Abstract
Objective
The authors sought to study activity in neural circuits that subserve the inhibition of a semi-involuntary motor behavior, eye blinking, in children and adults with Tourette syndrome and in healthy comparison subjects.
Method
Functional magnetic resonance imaging was used to scan 120 participants (51 with Tourette syndrome and 69 comparison subjects) as they either blinked normally or successfully inhibited eye blinking. The authors compared the blood-oxygen-level dependent signal during these two conditions across the Tourette and comparison groups.
Results
Relative to comparison subjects, patients with Tourette syndrome activated more strongly the frontal cortex and striatum during eye blink inhibition. Activation increased more with age in the dorsolateral and inferolateral prefrontal cortex and caudate nucleus in the Tourette group relative to comparison subjects. In addition, the Tourette group more strongly activated the middle frontal gyrus, dorsal anterior cingulate, and temporal cortices. The severity of tic symptoms in the Tourette group correlated inversely with activation in the putamen and inferolateral prefrontal cortex.
Conclusions
Frontostriatal activity is increased in persons with Tourette syndrome during the inhibition of eye blinks. Activation of frontostriatal circuits in this population may help to maintain regulatory control over semi-involuntary behaviors, whether these are tics or eye blinks.
Tics are sudden, repetitive, stereotyped movements or vocal utterances that are performed in response to somatosensory or environmental cues (1). Individuals often experience an urge to respond to these cues by performing a tic (2). Tics can be suppressed but not indefinitely, and their temporary suppression often exacerbates the proceeding urge. The frequent need to suppress tics can be exhausting and can have a profound effect on psychosocial functioning (1).
Prior anatomical imaging studies have documented reduced volumes of the caudate nucleus (3) and thinning of primary sensory, primary motor, and premotor cortices in children with Tourette syndrome (3, 4), suggesting the presence of hypoplasia throughout the motor portions of cortico-striatal-thalamo-cortical circuits. Electrophysiological and histological evidence for reduced GABA-ergic tone in these cortical and subcortical brain regions suggests the presence of reduced local inhibition of neural activity in these motor circuits (5, 6). Prior studies of voluntary tic suppression (7) and cortical volumes (8) have suggested that suppression of tics robustly activates the frontal cortex, and that this frequent activation in social settings may in turn produce a compensatory, neuroplastic hypertrophy of frontal cortices that helps to regulate activity within those motor circuits, thereby reducing tic symptoms (9). This neuroplastic hypertrophy of the frontal cortex in response to experiential need seems to be absent in adults who have persistent Tourette syndrome (8), suggesting that a failure to generate frontal hypertrophy may contribute to the persistence of tics in the minority of persons with Tourette syndrome whose symptoms fail to remit during adolescence (9, 10).
Although these prior studies have highlighted the importance of frontostriatal activation for the voluntary control of tic symptoms, the prior functional imaging study of tic suppression could not directly compare activation of circuits required for the suppression of tics in persons with Tourette syndrome with activation in healthy comparison subjects, since the comparison subjects by definition did not have tics to suppress. Therefore, activation of inhibitory circuits in persons with Tourette syndrome has been compared instead with activation in healthy comparison subjects during the performance of cognitive tasks that require the inhibition of prepotent responses. One study, for example, compared the brain activity of children and adults with and without Tourette syndrome during performance of the Stroop Word-Color Interference Task (11), which requires the inhibition of an automatic response tendency (reading) in favor of a less automatic response (color naming). The correlations of activation with age within frontostriatal circuits were greater in the Tourette group than in comparison subjects, suggesting the presence of altered maturation of frontostriatal circuits. Greater frontostriatal activity in the adults with Tourette syndrome was interpreted as helping them to maintain normal levels of task performance in the presence of a diminished inhibitory reserve in these circuits (8).
We sought to develop a functional imaging task that was similar to voluntary tic suppression in the sense that it would require participants to suppress a semi-involuntary behavior but would also permit performance by healthy participants, thereby allowing a direct comparison of activity in frontostriatal circuits across Tourette syndrome and comparison groups. One semi-involuntary behavior that is phenomenologically similar to tics is eye blinking. Blinks occur at a similar frequency and with similar forcefulness as most tics, and they can be voluntarily suppressed temporarily although not indefinitely. Difficulty in regulating the frequency and forcefulness of eye blinking is one of the earliest-developing tic symptoms (12); moreover, individuals with Tourette syndrome report that the somatosensory tension experienced during the sustained inhibition of eye blinking is similar to the premonitory urge experienced just prior to performing a tic. Functional imaging studies have shown that frontostriatal circuits are activated in healthy individuals during the inhibition of eye blinks (13), thus making the suppression of eye blinks an ideal candidate task for comparing frontostriatal activity in suppressing semi-involuntary behaviors across the Tourette and comparison participants. This comparison should allow determination of whether the functioning of frontostriatal circuits during the inhibition of a normal, semi-involuntary behavior is aberrant, therefore suggesting the presence of a generalized deficiency in inhibitory systems in Tourette syndrome, or whether aberrant inhibition represents a domain-specific deficiency in the inhibition of tics alone.
