Abstract
Human embryonic stem cells have been advanced as a source of insulin-producing cells that could potentially replace cadaveric-derived islets in the treatment of type 1 diabetes. To this end, protocols have been developed that promote the formation of pancreatic progenitors and endocrine cells from human pluripotent stem cells, encompassing both embryonic stem cells and induced pluripotent stem cells. In this review, we examine these methods and place them in the context of the developmental and embryological studies upon which they are based. In particular, we outline the stepwise differentiation of cells towards definitive endoderm, pancreatic endoderm, endocrine lineages and the emergence of functional beta-cells. In doing so, we identify key factors common to many such protocols and discuss the proposed action of these factors in the context of cellular differentiation and ongoing development. We also compare strategies that entail transplantation of progenitor populations with those that seek to develop fully functional hormone expressing cells in vitro. Overall, our survey of the literature highlights the significant progress already made in the field and identifies remaining deficiencies in developing a pluripotent stem cell based treatment for type 1 diabetes.
Keywords: diabetes, embryonic stem cells, differentiation, pancreas, insulin-producing cells, pluripotent cells, progenitor, transplantation
Abbreviations: BMP4 - bone morphogenetic protein 4; Cdh1 - E-cadherin; CXCR4 - chemokine (C-X-C motif) receptor 4; EMT - epithelial-mesenchymal transition; EphB3 - ephrin receptor B3; ESC - embryonic stem cell; FGF10 - fibroblast growth factor 10; FoxA2 - forkhead-box protein A2; GCG - glucagon; GFP - green fluorescence protein; GHRL - ghrelin; Gsc - goosecoid; GSIS - glucose stimulated insulin secretion; Hes1 - hairy and enhancer of split 1; hESC - human embryonic stem cell; HGF - hepatocyte growth factor; Hnf6 - hepatocyte nuclear factor 6; hPSC - human pluripotent stem cell; IDE1/2 - inducer of definite endoderm 1/2; IGF - insulin-like growth factor; INS - insulin; MafA - v-maf musculoaponeurotic fibrosarcoma oncogene homolog A; MEF - mouse embryonic fibroblast; Mixl1 - Mix-like 1 homeodomain protein; Ngn3 - neurogenin 3; Nkx6.1 - Nk6 homeobox protein 1; Oct4 - octamer binding transcription factor 4; PDX1 - pancreatic and duodenal homeobox 1; PI3K - phosphatidylinositide 3 kinase; PP - pancreatic polypeptide; PSC - pluripotent stem cell; RA - retinoic acid; RALDH - retinaldehyde dehydrogenase; SHH - sonic hedgehog; SOM - somatostatin; Sox17 - sex-determining region Y box 17; SST - somatostatin; TGFβ - transforming growth factor beta; Wnt3a - wingless-type MMTV integration site family member 3a
1. Introduction
Embryonic stem cells (ESCs) are immortal, pluripotent cells derived from the inner cell mass of the pre-implantation embryo that retain the capacity to differentiate into extra-embryonic and embryonic derivatives of all three germ layers [1, 2]. The isolation of human ESCs in 1998 [3] and their subsequently demonstrated broad differentiation capacity [4] provided an opportunity to develop in vitro models of post-implantation stages of early human development. Furthermore, the ability of hESCs to be differentiated towards specific cell types raised the possibility that hESC-derived cell types could form a platform for cell-based therapies in the future. This possibility has heightened interest in directing hESC differentiation in vitro to therapeutically relevant cell types, such as insulin-producing pancreatic beta-cells that could potentially replace cadaveric derived islets for the treatment of type 1 diabetes. This review examines protocols designed to differentiate pluripotent stem cells (PSCs) towards pancreatic endocrine cells and discusses how these methodologies relate to the developmental principles upon which they are based.
2. Embryonic stem cell differentiation to beta-cells
A number of studies have reported protocols for the derivation of pancreatic endoderm from differentiating human PSCs (hPSCs). One such protocol, published by D'Amour et al., incorporated combinations of growth factors that had been implicated in normal pancreatic development [5]. In this method, hESCs were guided from their undifferentiated state to insulin-expressing cells via a series of obligate intermediate cell types identified through developmental studies. Following this, many other groups have published methods for the generation of pancreatic cells from hPSCs. In general, these protocols follow the same ontogeny-based approach articulated by D'Amour and colleagues. Figure 1 summarizes features common to many of these protocols and provides an overview of the important developmental milestones as cells exit pluripotency and differentiate towards pancreatic endoderm.
Figure 1. Generation of pancreatic cells in vivo and in vitro.
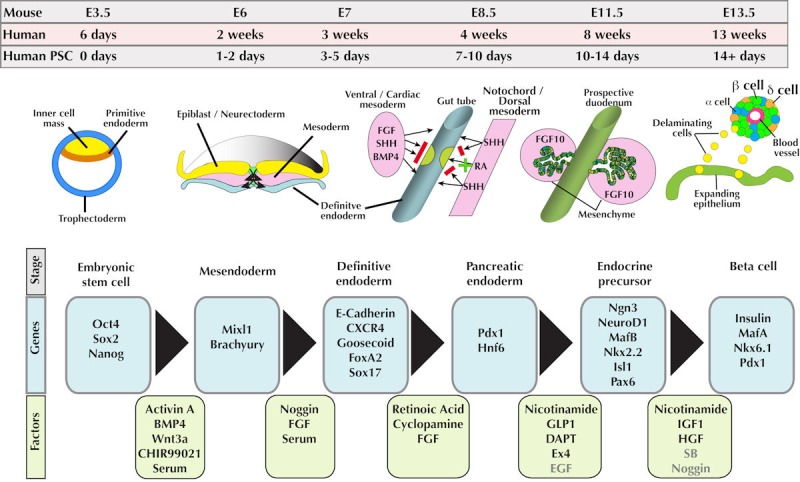
Upper lines show the comparative time frames of mouse and human pancreatic development in vivo, and the approximate time line of human pluripotent stem cell differentiation towards pancreatic lineages in vitro. Key morphogenetic events and developmental milestones corresponding to each time point are shown. Specific genes marking the above developmental milestones are boxed in blue. Green boxes indicate factors commonly used to promote differentiation of cells for each of the indicated steps. Note the juxtaposition of mesoderm and endoderm throughout several key stages of pancreatic development. Red bars indicate instances where signaling pathways need to be blocked to permit pancreatic development. Abbreviations: BMP4 - bone morphogenetic protein 4, CXCR4 - chemokine (C-X-C motif) receptor 4, DAPT - N-(N-(3,5-difluorophenacetyl)-L-alanyl)-S-phenylglycine t-butyl ester, EGF - epidermal growth factor, FGF10 - fibroblast growth factor 10, FoxA2 - forkhead-box protein A2, GLP1 - glucagon-like peptide 1, HGF - hepatocyte growth factor, Hnf6 - hepatocyte nuclear factor 6, Isl1 - insulin gene enhancer protein 1, MafA - v-maf musculoaponeurotic fibrosarcoma oncogene homolog A, MafB - v-maf musculoaponeurotic fibrosarcoma oncogene homolog B, Mixl1 - Mix-like 1 homeodomain protein, Ngn3 - neurogenin 3, Nkx2.2 – Nk2 homeobox protein 2, Nkx6.1 - Nk6 homeobox protein 1, Oct4 - octamer binding transcription factor 4, Pax6 - paired box gene 6, Pdx1 - pancreatic and duodenal homeobox 1, RA - retinoic acid, SHH - sonic hedgehog, Sox2 - sex-determining region Y box 2, Sox17 - sex-determining region Y box 17, Wnt3a - wingless-type MMTV integration site family member 3a.
