Abstract
For centuries, physicians have relied on touch to palpate tissue and detect abnormalities throughout the body. While this time-tested method has provided a simple diagnostic exam for large, superficial abnormalities, it does not permit quantifiable measurements of stiffness in deeper, small organs. Advances in noninvasive imaging to measure tissue rigidity represent important extensions of manual palpation techniques. Tissue fibrosis occurs with age in many organs; in the ovary, it is thought to be a marker of polycystic ovary syndrome (PCOS) and age-related idiopathic infertility, although quantitative assessment of fibrosis in this deep, abdominal tissue has not been possible. We used noninvasive methods to quantify ovarian tissue rigidity and clarify the role of tissue stiffness in reproductive health. With proper validation against accepted standards, noninvasive imaging techniques may become the quantitative counterpart to interior probing palpation methods and invasive (surgical) diagnoses, with applications across many clinical settings, including evaluation of adolescent and young adult ovarian function.
Keywords: elastography, nanoindentation, palpation, alginate, ovary
1. Introduction
Characterization of ovarian tissue rigidity is critical for understanding organ function and diseases such as polycystic ovary syndrome (PCOS) [1]. Unlike the breast or liver, however, the ovary is not typically biopsied for the diagnosis of PCOS, and clinicians must rely on external palpation to detect ovarian tissue abnormalities. Moreover, the ovary is not a regenerating tissue system, and biopsy of the ovarian tissue is thus not an option in young, normally cycling women. Noninvasive measures of ovarian function and rigidity would make it possible to more quantitatively assess mechanical properties of the ovary and diagnose diseases such as PCOS. Moreover, non-invasive measures of ovarian function in adolescents would be far superior to invasive techniques, especially those that require transvaginal probes.
Quantitative methods to measure the mechanical properties of deep, abdominal, soft tissues such as the ovary are still in their infancy [2]. Tissue size, heterogeneity, depth, and porosity present challenges when attempting to accurately assess the material properties of a tissue. Tissues are complex materials and contain fluid—up to 90% or more of total weight—which greatly influences their properties and renders most tissues nearly incompressible (ν≈0.50) [3]. Because of their solid-fluid nature, tissues are more accurately characterized in the frequency domain using viscoelastic material models [4]. Stiffness is measured and reported as the storage modulus (E′ or G′) in the frequency domain. Young’s modulus (E) and shear modulus (G) are related by the Poisson ratio for isotropic homogeneous materials [E=2G*(1+ν)], typically using a factor of 3 for soft materials such as tissues. Borrowing from characterization of traditional heterogeneous materials [5–7], contact or indentation-type measurement methods have been applied to tissues [8–11] to characterize spatially resolved properties. However, this indentation-type methodology is only applicable to in vitro studies and therefore represents a quantifiable, but micro-scale, palpation test that is not amenable to clinical assessment of the ovaries.
MRE is a novel technique that has been used to acoustically probe internal organs, such as the breast and liver, for early detection of cancer and cirrhosis [12]. This method combines mechanical wave propagation methods with magnetic resonance imaging (MRI). During MRE, a low-frequency sound wave (60–120 Hz) is sent into the body through a driver that is placed on the body, pointing towards the area of interest. The sound wave displaces the tissue and the tissue displacements are then measured via MRI and transformed into an equivalent stiffness image, or elastogram. MRE, like MRI, is noninvasive and well accepted by patients [2]. MRE can have up to sub-millimeter to millimeter resolution given the robust algorithm package capable of reducing artifacts and directional filtering of shear waves to eliminate shear wave interference. The resolution of MRE is typically one-fifth to one-half the resolution of the MRI image depending partially on the vibrational frequency of the acoustic wave. MRE represents a technological link between what has been accomplished previously with in vitro imaging and the ability to detect tissue abnormalities through traditional palpation. The ability to detect tissue stiffness abnormalities in vivo, which are measured as hard or soft domains on MRE, can provide important additional prognostic information and insight into the overall health of an individual patient.
