from condition to condition. In deciding whether a patient should be listed for transplantation, the most important question is whether undergoing transplantation offers a survival benefit to the patient. Simply put, the clinician needs to determine whether the benefit of the transplant outweighs the risk of avoiding the procedure.
The recommendation is to refer patients for transplantation when their 2-3 year survival rate is <50% or when their New York Heart Association (NYHA) functional class is III or IV. Because predicting the 2-3 year survival rate for these conditions is difficult, early referral is desirable.
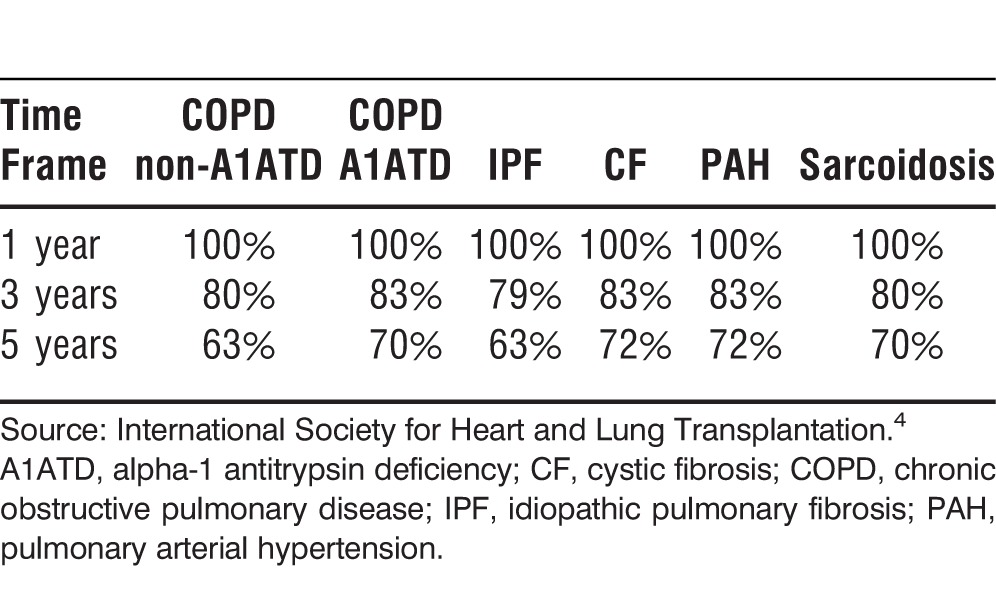
The conditions most commonly evaluated for transplantation include chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, cystic fibrosis, pulmonary hypertension, and sarcoidosis. Each of these conditions has a set of clinical parameters to guide the decision to refer and perform a transplant on the patient (Table 1).5 Also, the guidelines for these conditions can guide the decision to refer for transplant patients with other pulmonary conditions that share similar characteristics.
Table 1.
Guidelines for Evaluation and Transplantation for the Most Common Pulmonary Conditions
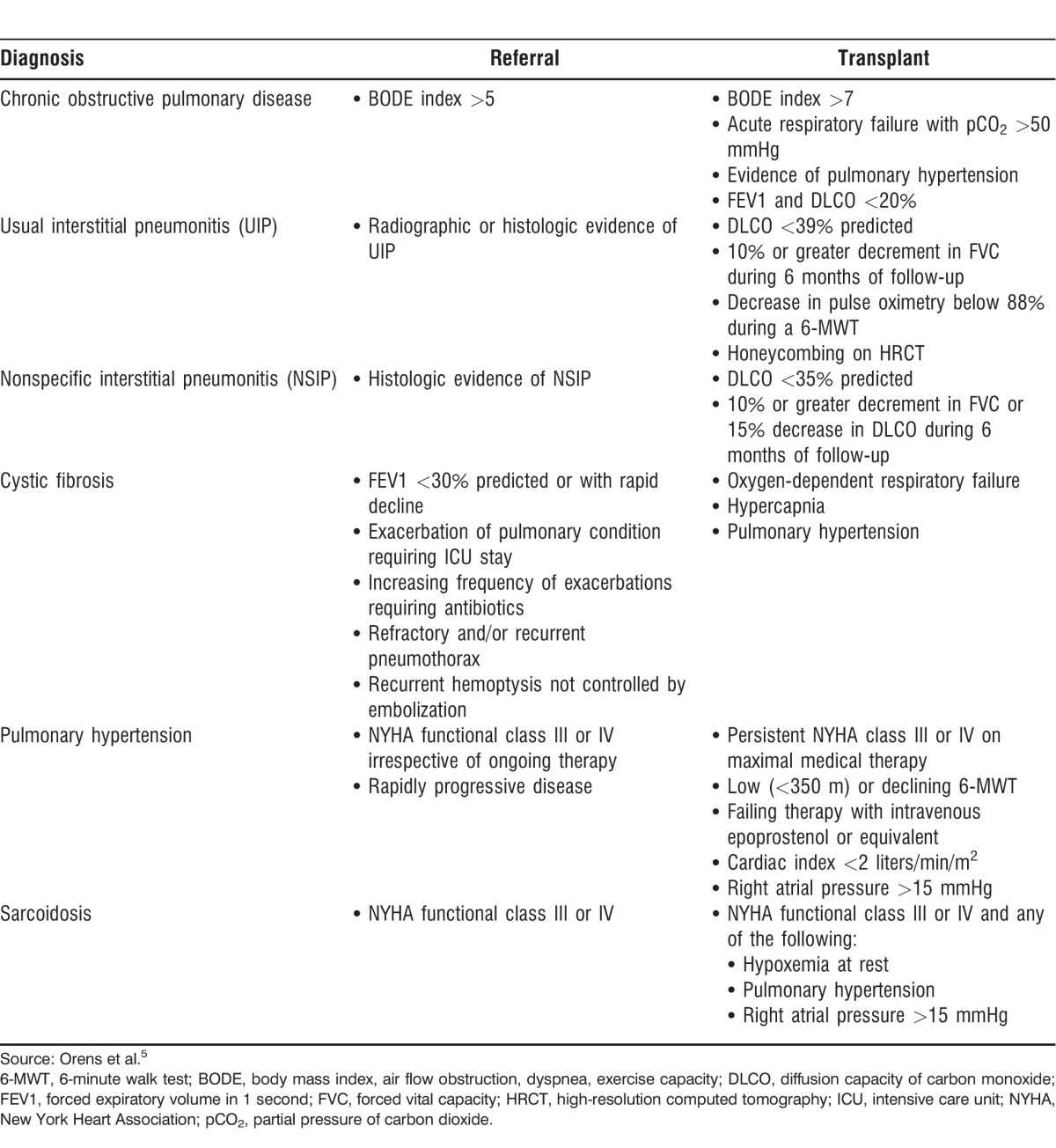
Chronic Obstructive Pulmonary Disease
Non–alpha-1-antitrypsin deficient COPD has remained the most common indication for lung transplantation, with approximately 34% of all lung transplants performed on this patient population.4 The body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) index is a tool that helps physicians decide when to start evaluating patients with COPD for transplantation because it gives physicians an estimate of the patient's 4-year survival.6 The recommendation for physicians is to refer patients with COPD to be evaluated for transplantation when their BODE index exceeds 5.5
The decision to list a patient with COPD for transplantation should be multifactorial. A BODE index >7 gives the patient an 18% survival rate within 4 years.6 Other risk factors that decrease survival in patients with COPD have been identified and serve as a guideline for transplantation irrespective of the BODE index.
Pulmonary Fibrosis
Interstitial lung diseases (ILDs) are the second most common indication for lung transplantation.4 These conditions carry a high mortality rate that is difficult to predict. Idiopathic pulmonary fibrosis (IPF) is the clinical manifestation of usual interstitial pneumonitis (UIP) when no other etiology has been identified. The forced vital capacity (FVC) is the strongest predictor of mortality in