3. Step 1: formation of definitive endoderm from pluripotent stem cells
The first steps in the process of making pancreatic cells from hPSCs involve the generation of mesendoderm and its subsequent differentiation towards definitive endoderm. Both in vitro and in vivo, mouse mesendoderm cells are marked by the expression of the transcription factors Mixl1 and Brachyury [6-8]. The commitment of this population into definitive endoderm is accompanied by the upregulation of three other transcription factors, Gsc, Sox17, and FoxA2 [9-12]. Mouse ESC differentiation experiments indicated that definitive endoderm can also be identified by the co-expression of two cell surface receptors: E-cadherin (Cdh1) and CXCR4 [13]. However, an important caveat with endoderm-associated markers is that their ability to identify definitive, as opposed to primitive endoderm is predicated on the earlier transit of cells through a stage where they express primitive streak (mesendoderm) genes, such as Mixl1 and Brachyury. This same progression is thought to occur in both human development and during human PSC differentiation.
In the majority of differentiation protocols, induction of definitive endoderm involves treating cells with high concentrations of activin A, a transforming growth factor beta (TGFβ) family member used in place of nodal, the endogenous driver of anterior mesendoderm formation within the developing embryo [14]. The use of activin A to mimic nodal signaling during hPSC differentiation is based upon a number of observations from in vivo and in vitro studies. Gene targeting experiments in mice demonstrated that nodal-null embryos failed to form a primitive streak, the morphological structure transiently present during gastrulation from which definitive endoderm and mesoderm are generated (reviewed in reference [15]). A series of experiments performed by Lu and Robertson showed that nodal played a crucial role in the establishment of the global antero-posterior axis, and that the level of nodal expression correlated with anteriorization of the mesendoderm [16].
Early work with mouse ESCs examined the induction of mesoderm and definitive endoderm by TGFβ family members. Kubo et al. evaluated the ability of activin A to induce mesoderm and endoderm in embryoid bodies using a serum-free differentiation system. They concluded that different concentrations of activin A induced different developmental outcomes: low concentrations of activin A favored ventral mesodermal fates, whereas high concentrations favored more dorsal mesoderm and endodermal lineages [17].
Subsequent studies demonstrated that PI3K signaling must be suppressed for hESCs to respond optimally to activin/nodal [18]. Compounds such as wortmannin, which inhibits PI3K signaling, promoted definitive endoderm formation [18], and have been used in conjunction with activin A to form definitive endoderm from hESCs [19]. Because of the expense and batch-to-batch variability associated with preparations of activin A, researchers have also sought to discover small molecule alternatives that promote hESC differentiation towards definitive endoderm. Borowiak and colleagues identified two such molecules, IDE1 and IDE2, which induced endoderm (in the presence of serum) with similar efficiencies to activin A. The specific target molecule for the compounds was not identified, although experiments indicated that activation of TGFβ signaling may be involved [20].
Many protocols also incorporate other factors that have also been implicated in fate decisions in the early embryo. For example, Wnt3a is frequently included during the earliest stages, presumably to enhance mesendoderm formation (see, for example, references [5, 21]). In these circumstances, Wnt3a is probably acting as a surrogate for Wnt3, a molecule expressed in the proximal epiblast prior to gastrulation, and then later in the primitive streak. Mice lacking Wnt3 fail to undergo gastrulation and blocking canonical Wnt signaling during ESC differentiation also blocks mesendoderm formation [22-24]. In this context, Bone and colleagues found that 1m, a small molecule inhibitor of the Wnt signaling suppressor, GSK-3, induced differentiation of hESCs towards definitive endoderm [25].
Another member of the TGFβ superfamily that is critical for mesendoderm formation is BMP4, which is expressed in the amnion, extra-embryonic mesoderm and posterior primitive streak during mouse development [26]. Mouse embryos lacking BMP4 fail to express mesendoderm-associated genes such as Brachyury and die during gastrulation, at approximately embryonic day (E) 6.5 [26]. A number of studies have reported the inclusion of low concentrations of BMP4 to enhance the endodermal differentiation effects of activin A (for example: [27-29]).
An alternative approach in many differentiation protocols is the inclusion of low levels of fetal calf serum during the mesendoderm induction regime. The role played by serum is unclear, but may relate to either its positive affect on cell survival [30], or the presence of growth factor activities, such as, but not limited to, BMP-like effects [31] or activin A [32].
4. Step 2: definitive endoderm to foregut/pancreatic endoderm
Following the induction of definitive endoderm by high levels of activin A, protocols then incorporate factors to direct this endoderm towards a pancreatic fate; a differentiation step marked by the expression of the pancreatic transcription factor PDX1. This is frequently achieved by treating cultures for a number of days with retinoic acid (RA) (Figure 1).
