Abstract
Pathological retinal neovascularization (RNV) is a common micro-vascular complication in several retinal diseases including retinopathy of prematurity, diabetic retinopathy, age-related macular degeneration and central vein occlusion. The current therapeutic modalities of RNV are invasive and although they may slow or halt the progression of the disease they are unlikely to restore normal acuity. Therefore, there is an urgent need to develop treatment modalities, which are less invasive and therefore associated with fewer procedural complications and systemic side effects.
This review article summarizes our understanding of the pathophysiology and current treatment of RNV in ischemic retinopathies; lists potential therapeutic targets; and provides a framework for the development of future treatment modalities.
Keywords: Retinopathy, Retinal neovascularization, VEGF, PEDF, Oxidative stress, Lipoxygenase, Inflammation, PPAR gamma
Pathological retinal neovascularization (RNV) is a common micro-vascular complication in several retinal diseases including retinopathy of prematurity, diabetic retinopathy, age-related macular degeneration and central vein occlusion. The current therapeutic modalities of RNV are invasive and although they may slow or halt the progression of the disease they are unlikely to restore normal acuity. Therefore, there is an urgent need to develop treatment modalities, which are less invasive and therefore associated with fewer procedural complications and systemic side effects.
This review article summarizes our understanding of the pathophysiology and current treatment of RNV in ischemic retinopathies; lists potential therapeutic targets; and provides a framework for the development of future treatment modalities.
Introduction
Retinal angiogenesis or neovascularization (RNV) is a cardinal feature in several diseases of retina as in retinopathy of prematurity (ROP), diabetic retinopathy (DR), age-related macular degeneration (AMD), and retinal vein occlusions. AMD and DR are common leading causes of legal blindness in >50 years old people and populations at working age, respectively [1,2]. The prevalence of DR and AMD is expected to increase in the next few decades due to significant increases in the number of population with obesity and diabetes as well as the number of aged population [3,4]. Patients with retinal neovascularization usually loss their daily activity independence, and this places a substantial economic burden on the family, the community, and the society as a whole. Thus, it is important to develop new and effective modalities to treat or prevent the development RNV. During the last decade, there was noticeable improvement in understanding of the underlying molecular and cellular mechanisms of RNV. This understanding was essential in the identification and introduction of new therapeutic intervention to suppress this process.
Eye is unique in having certain avascular tissues such as cornea, lens, vitreous and outer retina. Avascular feature of these ocular tissues is essential for the normal vision. Cornea, retina, and choroid are the most commonocular tissues to be invaded by pathological NV. The normal vascular pattern of the ocular tissue is firmly controlled by the delicate balance between the pro-angiogenic and angiostatic molecules. For example, contributing factors to pathological neovascularization such as hypoxia, ischemia, and inflammation have been shown to disrupt the normal balance in the retinal expression of vascular endothelial growth factor (VEGF) and pigment epithelium-derived factor (PEDF). VEGF and PEDF are known to be the key angiogenic and angiostatic factors in retina respectively (Fig. 1) [5–11]. Because of absence of pericytes and basement membrane, the new vessels become more fragile and leaky leading to hemorrhage and edema, which interrupt light transmission ending by vision loss. Although the pathogenesis of RNV had been heavily studied, the exact underlying mechanism is not yet well understood. This lack of understanding led to absence of effective therapeutic strategy for RNV at present despite laser treatment demonstrates some favorable therapeutic effects on retinal and choroidal NV. Here, we discuss the underlying mechanism of RNV and the current and future potential therapeutic targets which may represent efficient clinical agents and strategies.
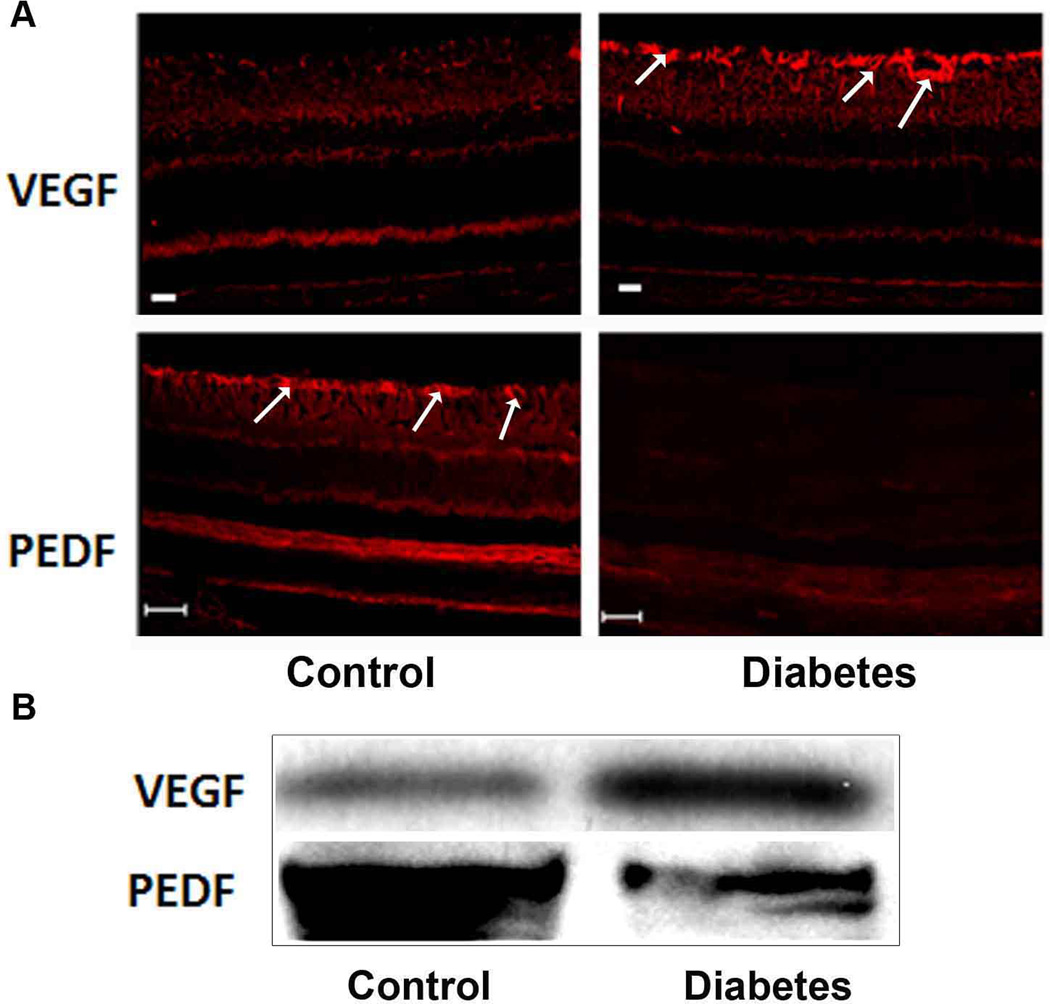
Fig. 1. Changes in the retinal levels of VEGF and PEDF during ischemic retinopathy.
Immunofluorescence reaction (A) using a specific antibodies to VEGF and PEDF shows intense VEGF and less PEDF immunoreactivity in retina of diabetic rats compared to the control. Note, the immunoreactivity of VEGF and PEDF was localized mainly in perivascular glial cells (arrows).This pattern was confirmed by Western blotting (B). Scale Bar is 20 µm.
Retinal neovascularization
Pathological RNV leads to diminution of vision and blindness. News vessels distort normal retinal architecture, cause significant damage to retinal tissue and vascular leakage. Furthermore, RNV is associated with breakdown of inner limiting membrane allowing the new vessels to sprout into the vitreous. This ultimately leads to fibrosis and retinal detachment. Endothelial cells of the normal retinal vessels have tight junctions that maintain normal inner blood–retinal barrier (BRB)function [12]. During RNV, the new retinal vessels become deficient in such endothelial tight junctions leading to retinal vascular hyperpermeability and inflammation that disrupt normal retinal tissue/function [13]. In the BRB, the main physical barrier is formed by retinal endothelial cells, but pericytes and retinal glia were found to contribute to the maintenance of the BRB as well [14–16]. Therefore, In addition to the loss of endothelial cell tight junction, loss of retinal pericytes during DR abrogates structural vascular integrity and contributes to retinal vascular leakage [12,17].
In retina new blood vessel develop by two distinct processes; one is vasculogenesis which refers to de novo new vessel formation from the angioblasts or endothelial precursor cells (EPC), and angiogenesis, which is the sprouting of new blood vessels from pre-existing ones. Although during the normal embryonic development retinal vessels develop mainly by vasculogenesis [18,19], angiogenesis also contributes to the embryonic vascular development and dependent on activation of VEGF receptor 2. In human fetal retina central retinal vessels form by vasculogenesis whereas peripheral retinal vessels in the inner retina and outer plexus capillaries develop by angiogenesis [20,21]. The undifferentiated and differentiated angioblasts have been reported in retinas of experimental animals in the first few postnatal weeks, suggesting that vasculogenesis also contributes to the postnatal remodeling of retinal vasculature [22]. Although pathological retinal NV such as DR, AMD and ROP is dependent mainly on the angiogenesis as a major form of new vessels formation, recent studies suggest that endothelial progenitor cells (EPCs) or vasculogenesis, is also contributing to the pathological RNV [22–26]. Consistent with the role of vasculogenesis in pathological RNV, EPCs isolated from adult peripheral blood have been shown to differentiate ex vivo to an endothelial phenotype [18], to incorporate into newly formed blood vessels and to express several of the known endothelial cell markers, such as LDL receptor and fetal liver kinase-1 (flk-1) [22,23,26,27]. Furthermore, EPCs were also noticed in the preretinal neovascular capillary tufts in pathological RNV induced by laser-induced venous occlusion and VEGF overexpression [23]. In addition to retinal NV, EPC derived from adult hematopoietic stem cells also contributed to the formation of laser-induced choroidal neovavscularization (CNV) [28]. Inhibition of stromal-derived factor-1 (SDF-1), which is key factor in recruiting EPCs to areas of neovascularization has been found to significantly reduce the size of the CNV lesions [26]. Taken together these findings suggest inhibition of the recruitment of circulating EPCs as a potential therapy for RNV. Moreover, enhancing recruitment of EPCs to ischemic retina during the early stage of DR may prevent the hypoxia-induced signaling pathway that leads to RNV.
Diabetic retinopathy
Diabetic retinopathy (DR) is a leading cause of blindness in people of the working age. The clinical course of retinopathy, anatomical changes, its pathogenesis and current treatment are described, followed by an overview of the emerging drug therapies for the potential treatment of this sight-threatening complication of diabetes. DR develops nearly in all patients with type 1 diabetes and more than 60% of patients with type 2 diabetes during the first two decades of disease [29]. Hyperglycemia triggers endothelial cell dysfunction and increases leukostasis leading to breakdown of the BRB and vascular hyperpermeability. This leakage results in diabetic macular edema, the most common cause of decreased visual acuity in diabetic patients. Later, capillary degeneration and ischemia develop which leads to uncontrolled neovascularization in attempt to compensate for the lack of blood flow [30–32] The current treatment modalities include timely laser photocoagulation, vitrectomy and repeated intraocular injections of anti-VEGF or steroids, are invasive and limited by considerable side effects and they may prevent progression of DR but do not restore the visual acuity. DR is classified clinically into two stages: non-proliferative (NPDR) and proliferative (PDR). NPDR is characterized by microaneurysms, ‘dot and blot’ or ‘splinter’ hemorrhages, and intraretinal microvascular abnormalities (IRMA, a form of intraretinal NV). Moreover, ‘cotton wool’ spots are also characteristic feature of NPDR, representing the non-perfused area which leads to inner retina ischemia. In addition to the changes in NPDR, PDR is characterized by RNV which develops to overcome the inner retina ischemia (Fig. 2). However, the new vessels are abnormal, fragile, leaky resulting in edema and hemorrhage into the vitreous. This leads to fibrous tissue proliferation and the subsequent tractional retinal detachment, which eventually causes blindeness in the late stage of PDR. Therefore, targeting retinal NV is very important strategy to improve the visual acuity in diabetic patients.
Fig. 2. Vascular changes in retina of patients with proliferative diabetic retinopathy (PDR).
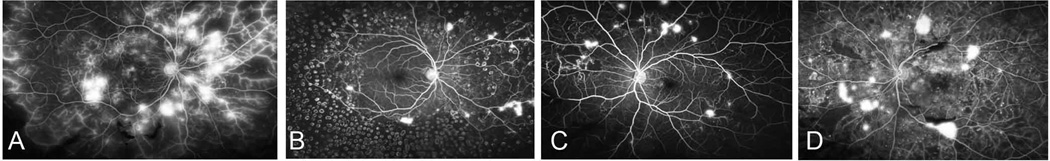
A, Extensive capillary non perfusion (CNP), leakage from NV (at disc and elsewhere) and staining of venous walls is consistent with severe retinal ischemia. B, there is extensive pan-retinal photogoagulation (PRP) and more pronounced reduction in leakage from NV following laser. C, PDR with extensive CNP, multiple micro aneurysms surround areas of CNP and leakage from islands of NV. D, there is blocked fluorescence from areas of pre-retinal hemorrhage, leakage from islands of NV, intraretinal fluorescein leakage with likely retinal edema and numerous micoaneurysms surround areas of CNP.
Of Note, hypoxia and HIF-1a signal pathways are crucial for the RNV during normal vascular development and pathological RNV (Fig.3) [33,34]. The development of RNV in DR is preceded by molecular and cellular changes that occur during early course of diabetes. Hyperglycemia triggers oxidative stress and pro-inflammatory signal pathways that leads to apoptosis of pericytes and endothelial cells. This leads to capillary degeneration and retinal ischemia that triggers hypoxia inducible factor-1a (HIF-1a) dependent VEGF expression and finally RNV [35–37](Fig.4).