this patient population. Flaherty et al showed that a decrease in absolute FVC of 10% or more in a 6-month period is the strongest predictor of mortality, followed by a similar decrease in 12 months.7 The 6-minute walk test (6-MWT) was also found to be a strong predictor of mortality in a large population of IPF patients undergoing a therapeutic trial.8 In that study, a decrease of more than 50 meters in 24 weeks was associated with a 4-fold increase in 1-year mortality. A prediction model that takes into consideration age, FVC, change in FVC, and hospitalizations has been published but has yet to be validated in larger populations of patients with IPF.9 The recommendation then is to refer patients with UIP (IPF or non-IPF) for transplantation as soon as the diagnosis is confirmed.
Nonspecific interstitial pneumonitis (NSIP) is a condition that is usually a manifestation of connective tissue diseases and is subdivided into cellular and fibrotic types. The cellular type of NSIP can have a favorable response to immunomodulating therapies and can also have better outcomes than the fibrotic type. Fibrotic NSIP has a less favorable response to therapy and follows a course similar to UIP.10,11 The recommendation is to refer patients with NSIP for transplant evaluation as soon as the diagnosis is confirmed.
The decision to list a patient with UIP or NSIP for transplant depends on the progression of the patient's disease. Early referral and listing are recommended, so the transplant center can provide close follow-up.
Cystic Fibrosis
Respiratory failure is the most common cause of mortality in patients with cystic fibrosis (CF).12 Bronchiectasis is the pulmonary condition this patient population develops. It is characterized by recurrent lower respiratory tract infections and progressive airway obstruction. Patients with CF are commonly colonized with highly virulent organisms such as multidrug-resistant Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus.
The timing for referring a patient for transplantation depends on the patient's pulmonary function as well as the progression of the disease. The recommendation for physicians is to refer a patient with CF when the patient's forced expiratory volume (FEV1) decreases below 30% predicted or when the patient has had more than 2 hospitalizations for exacerbations of bronchiectasis in a 12-month period. Both of these characteristics are associated with an increase in 2-year mortality for CF patients.12
The decision to list a patient with CF for transplantation depends on the progression of the disease and on the development of chronic respiratory failure.