Though imaging methods to date have been restricted to assessment of the larger organs such as the liver and lungs, recent developments in driver and software technology are making it possible to penetrate deeper to analyze organs like the ovary. To date, MRE results have not been quantitatively validated by established mechanical characterization methods and have not been applied specifically to assess ovarian rigidity. Our goal was to investigate MRE as a quantitative biomaterial characterization method that could be applied clinically to assess rigidity of the ovary in vivo. We first performed a rigorous validation of MRE on gels and tissues and then compared the results to those of in vitro studies using bovine ovaries. Finally, we examined the ability of MRE to distinguish between healthy and PCOS ovaries in vivo.
2. Materials and Methods
2.1. Animal tissue collection
Bovine ovaries were isolated from heifers (<2 years of age) following kosher slaughter from the Aurora Packing Company in Aurora, IL. The ovarian tissue was immediately stored at 4°C and transported by automobile to Northwestern University (1 hour).
2.2. Human tissue collection
Using an institutional review board (IRB)-approved, HIPAA-compliant protocol, 7 women were selected and gave consent to undergo clinical testing with MRE (see Supplementary Information). Each subject underwent multiple MRE scans to determine interscan variability.
2.3. Agar hydrogel sample processing
Granulated agar (Fisher BioReagents, Waltham, MA) was mixed with DDI water at 2.25 wt% for MRE, nanoindentation, and bulk experiments. The gels were cured using the liquid setting in a standard autoclave for 1 hour.
2.4. Bulk material characterization of gels
RSA III dynamic mechanical analysis (DMA) (TA Instruments, New Castle, DE) was used to test the tensile and compressive viscoelastic properties (E′, E″) of the agar hydrogel samples (phantoms). For rheometry, a series of 5 agar gels specimens were cut using a cylindrical die and tested for shear viscoelastic properties (G′, G″) using a Physic MCR-300 (Anton Paar, Graz, Austria). Specimens were preloaded with 0.2 N to ensure proper contact before samples were subjected to testing. The details of this protocol are included the Supplementary Information.
2.5. Nanoindentation
Indentation testing was performed using a Triboindenter (Hysitron, Minneapolis, MN) and a 100 μm radius spherical tip with a dynamic oscillatory load [4] of 1.0 μN at 10–200 Hz, at a sufficiently large contact depth of between 1.5 and 2 μm. For bovine ovary samples, nanoindentation tests were performed at 3 different sites on each ovary to test for heterogeneities: the cortex, the medulla, and the corpus luteum. At each site, a series of 5 different sites were tested (10 data points per site), sufficiently spaced by 50–200 μm. During testing, samples were partially submerged in Dulbecco’s phosphate-buffered saline to prevent dehydration. For nanoindentation testing, a universal surface energy term, similar in value to those defined for the agar hydrogels, was used to correct stiffness values to account for work of adhesion (see Supplementary Information).
2.6. Magnetic resonance elastography
Measurements of internal ovarian stiffness were obtained using MRE by determining a circular region of interest within the ovary margins to ensure that the surrounding tissue was not included in the stiffness measurement. This is a conservative approach that sacrifices the outer cortex region of the ovary and only captures information on the interior region. Images were taken with a field view of 275 mm × 400 mm and were acquired on a 141 × 256 matrix, yielding anatomic images with 1.9 mm × 1.5 mm resolution, corresponding to elastograms of 1.3 cm × 1.0 cm. Stiffness was computed using the MRE software package [13] (See Supplementary Information for more details on the stiffness extraction method).
