Abstract
The left temporal pole (LTP) has been posited to be a heteromodal hub for retrieving proper names for semantically unique entities. Previous investigations have demonstrated that LTP is important for retrieving names for famous faces and unique landmarks. However, whether such a relationship would hold for unique entities apprehended through stimulus modalities other than vision has not been well established, and such evidence is critical to adjudicate claims about the “heteromodal” nature of the LTP. Here, we tested the hypothesis that the LTP would be important for naming famous voices. Individuals with LTP lesions were asked to recognize and name famous persons speaking in audio clips. Relative to neurologically normal and brain damaged comparison participants, patients with LTP lesions were able to recognize famous persons from their voices normally, but were selectively impaired in naming famous persons from their voices. The current results extend previous research and provide further support for the notion that the LTP is a convergence region serving as a heteromodal hub for retrieving the names of semantically unique entities.
2. INTRODUCTION
Previous research has supported the idea that the left temporal pole (LTP) is a heteromodal hub for proper naming (i.e., naming semantically unique entities). In this case, we define the term “heteromodal” to mean the ability to name entities based on different stimulus modalities of presentation (i.e., from visual, auditory, or tactile modalities). The hypothesis about LTP derives from the idea that temporal polar regions serve as “convergence zones,” and the LTP in particular serves as a convergence region for retrieval of proper nouns. In general terms, the LTP has been proposed as a third-party mediation structure that brokers the processes of recognition (e.g., retrieving semantic and conceptual knowledge) and naming (lexical retrieval; see Damasio et al., 2004, and Tranel, 2009, for a more thorough explication of this theory (1–2)). It has also been suggested that this relationship is modality-independent (or “heteromodal”), i.e., that the LTP would broker the recognition-naming interface regardless of the sensory portal through which a stimulus arrived (3–4). However, this aspect of the theory has not been well studied, as most prior investigations have focused almost exclusively on visual stimuli. The current study makes an important and novel contribution by investigating proper name retrieval for auditory stimuli (famous voices).
Support for the notion that the LTP is a hub for retrieving the names of unique entities is derived from both lesion deficit based approaches (5–9), other neuropsychological investigations (10–11), electrical stimulation approaches (12), and from functional neuroimaging studies (1, 13–16). A relationship between the LTP and proper name retrieval has been documented for both famous persons and unique landmarks (14, 17).
Functional neuroimaging investigations have provided convergent evidence that more posterior left inferotemporal regions are important for naming non-unique entities in a heteromodal manner. Specifically, Tranel and colleagues, (18) utilized positron emission tomography (PET) imaging to demonstrate overlapping patterns of brain activation in left inferotemporal cortices to both visual and auditory stimulation when naming non-unique entities (e.g., tools and animals). Thus, naming abilities of other (non TP) portions of the temporal cortex appear to be heteromodally mediated. This finding raises the question of whether LTP will also serve as a heteromodal region (for naming unique entities). Thus, the notion is that the LTP serves as a heteromodal hub for naming unique entities, and individuals with LTP lesions will have a deficit in naming unique entities presented by means of varied stimulus modalities (e.g., visual, auditory).
3. HYPOTHESES
The theoretical formulation that the LTP serves as a heteromodal hub for naming unique entities requires further evidence beyond visual stimuli—viz., it is crucial to test whether the LTP would be required for naming stimuli presented via a different stimulus modality, e.g., audition. The current study extends previous work by conducting precisely such an investigation. We asked participants to identify and name famous persons from audio clips in which the famous persons were speaking, (i.e., to recognize and name famous voices). We tested the following specific hypotheses, derived from our theoretical framework: 1. Patients with damage to the left temporal pole (but not patients with damage elsewhere in the brain) would be impaired in their ability to name famous voices. 2. Patients with damage to the left temporal pole would not be impaired in their ability to recognize famous voices.
4. METHODS
4.1. Participants
The participants included 36 neurological patients with stable, focal brain lesions, and 20 neurologically/psychiatrically normal comparisons. The participants were selected from the Iowa Patient Registry (19), and do not have a history of mental retardation, learning disability, psychiatric disorder, substance abuse, or dementia. Overall mean participant age = 50.6 years (SD=13.0), range = 19–74. There were 31 women and 25 men. Group mean years of education = 15.1 (SD = 2.2), range = 11 – 20 years. All participants with neurological lesions were at minimum 6 months post neurological insult (mean = 8.6 years, SD = 7.6) when their neuroanatomical, neuropsychological, and experimental data were collected. Etiologies for the patients with lesions included surgical resection secondary to epilepsy (n = 16), stroke (n = 12), and surgical resection secondary to benign tumor (n = 8).