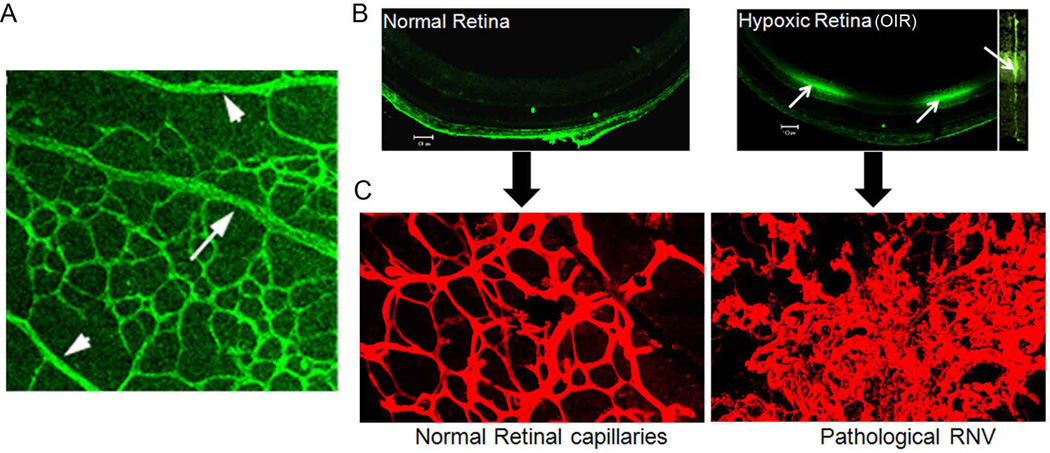
Fig. 3. Hypoxia derives normal and pathological retinal angiogenesis.
Flat mount retina of normal mouse labeled with vascular marker (A). There is marked capillary free zone around retinal arteries (arrowhead), while retinal veins are surrounded by dense capillary network (arrows). This was attributed to high oxygen tension in arteries and low oxygen tension in veins. The low oxygen tension in veins causes formation of the dense capillary network around them. Retinal sections from normal mouse or mouse with OIR (B). These mice were injected intraperitoneally with a hypoxyprobe which binds to tissue with low oxygen tension. Retinal section were then incubated with a specific antibody against the hypoxyprobe followed by Oregon green labeled secondary antibody. Note the intense hypoxyprobe immunoreactivity in OIR which was localized mainly in the inner nuclear layer (arrows). Magnification of the reactive area demonstrateed that hypoxia is localized mainly in Muller cells (insert). The hypoxic condition of the retina was associated with marked RNV compared to the normal retina (C).
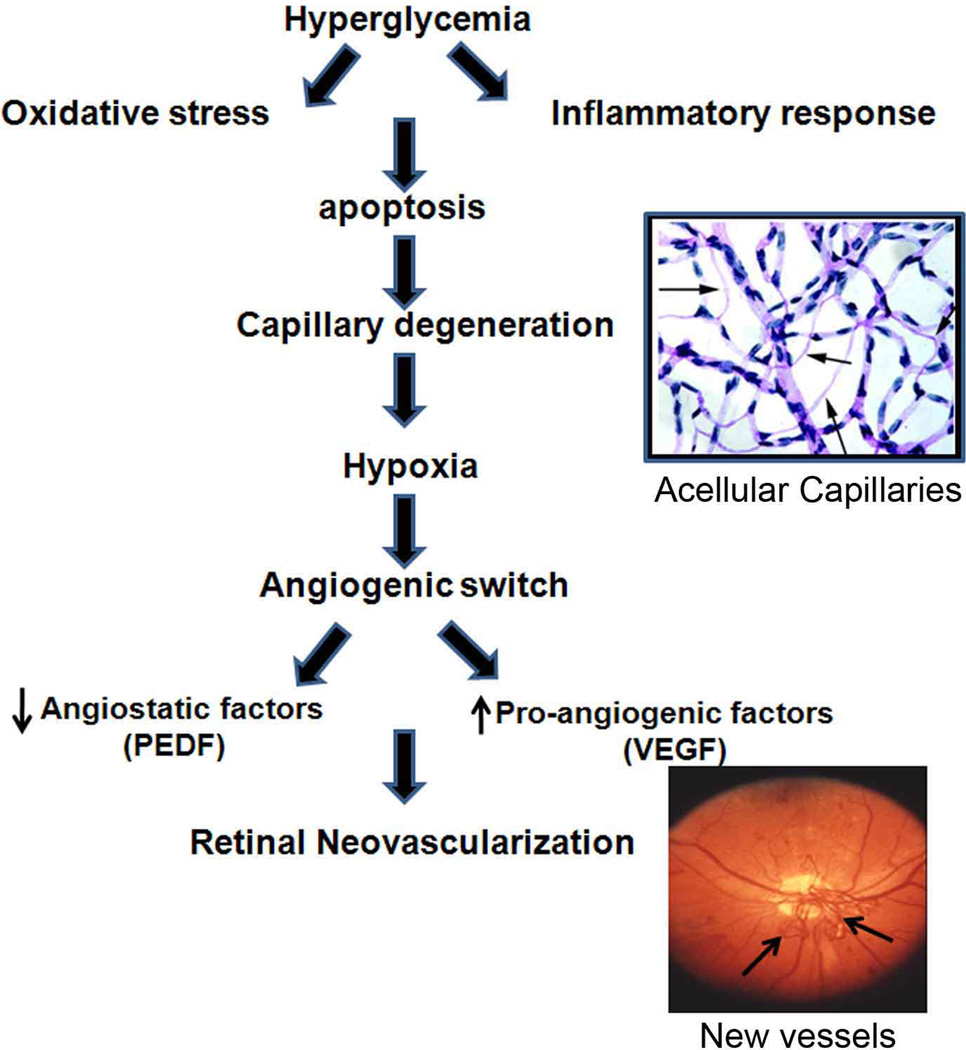
Fig. 4. Cascade of events that leads to RNV in DR.
The figure shows a proposed cascade of events which proceeds RNV in DR. Hyperglycemia induces oxidative stress and inflammatory response followed by accelerated death of retinal microvascular cells (pericytes and endothelial cells). This leads to development of acellular capillaries and retinal ischemia that triggers upregulation of VEGF expression and downregulation of PEDF and subsequent RNV.
Blood Retinal Barrier
Retinal functional/structural integrity is partially dependent on the presence of intact blood–retinal barrier (BRB). The integrity of BRB is important for normal retinal micovascular homeostasis and preventing leakage of harmful molecules into the retina. There are inner and outer BRB, breakdown of which contributes to the deteriorated visual function during DR and AMD respectively. The inner BRB (iBRB) consists of endothelial cells which rest on a basal lamina and supported tight junctions and the foot processes of glial cells as well as pericytes [14–16,38]. The outer BRB (oBRB) is formed by TJ between retinal pigment epithelium (RPE) cells which rest upon the Bruch's membrane. Neural retina is separated from the fenestrated choriocapillaris by Bruch’s membrane and nutrients are transported from the blood to the outer retina via the oBRB [39].
Interplay of retinal endothelial and glial cells during RNV
Molecular and physical Cross talk between ganglion cells, glial, and endothelial cells is a key factor in retinal vascular development. In particular, reciprocal feedback between endothelial cells and glial cells is crucial for proper vascular patterning. Hypoxia-induced VEGF expression in retinal glial cells plays a key role in formation of new retinal vessels [40]. The capacity of Müller cells to preserve the integrity of BRB function has been shown to be significantly reduced during hypoxic insult [41]. Pigment epithelium derived growth factor (PEDF) is secreted by Müller cells under normal conditions secrete [42] and counteracts the permeability and angiogenic actions of VEGF. Under hypoxia, Müller cells expression of PEDF is decreased leading to unopposed action of VEGF and hence vascular hyperpermeability and angiogenesis [43].
Selective ablation of Müller cells led to an imbalance between VEGF-A and PEDF and impaired retinal function due to photoreceptor apoptosis, blood-retinal barrier breakdown and intraretinal neovascularization. These findings support the thought that Müller glial dysfunction a key player in retinal neurovascular pathologies during ischemic retinopathies [44]. In the future, supplying glial cells may be a strategy that will allow the inhibition of pathological NV and vascular repair in avascular retina areas. The delivery of recombinant PEDF allows for the recovery of Müller cells, and thus creates the conditions favorable for the survival of nerve cells in loss of retinal homeostasis [45].
GROWTH FACTORS AND RETINAL NEOVASCULARIZATION
1. ANGIOGENIC FACTORS
The role of growth factors in the pathogenesis of pathological RNV is well established and was first proposed by Michaelson in 1954. Michaelson suggested that development of retinal vasculature is controlled by a retinal vasoformative factor, formation of which is dependent on the retinal metabolic needs [46]. Subsequent studies showed that the same vascular growth factor is also involved in the pathological RNV. There are several growth factors that found to contribute to RNV over the last two decades for instance VEGF, bFGF, IGF-1, PEDF, angiopoieteins, and epithelium growth factor (EGF). The next section will cover in detail some of these factors.
Vascular endothelial growth factor
There are several extensive series of clinical and preclinical investigations have confirmed the central role of VEGF in promoting ocular NV [47–54]. For example, clinical studies have reported increased ocular levels of VEGF in patients with retinopathy of prematurity [55], diabetic macular edema (DME) [56–59], and retinal vein occlusion [47]. Similarly, there was a significant increase in the macular expression of VEGF in patients with AMD [60] as well as in choroidal neovascular membranes [61–63].
VEGF is the key retinal growth factor which implicated in the normal and pathological retinal new vessels formation. It is a 46-kDa homodimeric with four isoforms (VEGF121, VEGF165, VEGF189, and VEGF206). They are produced via alternative mRNA splicing from the same gene [64]. The biological effects of VEGF require two receptors, VEGF-R1 (Flt-1) and VEGF-R2 (Flk-1/KDR).
The major sources of VEGF in retina are RPE and Müller cells while, endothelial cell is the main target of VEGF’s biological effect [65,66]. However, VEGF still can be produced by other cell types such as pericytes, astrocytes and ganglion cells [66–68]. Therapeutic laser photocoagulation decreases the levels of VEGF in the vitreous and aqueous humor of patients with PDR by 75% [47], suggesting the importance of retinal levels of VEGF for the development/regression of RNV. Similarly, the levels of VEGF Increased during diabetic macular edema (DME) and found to be correlated with the disease severity [59]. These findings indicate that VEGF is involved in the development of both vascular leakage and RNV during DR.
Recently, the link between the gene polymorphism of VEGF promoter and microvascular complications of diabetes was studied in a group of 267 UK diabetic Caucasian patients. The study suggested the VEGF-460 genotype, as an independent predictive factor for PDR, but not for diabetic nephropathy [69]. VEGF-460 genotype enhances the activity of VEGF promoter by 70% in comparison to wild type. Animal models of DR also demonstrated upregulation of VEGF expression which correlated with the early retinal vascular hyperpermeability [70]. This increase in vascular permeability was successfully prevented by specific VEGF-neutralizing soluble Flt/F(c) construct (VEGF TrapA(40) [71]. These coincidental VEGF upregulation and the breakdown of BRB were also observed in the relative long-term experimental diabetes [72]. Oxygen-induced retinopathy (OIR) (Fig.5) is an accepted animal model to study the molecular and cellular mechanisms of pathological RNV. The elevated retinal levels of VEGF during OIR have been correlated with the progression of NV [73]. The crucial role of VEGF in the pathogenesis of RNV has been further proved by the observation of a significant reduction in RNV in animals treated with a single intravitreal injection of the soluble VEGF receptor Flt or Flk chimeric proteins, which interferes with VEGF signaling via binding to VEGF with the same affinity as the native receptors [49,50]. Furthermore, Intravitreal injection of a neutralizing anti-VEGF aptamer, which specifically binds to VEGF 165 demonstrated remarkable inhibition of leukostasis and subsequent pathological RNV in OIR rats without interruption of the physiological NV [74]. On the other hand, both pathological and physiological RNV were significantly inhibited by VEGFR-1/Fc fusion protein, which blocks all VEGF isoforms. Recent studies reported that targeting KDR activation causes significant inhibitory effects on RNV and other ocular diseases associated with NV [75,76]. Taken together all these findings support the key role of VEGF in physiological and pathological RNV.
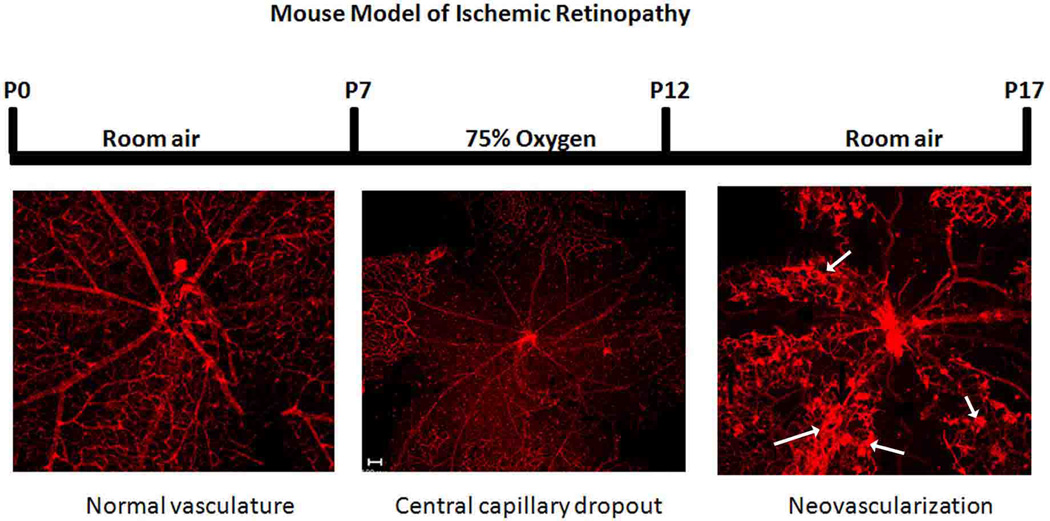
Fig. 5. Mouse model of oxygen-induced retinopathy.
To develop a model of pathological RNV, mice were exposed to high oxygen (75%) during the period between postnatal day7–12 (p7–p12) followed by room air for additional 5 days (p17). During high oxygen exposure period retinal vessels undergo vasobliteration and capillary dropout occurs in central retina (encircled area). This leads to relative hypoxia during room air exposure period. The relative hypoxia leads to overexpression of VEGF and subsequent RNV which reach maximum by p17 (arrows).