We therefore compared neural activity in individuals with Tourette syndrome and healthy comparison subjects as they inhibited eye blinking. Given the previously reported disturbances in frontostriatal circuits of adults with Tourette syndrome during performance of the Stroop task (11), we expected to detect a similar pattern of increased activity of frontal cortices in response to inhibitory demands in adults with persistent symptoms (8, 11), suggesting that they increase frontostriatal activity to compensate for reduced inhibitory reserve within frontal cortices.
Method
Participants
We recruited persons diagnosed with Tourette syndrome from the Tic Disorder Specialty Clinic at the Yale Child Study Center in New Haven, Conn., excluding persons who had an axis I disorder other than obsessive-compulsive disorder (OCD) or attention deficit hyperactivity disorder (ADHD) before the onset of Tourette syndrome (comorbid OCD: N=21, comorbid ADHD: N=11, both OCD and ADHD: N=4). We recruited comparison subjects from a telemarketing list of individuals residing in the same postal codes, excluding those who reported a history of tic disorder, OCD, or ADHD, or who met diagnostic criteria for any axis I disorder at the time of the interview. Additional exclusionary criteria for both groups were a lifetime history of substance abuse or head trauma or a full-scale IQ < 75.
We administered the Schedule for Tourette and Other Behavioral Disorders (14), as well as clinical evaluations, to establish diagnoses through a consensus procedure of expert clinicians (15). The Schedule for Tourette and Other Behavioral Disorders includes the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL [16]) for children and the Schedule for Affective Disorders and Schizophrenia (SADS [17]) for adults, as well as sections on Tourette syndrome and OCD for both. Ratings of current and worst-ever symptom severity of tic symptoms were obtained using the Yale Global Tic Severity Scale (18), the child or adult version of the Yale-Brown Obsessive Compulsive Scale (19, 20), and the ADHD rating scale (21). Socioeconomic status was assessed using the Hollingshead Index of Social Status (22), and IQ was measured using the Wechsler Abbreviated Scale of Intelligence (23). Additionally, we assessed performance on the Stroop interference task (24) and Conners Continuous Performance Task-II (25), both measures of inhibitory control (26). Tourette and comparison subjects were group-matched on demographic characteristics (Table 1).
TABLE 1.
Demographic and Clinical Characteristics of Study Participantsa
| Children |
Adults |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Tourette Syndrome (N=22) |
Healthy Comparison Subjects (N=21) |
Tourette Syndrome (N=29) |
Healthy Comparison Subjects (N=48) |
||||
| N | % | N | % | N | % | N | % | |
| Sexb | ||||||||
| Male | 19 | 86.4 | 12 | 57.1 | 17 | 58.6 | 21 | 43.7 |
| Female | 3 | 13.6 | 9 | 42.9 | 12 | 41.4 | 27 | 56.3 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 13.1 | 2.6 | 13.4 | 3.1 | 35.1 | 11.1 | 31.4 | 11.0 |
| Socioeconomic status | 50.8 | 9.6 | 48.9 | 11.8 | 43.9 | 10.1 | 48.4 | 12.2 |
| IQ (Wechsler Abbreviated Scale of Intelligence) | 116.0 | 12.1 | 116.1 | 13.4 | 117.3 | 14.9 | 120.7 | 13.7 |
| Motion indexc | 8.8 | 10.4 | 4.9 | 3.3 | 5.9 | 3.7 | 4.2 | 2.9 |
| Yale Global Tic Severity Scale scored | ||||||||
| Total current | 18.5 | 11.1 | 21.0 | 10.6 | ||||
| Total worst ever | 28.9 | 8.6 | 30.2 | 9.2 | ||||
| Yale-Brown Obsessive Compulsive Scalee | ||||||||
| Total current | 3.4 | 6.6 | 8.8 | 9.5 | ||||
| Total worst ever | 9.1 | 13.7 | 10.9 | 11.9 | ||||
| ADHD Rating Scale total score | ||||||||
| Current | 14.3 | 12.6 | 18.8 | 7.8 | ||||
| Pre-medicationf | 26.5 | 18.7 | 20.2 | 10.1 | ||||
A total of 63 participants with Tourette syndrome and 70 comparison subjects were scanned, but 12 with Tourette syndrome were excluded due to ghosting artifact (N=8) or excessive movement (N=4). Among these excluded participants, seven were male and five were female; four were children and eight were adults. Only one adult male comparison participant was excluded due to ghosting artifact.
Differences between groups were calculated using unpaired t tests. A significant difference was found in gender ratio in children (t= 2.20, df=41, p=0.03).
Calculated as the maximum motion of a 4×4×4mm3 cube at the center of the brain moving along a three-dimensional trajectory over time, where the trajectory was reconstructed from a series of six motion parameters (three translations and three rotations in a rigid-body transformation) that were generated during motion realignment of the fMRI time series. A significant between-group difference was found in motion index among adults (t=2.18, df=75, p=0.03). Regional activations in both groups assessed separately did not correlate significantly with motion index, and covarying with this motion index in our statistical analyses did not appreciably alter any of our findings.
Twenty-five Tourette patients had eye-blinking tics at the time of the study. Among them, 14 were children and 11 were adults.
OCD symptoms were significantly more severe at the time of scan for Tourette adults than for Tourette children (t=−2.29, df=49, p=0.02).