The brain lesion participants were separated into 2 groups: those whose lesions included the LTP (n = 18), and a brain damaged comparison (BDC) group which was comprised of individuals who had right hemisphere lesions only (n = 18, including 4 with right temporal pole lesions). In addition, we studied a neurologically normal participant group that had an average age = 48.0 years (SD = 15.3), and average education = 15.5 years (SD = 2.1). Average age for left temporal pole lesion patients was 52.7 years (SD = 11.5), range 29 – 67 years, with average years of education equal to 14.9 years (SD = 2.4). For the BDC group, average age was 51.4 years (SD = 11.8), range 34–68, and average years of education was 14.9 (SD = 2.0). Please see detailed demographic information in Table 1. Based on scores derived from the Geschwind-Oldfield Handedness Questionnaire, all of the LTP lesion participants were right handed (one reported +60 handedness, four reported +80 handedness, and the remaining thirteen reported being completely [+100] right handed). For our right hemisphere BDC group, two participants were left handed (−80, −70), and the remainder reported being right handed. One BDC participant reported +75 handedness, while the remaining fifteen participants reported being fully right handed (+100). Handedness data were not collected for the neurologically normal comparison group. We chose to exclude individuals with left hemisphere lesions (not including the temporal pole) and individuals with bilateral brain lesions due to the fact that lesions in a number of left hemispheric brain regions can result in varied degrees of naming deficits (see Semenza, Mondini, and Zettin, 1995; and Semenza, 2011, for reviews (20–21)). All participants completed informed consent procedures approved by the University of Iowa Human Subjects Committee and Institutional Review Board.
Table 1.
Demographic data and neuropsychological test performances of the three participant groups. Neuropsychological data are presented with raw score (standard deviation).
| Participant Group |
|||||
|---|---|---|---|---|---|
| Neurologically Normal |
Right Hemisphere Lesion |
Left Temporal Pole Lesion |
F/T value | Significance | |
| n | 20 | 18 | 18 | ||
| age | 48.0 (15.3) | 52.7 (11.5) | 51.4 (11.8) | 0.667 | .518 |
| education | 15.5 (2.1) | 14.9 (2.4) | 14.9 (2.0) | 0.380 | .686 |
| lesion chronicity | - | 9.4 (8.8) | 7.7 (6.4) | 0.667 | .509 |
| FSIQ | - | 104.7 (12.1) | 104.7 (11.2) | 0.000 | 1.00 |
| BNT | - | 57.6 (2.7) | 49.7 (13.2) | 2.501 | .017* |
| COWA | - | 39.7 (12.0) | 42.2 (13.3) | 0.592 | .558 |
| Token Test | - | 43.7 (0.8) | 42.8 (2.0) | 1.641 | .111 |
In order to assess for deficits in intellect or language that would lead to difficulties with the task, all patients with neurological lesions were administered the Wechsler Adult Intelligence Scale-IV (22), the Boston Naming Test (23), Controlled Oral Word Association Test, and the Token Test from the Multilingual Aphasia Examination (24).
4.2. Stimuli
Audio clips of dialogue from famous individuals were taken from public domain sources (e.g., http://www.youtube.com). The famous voices in these clips came from a variety of fields including entertainment, politics, news, and sports. The clips were screened for words or phrases that would betray the identity of the speaker, and clips that contained such features were eliminated from the experimental protocol. The clips were 12–17 seconds in duration (mean = 13.8 seconds, SD = 2.3 seconds). Clips were not included if they were drawn from a source that would be recognizable to many participants and potentially give away the identity of the speaker (e.g., from a well known movie or television show). The order of audio clip presentation was randomized.
The initial clip pool contained more than 100 stimuli. These examples were pilot screened with our group of 20 neurologically normal comparisons. The clips were included in the experimental protocol if at least 4 of these 20 participants were able to accurately name the famous person whose voice was in the clip. Using this method, the stimulus set was edited down to 60 audio clips. The data that are reported for the neurologically normal comparison group only include their performances on the final set of 60 audio clips (please see Appendix 1 for a listing of the speakers/names of the famous voices clips). Brain lesion participants were only presented with the final set of 60 audio clips. Total task time was approximately 30 minutes for the 60-item task. The protocol was completed by a trained administrator.