Pulmonary Hypertension
Pulmonary hypertension (PH) is characterized by an increase in pulmonary vascular resistance leading to progressive right ventricular failure. The World Health Organization (WHO) has 5 classifications for PH, depending on the etiology of the disease.13 Pulmonary arterial hypertension (PAH) is classified as WHO group 1. Current therapies for PAH are mostly reserved for patients in WHO group 1. Diagnoses under WHO group 1 that receive therapy include mostly idiopathic PAH and PAH associated with connective tissue diseases.
Before therapy with pulmonary vasodilators was available for PAH, median survival was 2.8 years from the time of diagnosis.5 With therapy, survival has improved to 80% at 2 years from diagnosis.14 The timing to referral for lung transplantation has changed from the moment of diagnosis to the moment when the patient is failing on maximal medical therapy. Given the complexity of these cases, many transplant centers will routinely follow the patient with the PH specialist to more accurately decide the time to list the patient for transplantation.
The identified risk factors that increase mortality in patients with PAH include worsening NYHA class, worsening 6-MWT, hospitalizations, and addition of prostanoids to therapy.14 When any of these risk factors are identified, the patient should be evaluated for transplantation. Listing the patient and transplanting depend on the presence of these risk factors in addition to the patient's hemodynamics.
Sarcoidosis
Sarcoidosis is a chronic granulomatous disease of unknown origin that affects multiple organ systems. Pulmonary involvement is present in 90% of patients with sarcoidosis, with about 30% of patients developing progressive pulmonary disease.15 ILD is the most common presentation of progressive pulmonary disease.
About 5%-14% of patients with sarcoidosis develop PAH. Sarcoidosis-associated PAH is categorized as WHO group 5 because it is still unclear whether the PAH is secondary to progressive parenchymal lung disease, compression of the central pulmonary vasculature by lymphadenopathies, left ventricular dysfunction, or a vasculopathy. Properly selected patients should have a good response to pulmonary vasodilator therapy.16
Timing for referral of patients with sarcoidosis depends on their functional status. The recommendation is to refer patients with NYHA class III-IV. Risk factors associated with increased mortality in patients with sarcoidosis include African American race, the presence of PH, and supplemental oxygen use.17
Connective Tissue Diseases
Connective tissue diseases present a special challenge to the transplant pulmonologist. Often these patients present with ILD (UIP, NSIP), obstructive lung disease (obliterative bronchiolitis), and/or PH. Respiratory symptoms can present before the onset of other systemic symptoms. When deciding whether to list these patients for transplant, special attention must be directed to the function of other organs during the evaluation. Examples include thorough evaluation of gastroesophageal function in patients with systemic sclerosis and skeletal muscle evaluation in patients with myositis.
Other Pulmonary Conditions
Patients with pulmonary conditions not mentioned above can also be referred for transplantation. Examples include lymphangioleiomyomatosis, pulmonary Langerhans cell histiocytosis, refractory asthma, fibrotic organizing pneumonia, and chronic hypersensitivity pneumonitis. These conditions have no specific guidelines for transplantation but share characteristics with more established conditions, and decisions can be made on a case-by-case basis.
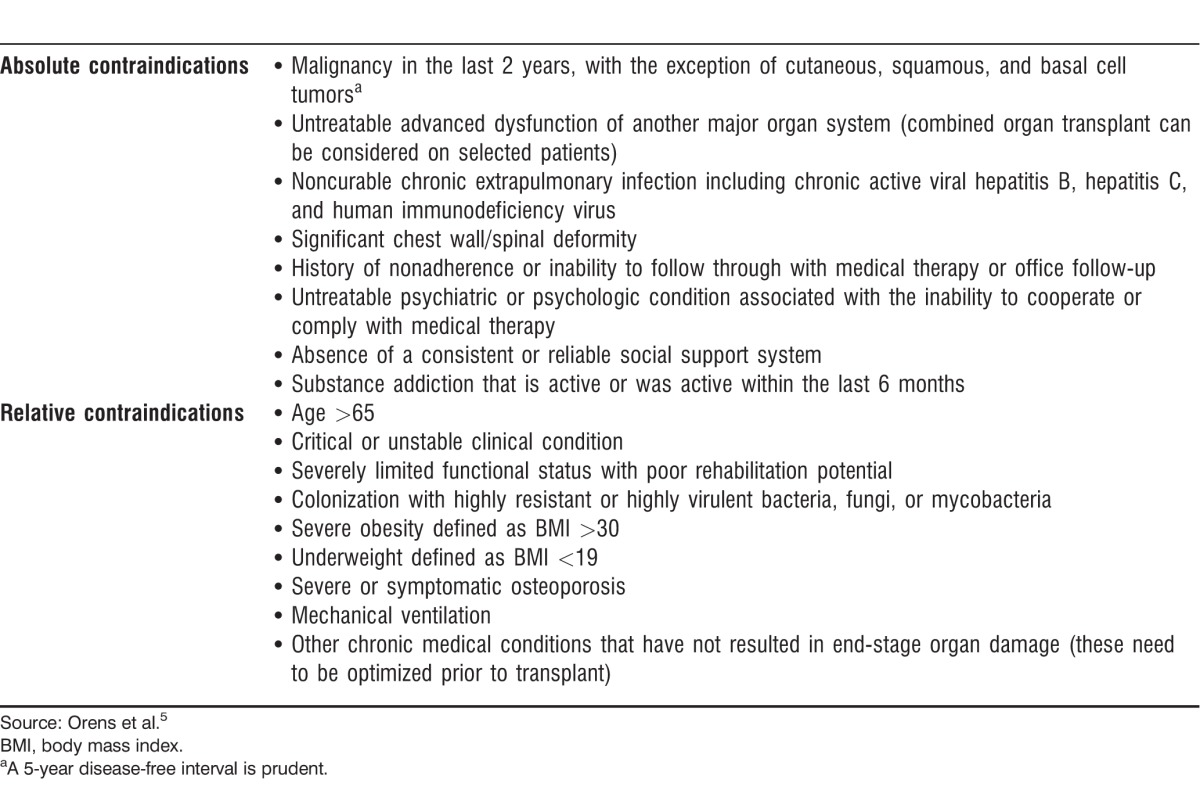
CONTRAINDICATIONS FOR LUNG TRANSPLANTATION
Selection of candidates for lung transplantation is a complex multidisciplinary process. Absolute and relative contraindications for lung transplantation have been identified to reduce morbidity and mortality in the posttransplant period and are summarized in Table 2.5 Variations occur among different lung transplant programs regarding the classification of absolute and relative contraindications.