It has been demonstrated that RA plays an essential role in the morphogenesis and organogenesis of a number of organs, including the pancreas (reviewed in reference [33]). Within the embryo, RA is synthesized from circulating retinol in a two-step reaction involving specific alcohol dehydrogenases and aldehyde dehydrogenases, known as retinaldehyde dehydrogenases (RALDHs) [34]. During gastrulation, Raldh2 is expressed in the mesendoderm before becoming localized to the lateral plate and paraxial mesoderm during segmentation [35]. Gain- and loss-of-function studies indicate that retinoid signaling is required for pancreatic specification in the zebrafish, Xenopus, quail, and mouse [34, 36, 37]. In zebrafish, for example, it has been shown that retinoid signaling is required for pancreas and liver specification, and that treatment with exogenous RA induces ectopic expression of pancreatic and liver markers [36]. In Xenopus, inhibition of retinoid signaling at the gastrula stage resulted in the loss of dorsal pancreas but had little effect on ventral pancreas development [38]. Further studies demonstrated that, whilst RA was sufficient to induce pancreatic-specific genes in the dorsal pancreas, it failed to do so in the ventral pancreas [39]. Similarly, in the quail, it was shown that RA deficient embryos lacked a dorsal pancreas. Additionally, in mice, it was demonstrated that RA signaling was sufficient to induce Pdx1 expression in anterior endoderm [34].
Based on such developmental studies, a number of groups showed that addition of exogenous RA could also promote the differentiation of mouse ESCs to Pdx1+ endoderm [40-42]. This finding was subsequently reproduced in human ESCs, where it was demonstrated that RA was required to convert posterior foregut endoderm to pancreatic endoderm [5]. The addition of exogenous RA to differentiating hESCs was also shown to up-regulate the subsequent expression of pancreatic-associated genes such as NEUROGENIN3 (NGN3), and hormones including INSULIN and GLUCAGON [5].
At ~E9.5 in the mouse, the splanchnic mesenchyme merges with the dorsal foregut evagination to form the dorsal pancreatic bud. A similar process is repeated with the ventral evaginations, resulting in the formation of a ventral pancreatic bud [43, 44]. At ~E16.5, gut rotation brings the ventral pancreas rudiment into juxtaposition with its dorsal counterpart, leading to fusion of the anlage [44, 45].
It is believed that the pancreas forms in a similar manner in the human embryo. Following gastrulation, the endoderm forms a flat sheet which then rotates to form a primitive gut tube. Following specification of the pancreatic endoderm within the regionalized gut tube, the dorsal bud appears at ~26 days of development and grows into the dorsal mesenchyme, opposite the hepatic diverticulum. The ventral bud appears several days later, and begins to grow into the ventral mesenchyme, just caudal to the gallbladder [46]. The epithelial cells forming both buds then continue to proliferate and branch, invading the surrounding mesenchyme. By week (W) 5, the ventral bud has commenced migrating posteriorly around the duodenum and by early in W6, lies adjacent to the dorsal pancreatic bud. The two pancreatic buds fuse a few days later [46].
In some studies, researchers have differentiated hESCs to a stage equivalent to the pancreatic endoderm before transplanting the cells into mice, to produce functional beta-cells at a later date in vivo. This strategy was first employed by Kroon and colleagues, who differentiated hESCs in vitro for 12 days to form PDX1+ pancreatic endoderm, prior to transplantation into immunodeficient non-diabetic mice. It proved necessary to allow the grafts to develop in vivo for a further 90 to 140 days post-transplant prior to selective ablation of the mouse beta-cells with streptozotocin (STZ), to determine whether the engrafted human pancreatic endoderm had differentiated into functional beta-cells. Not only did the engrafted cells secrete human insulin, but they also maintained normoglycemia until surgical graft removal, up to 88 days post-STZ treatment [47]. A number of other groups have subsequently followed a similar approach, as summarized in Table 1.
Table 1. Studies reporting on the differentiation of human ES cells to pancreatic endoderm and their subsequent transplantation.
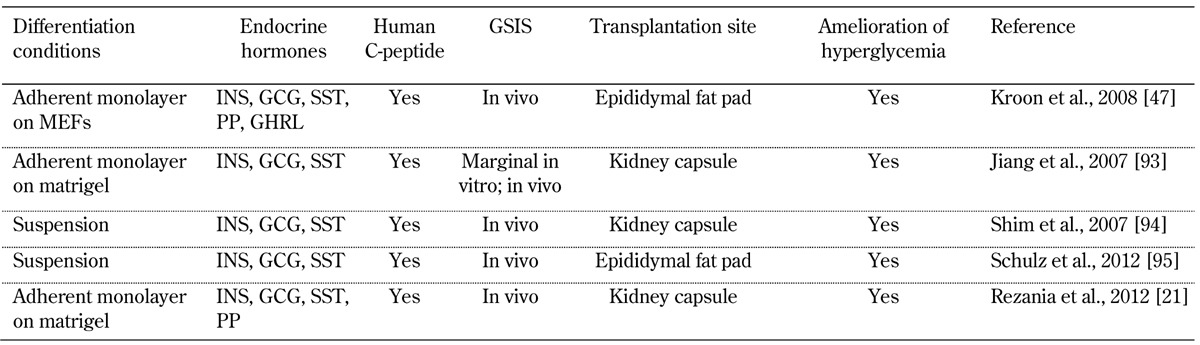
Legend: GCG - glucagon, GHRL - ghrelin, GSIS - glucose stimulated insulin secretion, INS - insulin, MEF - mouse embryonic fibroblast, PP - pancreatic polypeptide, SST - somatostatin.
Recently, Rezania and colleagues demonstrated that hESC-derived pancreatic progenitors could ameliorate diabetes in both diabetic mice and immunodeficient rats [21]. This is the first time that PSC-derived pancreatic cells have been shown to function in non-mouse animal models, an important proof of principle for future efforts to translate this approach into a clinical setting.
5. Step 3: pancreatic endoderm to endocrine precursor cells
Although the commitment of pancreatic endoderm to endocrine precursor cells is an obligate intermediate step during beta-cell formation, most differentiation protocols have not yet incorporated factors specifically designed to either promote or enhance this process. The transcription factor neurogenin 3 (Ngn3), is required for the development of endocrine cells in the pancreas [48]. However, this key gene is only expressed transiently, making it difficult to use as a marker to identify cells committing to an endocrine fate.
There are hints from developmental studies concerning which signaling pathways may need to be modulated to control endocrine precursor formation. In mouse experiments, impaired Notch receptor activation or signaling resulted in profound upregulation of Ngn3 gene expression, leading to premature endocrine cell differentiation at the expense of pancreatic progenitor expansion and exocrine cell differentiation [49, 50]. In contrast, active Notch signaling most likely maintains cells as undifferentiated progenitors that can contribute to proliferation, morphogenesis, and later differentiation events. In this sense, the function of Notch signaling during this process is analogous to its function during early mammalian neurogenesis [51, 52].