Twenty-one Tourette syndrome participants were taking medications at the time of the study, including typical neuroleptics (N=5), risperidone (N=4), α-adrenergic agonists (N=2), serotonin reuptake inhibitors (N=11), levothyroxine (N=2), and valproic acid (N=2). Medications were not mutually exclusive.
Behavioral Task
Prior to scanning, participants were introduced to the task using a practice procedure to ensure correct understanding of task instructions and ability to comply with task demands. Throughout the experiment, participants were instructed to keep their gaze focused on an “X” placed on a visible screen. At the beginning of each scanning session, participants blinked normally. After 40 seconds of scanning, the experimenter stated the word “stop,” at which point participants refrained from blinking for a 40-second period. The experimenter then stated the word “blink,” at which time participants returned to blinking normally. Two scanning sessions were run, each consisting of four 40-second blocks of eye blink inhibition and gaze fixation. A member of the study team observed the eyes of each participant in a mirror placed on the head coil to confirm successful inhibition of eye blinking at appropriate times.
Image Acquisition
Images were acquired on a Signa 1.5 Tesla LX scanner (GE Healthcare, Milwaukee) using a standard quadrature head coil. Head positioning in the magnet was standardized using the canthomeatal line. A T1-weighted sagittal localizing scan was used to position the axial images. The functional images were obtained using a T2*-sensitive gradient-recalled, single-shot, echo-planar pulse sequence (TR=1750 msec, TE=45 msec, 60° flip angle, single excitation per image, 20×40 cm field of view, and 64×128 matrix, providing a 3.1×3.1 mm in-plane resolution). Eight axial slices were acquired to correspond with axial slices in the z direction of Talairach space (27), with six slices extending superiorly six-ninths of the distance to the vertex and two slices inferiorly from the anterior commissure-posterior commissure (AC-PC) line. Slice thickness was a constant 7 mm. The skip between slices varied between 0.5 and 2 mm to maintain a strict correspondence to the Talairach coordinate system.
Image Preprocessing
Images were visually inspected during preprocessing and discarded if artifacts such as ghosting occurred or if the subject moved >0.5 pixels in any direction. Images were then motion-corrected using Yale software for three translational directions and three rotations (28). Corrected images underwent Gaussian spatial filtering (full width at half maximum of 6.3 mm). Drift of baseline image intensity was removed using an eighth-order high-pass Butterworth temporal filter with a frequency cutoff equal to three-fourths of the task frequency. The filter was run once forward and once backward to ensure no change in phase of task-related signal. T1-weighted axial anatomical images and corresponding echoplanar functional images were transformed into a common stereotactic space using a piece-wise linear warping to a common bounding box (27).
Hypothesis Testing
Our a priori hypothesis was that the correlation of age with activation during the inhibition of eye blinking would differ between Tourette and comparison groups. We assessed this effect using the diagnosis-by-age term within a linear-mixed model in which signal change for eye blink suppression versus blinking conditions served as a dependent measure, with diagnosis (patient or comparison subject) as a between-subject factor and with age and sex as covariates. We considered all main effects and interactions for inclusion in the model, using backward stepwise regression to eliminate terms, monitoring the hierarchical structure at each step to ensure inclusion of all possible lower-order component terms (29). We considered as significant any voxels that surpassed our thresholds for both t statistic (corresponding to a p value < 0.05) and cluster size of 25 adjacent voxels for the age-by-diagnosis interaction, a conjoint requirement that, based on an approximation formula, yielded a conservative significance threshold (p < 0.000005) (28). The volume of this cluster (3.1 mm×3.1 mm×7 mm×25 mm=1.68 cm3) was well below that of the caudate and lenticular nuclei (5–6 cm3), permitting detection of significant activation in those regions (3).
Exploratory Analyses
We evaluated the association of activation with symptom severity using a voxel-wise regression analysis that incorporated age and scores on the Yale Global Tic Severity Scale, Yale-Brown Obsessive Compulsive Scale, and ADHD rating scale separately. Similarly, because the Stroop, Continuous Performance Test, and eye blink inhibition represent similar constructs for the study of inhibitory control, we assessed the correlations of activation during eye blink inhibition with interference scores on the Stroop (26) and reaction times for correct hits on the Continuous Performance Test (25).
We also assessed the effects of medication on brain activation using a model that included only the participants with Tourette syndrome, employing as an independent variable a nominal measure of receiving versus not receiving a specific class of medication (α-antagonists, neuroleptics, selective serotonin reuptake inhibitors) at the time of study. We also employed these variables as statistical covariates in the linear model used for a priori hypothesis testing. The effects of co-occurring ADHD or OCD were assessed similarly.
Results
Hypothesis Testing
Main effects of diagnosis were detected in the right middle frontal gyrus, left dorsal anterior cingulate cortex, right middle temporal gyrus, and right superior temporal gyrus (Figure 1, column G; Table 2), suggesting increased activation of these areas in the Tourette group relative to comparison subjects. In addition, the superior frontal gyrus deactivated more in the Tourette group than in comparison subjects, whereas the posterior cingulate cortex deactivated more in the comparison subjects than in the Tourette group (Figure 1, columns F and G; Table 2). These effects were detected in voxels where the diagnosis-by-age effects were not significant, indicating that the group differences in activation were independent of age.