Erythropoietin (EPO)
EPO is produced mainly by the adult kidney to facilitate tissue oxygenation via regulation the erythrocyte mass in circulation [77,78]. EPO stimulates endothelial cell proliferation and migration, and enhance its survival through antiapoptotic mechanism suggesting EPO as a proangiogenic factor [79–81]. Several studies showed that EPO plays a role in the formation of RNV [78,82–84]. Tissue oxygen supply plays important role in regulating the EPO secretion and expression of its receptor in a variety of tissues including retina [82,84,85]. Retinal hypoxia induced simultaneous increase of EPO and VEGF expression [82] via activation of hypoxia-inducible factor 1 (HIF-1). Secretion of EPO is also regulated by several other stressors, such as hypoglycemia, increased intracellular calcium, inflammation and oxidative stress [85]. There is a positive cross talk between EPO and VEGF, which enhances endothelial cell proliferation [80]. For example, rhEPO significantly enhanced the expression of VEGF and VEGF receptors (KDR/flk-1 and flt-1) in endothelial cells. Additionally, rhEpo-induced endothelial cell proliferation was inhibited by anti-VEGF antibody. These findings suggest that EPO might be involved in hypoxia-induced VEGF expression [80]. In addition to its pro-angiogenic effect in retina, EPO demonstrated neuroprotective effects. It prevents ischemia-induced apoptosis of the photoreceptor and retinal ganglion cells [85]. In human, EPO found in high amount in the vitreous of patients with PDR compared to the non-diabetic patients [84,86]. Interestingly, EPO levels were significantly higher in active PDR in comparison to quiescent PDR. Simultaneous inhibition of both EPO and VEGF showed synergistic inhibitory effect on patients’ vitreous induced-endothelial proliferation and RNV in a murine model of OIR [84]. Thus, targeting more than one pro-angiogenic pathway may produce substantially greater clinical benefit. Recent study showed that single intravitreal injection of EPO (50ng/eye) protects against retinal vascular regression at the early stage of DR in streptozotocin-induced diabetic rats. This was associated with reduction in the levels of EPO receptor and VEGF suggesting that EPO may play a primary role against the progression of diabetic retinopathy by reducing blood vessel degeneration at a very early disease stage [87].
Insulin-like growth factor
Insulin-like growth factor is growth is involved in multiple biological effects in a variety of tissues. This includes cell growth, differentiation and transformation [88]. IGF-1 production in liver is regulated by growth hormone (GH). The expression of IGF-I and IGF-IR mRNA was reported to localize mainly in the neuroretinal layers, RPE, and choriocapillaries and retinal endothelial cells of human retina [89]. Decreased retinal levels of IGF-1 were reported to be associated with impaired retinal vessels formation suggesting the important role of IGF-1 in normal retinal vascular development [90]. Consistent with these findings, low retinal level of IGF-1 was noticed in ROP and this leads to cessation of vessel growth, retinal hypoxia, and subsequent VEGF production and NV [91]. Therefore, sufficient level of IGF-I is after birth leads to normal vessel development and prevention of ROP [92]. The key role of IGF-1 in retinal NV has been confirmed by using IGF-transgenic mice in which vascular features of retinopathy were noticed. For example, pericytes loss and basement membrane thickening of retinal capillaries were noticed in 2 month-old IGF-1 transgenic mice, while, dilatation of venules, abnormalities of intraretinal microvascular, and RNV were noticed in mice of 6 months of age and older [93]. Retinal NV was attributed to IGF-1-induced upregulation of VEGF in retinal glial cells.
Angiopoietin
Angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2) are members of a novel family of angiogenic factors that elicit their biological effects via binding to endothelial receptor tyrosine kinase (RTK), Tie-2 [94]. The angiopoietin–Tie2 system plays a crucial role in vascular homeostasis by regulating the cross talk between endothelial cells and pericytes as well as regulating VEGF signaling [94]. Ang-1 is a pericyte-derived signal that regulates endothelial cell maturation and integrity of endothelial cell barrier function [95]. Overexpression of retinal Ang-1 has been shown to significantly reduce VEGF-induced retinal vascular hyperpermeability, and to suppress the RNV in OIR and laser-induced CNV [96]. Interestingly, there was no changes in the normal retinal or choroidal vasculatures or retinal function as evaluated by electroretinography in the transgenic mice [96]. Therefore, Ang-1 has been suggested as a potential therapeutic target for DME and RNV.
Ang-2 is an endogenous inhibitor of the Ang-1/Tie-2 vascular function [94]. It plays a central role in both physiological and pathological angiogenesis [97–99]. Ang-2-deficient mice showed impairment of the development of superficial and deep retinal vasculatures which develop by vasculogenesis and angiogenesis respectively [97]. Consistent with the central role of the Ang-2/Tie-2 signaling in retinal NV the colocalization of Tie2, VEGF and Ang-2, was noticed in fibrovascular membrane of patients with ROP [100]. The expression of Ang-2 in retina of mouse model of OIR was correlated with the extent of angiogenesis and reached the peak at P17, the time of maximal RNV [101]. Additionally, RNV was significantly reduced by intraperitoneal injection of Tie-2 antagonist, muTek delta Fc. This was associated with a remarkable downregulation of matrix metalloproteinase-9 (MMP-9), suggesting that MMP plays a role in Ang-2-induced RNV [98].
Oxidative stress and RNV
Oxidative stress refers to the imbalance between reactive oxygen species (ROS) generation and the ability to scavenge ROS by endogenous antioxidative systems such as superoxide dismutase (SOD), catalase and glutathione peroxidase (GSH). Free radicals and ROS cause irreversible damage to cell membrane, DNA, lipids, proteins, and carbohydrates and disruption of cellular homeostasis [102]. High content of polyunsaturated fatty acids (PUFA) and oxygen uptake and glucose oxidation account for the high susceptibility of retina to oxidative stress compared to other tissues [103]. Accumulated evidences suggest oxidative stress as a key player in the pathogenesis of DR and its resistance to recover even after good glycemic control is reestablished (the metabolic memory phenomenon) [104]. Retina has several sources of ROS which include NADPH oxidase, advanced glycation end products (AGEs), mitochondrial oxidase, aldose reductase/polyol pathway, uncoupling of endothelial nitric oxide synthase (eNOS)and activation of PKC [105–111].
In preterm infants ROS generation increased and anti-oxidant systems is highly stressed and incompletely developed. This was correlated with increased retinal VEGF expression and RNV [112,113]. In OIR, NADPH was activated and ROS were increased in the retina and that correlated to the apoptosis of endothelial cells, which contributed to avascular retina [114] and to the subsequent RNV which was blocked the NADPH oxidase inhibitor, apocynin [107]. ROS generation is increased by VEGF stimulation, and activates VEGFR2 downstream signaling [115,116]. Thus, blocking ROS production attenuates RNV via regulating both VEGF expression and VEGFR2 downstream signaling pathway in OIR. Furthermore, our recent studies demonstrated that inhibition of NADPH oxidase or deletion of its catalytic subunit, NOX2 prevents the early inflammatory response and vascular hyperpermeability in experimental model of DR [106,108,117].
Luteolin a natural flavonoid and a potent candidate from Platycodon grandiflorum, which is widely used in Asian traditional herbal medicine [118] and has anti-oxidative activity [119]. Luteolin was found to inhibit hypoxia-induced VEGF expression and RNV in OIR without toxicity via inhibition of ROS generation [120,121]. Anti-oxidants that can inhibit the oxidative processes can protect retinal cells from ischemic/hypoxic insults, a crucial factor in inducing RNV during ischemic retinopathy. In particular, treatment using anti-oxidants such as vitamin E and lutein, inhibition of NADPH oxidase or related signaling pathways, and administration of catalase and SOD are possible therapeutic regimens for RNV [107,122]. It has recently been observed that mice with targeted deletion of superoxide dismutase 1 (Sod1−/−) mice show many of the features of AMD, including spontaneous development of choroidal NV [123,124]. Treatment of Sod1−/− mice with antioxidants significantly reduced ischemia-induced RNV [125]. Antioxidants also significantly suppressed NV in each of 3 experimental models of ocular NV, ischemia-induced RNV, VEGF-induced subretinal NV, and choroidal NV [125]. Inhibition of angiogenesis by antioxidants such as N-acetyl-L-cysteine (NAC) has been demonstrated both in vitro and in vivo. [126] suggesting use of antioxidants to inhibit pathological RNV via targeting VEGF signaling.
Lipid Metabolites in Retinal Neovascularization
Lipid metabolism and signaling is dysregulated in many vascular diseases such as diabetic retinopathy, retinopathy of prematurity and age-related macular degeneration. Arachidonic acid can be metabolized into eicosanoids via different enzymatic pathways including lipoxygenase (LOX), cyclooxygenase (COX) pathways into leukotrienes, hydroxyeicosatetraenoic acids (HETEs), and prostaglandins [127] which have been shown to promote angiogenesis [128,129]. Lipoxygenases are dioxygenases that catalyze oxygenation of arachidonic acid and other PUFAs. According to the site of oxygen insertion within arachidonic acid lipoxygenases are classified as 5-, 8-, 12-, or 15-LOX.
RNV during OIR was associated with decreased 15-LOX-1 expression, and retinal 15-LOX-1 levels were negatively correlated with the progression of RNV. RNV in OIR was significantly inhibited in the intravitreous injected Ad-15-LOX-1 mice. This was associated with significant decrease in the levels of VEGF-A suggesting that 15-LOX-1 may be a novel therapeutic target for ocular neovascularization diseases [130]. Moreover, 15-LOX-1 overexpression on retinal endothelial cells inhibited hypoxia-induced angiogenesis via blocking hypoxia-induced upregulation in VEGF family [131].
Diets enriched in ω-3 polyunsaturated fatty acids (PUFAs) significantly reduced pathological RNV in OIR, via suppression of microglial-derived tumor necrosis factor-α [132]. The protective effect of dietary ω-3 PUFAs against retinopathy was abrogatedin 5-lipoxygenase (5-LOX)-deficient mice. This protective effect was attributed to oxidation of the ω-3 PUFA lipid docosahexaenoic acid to 4-hydroxy-docosahexaenoic acid (4-HDHA) by 5-LOX. 4-HDHA has direct inhibitory effect on endothelial cell proliferation via PPARγdependent mechanism suggesting ω-3 PUFAs as a treatment for RNV either by itself or as a supplement to the current anti-VEGF therapy. Moreover, cyclooxygenase inhibitors such as aspirin and ibuprofen (but not LOX inhibitors, for instance zileuton) might be used without losing the protective effect of dietary ω-3 PUFA [133].
Our recent studies [134,135] also demonstrated the potential role of 12/15-LOX in the development of ischemic retinopathy and RNV via disrupting the balance in the retinal levels of VEGF and PEDF as well as inducing early inflammatory response. In the same studies we showed that RNV in OIR as well as PDR in human were associated with high levels of 12-LOX expression and its lipid products 12- and 15-HETEs, There was also significant increase in the amount of 5-HETE. Meanwhile inhibition of the LOX pathway by pharmacological inhibition with baicalein or 12-LOX deletion significantly abrogated RNV.
Taken together our and others’ studies lipoxygenase pathway could be a novel therapeutic target to prevent or at least ameliorate the RNV during ischemic retinopathies.
Role of inflammation and Immune Response in Ischemic Retinopathy
Retinas from diabetic humans or animals and high glucose-treated retinal cells demonstrated inflammatory response such as increased leukostasis, and hyperpermeability and this was correlated with the development of RNV [32,135,136]. The inflammatory response in retina during ischemic retinopathy has been liked to activation of NFκb signaling pathway with subsequent upregulation of cytokine levels such as IL-6, IL-1β and adhesion molecules such as ICAM-1 and VCAM-1 [106,108,117]. This suggests a crucial role of the early inflammatory response in the pathogenesis of microvascular dysfunction during DR including RNV. In addition to inflammatory response, it seems that immune response also might be implicated in the pathogenesis of ischemic retinopathy in particular the eye is an immune-privileged site. For example, the autosomal dominant neovascular inflammatory vitreoretinopathy (ADNIV) is a familial blinding disease in which the patient’s eye is infiltrated by T-cell (CD4and CD8 positive cells). Therefore, targeting T cells is suggested as a therapeutic modality to treat the ADNIV [137]. Furthermore, toll-like receptors (TLRs)-induced activation of the immune system is implicated in angiogenesis including oxygen-induced RNV [138]. Cytolytic immune attack was also suggested for treatment of exudative (wet) macular degeneration through using a fusion protein (ICON) composed of factor VII, the natural ligand for tissue factor, conjugated to the Fc domain of a human IgG1 immunoglobulin. It binds to the tissue factor expressed on neovascular endothelia leading to destruction of the neovascular tissue [139].
Accumulate evidence that correlates inflammatory/immune response to the ischemic retinopathy led to proposing several anti-inflammatory/immunosuppressive drugs to treat DR. For example, minocycline, corticosteroids and aspirin and salicylates are new suggested therapeutic modalities to treat DR.
ANGIOSTATIC FACTORS
Retinal angiogenesis is normally regulated by the delicate balance between angiogenic stimulators and inhibitors. Several studies demonstrated that the disruption of this balance is implicated in pathological angiogenesis [8,140,141]. Therefore, restoration of the balance by either providing angiostatic factors or inhibitors of angiogenic factors or both leads to inhibition of angiogenesis. There is agreement that this approach is a useful strategy in the prevention and treatment of pathological RNV. The presence of naturally occurring angiogenesis inhibitors in the vitreous fluid has been documented and suggested to be responsible for maintaining the avascular status of the vitreous body [142,143]. There are several angiogenic inhibitors have been identified in retina. Active antiangiogenic factors in the retina include endothelial cell specific inhibitors such as angiostatin, endostatin, and plasminogen kringle5 (K5), serine proteinase inhibitors such as PEDF, antagonists of angiogenic factor such as soluble soluble VEGFR-1 (Flt-1), thrombospondin-1 (TSP-1), interferon a, interferon g, and tissue inhibitor of metalloproteinases (TIMPs). Additionally, VEGF: PEDF ratio in the retina correlates with the progression of RNV in ischemic retinopathy [8,141,144]. Recent study by Mohan et al., reported that the mean vitreous and plasma levels of VEGF and EPO in patients with PDR were significantly higher than those in subjects without diabetes. Conversely, the vitreous and plasma levels of PEDF were significantly lower in the PDR patients compared to control subjects. Moreover, the protein expression of the VEGF and EPO in the retinal tissue was significantly higher in PDR and diabetes without complication groups compared to the controls. Moreover, the protein expression of PEDF was significantly lower in retinal tissues from diabetes patients without complications and in patients with PDR in comparison to the control group [145].