Other factors recently identified as playing key roles in controlling the balance between proliferation and differentiation include TGFβ2 and TGFβ3. Work from Guo et al. suggests that, in the mouse, TGFβ ligands can increase the number of pancreatic progenitors via a mechanism that is critically dependent of the duration of ligand signaling [53]. Whilst a short burst of TGFβ signaling promoted upregulation of Ngn3 and endocrine differentiation, prolonged TGFβ signaling led to continued expansion of the progenitor pool and attenuation of endocrine development [53].
As noted above, Ngn3 expression is a prerequisite for endocrine commitment. In the mouse, Ngn3 has a bimodal expression pattern with the first wave observed between E8.5 and E11. A second wave of expression is initiated at E12 and reaches its peak at E15.5 before rapid downregulation by E17.5 [54, 55]. Studies in the chick and mouse have shown that Ngn3 plays an essential role in the epithelial-mesenchymal transition (EMT) [56] associated with endocrine cell development, during which delaminating cells are marked by the expression of EphB3 [57]. Endocrine specification begins at ~E16.5 in the mouse, with the induction of Ngn3 in response to the repression of Notch signaling [49].
The mesenchyme associated with the developing mouse pancreas has also been shown to play a role in regulating both Ngn3 induction [58] and pancreatic epithelial cell proliferation [59]. Mouse knock-out studies have identified mesenchymally produced FGF10 as a key growth factor in this process, since FGF10-null mice exhibit severe pancreatic hypoplasia because of a striking reduction in the proliferation of the pancreatic epithelial progenitor cells [59]. In contrast, transgenic mice over-expressing FGF10 display pancreatic hyperplasia resulting from reduced differentiation and associated expansion of pancreatic progenitor cell numbers [60, 61]. A similar proliferative effect of FGF10 on pancreatic progenitor cells has been also been demonstrated in vitro in both isolated rat [62] and mouse [63] pancreatic epithelia. The addition of FGF10 to E10.5 mouse pancreatic epithelium increased the proliferation of pancreatic progenitors. Furthermore, this study also showed that FGF10 treatment maintained expression of Hes1, a downstream target of Notch1 signaling. Confirming this link between the FGF10 and Notch pathways, Miralles et al. showed that the inhibition of Notch signaling downregulated both Hes1 expression and decreased pancreatic progenitor cell proliferation in FGF10-treated epithelium. These results strongly suggest that Notch is required as a downstream mediator of FGF10 signaling in pancreatic progenitors [63]. A more recent study, performed by Sneddon et al., demonstrated the influence of pancreatic mesenchyme on progenitor cell proliferation [64]. In this study, the authors co-cultured mESC-derived Ngn3+ endocrine progenitor cells with mouse mesenchymal cell lines, observing a co-culture specific expansion of progenitor cells, without evidence of cell differentiation, following 6 days of culture, which was not seen when cells were cultured on either extra-cellular matrix or fibroblasts [64].
In addition to effects on pancreatic progenitor cell proliferation, it has also been shown that FGF10 enhanced expression of Ngn3 and increased the number of Ngn3+ pancreatic epithelial cells in the absence of mesenchyme [58, 65]. This regulatory circuit involving FGF10 and pancreatic mesenchyme is hypothesized to allow the maturation of the epithelial cells prior to Ngn3 expression, making cells competent to respond to downstream signals [58]. This hypothesis was supported by a study that used a conditional FGF10 gain-of-function model to demonstrate that the timing of FGF10 expression affects the competence of pancreatic progenitor cells to differentiate to different pancreatic lineages [66].
As noted above, mouse Ngn3 is expressed in a biphasic pattern – with the first wave of expression between E8.5 and E11, and a second wave beginning at E12 [55]. These two waves of expression correspond with the so-called primary and secondary transitions, events that represent two separate waves of endocrine cell differentiation. It has been postulated that only endocrine cells specified during the secondary transition form the definitive single-hormone positive cells in the adult pancreas [67]. In contrast, endocrine cells specified during the primary transition are not thought to contribute to the adult pancreas, instead playing a role in embryonic pancreatic function [68]. However, this paradigm is not supported by all studies of pancreatic cell development, some of which have demonstrated that endocrine cells (or their progenitors) formed during the primary transition can contribute to the adult pancreas [54].
During human pancreatic organogenesis, NGN3 expression is observed as early as W8, where it is co-expressed with other transcription factors such as PDX1, as well as the hormones INSULIN and GLUCAGON. As development proceeded, NGN3 expression was seen to decrease gradually, although it was still observable at W21 [69]. In contrast to the mouse, a biphasic expression pattern of NGN3 has not been reported. This may indicate that distinct primary and secondary transition events do not occur during human pancreatic organogenesis, or may simply reflect difficulties in obtaining appropriately staged human fetal tissues.
6. Step 4: commitment of endocrine precursors to beta-cells
The factors controlling the last step of differentiation, in which endocrine precursor cells are converted to insulin-expressing cells, are still incompletely characterized. Although some protocols incorporate factors implicated from developmental studies, most rely on the presence of nicotinamide, a small molecule shown to promote endocrine differentiation of fetal pancreatic precursors [70] and to increase expression of both insulin and the beta-cell-associated transcription factor, MafA [71].
In the human embryo, scattered insulin-expressing cells appear at W8. One study suggests that, at this point in development, expression of other hormones, such as glucagon or somatostatin, has not been initiated [72]. At W8.5, the first glucagon- and somatostatin-expressing cells are observed, followed at W10 by pancreatic polypeptide (PP)-expressing cells [73]. A proportion of these early hormone-expressing cells are polyhormonal. At W8, insulin-expressing cells have been reported to co-express glucagon at frequencies ranging from 10% to 92% [72-74], and to co-express somatostatin at frequencies ranging from less than 10% to 97% [72, 74]. Tri-hormone expressing cells have also been observed at varying frequencies at this time.
Cells expressing more than one hormone have frequently been observed during hPSC differentiation in vitro, with the proportion of cells expressing insulin alone or in conjunction with glucagon or somatostatin varying between studies (Figure 2). Experiments by D’Amour et al. and, more recently by Basford and colleagues, demonstrate that these in vitro derived polyhormonal INSULIN+ cells are functionally immature and lack the capacity for glucose-stimulated insulin release [5, 28].
Figure 2. Co-expression of endocrine hormones during pluripotent stem cell differentiation and fetal development.