2.7. Statistical analysis
Samples were compared using two-tailed Student’s t-tests. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Validation of MRE in agar hydrogel phantoms
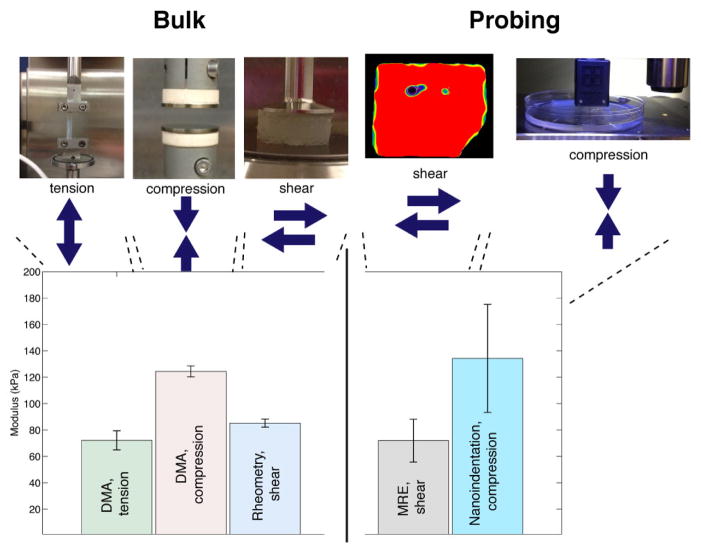
We first compared MRE, nanoindentation testing, and bulk mechanical methods applied to agar hydrogels, a common tissue mimic, using a suite of testing methods covering all strain modes (tension, compression, shear) and a wide range of probing volumes. We observed considerable agreement between MRE and bulk mechanical measurements (≈15% error) (Figure 1, left), thereby validating MRE against the standard testing methods. The minor variations in observed stiffness values can be attributed to testing differences, such as strain mode (tension/compression), sample geometries, or sample variation. Nanoindentation results on the agar hydrogels, corrected using a version of the Johnson-Kendall-Roberts (JKR) model [14, 15] (Figure 1, right), match those of the bulk methodologies, especially for compression (≈8% error).
Figure 1.
Comparison of elastic stiffness of an agar hydrogel using five different material characterization methods: three bulk methods (left) and two localized methods (right). Due to the incompressible nature of most isotropic hydrated materials, such as hydrogels and tissues, their shear modulus (G) is approximately 1/3 of the tensile or compressive modulus (E) [E=2*G*(1+nu)]; the conversion of the measured G to E is shown for easier comparison. Results are displayed for an agar gel at 2.25 wt% and averaged across a broad frequency range (1–100 Hz). Error bars displayed represent standard deviations. MRE results on the agar gel are taken from the gel phantoms used in the in vitro experiments (see also Fig. 2) and are visualized as the red portion of the image above. Nanoindentation results were corrected for tip adhesion effects using a Johnson, Kendall and Roberts model (JKR model) (see Supplementary Information).
3.2 Assessment of bovine ovarian stiffness
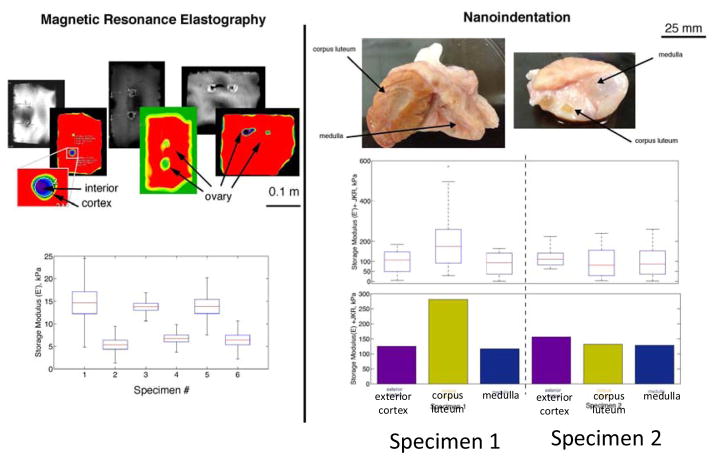
The cow ovary closely phenocopies human tissue in terms of size. Because ovaries from young women are rarely removed and never excised for research purposes, we used bovine ovaries as our proxy of human ovarian tissue. Two bovine ovaries were probed with nanoindentation methods. For in vitro MRE measurements, 3 pairs of bovine ovaries were embedded in an agar gel matrix, a standard method used to suspend tissue in a hydrated environment. Figure 2 shows a comparison of measurements from each technique and demonstrates the ability of nanoindentation to test local regions of a structure and resolve stiffness values.