FIGURE 1. Brain Activation During the Inhibition of Eye Blinks in Tourette Syndrome and Healthy Comparison Subjects a.
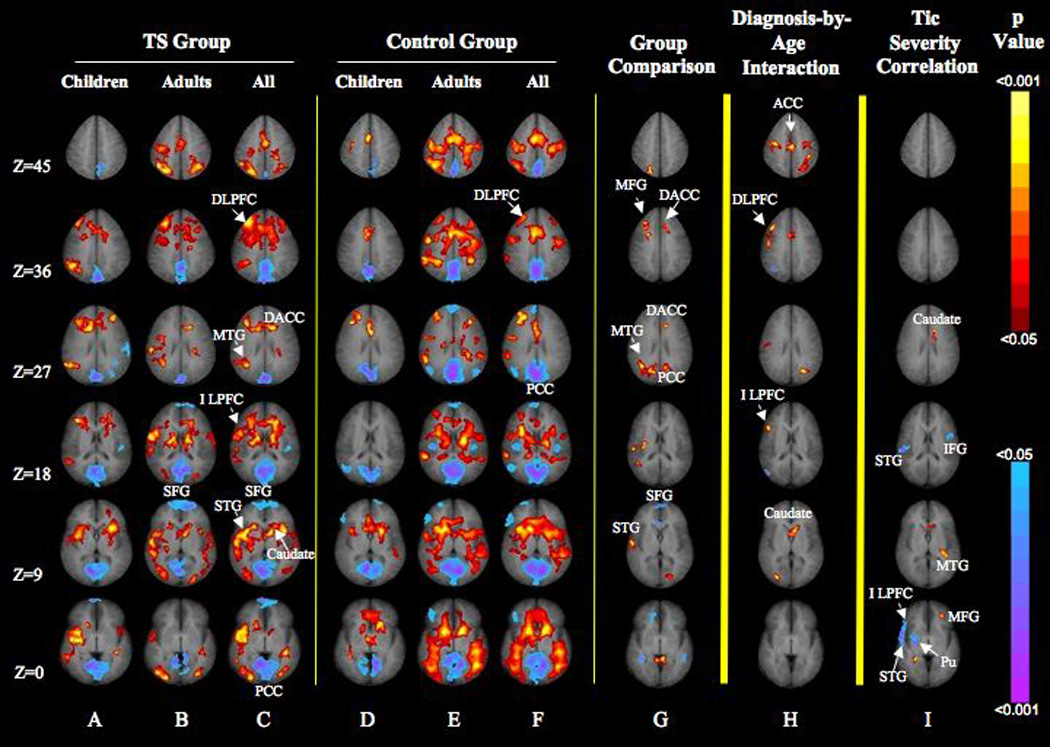
aImages are axial slices positioned “superiorly to inferiorly” (top to bottom) in radiological convention (the left side of the image represents the right side of the brain). Activation maps were generated using the general linear model in which fMRI signal change during the inhibition of eye blinking was the dependent variable, diagnosis was the independent variable, and age and sex were covariates. The model included a diagnosis-by-age interaction (p < 0.05, cluster size=25, a conjoint requirement that yielded a significance threshold of p < 0.000005). For columns A–F, increases in signal during the suppression of eye blinks are coded in red to yellow, and decreases are coded in blue to purple. In column G, greater activation in the Tourette syndrome group relative to comparison subjects is coded in red to yellow. Column I depicts the general linear model correlating signal change with the severity of tic symptoms in the Tourette group, while covarying for age and sex (p < 0.05, cluster size=25). Positive correlations are shown in red to yellow, and inverse correlations are in blue to purple. Activations in each group did not correlate significantly with motion indices. DACC=dorsal anterior cingulate cortex; STG=superior temporal gyrus; DLPFC=dorsolateral prefrontal cortex; ILPFC=inferolateral prefrontal cortex; SFG=superior frontal gyrus; MFG=middle frontal gyrus; IFG=inferior frontal gyrus; MTG=middle temporal gyrus; Pu=putamen; PCC=posterior cingulate cortex; ACC=anterior cingulate cortex.
TABLE 2.
Regions of Significant Activation Differences Between Tourette Syndrome and Healthy Comparison Subjects
| Effect and Region of Interest | Side | Talairach Coordinates |
Brodmann’s Area |
zscore | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Main effects of diagnosis | ||||||
| Middle frontal gyrusa | Right | −27 | 22 | 36 | 9 | 2.34 |
| Dorsal anterior cingulate cortex | Left | 11 | 19 | 27 | 32 | 2.92 |
| Left | 12 | 22 | 36 | 32 | 2.15 | |
| Middle temporal gyrus | Right | −24 | −58 | 27 | 39 | 2.80 |
| Superior temporal gyrus | Right | −52 | −13 | 9 | 22/41 | 2.54 |
| Superior frontal gyrus | Left/Right | 0.4 | 57 | 9 | 10 | −2.38 |
| Posterior cingulate cortex | Left | 8 | −58 | 27 | 23/31 | 2.61 |
| Diagnosis-by-age interaction | ||||||
| Dorsolateral prefrontal cortexa | Right | −37 | 18 | 36 | 9 | 2.58 |
| Inferolateral prefrontal cortex | Right | −45 | 16 | 18 | 45/46 | 2.48 |
| Anterior cingulate cortex | Left/Right | 0.2 | −6 | 45 | 24 | 3.13 |
| Caudate nucleus | Left | 5 | 10 | 9 | 2.72 | |
Despite a small overlap in activation between the middle frontal gyrus (of which 95 voxels were significant) and dorsolateral prefrontal cortex (of which 131 voxels were significant), the extent of each area was distinct, with only eight voxels shared between regions. The spatial independence of the main effects and interactions indicates that both effects can be interpreted.