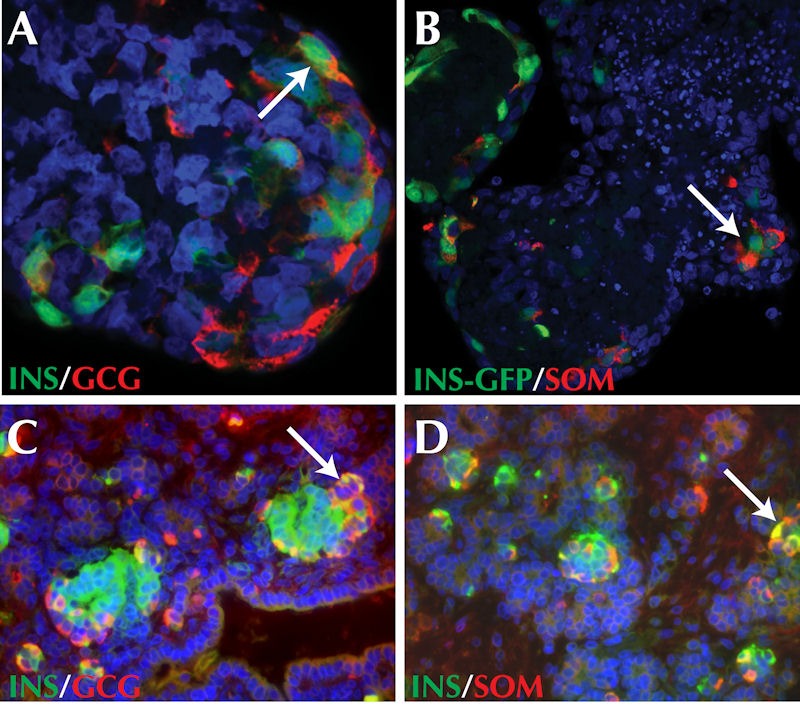
Polyhormonal cells are observed during both human embryonic stem cell differentiation and embryogenesis. A, B: INSULIN and GLUCAGON, as well as INSULIN and SOMATOSTATIN co-expression is observed in embryoid bodies following 25 days of human ESC differentiation. C, D: Similarly, INSULIN and GLUCAGON, as well as INSULIN and SOMATOSTATIN are co-expressed in approximately week 10 fetal pancreas. Arrows identify cells co-expressing the hormones indicated in each panel. Nuclei are identified by staining with DAPI (blue).
In contrast, PP-cells have only been observed as single-hormone expressing cells during human fetal development [72]. Endocrine cells that co-expressed more than one hormone decreased in frequency after W9 [72] and were not detected in preterm infant pancreata at W22 [75]. The variable proportion of hormone co-expressing cells observed in the developing human fetal pancreas may reflect difficulties in accurately staging fetal material, or inherent variability between the different regions of the pancreas analyzed. In terms of hESC pancreatic differentiation protocols, it is at this stage―where hormone expression, specifically insulin expression, has been observed―that some researchers have chosen to transplant cells to test for functionality. The reported outcomes are summarized in Table 2.
Table 2. Studies reportingon the differentiation of hESCs to insulin-expressing cells and their subsequent transplantation.
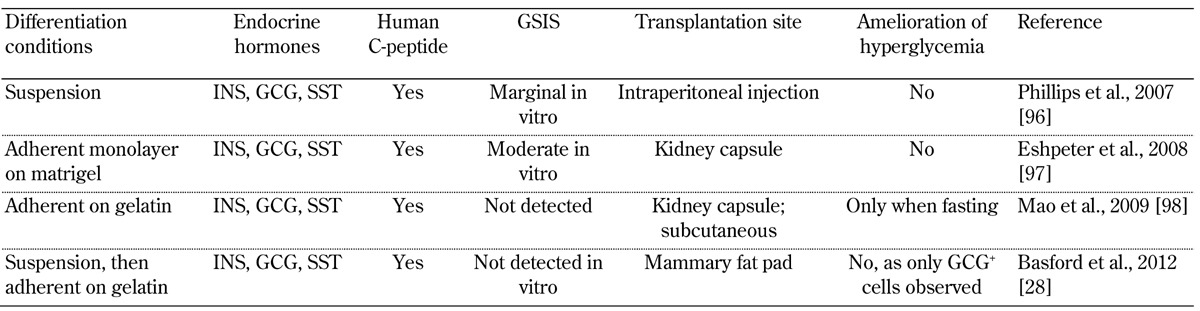
Legend: GCG - glucagon, GSIS - glucose stimulated insulin secretion, INS - insulin, SST - somatostatin.
Whilst the expression of insulin in the absence of other hormones remains a defining characteristic of beta-cells, the ability to regulate insulin release in response to glucose challenge is the key criterion that determines functionality. The transition from insulin expression to functionality is thought to require the expression of transcription factors such as Nkx6.1, MafB and MafA. Nkx6.1 expression is observed in most pancreatic epithelial cells in the mouse pancreas at E10.5 [76]. This broad expression is maintained until the start of the secondary transition at E13, after which expression becomes restricted. By E15.5, Nkx6.1 is only present in insulin-expressing cells and scattered ductal cells. In the adult, Nkx6.1 is exclusively found in insulin-expressing cells [77]. Nkx6.1 plays an important role in beta-cell specification and Nkx6.1-/- mice show a deficit in beta-cell numbers that can be observed as early as E14 [77]. In vitro, overexpression of Nkx6.1 in isolated rat islets resulted in an improvement in glucose stimulated insulin secretion (GSIS) and an increase in beta-cell replication [78]. Studies such as those described above have implicated Nkx6.1 as a marker of definitive beta-cells. However, in vivo, it has been demonstrated that Nkx6.1 overexpression cannot enhance either beta-cell GSIS or proliferation under either diabetic or non-diabetic conditions [79]. A similar expression pattern of NKX6.1 was observed during human pancreatic development. NKX6.1 was broadly expressed throughout the pancreatic epithelium from W9, and expression decreased in non-insulin expressing cells by W13. Similar to the mouse, NKX6.1 expression was restricted to beta-cells of the adult human pancreas [80].
Few groups who have differentiated human PSCs towards a beta-cell fate have analyzed their cultures for the expression of NKX6.1. Two early studies reported NKX6.1 RNA [81] and protein [5] expression from approximately day 15 of differentiation. However, neither report demonstrated co-expression of NKX6.1 with other markers of beta-cell differentiation. More recently, a number of groups have demonstrated the co-expression of NKX6.1 with either C-PEPTIDE [19], PDX1 [19, 82-84], or INSULIN [85]. However, despite co-expression with these other pancreatic genes, the differentiated cells either showed limited functionality [19], or functionality was not examined [83-85]. It is therefore unclear whether NKX6.1 co-expression with either PDX1 or C-PEPTIDE is sufficient to generate fully functional beta-cells.