Figure 2.
Comparison of probing/local measurements of stiffness in bovine ovaries suspended in agar hydrogel phantoms (matrices), using magnetic resonance elastography (MRE) and nanoindentation. Colorized MRE elastographs are scaled from min/max results of individual phantoms to visualize heterogeneity; quantified results for E′ (where E′=3G′) for the defined regions of interest (ROI) are shown as box and whisker plots for ovaries 1 through 6. The results illustrate heterogeneity within the ovary structure and a stiffened corpus luteum in ovary 1. For nanoindentation, the results are displayed as box-and-whisker plots (above) on a logarithmic scale and a simple average (below) on a linear scale. Resolving heterogeneity within the ovary was difficult with MRE, but this method was able to probe interior features and did not require a free surface for testing as is needed with nanoindentation.
We probed three regions of each ovary, but the results showed only one statistical outlier: the corpus luteum in ovary 1. We observed that the exterior cortex had statistically uniform mechanical properties, even in areas proximal to noticeable follicle structures. The lack of variation in stiffness of the interior features demonstrates that the ovary was fairly homogeneous, with the exception of a dominant corpus luteum, and the normal tissue was therefore extremely compliant (~ 100kPa or lower).
3.3 Comparison of ovary stiffness in human PCOS ovaries and matched controls
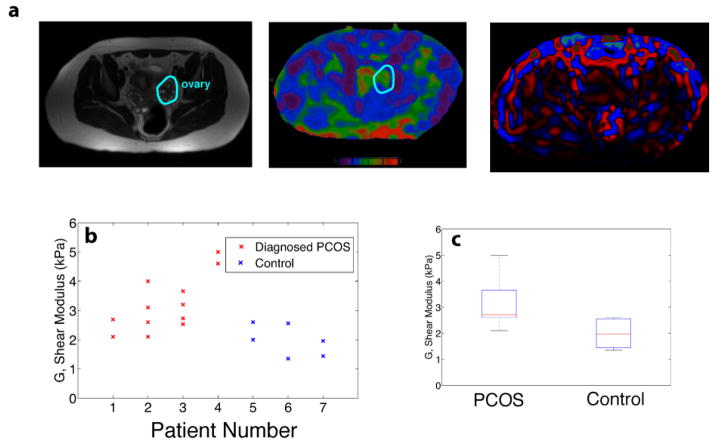
We next used MRE to explore differences in vivo in human ovaries of women with diagnosed PCOS [16] and age-matched controls. Representative MRI and elastograms from participants in the study are shown in Figure 3a, and illustrate the variability in tissue stiffness that exists naturally within the abdomen. This variability created noise and made isolation of the ovary difficult in practice because the organ is not perfectly isolated and lies underneath layers of air-filled bowel, fat, and other tissue structures. Despite this challenge, we found that patients with a diagnosis of PCOS had stiffer ovaries than those of age-matched controls (Figure 3b,c; p<0.05; two-sided student’s t-test).
Figure 3.
In vivo magnetic resonance elastography (MRE) studies were performed on seven clinical patients, four of whom had a previous diagnosis of PCOS. (a) T-2 anatomical images were cross-referenced with colorized MR elastograms (0–8 kPa, purple-red respectively) to identify a region of interest (ROI). The ROI was used to measure the shear stiffness, G′ (kPa). (b,c) However, results demonstrate an overall higher stiffness in the ovaries of women diagnosed with PCOS versus age-matched controls. MRE wave images are shown as last color image.