Significant age-by-diagnosis interactions were detected in several other regions, indicating the presence of age-specific differences in activation across groups. Interactions in right dorsolateral prefrontal cortex and right inferolateral prefrontal cortex (Figure 1, column H; Table 2) derived from an increase in mean percent signal change in these regions with advancing age in the Tourette group and a decline with age in the comparison subjects group (Figure 2). An interaction was also detected in the left caudate nucleus (Figure 1, column H; Table 2), where activation increased with age in the Tourette group more than it did in comparison subjects (Figure 2). Finally, an interaction with age was observed in the anterior cingulate cortex (Figure 1, column H; Table 2) that was attributable to increasing activation with advancing with age in the Tourette group but not in the comparison subjects (Figure 2).
FIGURE 2. Regions of Age-Specific Differences in Activation Between Tourette Syndrome and Healthy Comparison Subjects.
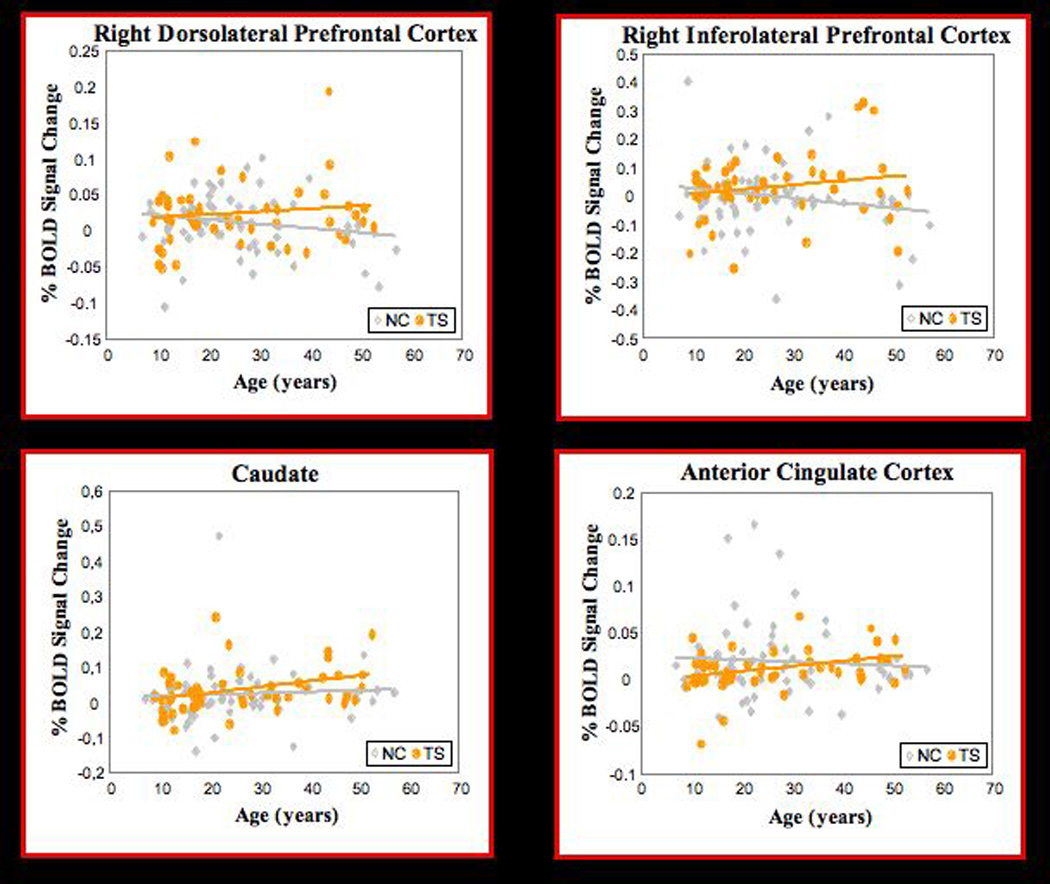
a Activation increased with age in persons with Tourette syndrome, declined in the healthy comparison subjects. Correlations of activation with age in the Tourette group (r=0.30, p=0.03) and comparison group (r=−0.22, p=0.07) were compared through Fisher z transformation (z=2.81), with the positive z score indicating a significantly larger correlation coefficient in the Tourette group relative to the comparison subjects (p=0.005).
b Activation increased with age in persons with Tourette syndrome, declined in the healthy comparison subjects. Correlations of activation with age in the Tourette group (r=0.37, p=0.008) and comparison group (r=−0.17, p=0.16) were compared through Fisher z transformation (z=2.92), with the positive z score indicating a significantly larger correlation coefficient in the Tourette group relative to the comparison subjects (p=0.003).
c Activation increased with age in persons with Tourette syndrome more than in the healthy comparison subjects. Correlations of activation with age in the Tourette group (r=0.34, p=0.01) and comparison group (r=0.20, p=0.09) were compared through Fisher z transformation (z=2.96), with the positive z score indicating a significantly larger correlation coefficient in the Tourette group relative to the comparison subjects (p=0.003).
d Activation increased with age in persons with Tourette syndrome, declined in the healthy comparison subjects. Correlations of activation with age in the Tourette group (r=0.51, p < 0.0001) and comparison group (r=−0.24, p=0.04) were compared through Fisher z transformation (z=4.25), with the positive z score indicating a significantly larger correlation coefficient in the Tourette group relative to the comparison subjects (p < 0.0001).