Pigment epithelium-derived factor
In 1987 Tombran-Tink and Johnson has identified the PEDF for the first time in human retinal RPE cells conditioned media [146]. PEDF is a 50-kDa glycoprotein which has similar sequence and structure homology to the family of serine proteinase inhibitor (Serpin). However, PEDF has not been shown inhibitory effect on known proteinases [147]. It demonstrated neurotrophic function by maintaining the normal development, structure and function of retinal neurons [146,148–151]. In addition to its neurotrophic function, PEDF also showed anti-angiogenic activity [152–154]. Ocular avascular tissues such as vitreous, cornea, and aqueous humor have high levels of PEDF in comparison to vascular tissues [155]. Several studies have confirmed the negative correlation between the levels of PEDF and pathological RNV in different experimental models of ischemic RNV [8,144,156]. In addition to inhibitory effect of PEDF on RNV, PEDF also has negative effect on retinal vascular permeability [157,158]. Furthermore, the correlation between the imbalance in VEGF/PEDF levels in the retina and ocular fluid and the pathogenesis of RNV was also evaluated in patients with DR and proliferative sickle cell retinopathy. In PDR levels of PEDF were significantly decreased and VEGF levels were significantly increased in the vitreous and aqueous humor, in comparison with the control subjects with normal blood glucose levels [145,159–161]. Interestingly restoration of PEDF levels in retina or vitreous of rats or patients was noticed after laser photocoagulation of retina [162,163] suggesting that therapeutic effect of laser photocoaugulation is partially attributed to the up-regulation of PEDF expression in the eye.
Peroxisome proliferator-activated receptor gamma (PPARγ)
Peroxisome proliferator-activated receptor gamma (PPARγ) is a member of a ligand activated nuclear receptor superfamily which plays a central role in several biological functions, such as glucose metabolism, adipogenesis and angiogenesis [164,165]. Previously PPARγ signaling pathway was shown to inhibit diabetes-induced vascular injury in retina [166] through inhibition of NFκΒ signaling pathway which includes leukocyte adhesion [167,168]. Our previous work on experimental diabetes and OIR showed that inhibition of NADPH oxidase or deletion of its catalytic subunit NOX2 results in increase PPARγ while and this was correlated to the decrease in the inflammatory response during DR and abrogation of RNV in OIR [117].
PPARγ pathway was found to be involved in the attenuation of progression of DR by herbal and traditional natural medicines or their active components [169] and also in the antiangiogenic effect of the omega3-PUFAs on RNV in OIR independent of vascular endothelial growth factor [170]. Moreover, PPARγligands have been shown to inhibit VEGF-induced choroidal angiogenesis in vitro and CNV in vivo [171]. These findings suggest that PPARγ ligands could be therapeutic strategy to prevent the development of RNV.
Treatment of Retinal Neovascularization
1. LASER THERAPY OF RNV
Laser therapy has been used successfully to reduce the risk of vision loss in posterior segment neovascularization such as ARMD and PDR. Laser photocoagulation therapy is used to treat choroidal neovascularization (CNV) in patients with exudative AMD. It has been a major advancement in treatment of exudative AMD. It involves the selective heating of ocular tissues through the absorption of a specific wave length of light by ocular pigments. This leads to thermal damage and denaturation of proteins and other large molecules and ROS are generated within the target vasculatures [172,173]. Photodynamic therapy (PDT) is a selective and localized therapy for CNV which causes oxidation of tissues by a photodynamic reaction. For this purpose, a photosensitizing dye such as verteporfin is first delivered to the target tissue followed by locally photo-excitation using a specific wavelength of light delivered via an ophthalmic laser. This leads to generation of ROS which induces platelet activation and thrombosis which ultimately reduces vascular permeability and causes involution of the lesion [174]. Another type of laser therapy for CNV is feeder vessels photocoagulation (FVP) which targets small number of intrachoroidal (feeder vessels) that supplies the entire CNV complex. This requires high speed idocyanine green angiography to localize the feeder vessels followed by delivery of laser energy to block the feeder vessels [175].
Panretinal photocoagulation (PRP) involves application of multifocal laser burns on retinal periphery sparing the macula. It reduces the risk of vision loss in the majority of patients with PDR. PRP is indicated for treatment of PDR that exhibits remarkable preretinal or disc NV with hemorrhage and some visual acuity may be already have been compromised. It involves inducing multifocal laser burns in the peripheral retina sparing the macula. Vitrectomy is often performed with PRP in patients with vitreous hemorrhage and or traction retinal detachment [176–178]. The underlying mechanism of inhibiting further retinal NV by PRP is still not well known. However there are several hypotheses such as direct destruction of retinal tissues responsible for producing angiogenic factors, reduction in metabolic demands of retina which reduces the relative hypoxia and generation of retinal anti-angiogenic molecules such as PEDF or TGFb. These thoughts have been supported by reduction of VEGF levels in vitreous, aqueous and plasma following PRP therapy [47,179].
2. ANTI-VEGF THERAPY
There is a strong evidence from animal studies demonstrated that VEGF is an optimal target for treatment of many ocular diseases in which neovascularization and increased vascular permeability is the key for its pathogenesis, including PDR. Four anti-VEGF pharmacologic agents are now commercially available and being in use in trials for treatment of diabetic macular edema (DME) and PDR. Pegaptanib (Macugen, OSI/Eyetech, Melville, NY, USA) is a modified 28-base RNA aptamer, which binds to VEGF 165 isoform inhibiting its vascular permeability effects [180]. Pegaptanib was tested in vitro which showed significance inhibition of VEGF induced vascular permeability [181]. It was also tested in OIR model, which showed inhibition of the pathological RNV without affecting the physiological NV [74]. Pegaptanib emerged as the first antiangiogenic drug which provide a significant statistically and clinically improvement in treatment of patients with neovascular AMD based on result of (VISION) trial. Many trials were done to test the safety and tolerability of the anti-VEGF in improving visual acuity in patients with macular edema [182,183]. It was found that pegaptanib is well tolerated at all administrative dose with transient adverse events due to injection procedure rather than the safety of the drug. On the other side, pegaptanib showed improvement in both DME and PDR patients in the form of increase in the visual acuity, beside reductions in central retinal thickness, with less need for macular laser photocoagulation [184].
Bevacizumab
(Avastin; Genetech, San Francisco, CA, USA) another anti-VEGF is a full length humanized monoclonal antibody, that is 93% of human origin and 7% murine [185]. It binds to all VEGF isoforms and inhibits VEGFR1 and VEGFR2 [186]. Bevacizumab has been approved by FDA for treatment of metastatic colorectal cancer and is being in phase III trials for treatment of advanced breast and renal cancer [187,188]. Due to the comparatively low cost and the availability of bevacizumab it was studied very well in the field of DME and PDR [189]. Bevacizumab showed promising results in decreasing leakage in neovascular lesions resulting in improvements in both visual acuity and central macular thickness [184]. Bevacizumab showed systemic side effects in cancer therapy such as arterial thromboembolism, hemorrhage, gastrointestinal perforation and hypertensive crisis [188,190]. Moreover, local delivery in the eye causes tractional retinal detachment (TRD) with the possibility of systemic drug absorption and side effects [191]. Therefore, long-term and well-designed studies involving multiple dosing are still needed.
Ranibizumab
(Lucentis; Genetech, Inc. South San Francisco, Calif) is fragmented humanized monoclonal antibody that also binds to all VEGF isoforms. Lucentis was approved by FDA for treatment of neovascular AMD [192]. Many trials were done in patients with choroidal neovascularization (CNV) secondary to AMD which shows that ranibizumab is well-tolerated in intravitrous injection with improvement in the visual acuity [193]. A recent trial of ranibizumab has shown a great success in treatment of DME for at least 2 years. In this study ranibizumab treatment of recurrent or persistent DME only as frequently as every 2 months for a period of 18 months resulted in maintenance of the excellent visual benefit that had been obtained after 6 months of a fixed regimen of ranibizumab injections. It was also associated with significant improvement in visual acuity of patients treated previously only with focal/grid laser photocoagulation [194].
The most recent anti-VEGF therapy the VEGF Trap- Eye (Regeneron Pharmaceuticals, Inc., Tarrytown, New 6 Journal of Ophthalmology York, NY, USA and Bayer Healthcare Pharmaceuticals, Berlin, Germany). VEGF Trap- Eye is a 115 kDA recombinant protein consisting of the VEGF-binding domains of human VEGFR-1 and VEGFR-2 fused to the Fc domain of human immunoglobulin-G1 [195]. Animal studies have shown that intravitreal injection of VEGF Trap-Eye has a promising results comparing to other anti-VEGF treatments, including higher binding affinity to VEGF-A and related proteins and longer half-life in the eye currently Trap-Eye is in phase III clinical trials [196,197].
Limitations of anti-VEGF therapy
Prevention of recurrence of RNV and retinal edema requires repeated laser therapy and administration of anti- VEGF [193,198–201] in particular, the ischemic and inflammatory triggers of these conditions are chronic and relatively obstinate [202–204]. Therefore, to prevent progression of new vessel formation or edema, therapeutic intervention should be able to continually block or target the pro-inflammatory and ischemic signaling. Efficient therapeutic approaches can be achieved via, repeated or less frequent administration of short or longer acting agents respectively or sustained delivery of a therapeutic agent. Moreover, it is important to facilitate effective access of the therapeutic agents to the affected tissues particularly the retina is difficult to be accessed. There are some limitations for both systemic and topical administration of therapeutic agents to guarantee effective amount to access retinal tissues. While, blood–retinal barriers represent limitation for the systemic agents as discussed before, there are several anatomical and metabolic barriers for topically applied agents to reach the retina. To overcome these limitations, therapeutic agents are currently injected intravitreally. However, intravitreal injection can be associated with, significant risk of retinal tear/detachment [205,206], endophthalmitis [207] and cataract [205,206]. Therefore, approaches like use of long acting medical agents or sustained release delivery are necessary to reduce the incidence of intravitreal injection complications. For example, VEGF Trap-Eye (Regeneron) which binds all isoforms of VEGF-A, VEGF-B and placental growth factor [208] has higher affinity to VEGF than bevacizumab [195] and effective at low concentration. This results in slower clearance and in turn less frequent intravitreal injection. VEGF Trap-Eye is currently in Phase III clinical trials [209].
There are several other factors in addition to VEGF are involved in the pathogenesis of RNV and CNV. For example, erythropoietin (Epo), insulin-like growth factor-1 (IGF-1), angiopoietin and stroma-derived factor-1 (SDF-1) have been proven to play important role and therefore, they became potential therapeutic targets to treat ocular NV.
3. RUBOXISTAURIN MESYLATE
Microvascular complications in diabetes is linked to a member of the serine threonine kinases family, protein kinase C beta (PKC-β), which shown to be involved in leukostatasis, increase in vascular permeability, basement membrane thickening and neovascularization [210–214]. Ruboxistaurin mesylate (LY333531, Eli Lilly & CO), is orally bioavailable competitive inhibitor of ATP which binds to PKC-β [215]. Although phase III results indicated no significant effect and their primary endpoint was not achieved, ruboxistaurin mesylate has been shown to reduce retinal vascular leakage in patients with DME [216]. Although an approvable letter was issued by FDA for ruboxistaurin in 2006, the medication is not yet clinically available [217].
4. siRNA
The scientific breakthrough, Interference RNA (iRNA), discovered by Fire and Mello et al. in 1998 [218] provided a major milestones in VEGF therapy. The first data out of human siRNA clinical trials was released by Sirna Therapeutics (formally Ribozyme Pharmaceuticals) in which they showed that single dose of siRNA -027, an anti-VEGFR1 siRNA stabilized the visual acuity of patients with AMD [219]. Sirna-027 has shown to reduce the levels of VEGFR1 mRNA in cultured endothelial cells and mouse model of RNV suggesting that VEGFR1 plays a key role in RNV and Sirna-027 could potentially reduce ischemic retinopathy [220].
5. SOMASTATIN
The ability of GH and IGF-1 to induce endothelial cell proliferation in DR has made both a target of therapeutic intervention. Somatostatin is a natural occurring hormone produced in the retina which can directly inhibit GH and IGF-1 [221]. Retinal NV was inhibited in a transgenic mouse model expressing an antagonist gene for GH and in mice treated with a GH inhibitor (MK678) in an ischemic model [222]. Therefore, inhibiting the GH-IGF pathway may serve as a therapeutic target in preventing RNV.
6. LISINOPRIL
There is strong evidence suggesting that renin-angiotensin system (RAS) system modulates microvascular function through the production of VEGF [223–225]. Lisinopril is an angiotensin-converting enzyme (ACE) inhibitor that has been shown to reduce the risk of developing DR in type 1 diabetic patients and slowing of the disease in patients with pre-existing DR [226]. Therefore, targeting RAS may serve as a potential therapeutic strategy for RNV.
7. MATRIX METALLOPROTEINASE (MMP) INHIBITORS
MMPs are known to play key roles in angiogenesis such as endothelial cell migration and tube formation and its inhibition may prevent neovascularization. Prinomastat (AG3340) is a selective inhibitor of MMP developed initially by Agouron Pharmaceuticals, Inc. [227,228]. In vivo studies showed that administering AG3340 intraperitoneally inhibits hypoxia induced RNV in mouse model of OIR [229].
8. ANTI-INFLAMMATORY DRUGS
8.1 Glucocorticoids (Corticosteroids)
One of the recent breakthroughs in the treatment of diabetic macular edema is the intravitreal triamicinolone acetonide (IVTA). Corticosteroids have shown to inhibit proliferation, migration and tube formation in vascular endothelial cells [230,231] and inhibit VEGF expression and inflammatory responses in various cell types [232–234]. These findings have laid the basis for several clinical studies with triamicinolone to prevent the development of PDR and RNV. However there are significant side effects which might develop with using the IVTA. For example, infectious endophthalmitis, subconjunctival hemorrhage, cataract formation and increased intraocular pressure were reported after IVTA [235]. Furthermore, viral retinitis was also reported following IVTA in patients with predisposing medical comorbidities [236].