Table 2.
Contraindications for Lung Transplantation
TRANSPLANTATION PROCEDURES
Three main types of surgical procedures can be performed on lung transplant candidates: single lung transplant (SLT), bilateral sequential lung transplant (BSLT), and heart-lung transplant (HLT). Procedure selection depends on recipient characteristics and donor availability.
BSLT is the procedure of choice, and the number of BSLTs performed per year continues to increase. In general, patients with chronic pulmonary infections (CF, bronchiectasis) should receive a BSLT to prevent infection of the graft from the contralateral native lung. Also, patients with PAH and severe secondary PH should receive BSLT to maximally decrease pulmonary vascular resistance and allow faster recovery of right ventricular function.
SLTs are offered in general to patients with COPD and ILDs (UIP, NSIP). The decision to offer an SLT is multifactorial. Many patients have had previous thoracic surgeries or pleurodeses, making a pneumonectomy difficult; therefore an SLT is performed. Previous cardiac surgeries such as coronary bypass with a left internal mammary artery may also preclude a left pneumonectomy. Organ availability also plays a role in the decision to perform an SLT. A single donor's lungs can be used for 2 recipients to maximize donor organ utilization. Also, some donors may have a damaged lung because of their mechanism of death, so only one lung can be utilized.
Early outcomes of BSLT and SLT are comparable. Survival with BSLT appears to be higher 3 years after the transplant. These data are retrospective and uncontrolled, making it difficult to choose one type of transplant over the other in situations where both procedures can be performed.4
An HLT is generally reserved for patients with Eisenmenger syndrome secondary to congenital heart disease. Many centers prefer HLT for patients with PAH with severe right ventricular dysfunction. HLT is also performed on patients who require a lung transplant and have severe left ventricular dysfunction or severe coronary artery disease. A review of BSLT compared to HLT for patients with PAH showed that HLT is the better choice for congenital heart disease.18 The review also showed that patients with PAH not associated with congenital heart disease had no difference in survival between BSLT and HLT. An important consideration when making the decision to list a patient for BSLT or HLT is that wait times are generally longer for HLT than BSLT because HLT falls under the heart transplant allocation policy instead of the lung transplant allocation policy.
WAITING LIST
In May 2005, the Organ Procurement and Transplantation Network (OPTN) adopted the lung allocation score (LAS) in the United States. The goal of the LAS is to allocate organs to decrease waiting list patient mortality while improving posttransplant survival. Before the adoption of the LAS, allocation of organs was based on time accrued on the waiting list; this system was beneficial to COPD patients, but patients with IPF had increased mortality.19 Factors that determine the LAS include diagnosis, FVC, oxygen requirements, and 6-MWT distance. The higher the LAS, the higher the likelihood the recipient will receive an organ offer, but a higher LAS also means that the patient's disease has advanced and the patient has a higher risk of dying on the waiting list than patients with lower scores.20
Criticism of the LAS system comes mainly from the PAH community. After the implementation of the LAS, waiting-list mortality decreased for all diagnoses except PAH.21 Patients with PAH are usually younger and have a preserved FVC that confers a lower LAS. Modifications to the LAS have been proposed that include using mean right atrial pressure >14 mmHg and a 6-MWT distance of <300 m in the calculation of the LAS in patients with PAH. These 2 variables are better discriminators of urgency in this patient population.22
The OPTN allocates organs for transplantation. Allocation is first by identical blood type and then by compatible blood type. The organ is allocated to the patient with the highest LAS within the blood type group.