In addition, the magnitude of activation in the right inferolateral prefrontal cortex (Brodmann’s area 47), right superior temporal gyrus (Brodmann’s area 22), left inferior frontal gyrus (Brodmann’s area 44), and right putamen correlated inversely with the severity of tics at the time of scan (Figure 1, column I), indicating that greater activity in these regions accompanied fewer symptoms in the Tourette group. Conversely, the left middle temporal gyrus (Brodmann’s area 39), left middle frontal gyrus (Brodmann’s area 46), and left caudate correlated positively with tic severity, indicating that more activation accompanied more severe tic symptoms in the Tourette group.
Exploratory Analyses
We did not detect significant correlations of activation during eye blink inhibition with measures of Stroop or Continuous Performance Test performance, and neither Stroop nor Continuous Performance Test scores correlated significantly with symptom severity (r=−0.14, p=0.37 and r=−0.01, p=0.94, respectively). Statistical covariation for the effects of medication and comorbidity had no appreciable effect on the findings from a priori hypothesis testing. No significant differences in activation were detected in persons with Tourette syndrome receiving medication compared with those not receiving medication, suggesting that medication effects did not contribute to our findings. Similarly, comparisons of those with and without co-occurring ADHD or OCD suggested that the presence of these conditions did not significantly affect our results (not shown). We also included the motion index in our analysis and found that regional activations in each group did not correlate significantly with this parameter. Moreover, the addition of motion index as a covariate in the maps had no effect on our findings. Finally, to investigate whether the presence or absence of eye blinking tics could have influenced our results, we included it as a statistical covariate in our activation maps, and we found no effects on group differences or age effects.
Discussion
Excessively frequent and forceful eye blinking is most commonly the first symptom to manifest in children with Tourette syndrome (30). Eye blinks are semi-involuntary behaviors that have a similar duration, frequency, and forcefulness to tics themselves, and persons with Tourette syndrome report that the subjective experience while inhibiting eye blinks is similar to their subjective experience while inhibiting tics. The temporal dynamics of tics and eye blinks are similar (31), and the frequencies of both are believed to be under the neuromodulatory control of dopamine (32). Thus, the inhibition of eye blinks provides an ideal task to study neural circuits that provide inhibitory control over semi-involuntary movements across Tourette and healthy participants.
Numerous imaging studies have shown that the frontal cortex plays a central role in the development of inhibitory motor control (33, 34) in both children (35) and adults (36). Other imaging studies have suggested that frontal control of tic behaviors is impaired in persons with Tourette syndrome, particularly adults who have persistent symptoms. Persons with Tourette syndrome, for example, have abnormalities in the structure and function of their prefrontal cortices (7, 8). Whereas children with Tourette have enlarged frontal cortices, adults have reduced frontal volumes (3). In addition, prefrontal volumes in both children and adults with Tourette syndrome correlate inversely with tic severity (3), suggesting that enlargement of frontal cortices in children may represent a plastic hypertrophy in response to their frequent need to suppress tics, which is known to activate the frontal cortex robustly (7). Plastic hypertrophy may thereby enhance inhibitory control over tics. Conversely, smaller prefrontal volumes in Tourette adults are thought to represent a failure of this plastic hypertrophy (8), limiting inhibitory reserve and requiring an increased magnitude and spatial extent of frontal activation to maintain a comparable level of performance on tasks requiring inhibitory control (11).
Consistent with this model of impaired inhibitory control in adults who have persistent symptoms, one prior study reported greater age-related increases in frontal activation during an inhibitory control task in persons with Tourette syndrome relative to that seen in healthy comparison subjects (11). These findings were interpreted as a functional response in Tourette adults that likely compensated for their reduced frontal and caudate volumes (11). We detected a similar age-by-diagnosis interaction in the right dorsolateral prefrontal cortex and right inferolateral prefrontal cortex (Figure 1, column H) that was driven by greater activation with advancing age in the Tourette group than in comparison subjects (Figure 2). These two studies included most of the same participants, demonstrating the within-subject reproducibility of this set of findings. These consistent findings across studies suggest that the exaggerated deployment of inhibitory control in Tourette adults likely extends beyond the more cognitive domain of the Stroop task and into the more purely motor domain of motoric inhibition.