8.2. Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
Aspirin and sodium salicylates prevent prostaglandins generation via inhibition of cyclo-oxygenase enzyme (COX2). COX-2 is found to be elevated in the hypoxic and diabetic retina. [237,238]. Nepafenac, a COX-1/2 inhibitor, is metabolized to amfenac when penetrated through the cornea and inhibit RNV in OIR through decreasing the production of VEGF [239]. It has also been found that capillary leukostasis and degeneration in retinas of experimental diabetic rodents can be prevented by administration of aspirin or sodium salicylate [240].
8.3. Minocycline
Minocyclin is a chemically modified tetracycline which demonstrated neuroprotective effect in cerebral ischemia and brain injuries [241,242]. It also inhibits the degeneration of retinal capillaries in experimental diabetic mice. Therefore it is expected to elicit beneficial effect again RNV [243]. Oral doxacyclin increases RPE FasL expression and reduces circulating and tissue-associated sFasL causing inhibition of ocular neovascularization [244].
9. AdPEDF
PEDF gene therapy using an adenovirus has shown success in preventing RNV in transgenic mice with increased VEGF expression and OIR mice model [153]. Currently, clinical trials are underway by GenVec to develop PEDF adenovirus (adPEDF) therapies for AMD through intravitreal and periocular delivery of adenovirus type 5.
10. ANGIOSTATIN AND ENDOSTATIN
Angiostatin and endostatin, are two naturally occurring anti-angiogenic proteins. Patients with high levels of VEGF and low endostatin levels were found to have a significantly great risk of progression of PDR after vitreous surgery [245]. Similarly, Moreover intravitreal injection of viral vector expressing mouse angiostatin suppressed RNV in Sprague-Dawley rat model of pre-retinal neovascularization induced by laser-induced retinal vein occlusion [246].
Expert opinion
The ultimate goal in treating ischemic retinopathies is to prevent the development of pathological RNV. The current treatment modalities including timely laser photocoagulation, vitrectomy and repeated intraocular injections of anti-VEGF or steroids, are invasive and limited by considerable side effects. Furthermore, they may prevent progression of ischemic retinopathy but do not restore the visual acuity. Identification of novel/effective therapeutic intervention depends on further elaboration of the underlying pathophysiology of the RNV. The central role of VEGF as a major player in RNV has been clinically confirmed and was the foundation for introducing the anti-VEGF therapy to treat RNV. However, there are several other new factors have been proven at least experimentally to contribute to the regulation of retinal microvascular function in health and disease. These new players include but not limited to PEDF, ephrin/Eph receptor, renin-angiotensin system, matrix metalloprotinases, IGF, PKC-b and stromal-derived factor (SDF-1)/CXCR4. Lipid metabolites and oxidative stress have been also shown to contribute to the RNV during DR and ROP. Pro-inflammatory pathways also play important role in pathogenesis of RNV during ischemic retinopathies. For example, many patients have evidence of retinal subclinical inflammation at the time they are diagnosed with diabetes. This means that pro-DR mechanisms arise early during the course of diabetes, although the nature of these mechanisms is not clearly evident. Hence, it is important to shift investigative efforts to focus on elucidating the mechanisms initiating and promoting the early changes in retinal function so that more effective therapeutic approaches may be developed with the goal of preventing (or at least minimizing) the development of DR or its progression to PDR. An understanding of the underlying mechanisms of RNV, as well as counterbalancing events, may ultimately disclose targeted therapeutic approaches with the potential to minimize the RNV and its retinal complications.
5-Year view
There is urgent need to improve the current pharmacological treatment modalities to improve the outcome of the retinal neovascular diseases. For treatment of RNV, the major challenge is to deliver effective amount of the drug over prolonged periods. Therefore, it is essential to develop an efficient drug delivery system to facilitate the transport of a relatively short lived drug from the anterior to the posterior eye segments or to provide local posterior or universal retinal concentrations of drug over long time periods (months to years). We believe that drug delivery will play an essential role in developing future anti-angiogenic therapy that offers substantial advantages over the current therapeutic strategies. There are several emerging methods to improve drug delivery to the posterior eye segment such as viral vectors, stem cell therapy and cellular delivery. Of note, improving local and targeted delivery of angiostatic agents should eliminate systemic drug administration and related side effects. Additional promising approach is to target master regulators of RNV such as HIF-1α or protein kinase CK2. Drug combination is another promising approach similar to drug therapy of cancer and AIDS. Combination therapy has additive effect and better outcome than the monotherapy. One of the promising strategies to prevent the development of RNV is the use of anti-inflammatory drugs. Moreover, Dietary supplement with antioxidants, lutein and ω-3 PUFA is another avenue of RNV therapy. Since injury of glial cells play crucial role in the development of pathological RNV, glial cells transplantation is also a potential therapeutic modality in the future.
Key Issues.
Pathological retinal neovascularization which detrimentally affect vision function is a cardinal feature in several retinal diseases, such as diabetic retinopathy, age-related macular degeneration, retinopathy of prematurity (ROP) and retinal vein occlusions
Pathological RNV in DR, AMD and ROP occurs mainly through angiogenesis. However endothelial progenitor cells (EPCs) or vasculogenesis, are also involved in this process. Both angiogenesis and vasculogenesis are dependent on the activation of VEGF signaling mechanism.
Hypoxia is the main driving force for both physiological and pathological vessel formation in retina through HIF-1α-dependent VEGF expression
Although the role of VEGF as a primary mediator of RNV has been clinically validated, several other growth factors contribute to RNV and suggested as potential therapeutic targets to prevent RNV including EPO, IGF-1, PKC-β, PEDF, angiopoietin, RAS. Lipid metabolites and oxidative stress have been also shown to contribute to the RNV during DR and ROP
The presence of an intact blood–retinal barrier (BRB) is essential for the structural and functional integrity of the retina. Vision is adversely affected in clinical conditions associated with BRB breakdown such as DR or AMD
Müller cell dysfunction contributes to retinal neuronal and vascular pathologies during ischemic retinopathies
Retinal angiogenesis is normally regulated by the delicate balance between angiogenic stimulators such as VEGF and inhibitors such as PEDF. The disruption of this balance is implicated in pathological RNV. Therefore, restoration of the balance is crucial to inhibit RNV
VEGF is an optimal target for treatment of many ocular associated with neovascularization and increased vascular permeability
Four anti-VEGF pharmacologic agents are now commercially available and being in use in trials for treatment of diabetic macular edema (DME) and PDR; Pegaptanib (Macugen), Bevacizumab (Avastin), ranibizumab (Lucentis) and VEGF Trap- Eye
The current treatment modalities of RNV including timely laser photocoagulation, vitrectomy and repeated intraocular injections of anti-VEGF or steroids, are invasive and limited by considerable side effects
The major challenge for treatment of RNV is to deliver effective amount of the drug over prolonged periods. Therefore, Drug Delivery will play an essential role in developing future anti-angiogenic therapy such as viral vectors, cellular delivery, and stem cell therapy that offers substantial advantages over the current therapeutic strategies.
Future potential therapeutic intervention to prevent/treat pathological RNV is the use of drug combination, anti-inflammatory drugs, glial cells transplantation, stem cell therapy or dietary supplement with antioxidants, lutein or ω-3 PUFA
The development of RNV in DR is preceded by molecular and cellular changes that occur during early course of diabetes including inflammation, apoptosis of pericytes and endothelial cells, leakage, capillary degeneration and retinal ischemia. Therefore, it is important to shift investigative efforts to focus on elucidating the mechanisms initiating and promoting the early changes in retinal function so that more effective therapeutic approaches may be developed with the goal of preventing (or at least minimizing) the development of DR or its progression to PDR
Acknowledgment
M Al-Shabrawey has received funding from NIH (1R01EY023315-01), Qatar National Research Fund (NPRP 4-1046-3-284) and Georgia Regents University’s Bridge Fund and Culver Vision Discovery Institute.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliation or financial involvement with any organization or entity with financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as
•Of Interest
••Of Considerable Interest
- 1.Buch H, Vinding T, Nielsen NV. Prevalence and causes of visual impairment according to World Health Organization and United States criteria in an aged, urban Scandinavian population: the Copenhagen City Eye Study. Ophthalmology. 2001;108(12):2347–2357. doi: 10.1016/s0161-6420(01)00823-5. [DOI] [PubMed] [Google Scholar]
- 2.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127(4):533–540. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 4. Saaddine JB, Honeycutt AA, Narayan KM, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005–2050. Arch Ophthalmol. 2008;126(12):1740–1747. doi: 10.1001/archopht.126.12.1740. ••Of Considerable Interest (5–11), these references provide insight into the mechanism of pathological neovascularization in general and in RNV in particular
- 5.Folkman J, Ingber D. Inhibition of angiogenesis. Semin Cancer Biol. 1992;3(2):89–96. [PubMed] [Google Scholar]
- 6.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267(16):10931–10934. [PubMed] [Google Scholar]
- 7.Cao Y. Endogenous angiogenesis inhibitors and their therapeutic implications. Int J Biochem Cell Biol. 2001;33(4):357–369. doi: 10.1016/s1357-2725(01)00023-1. [DOI] [PubMed] [Google Scholar]
- 8.Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489(2–3):270–276. doi: 10.1016/s0014-5793(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 9.Gao G, Ma J. Tipping the balance for angiogenic disorders. Drug Discov Today. 2002;7(3):171–172. doi: 10.1016/s1359-6446(01)02160-2. [DOI] [PubMed] [Google Scholar]
- 10.Ma JX, Zhang SX, Wang JJ. Down-regulation of angiogenic inhibitors: a potential pathogenic mechanism for diabetic complications. Curr Diabetes Rev. 2005;1(2):183–196. doi: 10.2174/1573399054022839. [DOI] [PubMed] [Google Scholar]
- 11.Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med (Berl) 1995;73(7):333–346. doi: 10.1007/BF00192885. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kim CS, Lee YM, Jo K, Shin SD, Kim JS. Methylglyoxal induces hyperpermeability of the blood-retinal barrier via the loss of tight junction proteins and the activation of matrix metalloproteinases. Graefes Arch Clin Exp Ophthalmol. 2012;250(5):691–697. doi: 10.1007/s00417-011-1912-5. [DOI] [PubMed] [Google Scholar]
- 13.Peng S, Gan G, Rao VS, Adelman RA, Rizzolo LJ. Effects of proinflammatory cytokines on the claudin-19 rich tight junctions of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2012;53(8):5016–5028. doi: 10.1167/iovs.11-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 15.Hosoya K, Fujita K, Tachikawa M. Involvement of reduced folate carrier 1 in the inner blood-retinal barrier transport of methyltetrahydrofolate. Drug Metab Pharmacokinet. 2008;23(4):285–292. doi: 10.2133/dmpk.23.285. [DOI] [PubMed] [Google Scholar]
- 16.Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Methods Mol Biol. 2011;686:133–148. doi: 10.1007/978-1-60761-938-3_5. [DOI] [PubMed] [Google Scholar]
- 17.Ejaz S, Chekarova I, Ejaz A, Sohail A, Lim CW. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obes Metab. 2008;10(1):53–63. doi: 10.1111/j.1463-1326.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 18.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 19. Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res. 2004;117:3–32. doi: 10.1007/978-1-4419-8871-3_1. •Of Interest (20– 28). These articles discussed various mechanisms for the development of new blood vessels in retina. This include role of glial cells and endothelial progenitor cells.