4. Discussion
In this paper, we compared established technologies and MRE to measure tissue material properties in vitro, and explored the ability of MRE to distinguish between healthy and diseased (e.g., PCOS) ovaries in vivo. MRE results were comparable to those of bulk material characterization methods on a standard engineering material, thereby validating MRE as an accurate and appropriate method to characterize hydrated materials. Studies of bovine ovaries in vitro showed some agreement between MRE and nanoindentation, though the magnitudes differed. These discrepancies are likely attributable to differences between interior probing (MRE) and surface probing (nanoindentation) methods, as well as loading mode differences, in which localized hydration state, surface adhesion and nonlinearities present in tissues can significantly influence results. Nanoindentation revealed some statistical variation inside the bovine ovary, in the case of the corpus luteum, and while MRE did not capture discrete variation, it was able to detect variations within an individual ovary. Localized stiffness variations provide evidence of slight heterogeneity within an overall fairly compliant and homogeneous tissue. Using the validated MRE stiffness measurements, our preliminary clinical results are the first published evidence of ovary stiffening in PCOS patients compared with healthy controls. These results provide the first in vivo validation of the hypotheses generated from previous in vitro studies regarding biomechanical regulation mechanisms within the ovary and the relationship between ovary stiffness and the presence of ovarian cysts in PCOS [17, 18].
4.1 MRE, nanoindentation testing, and bulk mechanical methods are comparable in agar hydrogel phantom testing
Nanoindentation techniques, both quasi-static and dynamic, have achieved similar moduli compared to traditional bulk methods for a wide range of polymer types [19–22]. Indentation-type characterization methods are commonly chosen for heterogeneous materials and can be tailored to a wide range of material types. Most often, contact-based methodologies resolve higher stiffnesses or moduli for soft materials, attributed to adhesion effects, surface confinement, and non-linear viscous effects [15, 23]. Aside from these effects, indentation-type testing has proven to be a consistent methodology for determining relative stiffness of a variety of material types [15, 22] and has unique spatial capabilities for mapping or isolating heterogeneity within materials [24, 25]. Adhesion effects have been well studied and are defined by the surface energies of the tip and sample and measured through pull-off experiments [15]. In our study, the agreement between MRE, nanoindentation and several bulk mechanical testing procedures applied to standardized agar samples demonstrates that MRE may be useful for probing the local stiffness of soft materials with complex geometries without the need for physical contact with the region of interest.
4.2 MRE and nanoindentation assess bovine ovary stiffness at different resolutions
In contrast to the controlled agar samples (phantoms), the nanoindentation stiffness results for the ovary samples were approximately an order of magnitude higher than the MRE results (140 kPa vs 13 kPa). In MRE, the regions of interest (ROI) are deliberately chosen as smaller than the ovary to avoid artifacts from the surrounding gel (see Supplementary Information); as such, the cortex material is excluded from the measurements, providing an underestimation of ovary stiffness. Additionally, nanoindentation tests of soft and irregular samples have been shown to overestimate moduli due to non-ideal sample geometry factors—such as surface curvature, tilted sample surface, and non-linear response—and tip-sample adhesion effects [11, 14, 26, 27]. The unique inherent challenges with tissue samples arise because of the need for physical contact to determine stiffness, a significant drawback in this particular case. The current challenges facing indentation techniques on ultra-soft materials—especially machine sensitivity, non-linear behavior, and adhesion effects—have slowed efforts to develop it as a standard method for assessing tissue stiffness in general. Nevertheless, while nanoindentation overestimated absolute values of the stiffnesses compared to shear-based methods, the technique produced results comparable to bulk compression (Figure 1 and Supplementary Information) and provided high spatial resolution and accurate relative differences in moduli.
In the case of soft tissues, the compliance of the sample approached the sensitivity limit of the instrumentation and noise was significant. Within the nanoindentation results, we observed compliant medulla and cortex regions as well as significant stiffening in the dominant corpus luteum structure in ovary 1, the only region that passed a two-sample t-test. Although some heterogeneity may exist, the current level of noise complicated this differentiation. Nanoindentation did show that primary structures, such as in ovary 1, were significantly stiffer than other regions of the ovary. MRE resolved some stiffness variations within the ovary as shown in the enlarged view of ovary 2; however, MRE did not match the spatial resolution capabilities of nanoindentation. Of course, what MRE lacks in spatial resolution must be weighed against its unique capabilities as an noninvasive clinical tool and its relative simplicity, features that make it an attractive diagnostic method for evaluating tissues such as the ovary, where biopsy is not possible and palpation is the only method available for assessment of tissue abnormalities.