Although the bulk of evidence together suggests that the greater activation of frontal cortices during the Stroop and eye blink tasks represents an attempt to compensate for the reduced plasticity of the prefrontal cortex in persons with Tourette syndrome, other interpretations can also account for greater activation of frontostriatal pathways in Tourette adults. Imaging, postmortem, and electrophysiological studies, for example, suggest the presence of hypoplasia and reduced GABA-ergic tone throughout motor portions of cortico-striatal-thalamo-cortical circuits in persons with Tourette syndrome (5, 6). Reduced GABA-ergic tone would increase the excitability of the motor pathways that presumably drive the generation of both tic symptoms and eye blinks. Greater excitability in these motor pathways could in turn require greater activation of frontostriatal pathways to suppress those behaviors. This explanation, however, would not readily account for the increased activation of frontostriatal pathways detected in Tourette syndrome adults during performance of a cognitive control task (11).
Our findings of increased activation of the dorsal anterior cingulate cortex and right anterior temporal cortex in the Tourette group relative to comparison subjects during eye blink inhibition is consistent with prior findings of increased activation of these areas during tic suppression in persons with Tourette syndrome (7). The dorsal portion of the anterior cingulate cortex is believed to subserve attention and monitoring functions during the performance of goal-directed tasks (37), and the right anterior temporal cortex subserves attention and the processing of visuospatial and somatosensory information (38). Thus, increased activity in these areas in the Tourette group may represent a greater reallocation of attentional resources than in comparison subjects during the suppression of eye blinks, perhaps reflecting a more generalized difficulty with motor inhibition across all ages in the Tourette group.
In addition to activating frontostriatal pathways more than comparison subjects, Tourette subjects deactivated the superior frontal gyrus more than comparison subjects during the suppression of eye blinks. The superior frontal gyrus is thought to support the processing of self-referential information and metacognitive representations of the actions of self and others (39). More prominent deactivation of the superior frontal gyrus in the Tourette group likely reflects a greater suppression of self-directed thought during the suppression of eye blinks, suggesting that this task is more demanding in persons with Tourette syndrome than in comparison subjects. In contrast, the Tourette group failed to deactivate the posterior cingulate cortex to the extent that healthy comparison subjects activated it. The posterior cingulate is thought to support the monitoring and recall of somatic and other sensory information (37). Failure to turn off this region sufficiently during eye blink suppression in the Tourette syndrome group may represent a disturbance in the ability to suppress somatic sensations associated with the urge to blink when persons with Tourette syndrome willfully suppress their eye blinks.
We detected inverse correlations between the current severity of tic symptoms and the magnitude of activation of the right inferolateral prefrontal cortex, right superior temporal gyrus, and right putamen during the inhibition of blinks, and we detected a positive correlation in the middle frontal gyrus and caudate. These correlations are consistent with those reported previously during tic suppression (7). The inverse correlations indicate that individuals with more severe tics, and by extension those who had greater difficulty suppressing tics, activated these regions less than did those who had less severe symptoms. The correlations provide further support for our claim that activation of frontostriatal circuits provides inhibitory control over semi-involuntary movements, whether these are tics or eye blinks. Greater functional disturbances in frontostriatal systems may therefore contribute to more severe symptoms.
These findings must be interpreted in light of several limitations of the study. First, variability in task compliance could produce differences in neural activity. To minimize this variability, a member of the study team directly observed each participant during the scan to confirm their success in suppressing blinks. Second, the presence of co-occurring illnesses and use of medications could have influenced our results. Weighing against this possibility, however, is that covarying for medication use and co-occurring illnesses had no discernible effects on our findings. Third, we were unable to quantify the number of eye blinks during the freely blinking control task because bursts of blinks occurred immediately following suppression blocks, rendering their enumeration impossible (31). The Tourette and comparison groups therefore may have differed in their frequency of eye blinking during the resting condition, thereby influencing estimates of task-related change in MRI signal. Quantitative studies of blink rates, however, generally have not reported abnormal blink rates in persons with Tourette syndrome (40). Finally, we did not include Tourette adults with remitted symptoms, and therefore we could not assess whether exaggerated frontostriatal activation generalizes to all adults with a lifetime history of Tourette syndrome or only to those whose symptoms persist. Despite these potential limitations, our study has important implications for understanding the developmental trajectory of brain functioning in Tourette syndrome. In particular, it provides new support for the hypothesis that disturbances in the maturation of frontostriatal systems that mediate inhibitory control can contribute to the development, persistence, and severity of tic symptoms.
Supplementary Material
Acknowledgments
Supported in part by NIMH grants MH-01232, MH-59139, MH-068318, and K02-74677; by NICHD grants HD-39667 and HD-051502; and by a grant from the Tourette Syndrome Association. Dr. Mazzone was supported by the Alexander Bodini Fellowship at the Italian Academy for Advanced Studies in America at Columbia University.
The authors thank James Leckman, M.D., Robert King, M.D., Larry Scahill, Ph.D., Diane Findley, Ph.D., and the Tourette Syndrome Association for their help with recruitment and John Gore, Ph.D., and Cheryl Lacadie, Ph.D., for their technical assistance.
Footnotes
All authors report no financial relationships with commercial interests.