4.3. Task
We used a protocol similar to the one we have used for previous naming experiments (25), with appropriate modifications to accommodate the fact that for the current study, participants were naming famous individuals based on their voices as opposed to naming famous individuals based on pictures of their faces. Participants were presented each audio clip in open field and given the following instructions:
“I am going to play some audio clips of the voices of some famous people. These people may be famous for being in the news, or sports, or entertainment. When I play the clip, tell me if the voice is familiar to you or not. If it is familiar try to name the person. If you cannot think of the name, try to describe the person as best you can by providing specific details about him or her.”
Participants were allowed to hear the clip a second time if requested. Consistent with previous recognition and naming experiments from our lab, recognition was defined as either accurate naming (which is taken as evidence of accurate identification), or, if naming was unsuccessful, accurate description of the individual such that another person could reasonably ascertain who the subject was describing. Naming was defined as production of a specific proper name corresponding to the individual speaker. For example, the following response “the former president whose wife was Secretary of State” would be judged as having correctly recognized the voice as that of Bill Clinton. A response such as “he’s a man from the South” would be judged as not having correctly recognized the voice.
4.4. Behavioral data analysis
Behavioral data analysis was completed with SPSS 21 software. T-tests, ANOVAs, and ANCOVAs were completed to examine between-group differences. Post-hoc tests for the ANOVA analyses were completed with Tukey’s HSD to examine between-group differences.
4.5. Lesion analysis
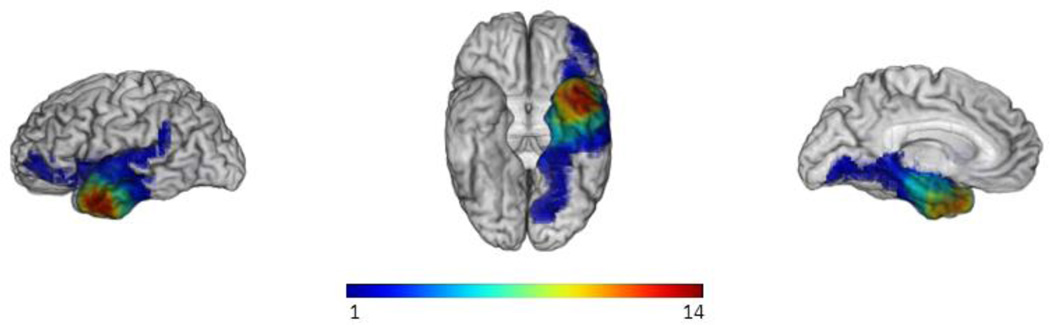
As noted, participants with brain lesions were separated into two groups: those whose brain lesions were located in the right hemisphere, and those whose lesions included the LTP. These lesions were identified from MRI scans (or CT scans, if an MRI was contraindicated for the patient). Please see Figure 1 for depiction of the lesion overlap in the LTP group.
Figure 1.
a) Lesion overlap map for our participants with left temporal pole lesions. Images depict the overlap (from left to right, respectively) from the lateral perspective, ventral perspective, and from a mesial sagittal perspective. The “hotter” colors (orange to red) depict a higher number of lesion overlaps.
5. RESULTS
5.1. Descriptive statistics
No significant between-group differences were noted in age or education (please see Table 1 for more information about participant demographics). Average time since lesion onset for the LTP group was 7.7 years (SD = 6.4), while average time since lesion onset for the BDC group was 9.4 years (SD = 8.8). The two groups did not differ in their lesion chronicity, t(34) = 0.667, p = .509.
Regarding neuropsychological performances, the BDC group performed normally on FSIQ (mean = 104.7, SD = 12.1), BNT (mean = 57.6, SD = 2.7), COWA (mean = 39.7, SD = 12.0), and Token Test (mean = 43. 7, SD = 0.8). The LTP group’s neuropsychological scores were as follows: FSIQ (mean = 104.7, SD = 11.2), BNT (mean = 49.7, SD = 13.2), COWA (mean = 42.2, SD = 13.3), and Token Test (mean = 42.8, SD = 2.0). A statistically significant difference was noted between the groups on the BNT [t(34) = 2.50, p = .02], but no other statistically significant differences were noted between the groups. Please see Table 1 for more detailed between-group statistical comparisons. Taken together, our patients with stable brain lesions did not have deficits in intellect or language that would confound the interpretation of their performances on the experimental task (see Results below and the Discussion for a fuller treatment of the Boston Naming Test performances).