4.3 Study limitations
Limitations of the study include that it is a pilot study with low sample numbers, and used a prototype MRE system (acoustic driver, pulse sequence, and reconstruction software). We found that depth penetration of the acoustic waves were patient-specific in some cases, yielding low mathematical confidence. We explicitly rejected some exams based on poor image quality, indicating several areas were protocol development is needed. Optimization of the system and protocol are currently in progress.
5. Conclusions
The advent of in vivo imaging technologies such as MRE has opened up the possibility for diagnostic and prognostic testing—specifically for diseases related to tissue stiffness—that is less invasive, less expensive, and more accurate and/or reliable. Our studies provide an initial demonstration of MRE for measuring tissue material properties in vivo; in particular, the capacity for deep probing of tissues such as ovaries. MRE represents a major improvement on current palpation techniques, which are generally skill driven and less quantitative. The need for non-invasive assessments of reproductive function is becoming increasingly important as the obesity epidemic changes ovarian function in adolescents where more interventional imaging techniques such as transvaginal sonography is not as appropriate.
The non-invasive MRE method also potentially expands the amount of the body available for probing without the need for biopsy, which is not an option for tissues such as the ovary. The technological advance of MRE not only provides us with a new tool that will permit a greater understanding of ovarian biology, but also offers new opportunities to noninvasively and quantitatively assess other internal organs for early fibrotic changes and evaluate the efficacy of new treatments for fibrotic diseases. Improvements in non-invasive assessment may impact patient outcomes as well as overall quality of care by allowing for earlier, more precise diagnosis and collection of additional data that can refine prognosis.
Supplementary Material
Acknowledgments
We are grateful to the women who participated in this study. We thank Dr. Stacey Tobin for editorial support. The authors thank Andrea Dunaif, M.D. for providing access to the Northwestern University PCOS patient population. This work was supported by National Institutes of Health Grants U54HD041857 (to L.D.S., F.M. T.K.W).
Footnotes
Disclosure Summary: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. Journal of assisted reproduction and genetics. 2011;28:3–6. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariappan YK, Glaser KJ, Ehman RL. Magnetic Resonance Elastography: A Review. Clinical Anatomy. 2010;23:497–511. doi: 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diallo MS, Appel E. Acoustic wave propagation in saturated porous media: reformulation of the Biot/Squirt flow theory. Journal of Applied Geophysics. 2000;44:313–25. [Google Scholar]
- 4.Loubet J, Lucas B, Oliver W. Some measurements of viscoelastic properties with the help of nanoindentation. In: Smith DT, editor. International Wrokshop on Instrumental Indentation; San Diego, CA. 1995; pp. 31–4. [Google Scholar]
- 5.Gu YZ, Li M, Wang J, Zhang ZG. Characterization of the interphase in carbon fiber/polymer composites using a nanoscale dynamic mechanical imaging technique. Carbon. 2010;48:3229–35. [Google Scholar]
- 6.Bruet BJF, Song J, Boyce MC, Ortiz C. Materials design principles of ancient fish armour. Nat Mater. 2008;7:748–56. doi: 10.1038/nmat2231. [DOI] [PubMed] [Google Scholar]
- 7.Wood CD, Palmeri MJ, Putz KW, Ho G, Barto R, Catherine Brinson L. Nanoscale structure and local mechanical properties of fiber-reinforced composites containing MWCNT-grafted hybrid glass fibers. Composites Science and Technology. 2012;72:1705–10. [Google Scholar]
- 8.Franke O, Göken M, Meyers MA, Durst K, Hodge AM. Dynamic nanoindentation of articular porcine cartilage. Materials Science and Engineering: C. 2011;31:789–95. [Google Scholar]