References
- 1.Spessot AL, Peterson BS. In: Tourette’s syndrome: a multifactorial, developmental psychopathology, in Developmental Psychopathology. 2nd ed. Cicchetti D, Cohen DJ, editors. Vol. 3. Hoboken, NJ: John Wiley & Sons; 2006. pp. 436–469. [Google Scholar]
- 2.Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette’s syndrome. Am J Psychiatry. 1993;150:98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- 3.Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, King RA, Leckman JF, Staib L. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 4.Sowell ER, Kan E, Yoshii J, Thompson PM, Bansal R, Xu D, Toga AW, Peterson BS. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci. 2008;11:637–639. doi: 10.1038/nn.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leckman JF, Vaccarino FM, Kalanithi PS, Rothenberger A. Annotation: Tourette syndrome: a relentless drumbeat–driven by misguided brain oscillations. J Child Psychol Psychiatry. 2006;47:537–550. doi: 10.1111/j.1469-7610.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 7.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 8.Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, Cohen DJ, Gore JC, Albert J, Webster R. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58:427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- 9.Spessot AL, Plessen KJ, Peterson BS. Neuroimaging of developmental psychopathologies: the importance of self-regulatory and neuroplastic processes in adolescence. Ann N Y Acad Sci. 2004;1021:86–104. doi: 10.1196/annals.1308.010. [DOI] [PubMed] [Google Scholar]
- 10.Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, Kim YS, Peterson BS. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- 11.Marsh R, Zhu H, Wang Z, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control in Tourette’s syndrome. Am J Psychiatry. 2007;164:955–966. doi: 10.1176/appi.ajp.164.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung HY, Chung SJ, Hwang JM. Tic disorders in children with frequent eye blinking. J AAPOS. 2004;8:171–174. doi: 10.1016/j.jaapos.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Tsubota K, Kwong KK, Lee TY, Nakamura J, Cheng HM. Functional MRI of brain activation by eye blinking. Exp Eye Res. 1999;69:1–7. doi: 10.1006/exer.1999.0660. [DOI] [PubMed] [Google Scholar]
- 14.Pauls DL, Hurst CR. Schedule for Tourette and Other Behavioral Syndromes. New Haven, Conn: Yale University Child Study Center; 1996. [Google Scholar]
- 15.Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Endicott J, Spitzer RL. A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 18.Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen J. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale, I: development, use, reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 20.Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 21.DuPaul GJ. Parent and teacher ratings of ADHD symptoms: psychometric properties in a community-based sample. J Clin Child Psychol. 1991;20:245–253. [Google Scholar]
- 22.Hollingshead AB. Four-Factor Index of Social Status. New Haven, Conn: Yale University, Department of Sociology; 1975. [Google Scholar]
- 23.Axelrod BN. Validity of the Wechsler Abbreviated Scale of Intelligence and other very short forms of estimating intellectual functioning. Assessment. 2002;9:17–23. doi: 10.1177/1073191102009001003. [DOI] [PubMed] [Google Scholar]
- 24.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 25.Conners CK. Conners’ Continuous Performance Test. Ontario, Canada: MultiHealth Systems; 1995. [Google Scholar]
- 26.Goldman-Rakic PS. In: Circuitry of primate prefrontal cortex and regulation of behavior by representational memory, in Handbook of Physiology, Section 1: The Nervous System. Plum F, Mountcastle V, editors. Vol. 5. Washington, DC: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- 27.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- 28.Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1993;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 29.Morrell CH, Pearson JD, Brant LJ. Linear transformations of linear mixed-effects models. Am Statistician. 1997;51:338–343. [Google Scholar]
- 30.Shapiro AK, Shapiro ES, Young JG, Feinberg TE. In: Signs, symptoms, and clinical course, in Gilles de la Tourette Syndrome. Shapiro AK, Shapiro ES, Young JG, Feinberg TE, editors. New York: Raven Press; 1988. pp. 127–193. [Google Scholar]
- 31.Peterson BS, Leckman JF. The temporal dynamics of tics in Gilles de la Tourette syndrome. Biol Psychiatry. 1998;44:1337–1348. doi: 10.1016/s0006-3223(98)00176-0. [DOI] [PubMed] [Google Scholar]
- 32.Elsworth JD, Lawrence MS, Roth RH, Taylor JR, Mailman RB, Nichols DE, Lewis MH, Redmond DE., Jr D1 and D2 dopamine receptors independently regulate spontaneous blink rate in the vervet monkey. J Pharmacol Exp Ther. 1991;259:595–600. [PubMed] [Google Scholar]
- 33.Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory, and Language. San Diego: Academic; 1994. [Google Scholar]
- 34.Stuss DT, Toth JP, Franchi D, Alexander MP, Tipper S, Craik FIM. Dissociation of attentional processes in patients with focal frontal and posterior lesions. Neuropsychologia. 1999;37:1005–1027. doi: 10.1016/s0028-3932(98)00158-4. [DOI] [PubMed] [Google Scholar]
- 35.Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Godefroy O, Rousseaux M. Divided and focused attention in patients with lesion of the prefrontal cortex. Brain Cogn. 1996;30:155–174. doi: 10.1006/brcg.1996.0010. [DOI] [PubMed] [Google Scholar]
- 37.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 38.Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 39.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 40.Karson CN, Kaufmann CA, Shapiro AK, Shapiro E. Eye-blink rate in Tourette’s syndrome. J Nerv Ment Dis. 1985;173:566–569. doi: 10.1097/00005053-198509000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