- 20.Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41(5):1217–1228. [PubMed] [Google Scholar]
- 21.Chan-Ling T, McLeod DS, Hughes S, et al. Astrocyte-endothelial cell relationships during human retinal vascular development. Invest Ophthalmol Vis Sci. 2004;45(6):2020–2032. doi: 10.1167/iovs.03-1169. [DOI] [PubMed] [Google Scholar]
- 22.Flower RW, McLeod DS, Lutty GA, Goldberg B, Wajer SD. Postnatal retinal vascular development of the puppy. Invest Ophthalmol Vis Sci. 1985;26(7):957–968. [PubMed] [Google Scholar]
- 23.Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8(6):607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 24.Abu El-Asrar AM, Nawaz MI, Kangave D, Siddiquei MM, Ola MS, Opdenakker G. Angiogenesis regulatory factors in the vitreous from patients with proliferative diabetic retinopathy. Acta Diabetol. 2011 doi: 10.1007/s00592-011-0330-9. [DOI] [PubMed] [Google Scholar]
- 25.Abu El-Asrar AM, Struyf S, Opdenakker G, Van Damme J, Geboes K. Expression of stem cell factor/c-kit signaling pathway components in diabetic fibrovascular epiretinal membranes. Mol Vis. 2010;16:1098–1107. [PMC free article] [PubMed] [Google Scholar]
- 26.Sengupta N, Caballero S, Mames RN, Timmers AM, Saban D, Grant MB. Preventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factors. Invest Ophthalmol Vis Sci. 2005;46(1):343–348. doi: 10.1167/iovs.04-0153. [DOI] [PubMed] [Google Scholar]
- 27.Ash JD, Overbeek PA. Lens-specific VEGF-A expression induces angioblast migration and proliferation and stimulates angiogenic remodeling. Dev Biol. 2000;223(2):383–398. doi: 10.1006/dbio.2000.9755. [DOI] [PubMed] [Google Scholar]
- 28.Sengupta N, Caballero S, Mames RN, Butler JM, Scott EW, Grant MB. The role of adult bone marrow-derived stem cells in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(11):4908–4913. doi: 10.1167/iovs.03-0342. [DOI] [PubMed] [Google Scholar]
- 29. Tarr JM, Kaul K, Wolanska K, Kohner EM, Chibber R. Retinopathy in diabetes. Adv Exp Med Biol. 2012;771:88–106. doi: 10.1007/978-1-4614-5441-0_10. ••Of Considerable Interest (30–32). These articles discuss the underlying mechanism of retinal vascular hyperpermeability
- 30.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274(33):23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 31.Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14(4):240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 32.Leal EC, Manivannan A, Hosoya K, et al. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48(11):5257–5265. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- 33.Di Fiore JM, Kaffashi F, Loparo K, et al. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr Res. 2012;72(6):606–612. doi: 10.1038/pr.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimm C, Willmann G. Hypoxia in the eye: a two-sided coin. High Alt Med Biol. 2012;13(3):169–175. doi: 10.1089/ham.2012.1031. [DOI] [PubMed] [Google Scholar]
- 35.Steinle JJ. Retinal endothelial cell apoptosis. Apoptosis. 2012;17(12):1258–1260. doi: 10.1007/s10495-012-0777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li SY, Fu ZJ, Lo AC. Hypoxia-induced oxidative stress in ischemic retinopathy. Oxid Med Cell Longev. 2012;2012:426769. doi: 10.1155/2012/426769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arden GB, Sivaprasad S. The pathogenesis of early retinal changes of diabetic retinopathy. Doc Ophthalmol. 2012;124(1):15–26. doi: 10.1007/s10633-011-9305-y. [DOI] [PubMed] [Google Scholar]
- 38.Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A. Muller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol. 2007;245(5):627–636. doi: 10.1007/s00417-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 39.Klaassen I, Hughes JM, Vogels IM, Schalkwijk CG, Van Noorden CJ, Schlingemann RO. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Exp Eye Res. 2009;89(1):4–15. doi: 10.1016/j.exer.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Kubota Y, Suda T. Feedback mechanism between blood vessels and astrocytes in retinal vascular development. Trends Cardiovasc Med. 2009;19(2):38–43. doi: 10.1016/j.tcm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 41. Tretiach M, Madigan MC, Wen L, Gillies MC. Effect of Muller cell co-culture on in vitro permeability of bovine retinal vascular endothelium in normoxic and hypoxic conditions. Neurosci Lett. 2005;378(3):160–165. doi: 10.1016/j.neulet.2004.12.026. •Of Interest (42 & 43), these articles discuss the role of PEDF in RNV
- 42.Eichler W, Yafai Y, Keller T, Wiedemann P, Reichenbach A. PEDF derived from glial Muller cells: a possible regulator of retinal angiogenesis. Exp Cell Res. 2004;299(1):68–78. doi: 10.1016/j.yexcr.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002;43(3):821–829. [PubMed] [Google Scholar]
- 44.Coorey NJ, Shen W, Chung SH, Zhu L, Gillies MC. The role of glia in retinal vascular disease. Clin Exp Optom. 2012;95(3):266–281. doi: 10.1111/j.1444-0938.2012.00741.x. [DOI] [PubMed] [Google Scholar]
- 45.Robaszkiewicz J, Chmielewska K, Figurska M, Wierzbowska J, Stankiewicz A. Muller glial cells--the mediators of vascular disorders with vitreomacular interface pathology in diabetic maculopathy. Klin Oczna. 2010;112(10–12):328–332. [PubMed] [Google Scholar]
- 46. Michaelson IC, Herz N, Lewkowitz E, Kertesz D. Effect of increased oxygen on the development of the retinal vessels; an experimental study. Br J Ophthalmol. 1954;38(10):577–587. doi: 10.1136/bjo.38.10.577. •Of Interest (47–50), these articles provide important insight into the role of VEGF in RNV
- 47.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 48.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 49.Miller JW, Adamis AP, Shima DT, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994;145(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- 50. Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113(12):1538–1544. doi: 10.1001/archopht.1995.01100120068012. ••Of Considerable Interest (51–52), these articles are among the first to demonstrate the importance of targeting VEGF to prevent RNV
- 51.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92(23):10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krzystolik MG, Afshari MA, Adamis AP, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120(3):338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- 53.Tolentino MJ, Miller JW, Gragoudas ES, Chatzistefanou K, Ferrara N, Adamis AP. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol. 1996;114(8):964–970. doi: 10.1001/archopht.1996.01100140172010. [DOI] [PubMed] [Google Scholar]
- 54.Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103(11):1820–1828. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- 55.Lashkari K, Hirose T, Yazdany J, McMeel JW, Kazlauskas A, Rahimi N. Vascular endothelial growth factor and hepatocyte growth factor levels are differentially elevated in patients with advanced retinopathy of prematurity. Am J Pathol. 2000;156(4):1337–1344. doi: 10.1016/S0002-9440(10)65004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Funatsu H, Yamashita H, Ikeda T, Nakanishi Y, Kitano S, Hori S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. Am J Ophthalmol. 2002;133(4):537–543. doi: 10.1016/s0002-9394(02)01323-5. [DOI] [PubMed] [Google Scholar]
- 57.Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110(9):1690–1696. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- 58.Funatsu H, Yamashita H, Sakata K, et al. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology. 2005;112(5):806–816. doi: 10.1016/j.ophtha.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 59.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133(1):70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 60.Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81(2):154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37(5):855–868. [PubMed] [Google Scholar]
- 62.Frank RN, Amin RH, Eliott D, Puklin JE, Abrams GW. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996;122(3):393–403. doi: 10.1016/s0002-9394(14)72066-5. [DOI] [PubMed] [Google Scholar]
- 63. Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–126. ••Of Considerable Interest (64 & 65). These are important articles to discuss the importance of VEGF in RNV
- 64.Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991;47(3):211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- 65.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92(3):905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dorey CK, Aouididi S, Reynaud X, Dvorak HF, Brown LF. Correlation of vascular permeability factor/vascular endothelial growth factor with extraretinal neovascularization in the rat. Arch Ophthalmol. 1996;114(10):1210–1217. doi: 10.1001/archopht.1996.01100140410008. [DOI] [PubMed] [Google Scholar]
- 67.Pe'er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995;72(6):638–645. [PubMed] [Google Scholar]
- 68.Lu M, Amano S, Miyamoto K, et al. Insulin-induced vascular endothelial growth factor expression in retina. Invest Ophthalmol Vis Sci. 1999;40(13):3281–3286. [PubMed] [Google Scholar]
- 69.Ray D, Mishra M, Ralph S, Read I, Davies R, Brenchley P. Association of the VEGF gene with proliferative diabetic retinopathy but not proteinuria in diabetes. Diabetes. 2004;53(3):861–864. doi: 10.2337/diabetes.53.3.861. [DOI] [PubMed] [Google Scholar]
- 70.Braun L, Kardon T, Reisz-Porszasz ZS, Banhegyi G, Mandl J. The regulation of the induction of vascular endothelial growth factor at the onset of diabetes in spontaneously diabetic rats. Life Sci. 2001;69(21):2533–2542. doi: 10.1016/s0024-3205(01)01327-3. [DOI] [PubMed] [Google Scholar]
- 71.Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42(10):2408–2413. [PubMed] [Google Scholar]
- 72.Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest. 1996;74(4):819–825. [PubMed] [Google Scholar]
- 73. Hu J, Song X, He YQ, et al. Heparanase and vascular endothelial growth factor expression is increased in hypoxia-induced retinal neovascularization. Invest Ophthalmol Vis Sci. 2012;53(11):6810–6817. doi: 10.1167/iovs.11-9144. •Of Interest (74). This article highlighted the pro-inflammatory effect of VEGF during DR
- 74.Ishida S, Usui T, Yamashiro K, et al. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44(5):2155–2162. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- 75.Kinose F, Roscilli G, Lamartina S, et al. Inhibition of retinal and choroidal neovascularization by a novel KDR kinase inhibitor. Mol Vis. 2005;11:366–373. [PubMed] [Google Scholar]
- 76.McLeod DS, Taomoto M, Otsuji T, Green WR, Sunness JS, Lutty GA. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43(6):1986–1993. [PubMed] [Google Scholar]
- 77.Krantz SB. Erythropoietin. Blood. 1991;77(3):419–434. [PubMed] [Google Scholar]
- 78.Erbayraktar S, Yilmaz O, Gokmen N, Brines M. Erythropoietin is a multifunctional tissue-protective cytokine. Curr Hematol Rep. 2003;2(6):465–470. [PubMed] [Google Scholar]
- 79.Carlini RG, Reyes AA, Rothstein M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int. 1995;47(3):740–745. doi: 10.1038/ki.1995.113. [DOI] [PubMed] [Google Scholar]
- 80.Alvarez Arroyo MV, Castilla MA, Gonzalez Pacheco FR, et al. Role of vascular endothelial growth factor on erythropoietin-related endothelial cell proliferation. J Am Soc Nephrol. 1998;9(11):1998–2004. doi: 10.1681/ASN.V9111998. [DOI] [PubMed] [Google Scholar]
- 81.Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002;11(6):863–871. doi: 10.1089/152581602321080529. [DOI] [PubMed] [Google Scholar]
- 82.Grimm C, Wenzel A, Groszer M, et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8(7):718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 83.Morita M, Ohneda O, Yamashita T, et al. HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003;22(5):1134–1146. doi: 10.1093/emboj/cdg117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watanabe D, Suzuma K, Matsui S, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353(8):782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 85. Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293(1):90–95. doi: 10.1001/jama.293.1.90. ••Of Considerable Interest (86). This article is important article to discuss the signal pathways of RNV during DR
- 86.Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353(8):839–841. doi: 10.1056/NEJMe058142. [DOI] [PubMed] [Google Scholar]
- 87.Mitsuhashi J, Morikawa S, Shimizu K, Ezaki T, Yasuda Y, Hori S. Intravitreal injection of erythropoietin protects against retinal vascular regression at the early stage of diabetic retinopathy in streptozotocin-induced diabetic rats. Exp Eye Res. 2013;106:64–73. doi: 10.1016/j.exer.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24(3):435–444. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 89.Lambooij AC, van Wely KH, Lindenbergh-Kortleve DJ, Kuijpers RW, Kliffen M, Mooy CM. Insulin-like growth factor-I and its receptor in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44(5):2192–2198. doi: 10.1167/iovs.02-0410. [DOI] [PubMed] [Google Scholar]
- 90.Hellstrom A, Carlsson B, Niklasson A, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87(7):3413–3416. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]
- 91.Hellstrom A, Perruzzi C, Ju M, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A. 2001;98(10):5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith LE. Pathogenesis of retinopathy of prematurity. Growth Horm IGF Res. 2004;14(Suppl A):S140–S144. doi: 10.1016/j.ghir.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 93.Ruberte J, Ayuso E, Navarro M, et al. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J Clin Invest. 2004;113(8):1149–1157. doi: 10.1172/JCI19478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nourhaghighi N, Teichert-Kuliszewska K, Davis J, Stewart DJ, Nag S. Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Lab Invest. 2003;83(8):1211–1222. doi: 10.1097/01.lab.0000082383.40635.fe. [DOI] [PubMed] [Google Scholar]
- 95.Valable S, Montaner J, Bellail A, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25(11):1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- 96.Nambu H, Nambu R, Oshima Y, et al. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004;11(10):865–873. doi: 10.1038/sj.gt.3302230. [DOI] [PubMed] [Google Scholar]
- 97.Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192(2):182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 98.Das A, Fanslow W, Cerretti D, Warren E, Talarico N, McGuire P. Angiopoietin/Tek interactions regulate mmp-9 expression and retinal neovascularization. Lab Invest. 2003;83(11):1637–1645. doi: 10.1097/01.lab.0000097189.79233.d8. [DOI] [PubMed] [Google Scholar]
- 99.Oshima Y, Deering T, Oshima S, et al. Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J Cell Physiol. 2004;199(3):412–417. doi: 10.1002/jcp.10442. [DOI] [PubMed] [Google Scholar]
- 100.Umeda N, Ozaki H, Hayashi H, Miyajima-Uchida H, Oshima K. Colocalization of Tie2, angiopoietin 2 and vascular endothelial growth factor in fibrovascular membrane from patients with retinopathy of prematurity. Ophthalmic Res. 2003;35(4):217–223. doi: 10.1159/000071173. [DOI] [PubMed] [Google Scholar]