5.2. Task performance
For the Famous Voices task, we calculated the percentage of voices that each participant reported that they recognized and for which they were able to describe the speaker in such a way that the person would be uniquely identifiable. These data are reported as % of voices recognized. If the participant accurately named the speaker, this was counted as a correct naming response. The sum total across items named accurately is reported as % of voices named. If it was the case that the participant accurately named the speaker prior to reporting recognition, this was counted as both correct recognition and correct naming. In short, the naming score uses a participant-specific denominator tied to the number of stimuli the participant recognizes accurately, meaning that participants are not penalized for failing to name stimuli that they do not recognize.
Each participant group recognized just under half of the voices in the clips (neurologically normal participants recognition = 48.00%, SD = 14.70, 95% CI [41.12, 54.88]; BDC recognition = 47.89%, SD = 16.1, 95% CI [39.91, 55.87]; LTP recognition = 48.67%, SD = 14.78, 95% CI [41.31, 56.02]). An ANOVA demonstrated no significant between group differences in recognition, F(2,53) = .014, p = .986, ηp2 = .001. Of the voices that were recognized, neurologically normal participants were able to name 95.12%, SD = 6.04, 95% CI [92.30, 97.95]; BDC naming = 86.61%, SD = 9.16, 95% CI [82.05, 91.17]; and LTP naming = 66.17%, SD = 19.29, 95% CI [56.57, 75.76]. An ANOVA demonstrated an overall group effect for naming, F(2,53) = 25.949, p < .000, ηp2 = . 495. Tukey HSD post-hoc tests demonstrated statistically significant differences between the LTP lesion participants and neurologically normal participants (p < .000), as well as between the LTP lesion participants and the BDC lesion participants (p < .000). Non-significant differences were noted between the neurologically normal participants and the BDC participants (p = .105). For each group, non-significant correlations were noted between participant age and their performance on the voice naming and recognition tasks. LTP lesion participant age did not correlate significantly with voice recognition, r = −.323, p = .191, nor did it correlate significantly with voice naming, r = −.135, p = .595. BDC participant age also did not correlate significantly with voice recognition, r = −.141, p = .576, or with voice naming, r = −.177, p = .481. An ANCOVA was performed to assess whether a general deficit in naming for the LTP lesion group was responsible for that group’s deficit in voice naming. BNT performance did account for a significant portion of the variance on the task F(1,33) = 21.57, p = .000, ηp2 = .395. However, even when BNT performance was covaried out, the LTP lesion group still demonstrated impaired voice naming ability relative to patients with right hemisphere lesions F(1,33) = 8.40, p = .007, ηp2 = .203.
Of note, of our 4 right hemisphere BDC participants with right temporal pole lesions, all had normal performance in recognizing the famous voices relative to other groups. Two of these participants had normal naming relative to the other groups. One of these participants had borderline naming of famous voices (Z = 1.5 below the group means), and one had impaired naming of famous voices (Z = −4.0 below the group means).
5.3 Comparison to other proper naming tasks
Most of the patients in the current investigation had also completed different recognition and naming tests that have been described in prior investigations from our laboratory (e.g., the famous faces and landmarks tests: 2, 3, 17). Of the 18 LTP patients in the current study, 17 had also completed the Famous Faces Test or the Landmark Test, or both. Of these 17 patients, 12 had impaired (or borderline) naming performances on two or more of the three tests. In other words, 12/17 or 71% of the LTP patients who had completed ≥2 tests were defective on ≥2 tests in terms of their naming performance. Of the 18 BDC patients in the current study, all 18 had also completed the Famous Faces Test or the Landmark Test, or both. Of these, 2 had impaired (or borderline) naming performances on two or more of the three tests. In other words, 2/18 or 11% of the RH patients who had completed ≥2 tests were defective on ≥2 tests in terms of their naming performance.
On the Famous Faces Test, both participant groups recognized approximately 76% of the faces, and statistical differences were not noted [right hemisphere lesion M = 76.0, (SD = 15.3); LTP lesion M = 75.9 (SD = 15.0); t(33) = .011, p = .991]. There was a difference in naming on the Famous Faces Test between these groups [BDC M = 83.8 (SD = 9.9); LTP lesion M = 69.0, (SD = 18.9); t(33) = 2.938, p = .006]. Regarding the Famous Landmarks Test, between group differences were not noted in recognition [BDC M = 68.0, (SD = 14.9); LTP lesion M = 59.6 (SD = 19.5); t(29) = 1.352, p = .187]. Significant between group differences were noted in naming on the Famous Landmarks Test between the two groups [BDC M = 89.2 (SD = 7.5); LTP lesion M = 73.1, (SD = 15.4); t(29) = 3.722, p = .001]
Additionally, Z-score discrepancies were calculated to compare the performances of the BDC and LTP groups on each of the three tasks. Small differences were noted in each of the recognition tests: Famous Voice recognition discrepancy = − 0.05, Famous Face recognition discrepancy = 0.01, and Famous Landmark recognition discrepancy = 0.47. Larger Z-score discrepancies were seen in the naming tests between the BDC and LTP participants: Famous Voice naming discrepancy = 3.40, Famous Face naming discrepancy = 1.35, and Famous Landmark naming discrepancy = 2.01. These data are summarized in Table 3.