- 9.Mattice JM, Lau AG, Oyen ML, Kent RW. Spherical indentation load-relaxation of soft biological tissues. Journal of Materials Research. 2006;21:2003–10. [Google Scholar]
- 10.Nayar VT, Weiland JD, Hodge AM. Characterization of porcine sclera using instrumented nanoindentation. Materials Science and Engineering: C. 2011;31:796–800. [Google Scholar]
- 11.Ebenstein DM, Pruitt LA. Nanoindentation of biological materials. Nano Today. 2006;1:26–33. [Google Scholar]
- 12.Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, et al. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Medical Image Analysis. 2001;5:237–54. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 13.Rouviere O, Yin M, Dresner MA, Rossman PJ, Burgart LJ, Fidler JL, et al. MR elastography of the liver: Preliminary results. Radiology. 2006;240:440–8. doi: 10.1148/radiol.2402050606. [DOI] [PubMed] [Google Scholar]
- 14.Johnson KL, Kendall K, Roberts AD. Surface Energy and Contact of Elastic Solids. Proc R Soc Lon Ser-A. 1971;324:301. [Google Scholar]
- 15.Carrillo F, Gupta S, Balooch M, Marshall SJ, Marshall GW, Pruitt L, et al. Nanoindentation of polydimethylsiloxane elastomers: Effect of crosslinking, work of adhesion, and fluid environment on elastic modulus (vol 20, pg 2820, 2005) Journal of Materials Research. 2006;21:535–7. [Google Scholar]
- 16.Mutharasan P, Galdones E, Bernabe BP, Garcia OA, Jafari N, Shea LD, et al. Evidence for Chromosome 2p16. 3 Polycystic Ovary Syndrome Susceptibility Locus in Affected Women of European Ancestry. J Clin Endocr Metab. 2013;98:E185–E90. doi: 10.1210/jc.2012-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu M, Banc A, Woodruff TK, Shea LD. Secondary Follicle Growth and Oocyte Maturation by Culture in Alginate Hydrogel Following Cryopreservation of the Ovary or Individual Follicles. Biotechnology and Bioengineering. 2009;103:378–86. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biology of Reproduction. 2006;75:916–23. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 19.VanLandingham MR, Villarrubia JS, Guthrie WF, Meyers GF. Nanoindentation of polymers: An overview. Macromol Symp. 2001;167:15–43. [Google Scholar]
- 20.White CC, Drzal PL, VanLandingham MR. Viscoelastic characterization of polymers using dynamic instrumented indentation. Mater Res Soc Symp P. 2005;841:187–92. [Google Scholar]
- 21.White CC, Vanlandingham MR, Drzal PL, Chang NK, Chang SH. Viscoelastic characterization of polymers using instrumented indentation. II. Dynamic testing. J Polym Sci Pol Phys. 2005;43:1812–24. [Google Scholar]
- 22.Nayar VT, Weiland JD, Nelson CS, Hodge AM. Elastic and viscoelastic characterization of agar. Journal of the Mechanical Behavior of Biomedical Materials. 2012;7:60–8. doi: 10.1016/j.jmbbm.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Shi XH, Zhao YP. Comparison of various adhesion contact theories and the influence of dimensionless load parameter. J Adhes Sci Technol. 2004;18:55–68. [Google Scholar]
- 24.Wood CD, Palmeri MJ, Ho G, Putz KW, Barto R, Brinson C. Nanoscale structure and local mechanical properties of fiber-reinforced composites containing MWCNT-grafted hybrid glass fibers. Composites Science and Technology. 2012 [Google Scholar]
- 25.Bruet BJF, Song J, Boyce MC, Ortiz C. Materials design principles of ancient fish armour. Nat Mater. 2008;7:748–56. doi: 10.1038/nmat2231. [DOI] [PubMed] [Google Scholar]
- 26.Rettler E, Hoeppener S, Sigusch BW, Schubert US. Mapping the mechanical properties of biomaterials on different length scales: depth-sensing indentation and AFM based nanoindentation. J Mater Chem B. 2013;1:2789–806. doi: 10.1039/c3tb20120a. [DOI] [PubMed] [Google Scholar]
- 27.Kohn JC, Ebenstein DM. Eliminating adhesion errors in nanoindentation of compliant polymers and hydrogels. Journal of the Mechanical Behavior of Biomedical Materials. 2013;20:316–26. doi: 10.1016/j.jmbbm.2013.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.