MafB and MafA are transcription factors expressed in the pancreas in a temporospatially regulated fashion, in both mouse and human. In the mouse, MafB expression is first observed at ~E10.5 in the epithelium of the pancreas [86]. MafB expression is found in both insulin+ and glucagon+ cells during the primary transition, as well as in Ngn3+ pancreatic progenitor cells, before becoming restricted to the adult glucagon+ (alpha) cells [86, 87]. In contrast, MafA expression is observed later during development, at ~E13.5. The spatial distribution of MafA expression also differs from MafB, as expression of MafA is only observed in the insulin+ cells specified during the secondary transition [88]. Additionally, MafB and MafA play different functional roles during beta-cell differentiation. Only MafB is required for beta-cell development in the mouse; beta-cell numbers are not affected in mice lacking pancreatic MafA expression. However, it is possible that MafB is able to compensate for loss of MafA in this context [87]. By comparison, MafA has been shown to control the glucose-responsive transcription of insulin and other associated genes in definitive beta-cells [89, 90]. The impaired glucose tolerance and defects in insulin secretion in MafA-/- mice emphasize the importance of MafA in maintaining an appropriate GSIS [91].
In contrast to the similarities between the expression patterns of transcription factors such as NKX6.1 in mice and humans, the patterns of MAFA and MAFB expression in the human differ to those in the mouse, both spatially and temporally. In a study by Riedel and colleagues, MAFA expression was observed throughout the developing pancreatic epithelium, including in the developing endocrine cells, from W9 [80]. In INSULIN+ cells, strong nuclear expression of MAFA was observed, whereas, weaker expression was seen throughout the remaining epithelium. From W13, MAFA expression was downregulated, with weak expression restricted to the INSULIN+ cells followed by loss of MAFA expression by W21. However, nuclear localized beta-cell specific MAFA expression was observed again in the adult human pancreas, similar to that observed in the mouse [80]. In contrast to these findings, another study found MAFA transcripts increased from W9, with expression maintained until at least W23, albeit at levels lower than those found in the adult [92]. Collectively, these two studies raise the possibility that expression of MAFA may be modulated at both the transcriptional and post-transcriptional level during human pancreatic development.
In the study by Riedel and colleagues, the expression pattern of MAFB resembled that of MAFA at W9 in both the INSULIN+ cells and in the developing pancreatic epithelium. However, by W14, expression of MAFB had become restricted to INSULIN+ and GLUCAGON+ cells. This pattern was then maintained throughout development with expression of MAFB retained in the both alpha and beta-cells in the adult [80]. In contrast, MafB expression has not been observed in insulin>+ cells of the adult mouse islet [86].
It remains to be seen whether the expression of these transcription factors in differentiating PSCs will be the key to generating functional beta-cells in vitro. Alternatively, it is possible that unrelated post-transcriptional and post-translational events are the critical determinants of beta-cell maturation. In either case, further investigation into the regulation of beta-cell maturation in vivo will be required to design rational strategies for generating functional beta-cells from PSCs in vitro. Indeed, further study of the processes and mechanisms controlling pancreatic ontogeny are still likely to provide the best road map for optimizing all of the steps required to direct pluripotent stem cells to become pancreatic beta-cells.
7. Summary
Over the past 7 years, a number of thematically related methods for the generation of pancreatic progenitor cells have been reported. Many of these protocols derive pancreatic progenitors in vitro and then subsequently transplant these progenitors to allow the further development of functional beta-cells that display glucose-stimulated insulin release. The use of pancreatic progenitors as a platform for the treatment of type 1 diabetes has a number of advantages over the use of more differentiated INSULIN+ cells. The first and foremost of these is that cues required for the final steps of differentiation do not need to be identified, or understood, to achieve a therapeutic endpoint.
In contrast to the studies with pancreatic progenitors, insulin-expressing cells derived wholly from in vitro cells do not generally display an ability to release insulin in response to glucose in vivo. In fact, several groups have reported the loss of insulin+ cells following transplantation and their replacement with alternative cell types (Table 2). The fact that adult islets maintain a stable phenotype following transplantation suggests that INSULIN+ cells generated in vitro require further instructions before they achieve functionality akin to that of mature beta-cells.
Ultimately, the question whether progenitors or fully differentiated and functional beta-cells are the optimal approach for a cell-based therapy in type 1 diabetes can only be answered when such cell populations are transplanted into humans. In the interim, efforts to refine further the generation of both progenitors and fully functional beta-cells will remain an area of intense ongoing research.
Disclosures: The authors report no conflict of interests.
Acknowledgments
This work was supported by grants from the Australian Stem Cell Centre, Stem Cells Australia, The Juvenile Diabetes Research Foundation, and the National Health and Medical Research Council of Australia (NHMRC). AGE and EGS are Senior Research Fellows of the NHMRC.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 5.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- 7.Pearce JJ, Evans MJ. Mml, a mouse Mix-like gene expressed in the primitive streak. Mech Dev. 1999;87:189–192. doi: 10.1016/s0925-4773(99)00135-5. [DOI] [PubMed] [Google Scholar]
- 8.Robb L, Hartley L, Begley CG, Brodnicki TC, Copeland NG, Gilbert DJ, Jenkins NA, Elefanty AG. Cloning, expression analysis, and chromosomal localization of murine and human homologues of a Xenopus mix gene. Dev Dyn. 2000;219:497–504. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1070>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Blum M, Gaunt SJ, Cho KW, Steinbeisser H, Blumberg B, Bittner D, De Robertis EM. Gastrulation in the mouse: the role of the homeobox gene goosecoid. Cell. 1992;69:1097–1106. doi: 10.1016/0092-8674(92)90632-m. [DOI] [PubMed] [Google Scholar]
- 10.Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 13.Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 14.Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 15.Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- 16.Bort R, Martinez-Barbera JP, Beddington RS, Zaret KS. Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development. 2004;131:797–806. doi: 10.1242/dev.00965. [DOI] [PubMed] [Google Scholar]