Table 3.
Within the same patient population, comparisons to recognition and naming of other categories of stimuli. The Voice recognition and naming data are the same as presented in Table 2. For right hemisphere patients who completed the Famous Voices Test, N=18 had also completed the Famous Faces Test, and N=16 had also completed the Famous Landmarks Test. For patients with left temporal pole lesions who completed the Famous Voices protocol, N=17 had completed the Famous Faces Test, and N=15 had completed the Famous Landmarks Test.
| Participant Group |
||||||
|---|---|---|---|---|---|---|
| Task Performance | Neurologically Normal |
Right Hemisphere Lesion |
Left Temporal Pole Lesion |
F/T value |
Significance | Z-score difference b/w LTP and BDC |
| % voice recognition | 48.0 (14.7) | 47.9 (16.1) | 48.7 (14.8) | 0.014 | .986 | −0.05 |
| % voice naming | 95.1 (6.0) | 86.6 (9.2) | 66.2 (19.3) | 25.949 | .000 | 3.40 |
| % face recognition | 85.0 (15.0)* | 76.0 (15.3) | 75.9 (15.0) | 0.011 | .991 | 0.01 |
| % face naming | 85.0 (11.1)** | 83.8 (9.9) | 69.0 (18.9) | 2.890 | .008 | 1.35 |
| % landmark recognition | 62.0 (18.0)** | 68.0 (14.9) | 59.6 (19.5) | 1.340 | .192 | 0.47 |
| % landmark naming | 88.0 (8.0)** | 89.2 (7.5) | 73.1 (15.4) | 3.646 | .002 | 2.01 |
Previously published data. Different participant sample. N = 90. Taken from Tranel (2006).
Previously published data. Different participant sample. N = 68. Taken from Tranel (2006).
6. DISCUSSION
The current results provide support for our hypothesis that the left temporal pole (LTP) is a heteromodal hub that is important for naming unique entities, and that this holds across different stimulus modalities. In this investigation, participants with left temporal pole lesions demonstrated defective naming of famous voices, relative to a right hemispheric brain lesion group and a neurologically normal comparison group. The three groups did not differ in their ability to recognize the famous voices. This finding, in conjunction with previous work (1, 2, 6, 15), provides further support for the notion that the left temporal pole plays an important role in naming unique entities. Several previous investigations have documented the importance of the left temporal pole for naming unique entities through visual stimulation. However, to our knowledge, this is the first investigation to show that individuals with left temporal pole lesions also have a deficit in naming unique entities (famous persons) by way of an auditory stimulus (voice clips).
The left temporal pole has been posited to serve as a convergence zone (1) that brokers the relationships between semantic/conceptual information and for word form (lexical retrieval). The current results expand upon previous investigations from our laboratory that have demonstrated that infero-lateral temporal regions are important for naming non-unique entities (i.e., tools and animals) from both visual and auditory stimulation (18). Together, these results suggest that multiple distinct left temporal regions are important for lexical retrieval for different categories of entities, and that this process appears to be multi-modal (heteromodal). Of note, one important point to be made here is that there is a difference between naming voices of famous people and naming sounds that are made by some tools and animals. More specifically, there are words that exist to describe the sounds make by certain animals (e.g., a dog “barks”, and a cow “moos”), but these words do not denote the name of the animal. This stands in contrast to voice naming in that there is no word that denotes the sound that is different than the speaker’s name, aside from a generic descriptor such as “voice.”