How much time the wait-listed patient will wait depends on several factors: blood type, LAS, age, sex, and transplant center. Nationally, the median time on the waiting list is 3.6 months. Blood type is an important factor in waiting times.23 For example, the most commonly listed blood type is O (49.5%), so when a blood type O donor organ becomes available, the organ is usually allocated to the patient with the highest LAS, and patients with a lower LAS therefore continue to wait. In contrast, blood type AB patients are the least commonly listed (2.2%), so when a blood group AB donor organ becomes available, it is possible that only one patient is on the center's AB list. That patient will have a shorter waiting time than patients with other blood types.23
SURVIVAL
The ISHLT and the Scientific Registry of Transplant Recipients (SRTR) report survival rates after lung transplantation. The main difference between these databases is that the ISHLT reports data from all centers that voluntarily report the data worldwide, while the SRTR reports on all lung transplants performed in the United States under the oversight of the OPTN.
Data from the ISHLT show that the median survival after lung transplantation was 5.6 years during 1994-2011. Unadjusted survival rates at 1, 3, and 5 years are approximately 79%, 64%, and 53%, respectively. Survival also varies by underlying diagnosis and age. Patients with CF and PAH have a higher conditional survival than patients with COPD or IPF. Younger patients have a higher overall survival rate (Tables 3-5).4
Table 3.
Approximate Survival After Lung Transplantation by Recipient Diagnosis, 1990-2011
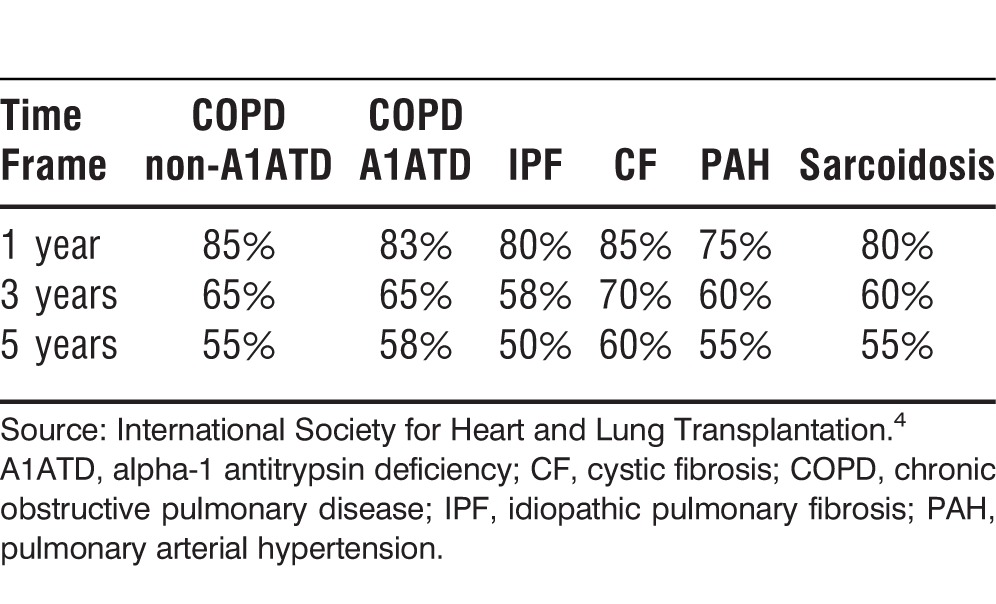
Table 5.
Approximate Survival After Lung Transplantation by Recipient Age, 1990-2011
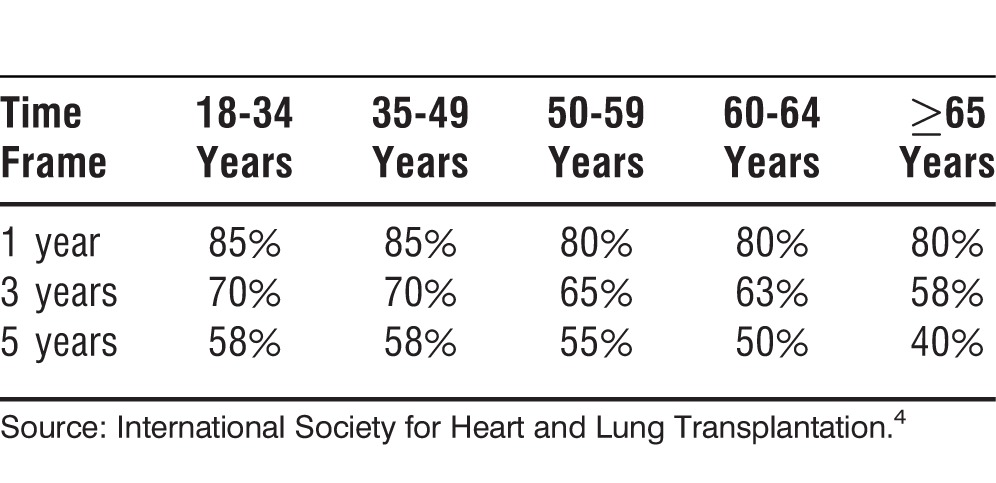
Table 4.