- 17.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 18.McLean AB, D'Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, Sheppard AM, Liu H, Xu Y, Baetge EE. et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 20.Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O'Neil JJ. et al. Maturation of Human Embryonic Stem Cell-Derived Pancreatic Progenitors Into Functional Islets Capable of Treating Pre-existing Diabetes in Mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 23.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson SA, Schiesser J, Stanley EG, Elefanty AG. Differentiating embryonic stem cells pass through 'temporal windows' that mark responsiveness to exogenous and paracrine mesendoderm inducing signals. Plos one. 2010;5:e10706. doi: 10.1371/journal.pone.0010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bone HK, Nelson AS, Goldring CE, Tosh D, Welham MJ. A novel chemically directed route for the generation of definitive endoderm from human embryonic stem cells based on inhibition of GSK-3. J Cell Sci. 2011;124:1992–2000. doi: 10.1242/jcs.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 27.Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB. et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basford CL, Prentice KJ, Hardy AB, Sarangi F, Micallef SJ, Li X, Guo Q, Elefanty AG, Stanley EG, Keller G. et al. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012;55:358–371. doi: 10.1007/s00125-011-2335-x. [DOI] [PubMed] [Google Scholar]
- 29.Teo AK, Ali Y, Wong KY, Chipperfield H, Sadasivam A, Poobalan Y, Tan EK, Wang ST, Abraham S, Tsuneyoshi N. et al. Activin and BMP4 synergistically promote formation of definitive endoderm in human embryonic stem cells. Stem Cells. 2012;30:631–642. doi: 10.1002/stem.1022. [DOI] [PubMed] [Google Scholar]
- 30.Ling Z, Hannaert JC, Pipeleers D. Effect of nutrients, hormones and serum on survival of rat islet beta cells in culture. Diabetologia. 1994;37:15–21. doi: 10.1007/BF00428772. [DOI] [PubMed] [Google Scholar]
- 31.Chen NX, Duan D, O'Neill KD, Wolisi GO, Koczman JJ, Laclair R, Moe SM. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006;70:1046–1053. doi: 10.1038/sj.ki.5001663. [DOI] [PubMed] [Google Scholar]
- 32.Sakai R, Shiozaki M, Tabuchi M, Eto Y. The measurement of activin/EDF in mouse serum: evidence for extragonadal production. Biochem Biophys Res Commun. 1992;188:921–926. doi: 10.1016/0006-291x(92)91143-e. [DOI] [PubMed] [Google Scholar]
- 33.Rhinn M, Dolle P. Retinoic acid signalling during development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 34.Martin M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dolle P, Gradwohl G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284:399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 35.Stafford D, White RJ, Kinkel MD, Linville A, Schilling TF, Prince VE. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949–956. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- 36.Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- 37.Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;232:950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–160. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 39.Pan FC, Chen Y, Bayha E, Pieler T. Retinoic acid-mediated patterning of the pre-pancreatic endoderm in Xenopus operates via direct and indirect mechanisms. Mech Dev. 2007;124:518–531. doi: 10.1016/j.mod.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Micallef SJ, Janes ME, Knezevic K, Davis RP, Elefanty AG, Stanley EG. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 2005;54:301–305. doi: 10.2337/diabetes.54.2.301. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y, Hou L, Tang F, Jiang W, Wang P, Ding M, Deng H. Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three-step approach with activin A and all-trans retinoic acid. Stem Cells. 2005;23:656–662. doi: 10.1634/stemcells.2004-0241. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nature Prot. 2006;1:495–507. doi: 10.1038/nprot.2006.71. [DOI] [PubMed] [Google Scholar]
- 43.Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111–127. doi: 10.1101/gad.859401. [DOI] [PubMed] [Google Scholar]
- 44.Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 45.Seymour PA, Bennett WR, Slack JM. Fission of pancreatic islets during postnatal growth of the mouse. J Anat. 2004;204:103–116. doi: 10.1111/j.1469-7580.2004.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore KL, Persaud TV, Shiota K. Colour atlas of clinical embryology. W.B. Saunders; Philadelphia: 1995. [Google Scholar]
- 47.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 48.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 50.Jacquemin P, Durviaux SM, Jensen J, Godfraind C, Gradwohl G, Guillemot F, Madsen OD, Carmeliet P, Dewerchin M, Collen D. et al. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol Cell Biol. 2000;20:4445–4454. doi: 10.1128/mcb.20.12.4445-4454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis J. Neurogenic genes and vertebrate neurogenesis. Curr Opin Neurobiol. 1996;6:3–10. doi: 10.1016/s0959-4388(96)80002-x. [DOI] [PubMed] [Google Scholar]
- 52.Beatus P, Lendahl U. Notch and neurogenesis. J Neurosci Res. 1998;54:125–136. doi: 10.1002/(SICI)1097-4547(19981015)54:2<125::AID-JNR1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 53.Guo T, Landsman L, Li N, Hebrok M. Factors Expressed by Murine Embryonic Pancreatic mesenchyme enhance generation of insulin-producing cells from hESCs. Diabetes. 2013;62(5):1581–1592. doi: 10.2337/db12-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 55.Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Dev Dyn. 2008;237:3270–3279. doi: 10.1002/dvdy.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gouzi M, Kim YH, Katsumoto K, Johansson K, Grapin-Botton A. Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev Dyn. 2011;240:589–604. doi: 10.1002/dvdy.22544. [DOI] [PubMed] [Google Scholar]
- 57.Villasenor A, Marty-Santos L, Dravis C, Fletcher P, Henkemeyer M, Cleaver O. EphB3 marks delaminating endocrine progenitor cells in the developing pancreas. Dev Dyn. 2012;241:1008–1019. doi: 10.1002/dvdy.23781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duvillie B, Attali M, Bounacer A, Ravassard P, Basmaciogullari A, Scharfmann R. The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes. 2006;55:582–589. doi: 10.2337/diabetes.55.03.06.db05-0839. [DOI] [PubMed] [Google Scholar]
- 59.Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- 60.Hart A, Papadopoulou S, Edlund H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev Dyn. 2003;228:185–193. doi: 10.1002/dvdy.10368. [DOI] [PubMed] [Google Scholar]
- 61.Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev Biol. 2003;264:323–338. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 62.Miralles F, Serup P, Cluzeaud F, Vandewalle A, Czernichow P, Scharfmann R. Characterization of beta cells developed in vitro from rat embryonic pancreatic epithelium. Dev Dyn. 1999;214:116–126. doi: 10.1002/(SICI)1097-0177(199902)214:2<116::AID-AJA2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 63.Miralles F, Lamotte L, Couton D, Joshi RL. Interplay between FGF10 and Notch signalling is required for the self-renewal of pancreatic progenitors. Int J Dev Biol. 2006;50:17–26. doi: 10.1387/ijdb.052080fm. [DOI] [PubMed] [Google Scholar]