6.1. Comparison to other proper naming tasks
Although not the primary focus of the current investigation, we have also included comparisons within the same subjects on other proper naming tests (Famous Faces, Famous Landmarks). As reported in the Results, most of the brain-damaged participants in the current study have completed recognition and naming tasks involving famous faces, unique landmarks, and famous voice, and we have the opportunity to compare performances across these different stimulus categories. The LTP and BDC participant groups performed similarly across all three tasks with respect to recognition and naming—the basic pattern being that the LTP group demonstrated greater difficulty with naming in all three tasks relative to BDC group, while there were no notable between-group differences in recognition performances. The Z-score discrepancy differences provide further support for this notion, and the greatest discrepancy was noted on the Famous Voices test. This finding is similar to the results of Tranel et al. (18), who found that naming non-unique entities via auditory stimulation was more difficult than naming the same objects via visual stimulation. Together, these results provide further support for the notion that patients with left temporal pole lesions have deficits in naming unique entities, regardless of the modality of input.
6.2. Alternate viewpoints for the role of the left temporal pole
There are alternate hypotheses on the role of the left temporal pole region in naming and semantic representation. A characterization of these alternate hypotheses can be found in Tranel and colleagues (25). One prominent hypothesis for the role of the left anterior temporal lobe region is that it serves as a hub for semantic information. For example, Tsapkini and colleagues (26) evaluated individuals with acute LTP lesions, and found that lesion size (infarct volume) was the only predictive factor that impacted naming abilities. The authors state that these results support the notion that the temporal pole regions seem to be a part of a network for naming and semantic representation, but is not solely responsible for these capacities. The hub and spoke model serves as an expansion of this idea (for example, see (27)). A distributed semantic network hypothesis was also demonstrated in a lesion investigation (28). Others have posited that both temporal poles are involved in semantic representation, but with a preferential responsibility of the left temporal pole for naming, and a particular tie to semantic integration for the right temporal pole (29–30). The current results do not disconfirm any of these hypotheses, as we focused mainly on patients with left temporal pole lesions (and did not have a large enough group of right temporal pole patients to make definitive claims). Future investigations could include participants with lesions of other left hemispheric regions, and patients with right temporal pole lesions, in order to more directly address these hypotheses. Nevertheless, our results do suggest that the left temporal pole is important for naming of unique entities, and that lesions to this region may disrupt the “hub” in the hub and spoke model.
Others have suggested that the anterior temporal pole regions serve a specific role in social cognition and social judgments, in addition to their role in processing or representing semantic knowledge (31). Support for this idea comes from Olson, Ross and colleagues who have employed several different techniques such as fMRI (32–33) and direct electrical current stimulation (12, 34) to evaluate the role of the anterior temporal lobes in a naming task. Our results do not disconfirm these hypotheses, as the current investigation did not directly address social cognition and judgments.
6.3. Limitations and future directions
One minor limitation with the current data is that the individuals with left temporal pole damage demonstrated a statistically significant weakness in naming ability on the Boston Naming Test. This deficit is not unexpected, given the association between left temporal lesions and naming ability in general, as well as the fact that lesions for some of the participants extended posteriorly to non-polar regions. However, with more detailed analysis, we see that the LTP lesion group’s deficit in famous voice naming is not a function of a naming deficit in general when individual participant data are analyzed. Specifically, 12 of the 18 LTP lesion participants have normal scores on the BNT, 1 has borderline impaired naming on the BNT, and the remaining 5 have impaired naming on the BNT. In contrast, on the famous voice naming task, only 1 LTP lesion patient has normal performance, 2 have borderline impaired naming performance, and 15 of the 18 LTP lesion patients have impaired performance on the task relative to the neurologically normal group. Furthermore, statistical analyses demonstrate that when BNT performance is covaried out, there remain between group differences on task performance (as reported in the Results).
Additionally, the current data serve as a starting point, as they only show an association between the left temporal pole and naming of famous voices. As alluded to earlier, future studies could include other patients with left hemisphere lesions to address whether the left temporal polar region has a specific association with naming famous voices.
6.4. Summary
Taken together, the current results provide further evidence that the left temporal pole serves as a heteromodal hub for naming unique entities. While this has been demonstrated in numerous past investigations of naming faces and landmarks by way of visual stimulation, to our knowledge this is the first investigation to document the importance of the left temporal pole in naming of unique entities through auditory stimulation. Thus, the importance of the left temporal pole for naming unique entities appears to be heteromodal in nature.
Table 2.