Approximate Survival After Lung Transplantation by Recipient Diagnosis Conditional on Survival to 1 Year, 1990-2011
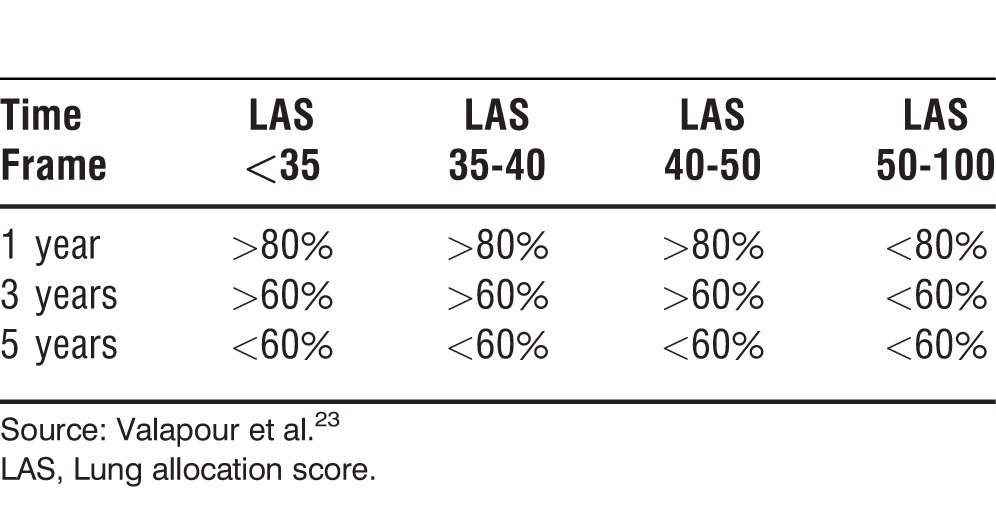
The SRTR reports similar survival rates but also adds the relationship between the LAS and patient survival. The data show that having a LAS <50 confers a 1-year survival rate >80%, but a LAS >50 is associated with a 1-year survival <80% (Table 6).23
Table 6.
Approximate Survival After Lung Transplantation by LAS at the Moment of Transplant, 2005-2006
COMPLICATIONS
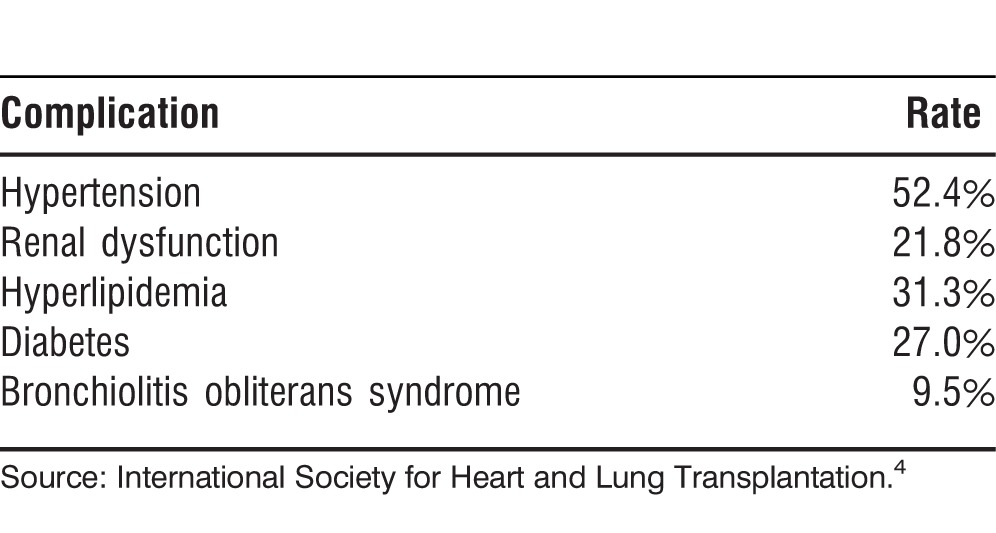
The most common complications after lung transplantation are the result of immunosuppressive therapy. Like other solid-organ recipients, patients after lung transplantation receive triple immunosuppression with a calcineurin inhibitor (tacrolimus or cyclosporine), a purine synthesis inhibitor (mycophenolate mofetil or azathioprine), and corticosteroids. If too much immunosuppression is administered, the patient has a higher risk of developing infectious complications, renal dysfunction, bone marrow toxicity, diabetes, and malignancies. On the contrary, if immunosuppression is lessened to decrease adverse reactions, the risk of graft rejection increases. A fine balance must be achieved with each patient to avoid graft loss and toxicities. Table 7 shows the incidence of the most common complications after lung transplantation.4
Table 7.