- 101.Das A, McGuire PG. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog Retin Eye Res. 2003;22(6):721–748. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 102.Mohr S, Xi X, Tang J, Kern TS. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51(4):1172–1179. doi: 10.2337/diabetes.51.4.1172. [DOI] [PubMed] [Google Scholar]
- 103.Anderson RE, Rapp LM, Wiegand RD. Lipid peroxidation and retinal degeneration. Curr Eye Res. 1984;3(1):223–227. doi: 10.3109/02713688408997203. [DOI] [PubMed] [Google Scholar]
- 104.Kowluru RA. Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes. 2003;52(3):818–823. doi: 10.2337/diabetes.52.3.818. [DOI] [PubMed] [Google Scholar]
- 105. Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006;47(4):1594–1599. doi: 10.1167/iovs.05-1276. •Of Interest. these articles demonstrated the crucial role of oxidative stress in particular NADPH oxidase in the pathogenesis of ischemic retinopathy
- 106.Al-Shabrawey M, Bartoli M, El-Remessy AB, et al. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(7):3231–3238. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Shabrawey M, Bartoli M, El-Remessy AB, et al. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005;167(2):599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al-Shabrawey M, Rojas M, Sanders T, et al. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2008;49(7):3239–3244. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miwa K, Nakamura J, Hamada Y, et al. The role of polyol pathway in glucose-induced apoptosis of cultured retinal pericytes. Diabetes Res Clin Pract. 2003;60(1):1–9. doi: 10.1016/s0168-8227(02)00248-6. [DOI] [PubMed] [Google Scholar]
- 110.Al-Shabrawey M, El-Remessy A, Gu X, et al. Normal vascular development in mice deficient in endothelial NO synthase: possible role of neuronal NO synthase. Mol Vis. 2003;9:549–558. [PubMed] [Google Scholar]
- 111.Stitt AW. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol. 2003;75(1):95–108. doi: 10.1016/s0014-4800(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 112.Lelkes PI, Hahn KL, Sukovich DA, Karmiol S, Schmidt DH. On the possible role of reactive oxygen species in angiogenesis. Adv Exp Med Biol. 1998;454:295–310. doi: 10.1007/978-1-4615-4863-8_35. [DOI] [PubMed] [Google Scholar]
- 113.Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol. 2003;188(1):203–208. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- 114.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008;49(4):1591–1598. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71(2):226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 116.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266(1):37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tawfik A, Sanders T, Kahook K, Akeel S, Elmarakby A, Al-Shabrawey M. Suppression of retinal peroxisome proliferator-activated receptor gamma in experimental diabetes and oxygen-induced retinopathy: role of NADPH oxidase. Invest Ophthalmol Vis Sci. 2009;50(2):878–884. doi: 10.1167/iovs.08-2005. [DOI] [PubMed] [Google Scholar]
- 118.Jang DS, Lee YM, Jeong IH, Kim JS. Constituents of the flowers of Platycodon grandiflorum with inhibitory activity on advanced glycation end products and rat lens aldose reductase in vitro. Arch Pharm Res. 2010;33(6):875–880. doi: 10.1007/s12272-010-0610-x. [DOI] [PubMed] [Google Scholar]
- 119.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36(7):838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 120.Anso E, Zuazo A, Irigoyen M, Urdaci MC, Rouzaut A, Martinez-Irujo JJ. Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism. Biochem Pharmacol. 2010;79(11):1600–1609. doi: 10.1016/j.bcp.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 121.Park SW, Cho CS, Jun HO, et al. Anti-angiogenic effect of luteolin on retinal neovascularization via blockade of reactive oxygen species production. Invest Ophthalmol Vis Sci. 2012;53(12):7718–7726. doi: 10.1167/iovs.11-8790. [DOI] [PubMed] [Google Scholar]
- 122.Akeel S, El-Awady A, Hussein K, et al. Recombinant bone morphogenetic protein-2 induces up-regulation of vascular endothelial growth factor and interleukin 6 in human pre-osteoblasts: role of reactive oxygen species. Arch Oral Biol. 2012;57(5):445–452. doi: 10.1016/j.archoralbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 123.Dong A, Shen J, Krause M, et al. Superoxide dismutase 1 protects retinal cells from oxidative damage. J Cell Physiol. 2006;208(3):516–526. doi: 10.1002/jcp.20683. [DOI] [PubMed] [Google Scholar]
- 124.Imamura Y, Noda S, Hashizume K, et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103(30):11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dong A, Xie B, Shen J, et al. Oxidative stress promotes ocular neovascularization. J Cell Physiol. 2009;219(3):544–552. doi: 10.1002/jcp.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398(6726):381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 127.Wang D, Wang H, Brown J, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203(4):941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nie D, Hillman GG, Geddes T, et al. Platelet-type 12-lipoxygenase in a human prostate carcinoma stimulates angiogenesis and tumor growth. Cancer Res. 1998;58(18):4047–4051. [PubMed] [Google Scholar]
- 129.Tang DG, Renaud C, Stojakovic S, Diglio CA, Porter A, Honn KV. 12(S)-HETE is a mitogenic factor for microvascular endothelial cells: its potential role in angiogenesis. Biochem Biophys Res Commun. 1995;211(2):462–468. doi: 10.1006/bbrc.1995.1836. [DOI] [PubMed] [Google Scholar]
- 130.Li Z, He T, Du K, et al. Overexpression of 15-lipoxygenase-1 in oxygen-induced ischemic retinopathy inhibits retinal neovascularization via downregulation of vascular endothelial growth factor-A expression. Mol Vis. 2012;18:2847–2859. [PMC free article] [PubMed] [Google Scholar]
- 131. Yan Y, He T, Shen Y, et al. Adenoviral 15-lipoxygenase-1 gene transfer inhibits hypoxia-induced proliferation of retinal microvascular endothelial cells in vitro. Int J Ophthalmol. 2012;5(5):562–569. doi: 10.3980/j.issn.2222-3959.2012.05.04. •Of Interest (132–135). These articles pointed to the importance of lipid metabolites in the pathogenesis of ischemic retinopathy
- 132.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7):868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sapieha P, Stahl A, Chen J, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3(69):69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Al-Shabrawey M, Mussell R, Kahook K, et al. Increased expression and activity of 12-lipoxygenase in oxygen-induced ischemic retinopathy and proliferative diabetic retinopathy: implications in retinal neovascularization. Diabetes. 2011;60(2):614–624. doi: 10.2337/db10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Othman A, Ahmad S, Megyerdi S, et al. 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: contribution of NADPH oxidase. PLoS One. 2013;8(2):e57254. doi: 10.1371/journal.pone.0057254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158(1):147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mahajan VB, Vallone JG, Lin JH, et al. T-cell infiltration in autosomal dominant neovascular inflammatory vitreoretinopathy. Mol Vis. 2010;16:1034–1040. [PMC free article] [PubMed] [Google Scholar]
- 138.He C, Sun Y, Ren X, et al. Angiogenesis mediated by toll-like receptor 4 in ischemic neural tissue. Arterioscler Thromb Vasc Biol. 2013;33(2):330–338. doi: 10.1161/ATVBAHA.112.300679. [DOI] [PubMed] [Google Scholar]
- 139.Tezel TH, Bodek E, Sonmez K, et al. Targeting tissue factor for immunotherapy of choroidal neovascularization by intravitreal delivery of factor VII-Fc chimeric antibody. Ocul Immunol Inflamm. 2007;15(1):3–10. doi: 10.1080/09273940601147760. [DOI] [PubMed] [Google Scholar]
- 140.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36(2):127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 141.Gao G, Li Y, Gee S, et al. Down-regulation of vascular endothelial growth factor and up-regulation of pigment epithelium-derived factor: a possible mechanism for the anti-angiogenic activity of plasminogen kringle 5. J Biol Chem. 2002;277(11):9492–9497. doi: 10.1074/jbc.M108004200. [DOI] [PubMed] [Google Scholar]
- 142.Lutty GA, Thompson DC, Gallup JY, Mello RJ, Patz A, Fenselau A. Vitreous: an inhibitor of retinal extract-induced neovascularization. Invest Ophthalmol Vis Sci. 1983;24(1):52–56. [PubMed] [Google Scholar]
- 143.Lutty GA, Mello RJ, Chandler C, Fait C, Bennett A, Patz A. Regulation of cell growth by vitreous humour. J Cell Sci. 1985;76:53–65. doi: 10.1242/jcs.76.1.53. [DOI] [PubMed] [Google Scholar]
- 144.Gao G, Li Y, Fant J, Crosson CE, Becerra SP, Ma JX. Difference in ischemic regulation of vascular endothelial growth factor and pigment epithelium--derived factor in brown norway and sprague dawley rats contributing to different susceptibilities to retinal neovascularization. Diabetes. 2002;51(4):1218–1225. doi: 10.2337/diabetes.51.4.1218. [DOI] [PubMed] [Google Scholar]
- 145.Mohan N, Monickaraj F, Balasubramanyam M, Rema M, Mohan V. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J Diabetes Complications. 2012;26(5):435–441. doi: 10.1016/j.jdiacomp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 146.Tombran-Tink J, Johnson LV. Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest Ophthalmol Vis Sci. 1989;30(8):1700–1707. [PubMed] [Google Scholar]
- 147.Becerra SP, Sagasti A, Spinella P, Notario V. Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem. 1995;270(43):25992–25999. doi: 10.1074/jbc.270.43.25992. [DOI] [PubMed] [Google Scholar]
- 148.Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53(3):411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 149.Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1993;90(4):1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Becerra SP. Structure-function studies on PEDF. A noninhibitory serpin with neurotrophic activity. Adv Exp Med Biol. 1997;425:223–237. [PubMed] [Google Scholar]
- 151.Araki T, Taniwaki T, Becerra SP, Chader GJ, Schwartz JP. Pigment epithelium-derived factor (PEDF) differentially protects immature but not mature cerebellar granule cells against apoptotic cell death. J Neurosci Res. 1998;53(1):7–15. doi: 10.1002/(SICI)1097-4547(19980701)53:1<7::AID-JNR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 152.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285(5425):245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 153. Mori K, Duh E, Gehlbach P, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188(2):253–263. doi: 10.1002/jcp.1114. ••Of Considerable Interest (154). The article demonstrated that PEDF can prevent the development of pathological RNV
- 154.Stellmach V, Crawford SE, Zhou W, Bouck N. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci U S A. 2001;98(5):2593–2597. doi: 10.1073/pnas.031252398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci. 2003;4(8):628–636. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- 156.Zhang SX, Ma JX, Sima J, et al. Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am J Pathol. 2005;166(1):313–321. doi: 10.1016/S0002-9440(10)62255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sheikpranbabu S, Haribalaganesh R, Lee KJ, Gurunathan S. Pigment epithelium-derived factor inhibits advanced glycation end products-induced retinal vascular permeability. Biochimie. 2010;92(8):1040–1051. doi: 10.1016/j.biochi.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 158.Sheikpranbabu S, Ravinarayanan H, Elayappan B, Jongsun P, Gurunathan S. Pigment epithelium-derived factor inhibits vascular endothelial growth factor-and interleukin-1beta-induced vascular permeability and angiogenesis in retinal endothelial cells. Vascul Pharmacol. 2010;52(1–2):84–94. doi: 10.1016/j.vph.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 159.Ogata N, Nishikawa M, Nishimura T, Mitsuma Y, Matsumura M. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am J Ophthalmol. 2002;134(3):348–353. doi: 10.1016/s0002-9394(02)01568-4. [DOI] [PubMed] [Google Scholar]
- 160.Boehm BO, Lang G, Volpert O, et al. Low content of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia. 2003;46(3):394–400. doi: 10.1007/s00125-003-1040-9. [DOI] [PubMed] [Google Scholar]
- 161.Kim SY, Mocanu C, McLeod DS, et al. Expression of pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in sickle cell retina and choroid. Exp Eye Res. 2003;77(4):433–445. doi: 10.1016/s0014-4835(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 162.Ogata N, Tombran-Tink J, Jo N, Mrazek D, Matsumura M. Upregulation of pigment epithelium-derived factor after laser photocoagulation. Am J Ophthalmol. 2001;132(3):427–429. doi: 10.1016/s0002-9394(01)01021-2. [DOI] [PubMed] [Google Scholar]
- 163.Spranger J, Osterhoff M, Reimann M, et al. Loss of the antiangiogenic pigment epithelium-derived factor in patients with angiogenic eye disease. Diabetes. 2001;50(12):2641–2645. doi: 10.2337/diabetes.50.12.2641. [DOI] [PubMed] [Google Scholar]
- 164.Yang J, Williams RS, Kelly DP. Bcl3 interacts cooperatively with peroxisome proliferator-activated receptor gamma (PPARgamma) coactivator 1alpha to coactivate nuclear receptors estrogen-related receptor alpha and PPARalpha. Mol Cell Biol. 2009;29(15):4091–4102. doi: 10.1128/MCB.01669-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shalom-Barak T, Nicholas JM, Wang Y, et al. Peroxisome proliferator-activated receptor gamma controls Muc1 transcription in trophoblasts. Mol Cell Biol. 2004;24(24):10661–10669. doi: 10.1128/MCB.24.24.10661-10669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Muranaka K, Yanagi Y, Tamaki Y, et al. Effects of peroxisome proliferator-activated receptor gamma and its ligand on blood-retinal barrier in a streptozotocin-induced diabetic model. Invest Ophthalmol Vis Sci. 2006;47(10):4547–4552. doi: 10.1167/iovs.05-1432. [DOI] [PubMed] [Google Scholar]
- 167.Lee KS, Kim SR, Park SJ, et al. Peroxisome proliferator activated receptor-gamma modulates reactive oxygen species generation and activation of nuclear factor-kappaB and hypoxia-inducible factor 1alpha in allergic airway disease of mice. J Allergy Clin Immunol. 2006;118(1):120–127. doi: 10.1016/j.jaci.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 168.Sung B, Park S, Yu BP, Chung HY. Amelioration of age-related inflammation and oxidative stress by PPARgamma activator: suppression of NF-kappaB by 2,4-thiazolidinedione. Exp Gerontol. 2006;41(6):590–599. doi: 10.1016/j.exger.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 169.Song MK, Roufogalis BD, Huang TH. Modulation of diabetic retinopathy pathophysiology by natural medicines through PPAR-gamma-related pharmacology. Br J Pharmacol. 2012;165(1):4–19. doi: 10.1111/j.1476-5381.2011.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stahl A, Sapieha P, Connor KM, et al. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ Res. 2010;107(4):495–500. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Murata T, He S, Hangai M, et al. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41(8):2309–2317. [PubMed] [Google Scholar]
- 172.Dougherty TJ, Marcus SL. Photodynamic therapy. Eur J Cancer. 1992;28A(10):1734–1742. doi: 10.1016/0959-8049(92)90080-l. [DOI] [PubMed] [Google Scholar]