Performance on the famous voices naming task. % Voices Named is equal to the percentage of voices named that had been recognized accurately.
| Participant Group |
|||||
|---|---|---|---|---|---|
| Voice Task Performance |
Neurologically Normal |
Right Hemisphere Lesion |
Left Temporal Pole Lesion |
F value | Significance |
| n | 20 | 18 | 18 | ||
| % voice recognition | 48.0 (14.7) | 47.9 (16.1) | 48.7 (14.8) | 0.014 | .986 |
| % voice naming | 95.1 (6.0) | 86.6 (9.2) | 66.2 (19.3) | 25.949 | .000** |
LTP lesion participants were significantly worse at naming than both neurologically normal and BDC groups
ACKNOWLEDGEMENTS
This work was supported by NINDS PO1 NS19632. Thank you also to Natasha Feuerbach for her help in creation of the famous voice stimuli.
Appendix 1
Famous voices included in the experiment.
Alan Alda
Muhammad Ali
Woody Allen
Roseanne Barr
Tom Brokaw
George Burns
George H.W. Bush
George W. Bush
Johnny Carson
Jimmy Carter
Johnny Cash
Winston Churchill
Bill Clinton
Hillary Clinton
George Clooney
Katie Couric
Bill Cosby
Kevin Costner
Walter Cronkite
Princess Diana
Bob Dole
Dwight Eisenhower
Sally Field
Michael J. Fox
Morgan Freeman
Whoopi Goldberg
Al Gore
Gene Hackman
Tom Hanks
Bob Hope
Ron Howard
Anthony Hopkins
Michael Jackson
Peter Jennings
Michael Jordan
John F. Kennedy
Martin Luther King Jr.
Ted Koppel
Jay Leno
David Letterman
Rush Limbaugh
John Madden
Ed McMahon
Jack Nicholson
Richard Nixon
Barack Obama
Rosie O’Donnell
Al Pacino
Dolly Parton
Ross Perot
Regis Philbin
Brad Pitt
Dan Rather
Robert Redford
Arnold Schwarzenegger
Jerry Seinfeld
Barbara Streisand
Mother Teresa
Lily Tomlin
Barbara Walters
REFERENCES
- 1.Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio AR. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Tranel D. The left temporal pole is important for retrieving words for unique concrete entities. Aphasiology. 2009;23(7–8):867–884. doi: 10.1080/02687030802586498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tranel D, Enekwechi N, Manzel K. A test for measuring recognition and naming of landmarks. J Clin Exp Neuropsychol. 2005;27(1):102–126. doi: 10.1080/138033990513663. [DOI] [PubMed] [Google Scholar]
- 4.Drane DL, Ojemann JG, Phatak V, Loring DW, Gross RE, Hebb AO, Silbergeld DL, Miller JW, Voets NL, Saindane AM, Baralou L, Ojemann KJ, Tranel D. Famous face identification in temporal lobe epilepsy: Support for a multimodal integration model of semantic memory. Cortex. 2013;49(6):1648–1667. doi: 10.1016/j.cortex.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damasio AR, Damasio H, Tranel D, Brandt J. Neural regionalization of knowledge access: Preliminary evidence. Cold Spring Harb Symp Quant Biol. 1990;55:1039–1047. doi: 10.1101/sqb.1990.055.01.098. [DOI] [PubMed] [Google Scholar]
- 6.Drane DL, Ojemann GA, Aylward E, Ogemann JG, Johnson LC, Silbergeld DL, Miller JW, Tranel D. Category-specific naming and recogntion deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46(5):1242–1255. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miceli G, Capasso R, Daniele A, Esposito T, Magarelli M, Tomaiuolo F. Selective deficit for people’s names following left temporal damage: An impairment of domain-specific conceptual knowledge. Cogn Neuropsychol. 2000;17(6):489–516. doi: 10.1080/02643290050110629. [DOI] [PubMed] [Google Scholar]
- 8.Seidenberg M, Griffith R, Sabsevitz D, Moran M, Haltiner A, Bell B, Swanson S, Hammeke T, Hermann B. Recognition and identification of famous faces in patients with unilateral temporal lobe epilepsy. Neuropsychologia. 2002;40(4):446–456. doi: 10.1016/s0028-3932(01)00096-3. [DOI] [PubMed] [Google Scholar]
- 9.Tranel D, Damasio H, Damasio AR. Anomia: Neuroanatomical and cognitive correlates. In: Goodglass H, Wingfield A, editors. On the neurology of naming. New York: Academic Press; 1997. pp. 65–90. [Google Scholar]
- 10.Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61(1):81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- 11.Viskontas IV, McAndrews MP, Moscovitch M. Memory for famous people in patients with unilateral temporal lobe epilepsy and excisions. Neuropsychology. 2002;16:472–480. [PubMed] [Google Scholar]