Most Common Complications Within 1 Year of Lung Transplantation, 2003-2012
The major complication of lung transplantation is the development of chronic lung allograft dysfunction. Three phenotypes are described, but the most common is bronchiolitis obliterans syndrome (BOS). BOS is characterized by a progressive loss of lung function that leads to severe obstructive disease and respiratory failure. Risk factors identified for development of BOS include repeated episodes of acute rejection, infections, and chronic aspiration.24 No proven therapies for BOS are available. Retransplantation is an option but is a strong risk factor for 1-year mortality.4
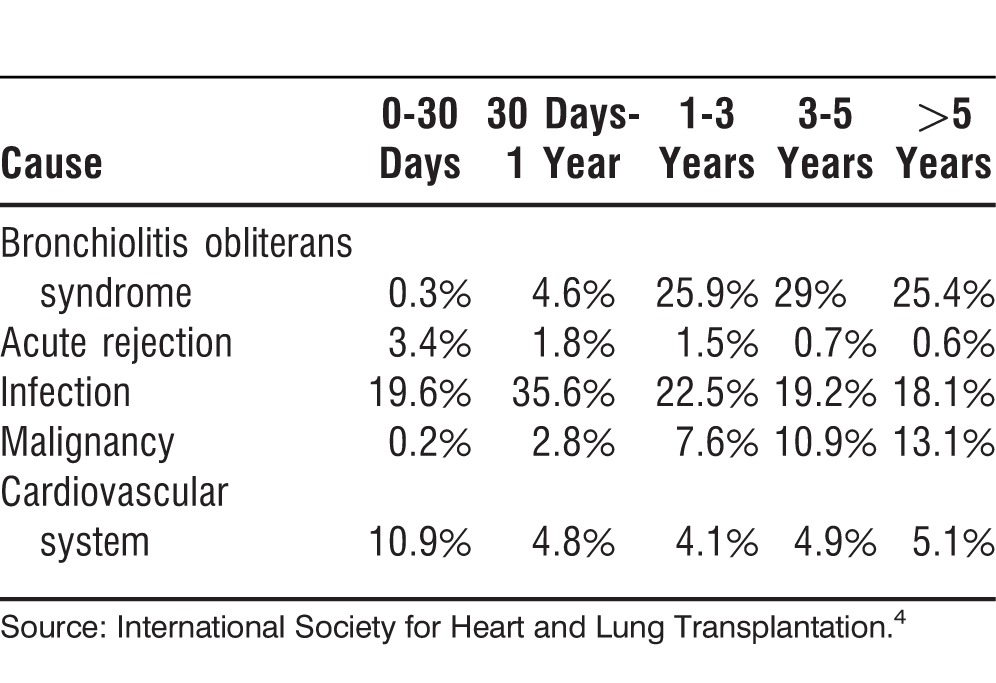
The 2 most common causes of death after lung transplantation are infections and BOS. Infections are seen most commonly in the first 12 months after a transplant, and BOS is more common after the first year.4 Table 8 shows the most common causes of death after lung transplantation.4
Table 8.
Most Common Causes of Death After Lung Transplantation
HEALTH-RELATED QUALITY OF LIFE
Studies of health-related quality of life (HRQL) after lung transplantation have yielded heterogeneous results. A systematic review of the published literature on HRQL after lung transplantation revealed an overall improvement in physical health and functioning. Patients' HRQL did not decrease compared to pretransplant, but their improvement did not reach the HRQL of a normal population.25
A prospective study that used the Medical Outcomes Study 36-Item Short Form Health Survey, version 2 (SF-36) to evaluate quality of life after lung transplantation showed a significant increase in the physical component but not in the mental component score. The results of the mental component were unchanged from baseline pretransplant scores; this lack of change raises the question of how much attention is paid to the psychological well-being of lung transplant patients in the pretransplant and posttransplant periods.26
The development of BOS has also been implicated in decreases in HRQL after lung transplantation. This association was demonstrated in a small prospective observational study that showed the development of BOS decreased the patient's quality of life but interestingly did not decrease the patient's autonomy.27
COST
The 2011 Milliman report on transplant cost estimated the costs of an SLT and a BSLT. The estimated amounts are the sum of the billed charges during the following periods: 30 days pretransplant, organ procurement, transplant admission, physician during transplant, 180 days posttransplant discharge (visits, diagnostics, readmissions), and immunosuppression and other prescriptions (within 180 days after transplant discharge). The estimated cost for an SLT is $561,200 and for a BSLT is $797,300. The authors of the report could not estimate how much a transplant costs a patient because the cost to the patient depends on specific insurance coverage.28
Some authors have addressed the cost effectiveness of the procedure. Results of the analyses vary. Analyses show that the difference in posttransplant survival seen with different pulmonary diseases causes variations in cost effectiveness measured by quality-adjusted life years.29 The research shows that the posttransplant monthly cost to the patient is initially higher compared to the pretransplant monthly cost, but the posttransplant cost decreases over time, becoming more manageable to the patient than the pretransplant cost.30
CONCLUSION
Lung transplantation is a therapeutic option for patients with various lung diseases. Timely referral and careful selection of candidates for lung transplantation maximize the outcomes of the procedure, including a longer lifespan with improved physical health. These patients need careful attention after the transplant to avoid the development of serious medical and psychosocial complications that can decrease their survival.