- 64.Sneddon JB, Borowiak M, Melton DA. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature. 2012;491:765–768. doi: 10.1038/nature11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Attali M, Stetsyuk V, Basmaciogullari A, Aiello V, Zanta-Boussif MA, Duvillie B, Scharfmann R. Control of beta-cell differentiation by the pancreatic mesenchyme. Diabetes. 2007;56:1248–1258. doi: 10.2337/db06-1307. [DOI] [PubMed] [Google Scholar]
- 66.Kobberup S, Schmerr M, Dang ML, Nyeng P, Jensen JN, MacDonald RJ, Jensen J. Conditional control of the differentiation competence of pancreatic endocrine and ductal cells by Fgf10. Mech Dev. 2010;127:220–234. doi: 10.1016/j.mod.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- 68.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 69.Lyttle BM, Li J, Krishnamurthy M, Fellows F, Wheeler MB, Goodyer CG, Wang R. Transcription factor expression in the developing human fetal endocrine pancreas. Diabetologia. 2008;51:1169–1180. doi: 10.1007/s00125-008-1006-z. [DOI] [PubMed] [Google Scholar]
- 70.Otonkoski T, Beattie GM, Mally MI, Ricordi C, Hayek A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Invest. 1993;92:1459–1466. doi: 10.1172/JCI116723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye DZ, Tai MH, Linning KD, Szabo C, Olson LK. MafA expression and insulin promoter activity are induced by nicotinamide and related compounds in INS-1 pancreatic beta-cells. Diabetes. 2006;55:742–750. doi: 10.2337/diabetes.55.03.06.db05-0653. [DOI] [PubMed] [Google Scholar]
- 72.Polak M, Bouchareb-Banaei L, Scharfmann R, Czernichow P. Early pattern of differentiation in the human pancreas. Diabetes. 2000;49:225–232. doi: 10.2337/diabetes.49.2.225. [DOI] [PubMed] [Google Scholar]
- 73.Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. J Histochem Cytochem. 2009;57:811–824. doi: 10.1369/jhc.2009.953307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. J Endocrinol. 2004;181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- 75.De Krijger RR, Aanstoot HJ, Kranenburg G, Reinhard M, Visser WJ, Bruining GJ. The midgestational human fetal pancreas contains cells coexpressing islet hormones. Dev Biol. 1992;153:368–375. doi: 10.1016/0012-1606(92)90121-v. [DOI] [PubMed] [Google Scholar]
- 76.Rudnick A, Ling TY, Odagiri H, Rutter WJ, German MS. Pancreatic beta cells express a diverse set of homeobox genes. Proc Nat Acad Sci USA. 1994;91:12203–12207. doi: 10.1073/pnas.91.25.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 78.Schisler JC, Fueger PT, Babu DA, Hohmeier HE, Tessem JS, Lu D, Becker TC, Naziruddin B, Levy M, Mirmira RG. et al. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol. 2008;28:3465–3476. doi: 10.1128/MCB.01791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schaffer AE, Yang AJ, Thorel F, Herrera PL, Sander M. Transgenic overexpression of the transcription factor Nkx6.1 in beta-cells of mice does not increase beta-cell proliferation, beta-cell mass, or improve glucose clearance. Mol Endocrinol. 2011;25:1904–1914. doi: 10.1210/me.2011-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riedel MJ, Asadi A, Wang R, Ao Z, Warnock GL, Kieffer TJ. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia. 2012;55:372–381. doi: 10.1007/s00125-011-2344-9. [DOI] [PubMed] [Google Scholar]
- 81.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 82.Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, Richardson M, Carpenter MK, D'Amour KA, Kroon E. et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- 83.Van Hoof D, Mendelsohn AD, Seerke R, Desai TA, German MS. Differentiation of human embryonic stem cells into pancreatic endoderm in patterned size-controlled clusters. Stem cell Res. 2011;6:276–285. doi: 10.1016/j.scr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Xu X, Browning VL, Odorico JS. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech Dev. 2011;128:412–427. doi: 10.1016/j.mod.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Micallef SJ, Li X, Schiesser JV, Hirst CE, Yu QC, Lim SM, Nostro MC, Elliott DA, Sarangi F, Harrison LC. et al. INS(GFP/w) human embryonic stem cells facilitate isolation of in vitro derived insulin-producing cells. Diabetologia. 2012;55:694–706. doi: 10.1007/s00125-011-2379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Artner I, Le Lay J, Hang Y, Elghazi L, Schisler JC, Henderson E, Sosa-Pineda B, Stein R. MafB: an activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes. 2006;55:297–304. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- 87.Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsuoka TA, Artner I, Henderson E, Means A, Sander M, Stein R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Nat Acad Sci USA. 2004;101:2930–2933. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol Cell Biol. 2003;23:6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang H, Brun T, Kataoka K, Sharma AJ, Wollheim CB. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia. 2007;50:348–358. doi: 10.1007/s00125-006-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K. et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarkar SA, Kobberup S, Wong R, Lopez AD, Quayum N, Still T, Kutchma A, Jensen JN, Gianani R, Beattie GM. et al. Global gene expression profiling and histochemical analysis of the developing human fetal pancreas. Diabetologia. 2008;51:285–297. doi: 10.1007/s00125-007-0880-0. [DOI] [PubMed] [Google Scholar]
- 93.Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J, Qing T, Sun X, Zhang P, Ding M. et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333–344. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- 94.Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R, Kim JH. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–1238. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 95.Schulz TC, Young HY, Agulnick AD, Babin MJ, Baetge EE, Bang AG, Bhoumik A, Cepa I, Cesario RM, Haakmeester C. et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. Plos One. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Phillips BW, Hentze H, Rust WL, Chen QP, Chipperfield H, Tan EK, Abraham S, Sadasivam A, Soong PL, Wang ST. et al. Directed differentiation of human embryonic stem cells into the pancreatic endocrine lineage. Stem Cells Dev. 2007;16:561–578. doi: 10.1089/scd.2007.0029. [DOI] [PubMed] [Google Scholar]
- 97.Eshpeter A, Jiang J, Au M, Rajotte RV, Lu K, Lebkowski JS, Majumdar AS, Korbutt GS. In vivo characterization of transplanted human embryonic stem cell-derived pancreatic endocrine islet cells. Cell Prolif. 2008;41:843–858. doi: 10.1111/j.1365-2184.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mao GH, Chen GA, Bai HY, Song TR, Wang YX. The reversal of hyperglycaemia in diabetic mice using PLGA scaffolds seeded with islet-like cells derived from human embryonic stem cells. Biomaterials. 2009;30:1706–1714. doi: 10.1016/j.biomaterials.2008.12.030. [DOI] [PubMed] [Google Scholar]