- 12.Ross LA, McCoy D, Coslett HB, Olson IR, Wolk DA. Improved proper name recall in aging after electrical stimulation of the anterior temporal lobes. Front Aging Neurosci. 2011;3:16. doi: 10.3389/fnagi.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- 14.Gorno-Tempini ML, Price CJ. Identification of famous faces and buildings: A functional neuroimaging study of semantically unique items. Brain. 2001;124:2087–2097. doi: 10.1093/brain/124.10.2087. [DOI] [PubMed] [Google Scholar]
- 15.Grabowski TJ, Damasio H, Tranel D, Boles-Ponto LL, Hichwa RD, Damasio AR. A role for the left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13(4):199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsukiura T, Fujii T, Fukatsu R, Otsuki T, Okuda J, Umetsu A, Suzuki K, Tabuchi M, Yanagawa I, Nagasaka T, Kawashima R, Fukuda H, Takahashi S, Yamadori A. Neural basis of the retrieval of people’s names: Evidence from brain-damaged patients and fMRI. J Cogn Neurosci. 2002;14(6):922–937. doi: 10.1162/089892902760191144. [DOI] [PubMed] [Google Scholar]
- 17.Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20(1):1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Tranel D, Grabowski TJ, Lyon J, Damasio H. Naming the same entities from visual or from auditory stimulation engages similar regions of the left inferotemporal cortices. J Cogn Neurosci. 2005;17(8):1293–1305. doi: 10.1162/0898929055002508. [DOI] [PubMed] [Google Scholar]
- 19.Tranel D. Theories of clinical neuropsychology and brain-behavior relationships: Luria and beyond. In: Morgan JE, Ricker JH, editors. Textbook of Clinical Neuropsychology. New York: Taylor and Francis; 2007. pp. 27–37. [Google Scholar]
- 20.Semenza C, Mondini S, Zettin M. The neuroanatomical basis of proper name processing: A critical review. Neurocase. 1995;1(2):183–188. [Google Scholar]
- 21.Semenza C. Naming with proper names: The left temporal pole theory. Behav Neurol. 2011;24(4):277–284. doi: 10.3233/BEN-2011-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition. San Antonio, TX: Pearson Assessment; 2008. [Google Scholar]
- 23.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lee & Febiger; 1983. [Google Scholar]
- 24.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination, Third Edition. Iowa City, IA: AJA Associates; [Google Scholar]
- 25.Tranel D, Feinstein J, Manzel K. Further lesion evidence for the neural basis of conceptual knowledge for persons and other concrete entities. J Neuropsychol. 2008;2:301–320. doi: 10.1348/174866407x227033. [DOI] [PubMed] [Google Scholar]
- 26.Tsapkini K, Frangakis CE, Hillis AE. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain. 2011;134:3094–3105. doi: 10.1093/brain/awr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambon-Ralph MA, Ehsan S, Baker GA, Rogers TT. Semantic memory is impaired in patients with unilateral anterior temporal lobe resection for temporal lobe epilepsy. Brain. 2012;135:242–258. doi: 10.1093/brain/awr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert NA, Swain MA, Miller LA, Caine D. Exploring the neural organization of person-related knowledge: Lateralization of lesion, category specificity, and stimulus modality effects. Neuropsychology. 2006;20(3):346–354. doi: 10.1037/0894-4105.20.3.346. [DOI] [PubMed] [Google Scholar]
- 29.Gainotti G. Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: A systematic review. Neuropsychologia. 2007;45:1591–1607. doi: 10.1016/j.neuropsychologia.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Gainotti G. What the study of voice recognition in normal subjects and brain-damaged patients tells us about models of familiar people recognition. Neuropsychologia. 2011;49:2273–2282. doi: 10.1016/j.neuropsychologia.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: A review and theoretical framework. Soc Cogn Affect Neurosci. 2013;8(2):123–133. doi: 10.1093/scan/nss119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross LA, Olson IR. Social cognition and the anterior temporal lobes. Neuroimage. 2010;49(4):3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skipper LM, Ross LA, Olson IR. Sensory and semantic category subdivisions within the anterior temporal lobes. Neuropsychologia. 2011;49:3419–3429. doi: 10.1016/j.neuropsychologia.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross LA, McCoy D, Wolk DA, Coslett HB, Olson IR. Improved proper name recall in aging after electrical stimulation of the anterior temporal lobes. Neuropsychologia. 2010;48:3671–3674. doi: 10.1016/j.neuropsychologia.2010.07.024. [DOI] [PubMed] [Google Scholar]