Footnotes
The author has no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Hardy JD, Eraslan S, Webb WR. Transplantation of the lung. Ann Surg. 1964 Sep;160(3):440–448. doi: 10.1097/00000658-196409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med. 1982 Mar 11;306(10):557–564. doi: 10.1056/NEJM198203113061001. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JK, Mehra MR, West LJ. History of International Heart and Lung Transplantation. Philadelphia, PA: Elsevier;; 2010. [Google Scholar]
- 4.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report—2013; focus theme: age. J Heart Lung Transplant. 2013 Oct;32(10):965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006 Jul;25(7):745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2007 Mar 4;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003 Sep 1;168(5):543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 8.du Bois RM, Weycker D, Albera C, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011 May 1;183(9):1231–1237. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 9.du Bois RM, Weycker D, Albera C, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011 Aug 15;184(4):459–466. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 10.Glaspole I, Goh NS. Differentiating between IPF and NSIP. Chron Respir Dis. 2010 Aug;7(3):187–195. doi: 10.1177/1479972310376205. [DOI] [PubMed] [Google Scholar]
- 11.Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol. 2000 Jan;24(1):19–33. doi: 10.1097/00000478-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002 Dec 15;166(12 Pt 1):1550–1555. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 13.McGlothlin D. Classification of pulmonary hypertension. Heart Fail Clin. 2012 Jul;8(3):301–317. doi: 10.1016/j.hfc.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL Registry Analysis. Chest. 2013 Nov 1;144(5):1521–1529. doi: 10.1378/chest.12-3023. [DOI] [PubMed] [Google Scholar]
- 15.Mihailovic-Vucinic V, Jovanovic D. Pulmonary sarcoidosis. Clin Chest Med. 2008 Sep;29(3):459–473. viii–ix. doi: 10.1016/j.ccm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Cordova FC, D'Alonzo G. Sarcoidosis-associated pulmonary hypertension. Curr Opin Pulm Med. 2013 Sep;19(5):531–537. doi: 10.1097/MCP.0b013e328363f4a3. [DOI] [PubMed] [Google Scholar]
- 17.Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest. 2003 Sep;124(3):922–928. [PubMed] [Google Scholar]
- 18.Olland A, Falcoz PE, Canuet M, Massard G. Should we perform bilateral-lung or heart-lung transplantation for patients with pulmonary hypertension? Interact Cardiovasc Thorac Surg. 2013 Jul;17(1):166–170. doi: 10.1093/icvts/ivt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006 May;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 20.Russo MJ, Worku B, Iribarne A, et al. Does lung allocation score maximize survival benefit from lung transplantation? J Thorac Cardiovasc Surg. 2011 May;141(5):1270–1277. doi: 10.1016/j.jtcvs.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Shiboski SC, Golden JA, et al. Impact of the lung allocation score on lung transplantation for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009 Sep 1;180(5):468–474. doi: 10.1164/rccm.200810-1603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benza RL, Miller DP, Frost A, Barst RJ, Krichman AM, McGoon MD. Analysis of the lung allocation score estimation of risk of death in patients with pulmonary arterial hypertension using data from the REVEAL Registry. Transplantation. 2010 Aug 15;90(3):298–305. doi: 10.1097/TP.0b013e3181e49b83. [DOI] [PubMed] [Google Scholar]
- 23.Valapour M, Paulson K, Smith JM, et al. OPTN/SRTR 2011 Annual Data Report: lung. Am J Transplant. 2013 Jan;13(Suppl 1):149–177. doi: 10.1111/ajt.12024. [DOI] [PubMed] [Google Scholar]
- 24.Knoop C, Estenne M. Chronic allograft dysfunction. Clin Chest Med. 2011 Jun;32(2):311–326. doi: 10.1016/j.ccm.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer JP, Chen J, Blanc PD, Leard LE, Kukreja J, Chen H. A thematic analysis of quality of life in lung transplant: the existing evidence and implications for future directions. Am J Transplant. 2013 Apr;13(4):839–850. doi: 10.1111/ajt.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finlen Copeland CA, Vock DM, Pieper K, Mark DB, Palmer SM. Impact of lung transplantation on recipient quality of life a serial, prospective, multicenter analysis through the first posttransplant year. Chest. 2013 Mar;143(3):744–750. doi: 10.1378/chest.12-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerbase MW, Soccal PM, Spiliopoulos A, Nicod LP, Rochat T. Long-term health-related quality of life and walking capacity of lung recipients with and without bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2008 Aug;27(8):898–904. doi: 10.1016/j.healun.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Bentley TS, Hanson SG. Milliman, Inc; Apr, 2011. 2011 U.S. organ and tissue transplant cost estimates and discussion. http://us.milliman.com/insight/research/health/2011-U_ S_-organ-and-tissue-transplant-cost-estimates-and-discussion/. Accessed July 22, 2014. [Google Scholar]
- 29.Groen H, van der Bij W, Koëter GH, TenVergert EM. Cost-effectiveness of lung transplantation in relation to type of end-stage pulmonary disease. Am J Transplant. 2004 Jul;4(7):1155–1162. doi: 10.1111/j.1600-6143.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 30.Vasiliadis HM, Collet JP, Penrod JR, Ferraro P, Poirier C. A cost-effectiveness and cost-utility study of lung transplantation. J Heart Lung Transplant. 2005 Sep;24(9):1275–1283. doi: 10.1016/j.healun.2004.10.012. [DOI] [PubMed] [Google Scholar]