- 173.Kaplan MJ, Somers RG, Greenberg RH, Ackler J. Photodynamic therapy in the management of metastatic cutaneous adenocarcinomas: case reports from phase 1/2 studies using tin ethyl etiopurpurin (SnET2) J Surg Oncol. 1998;67(2):121–125. doi: 10.1002/(sici)1096-9098(199802)67:2<121::aid-jso9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 174.Ben-Hur E, Orenstein A. The endothelium and red blood cells as potential targets in PDT-induced vascular stasis. Int J Radiat Biol. 1991;60(1–2):293–301. doi: 10.1080/09553009114552041. [DOI] [PubMed] [Google Scholar]
- 175.Flower RW. Optimizing treatment of choroidal neovascularization feeder vessels associated with age-related macular degeneration. Am J Ophthalmol. 2002;134(2):228–239. doi: 10.1016/s0002-9394(02)01579-9. [DOI] [PubMed] [Google Scholar]
- 176.Ho T, Smiddy WE, Flynn HW., Jr Vitrectomy in the management of diabetic eye disease. Surv Ophthalmol. 1992;37(3):190–202. doi: 10.1016/0039-6257(92)90137-i. [DOI] [PubMed] [Google Scholar]
- 177.Arrigg PG, Cavallerano J. The role of vitrectomy for diabetic retinopathy. J Am Optom Assoc. 1998;69(11):733–740. [PubMed] [Google Scholar]
- 178.Sharma S, Brown GC, Brown MM, Hollands H, Shah GK. The cost-effectiveness of photodynamic therapy for fellow eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2001;108(11):2051–2059. doi: 10.1016/s0161-6420(01)00764-3. [DOI] [PubMed] [Google Scholar]
- 179.Shinoda K, Ishida S, Kawashima S, et al. Clinical factors related to the aqueous levels of vascular endothelial growth factor and hepatocyte growth factor in proliferative diabetic retinopathy. Curr Eye Res. 2000;21(2):655–661. [PubMed] [Google Scholar]
- 180.Drolet DW, Nelson J, Tucker CE, et al. Pharmacokinetics and safety of an anti-vascular endothelial growth factor aptamer (NX1838) following injection into the vitreous humor of rhesus monkeys. Pharm Res. 2000;17(12):1503–1510. doi: 10.1023/a:1007657109012. [DOI] [PubMed] [Google Scholar]
- 181.Ruckman J, Green LS, Beeson J, et al. 2'-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998;273(32):20556–20567. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 182.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 183.Cunningham ET, Jr, Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112(10):1747–1757. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 184.Nicholson BP, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):915–930. doi: 10.1007/s00417-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 185.Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57(20):4593–4599. [PubMed] [Google Scholar]
- 186.Ellis LM, Curley SA, Grothey A. Surgical resection after downsizing of colorectal liver metastasis in the era of bevacizumab. J Clin Oncol. 2005;23(22):4853–4855. doi: 10.1200/JCO.2005.23.754. [DOI] [PubMed] [Google Scholar]
- 187.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23(4):792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 188. Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21(16):3127–3132. doi: 10.1200/JCO.2003.02.122. ••Of Considerable Interest (189–200). These articles discussed clinical application of anti-VEGF therapy in treating macular edema and pathological neovascularization
- 189.Abouammoh M, Sharma S. Ranibizumab versus bevacizumab for the treatment of neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2011;22(3):152–158. doi: 10.1097/ICU.0b013e32834595d0. [DOI] [PubMed] [Google Scholar]
- 190.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 191.Arevalo JF, Fromow-Guerra J, Sanchez JG, et al. Primary intravitreal bevacizumab for subfoveal choroidal neovascularization in age-related macular degeneration: results of the Pan-American Collaborative Retina Study Group at 12 months follow-up. Retina. 2008;28(10):1387–1394. doi: 10.1097/IAE.0b013e3181884ff4. [DOI] [PubMed] [Google Scholar]
- 192.Willard AL, Herman IM. Vascular complications and diabetes: current therapies and future challenges. J Ophthalmol. 2012;2012:209538. doi: 10.1155/2012/209538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 194.Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146–2151. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 195.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46(2):726–733. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 197.Rakic JM, Zelinkova M, Comhaire-Poutchinian Y, Galand A, Duchateau E. Treatment of Graves macular edema with intravitreal injection of corticosteroids. Bull Soc Belge Ophtalmol. 2003;(288):43–48. [PubMed] [Google Scholar]
- 198.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 199.Kaiser PK. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 5-year results of two randomized clinical trials with an open-label extension: TAP report no. 8. Graefes Arch Clin Exp Ophthalmol. 2006;244(9):1132–1142. doi: 10.1007/s00417-005-0199-9. [DOI] [PubMed] [Google Scholar]
- 200.Kaiser PK. Antivascular endothelial growth factor agents and their development: therapeutic implications in ocular diseases. Am J Ophthalmol. 2006;142(4):660–668. doi: 10.1016/j.ajo.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 201.Essex RW, Tufail A, Bunce C, Aylward GW. Two-year results of surgical removal of choroidal neovascular membranes related to non-age-related macular degeneration. Br J Ophthalmol. 2007;91(5):649–654. doi: 10.1136/bjo.2005.089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28(5):348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 203.Leal EC, Santiago AR, Ambrosio AF. Old and new drug targets in diabetic retinopathy: from biochemical changes to inflammation and neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2005;4(4):421–434. doi: 10.2174/1568007054546162. [DOI] [PubMed] [Google Scholar]
- 204.Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009;4(1):e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Simo R, Hernandez C. Intravitreous anti-VEGF for diabetic retinopathy: hopes and fears for a new therapeutic strategy. Diabetologia. 2008;51(9):1574–1580. doi: 10.1007/s00125-008-0989-9. [DOI] [PubMed] [Google Scholar]
- 206.Wirostko B, Wong TY, Simo R. Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res. 2008;27(6):608–621. doi: 10.1016/j.preteyeres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 207.Day S, Acquah K, Mruthyunjaya P, Grossman DS, Lee PP, Sloan FA. Ocular complications after anti-vascular endothelial growth factor therapy in Medicare patients with age-related macular degeneration. Am J Ophthalmol. 2011;152(2):266–272. doi: 10.1016/j.ajo.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology. 2002;143(7):2797–2807. doi: 10.1210/endo.143.7.8886. [DOI] [PubMed] [Google Scholar]
- 209.Ni Z, Hui P. Emerging pharmacologic therapies for wet age-related macular degeneration. Ophthalmologica. 2009;223(6):401–410. doi: 10.1159/000228926. [DOI] [PubMed] [Google Scholar]
- 210.Danis RP, Bingaman DP, Jirousek M, Yang Y. Inhibition of intraocular neovascularization caused by retinal ischemia in pigs by PKCbeta inhibition with LY333531. Invest Ophthalmol Vis Sci. 1998;39(1):171–179. [PubMed] [Google Scholar]
- 211.Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996;37(5):886–897. [PubMed] [Google Scholar]
- 212.Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46(9):1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 213.Feke GT, Buzney SM, Ogasawara H, et al. Retinal circulatory abnormalities in type 1 diabetes. Invest Ophthalmol Vis Sci. 1994;35(7):2968–2975. [PubMed] [Google Scholar]
- 214.Xu X, Zhu Q, Xia X, Zhang S, Gu Q, Luo D. Blood-retinal barrier breakdown induced by activation of protein kinase C via vascular endothelial growth factor in streptozotocin-induced diabetic rats. Curr Eye Res. 2004;28(4):251–256. doi: 10.1076/ceyr.28.4.251.27834. [DOI] [PubMed] [Google Scholar]
- 215.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. Inhibition of protein kinase Cbeta prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002;90(1):107–111. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]
- 216. Strom C, Sander B, Klemp K, Aiello LP, Lund-Andersen H, Larsen M. Effect of ruboxistaurin on blood-retinal barrier permeability in relation to severity of leakage in diabetic macular edema. Invest Ophthalmol Vis Sci. 2005;46(10):3855–3858. doi: 10.1167/iovs.05-0096. ••Of Considerable Interest (217). These articles discussed the beneficial effect of PKC inhibition to treat DR
- 217.Schwartz SG, Flynn HW, Jr, Aiello LP. Ruboxistaurin mesilate hydrate for diabetic retinopathy. Drugs Today (Barc) 2009;45(4):269–274. doi: 10.1358/dot.2009.45.4.1354195. [DOI] [PubMed] [Google Scholar]
- 218.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 219.Kaiser PK, Symons RC, Shah SM, et al. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol. 2010;150(1):33–39. e32. doi: 10.1016/j.ajo.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 220.Shen J, Samul R, Silva RL, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13(3):225–234. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- 221.Ceda GP, Hoffman AR, Silverberg GD, Wilson DM, Rosenfeld RG. Regulation of growth hormone release from cultured human pituitary adenomas by somatomedins and insulin. J Clin Endocrinol Metab. 1985;60(6):1204–1209. doi: 10.1210/jcem-60-6-1204. [DOI] [PubMed] [Google Scholar]
- 222.Smith LE, Kopchick JJ, Chen W, et al. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science. 1997;276(5319):1706–1709. doi: 10.1126/science.276.5319.1706. [DOI] [PubMed] [Google Scholar]
- 223.Gilbert RE, Kelly DJ, Cox AJ, et al. Angiotensin converting enzyme inhibition reduces retinal overexpression of vascular endothelial growth factor and hyperpermeability in experimental diabetes. Diabetologia. 2000;43(11):1360–1367. doi: 10.1007/s001250051539. [DOI] [PubMed] [Google Scholar]
- 224.Williams B, Baker AQ, Gallacher B, Lodwick D. Angiotensin II increases vascular permeability factor gene expression by human vascular smooth muscle cells. Hypertension. 1995;25(5):913–917. doi: 10.1161/01.hyp.25.5.913. [DOI] [PubMed] [Google Scholar]
- 225.Chua CC, Hamdy RC, Chua BH. Upregulation of vascular endothelial growth factor by angiotensin II in rat heart endothelial cells. Biochim Biophys Acta. 1998;1401(2):187–194. doi: 10.1016/s0167-4889(97)00129-8. [DOI] [PubMed] [Google Scholar]
- 226.Goa KL, Haria M, Wilde MI. Lisinopril. A review of its pharmacology and use in the management of the complications of diabetes mellitus. Drugs. 1997;53(6):1081–1105. doi: 10.2165/00003495-199753060-00010. [DOI] [PubMed] [Google Scholar]
- 227.Scatena R. Prinomastat, a hydroxamate-based matrix metalloproteinase inhibitor. A novel pharmacological approach for tissue remodelling-related diseases. Expert Opin Investig Drugs. 2000;9(9):2159–2165. doi: 10.1517/13543784.9.9.2159. [DOI] [PubMed] [Google Scholar]
- 228.Shalinsky DR, Brekken J, Zou H, et al. Broad antitumor and antiangiogenic activities of AG3340, a potent and selective MMP inhibitor undergoing advanced oncology clinical trials. Ann N Y Acad Sci. 1999;878:236–270. doi: 10.1111/j.1749-6632.1999.tb07689.x. [DOI] [PubMed] [Google Scholar]
- 229. Garcia C, Bartsch DU, Rivero ME, et al. Efficacy of Prinomastat) (AG3340), a matrix metalloprotease inhibitor, in treatment of retinal neovascularization. Curr Eye Res. 2002;24(1):33–38. doi: 10.1076/ceyr.24.1.33.5429. •Of Interest (230 and 231). These articles demonstrated the potential therapeutic effect of corticosteroid in DR.
- 230.Spandau UH, Sauder G, Schubert U, Hammes HP, Jonas JB. Effect of triamcinolone acetonide on proliferation of retinal endothelial cells in vitro and in vivo. Br J Ophthalmol. 2005;89(6):745–747. doi: 10.1136/bjo.2004.052563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang YS, Friedrichs U, Eichler W, Hoffmann S, Wiedemann P. Inhibitory effects of triamcinolone acetonide on bFGF-induced migration and tube formation in choroidal microvascular endothelial cells. Graefes Arch Clin Exp Ophthalmol. 2002;240(1):42–48. doi: 10.1007/s00417-001-0398-y. [DOI] [PubMed] [Google Scholar]
- 232.Koedam JA, Smink JJ, van Buul-Offers SC. Glucocorticoids inhibit vascular endothelial growth factor expression in growth plate chondrocytes. Mol Cell Endocrinol. 2002;197(1–2):35–44. doi: 10.1016/s0303-7207(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 233.Gille J, Reisinger K, Westphal-Varghese B, Kaufmann R. Decreased mRNA stability as a mechanism of glucocorticoid-mediated inhibition of vascular endothelial growth factor gene expression by cultured keratinocytes. J Invest Dermatol. 2001;117(6):1581–1587. doi: 10.1046/j.0022-202x.2001.01573.x. [DOI] [PubMed] [Google Scholar]
- 234.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341(2–3):309–315. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 235.Jamrozy-Witkowska A, Kowalska K, Jankowska-Lech I, Terelak-Borys B, Nowosielska A, Grabska-Liberek I. Complications of intravitreal injections--own experience. Klin Oczna. 2011;113(4–6):127–131. [PubMed] [Google Scholar]
- 236.Shah AM, Oster SF, Freeman WR. Viral retinitis after intravitreal triamcinolone injection in patients with predisposing medical comorbidities. Am J Ophthalmol. 2010;149(3):433–440. e431. doi: 10.1016/j.ajo.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wilkinson-Berka JL, Alousis NS, Kelly DJ, Gilbert RE. COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003;44(3):974–979. doi: 10.1167/iovs.02-0392. [DOI] [PubMed] [Google Scholar]
- 238. Carmo A, Cunha-Vaz JG, Carvalho AP, Lopes MC. Nitric oxide synthase activity in retinas from non-insulin-dependent diabetic Goto-Kakizaki rats: correlation with blood-retinal barrier permeability. Nitric Oxide. 2000;4(6):590–596. doi: 10.1006/niox.2000.0312. ••Of Interest (239–243). These articles demonstrated the beneficial effect of anti-inflammatory therapy in neurovascular injury
- 239.Takahashi K, Saishin Y, Mori K, et al. Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci. 2003;44(1):409–415. doi: 10.1167/iovs.02-0346. [DOI] [PubMed] [Google Scholar]
- 240.Zheng Z, Schwab S, Grau A, Berger C. Neuroprotection by early and delayed treatment of acute stroke with high dose aspirin. Brain Res. 2007;1186:275–280. doi: 10.1016/j.brainres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 241.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96(23):13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26(4):515–521. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Baptiste DC, Hartwick AT, Jollimore CA, Baldridge WH, Seigel GM, Kelly ME. An investigation of the neuroprotective effects of tetracycline derivatives in experimental models of retinal cell death. Mol Pharmacol. 2004;66(5):1113–1122. doi: 10.1124/mol.104.001081. [DOI] [PubMed] [Google Scholar]
- 244.Roychoudhury J, Herndon JM, Yin J, Apte RS, Ferguson TA. Targeting immune privilege to prevent pathogenic neovascularization. Invest Ophthalmol Vis Sci. 2010;51(7):3560–3566. doi: 10.1167/iovs.09-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Funatsu H, Yamashita H, Noma H, et al. Outcome of vitreous surgery and the balance between vascular endothelial growth factor and endostatin. Invest Ophthalmol Vis Sci. 2003;44(3):1042–1047. doi: 10.1167/iovs.02-0374. [DOI] [PubMed] [Google Scholar]
- 246.Lai CC, Wu WC, Chen SL, et al. Recombinant adeno-associated virus vector expressing angiostatin inhibits preretinal neovascularization in adult rats. Ophthalmic Res. 2005;37(1):50–56. doi: 10.1159/000083040. [DOI] [PubMed] [Google Scholar]