Abstract
Nanoparticles are designed to selectively deliver therapeutics and diagnostics to tumors. Their size, shape, charge, material, coating and cargo, determine their individual functionalities. A systems approach could help predict the behavior of trillions of nanoparticles interacting in complex tumor environments. Engineering these nanosystems may lead to biomimetic strategies where interactions between nanoparticles and their environment give rise to cooperative behaviors typically seen in natural self-organized systems. Examples include nanoparticles that communicate the location of a tumor to amplify tumor homing, or self-assemble and disassemble to optimize nanoparticle transport. The challenge is to discover which nanoparticle designs lead to a desired systems behavior. To this end, novel nanomaterials, deep biological understanding, and computational tools are emerging as the next frontier.
Keywords: Swarming, Cooperation, Nanoparticles, Systems Nanotechnology, Cancer
Cooperative nanosystems
Cancer is one of the leading causes of death worldwide. To address cancer, bioengineers are designing nanoparticles that can deliver treatments and diagnostics selectively to tumors [1, 2]. Their size, typically between 5nm and 500nm, allows them to escape the leaky vessels in tumors [3]. Focus has been on engineering the functionalities of individual nanoparticles to: improve transport [4], target the tumor vasculature [5, 6] or extracellular matrix [7], deliver therapeutics [8, 9], diagnostics [10] or heat [11, 12] to the tumor environment, and reprogram cancer cells [13] or the immune system [14].
The behavior of each nanoparticle depends on its design and the resulting interactions in the body. The collective behavior of trillions of such nanoparticles interacting in a complex tumor environment can define their success as diagnostic or treatment agents [15]. Predicting and engineering these collective behaviors is empirical and not intuitive. For example, nanoparticles that are optimized to strongly bind and accumulate in cancer cells mostly accumulate in the first cells they encounter after leaking into the tumor environment. The resulting collective behavior is poor tissue penetration, with deep seeded tumor cells left untreated, otherwise called a binding site barrier [16–18]. Weaker nanoparticle binding, although detrimental to the function of the individual nanoparticle, could lead to a better system outcome. Further engineering these behaviors could result in emergent cooperative behaviors typically seen in self-organized systems.
Self-organized systems in nature, including social insects, animals, humans and cells, are able to perform complex behaviors, such as amplification, optimization, mapping, structure assembly, collective motion, synchronization and decision making, through the local interactions of many simple agents and their environment [19–22]. The field of swarm robotics [23, 24] has long taken inspiration from nature to engineer minimal robots that use simple rules to interact with their neighbors and local environment to solve complex real world problems [25–27]. Similarly, behaviors demonstrated in nanomedicine include: self-assembly of nanoparticles to anchor imaging agents in tumors [28, 29], disassembly of nanoparticles to increase tissue penetration [30, 31], nanoparticles that compute the state of a tumor [32], nanoparticle-based remodeling of tumor environments to improve secondary nanoparticle transport [33], or nanoparticle signaling of tumor location to amplify the accumulation of other nanoparticles in tumors [34].
Even for simple nanoparticle designs, engineering and predicting the collective behavior of large numbers of nanoparticles that interact with complex tumor environments is non-intuitive. Through a systems approach, bioengineers could automatically explore nanoparticle designs using crowdsourcing (http://nanodoc.org) and machine learning [35]. Bioengineers could: model the resulting collective behavior in simulation [4, 16, 17, 36], automatically test the best candidates experimentally through fast prototyping of both the nanoparticles [37, 38] and their environment [39], and finally validate the collective behaviors in vivo with feedback provided by high-resolution imaging [40]. Expertise in nanomaterials, deep understanding of cancer biology, and advances in the modeling and automation of nanosystems are making possible the first step in this direction.
Lessons learned from the design of cooperative nanosystems could also prove useful in the engineering of natural swarmers, such as cells of the immune system [41] or synthetic bacteria [42], to improve tumor treatment and diagnostics. Overall, a systems approach to understand and engineer self-organized systems has the potential to result in behaviors that go beyond the functionalities of the individual agents and towards efficient, modular and predictable outcomes.
Nanoparticle behaviors
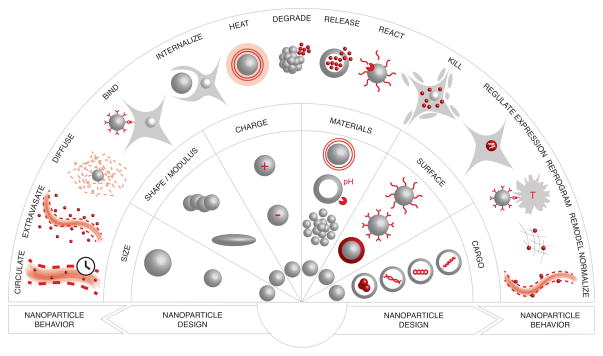
A large variety of nanoparticles have been engineered to deliver therapies and diagnostics to tumors. The design of a nanoparticle, and its interactions with the environment, defines its behavior. The composition of each nanoparticle can be summarized by its main features in terms of initial size, shape, charge, coating, cargo, and material, the combination of which determine its ability to move and interact with the environment (Figure 1) [43, 44].
Figure 1.
Nanoparticle Behaviors. Nanoparticle designs, in terms of size, shape, modulus, charge, material, surface and cargo, as well as their interactions in the body, determine their individual behavior.
The size of a nanoparticle has been shown to influence its circulation time, extravasation, interstitial diffusion and ability to be internalized by cells [45, 46]. Nanoparticles smaller than 5nm tend to escape the blood stream into tissues throughout the body [47, 48]. They are rapidly filtered by the kidneys and cleared in the urine. As a result, small nanoparticles have a short circulation time (on the order of minutes), rapid entry into tumors, and fast diffusion through the tumor tissue. Nanoparticles that reach the edge of a solid tumor can be carried out due to the gradient in hydrostatic pressure caused by the tumor physiology relative to the surrounding healthy tissue, thereby making their retention more challenging [3]. Nanoparticles larger than 5nm and up to 500 nm are, in some cases, able to remain in circulation for a longer time and accumulate in solid tumors by escaping through the enlarged pores in angiogenic vessels. Such passive targeting of nanoparticles due to porous angiogenic endothelium and dysfunctional lymphatic drainage, observed in some tumors, has been called the enhanced permeability and retention (EPR) effect [49]. Once in the tumor, the slow diffusion of larger nanoparticles and the difficulty of navigating the extracellular matrix tend to limit their ability to penetrate tumor tissue [50]. The size of a nanoparticle also limits its ability to be internalized by cells, and data indicates that different sizes could be trafficked via different endocytic pathways [44].
The shape and modulus of nanoparticles can also impact cellular uptake [51]. Nanoparticles with high aspect ratios, and particles that are rigid, have been shown to accumulate more slowly in macrophages than smaller, flexible particles, thereby reducing their clearance time from the blood. In situations where uptake is important, such as in tumor environments, spherical nanoparticles have thus far proven more efficient.
The charge of a nanoparticle also impacts its circulation time. Charged nanoparticles are rapidly opsonized and cleared by the immune system [52]. Passivating coatings such as polyethylene glycol have been used to shield the charge of nanoparticles, improve their circulation time on the order of days, and result in their accumulation in tumor tissue [43]. Similarly, phagocytic uptake can be minimized by coating nanoparticles with a “self-peptide” derived from the human “don’t eat me” receptor, CD47 [53]. Once in tumor tissue, charged nanoparticles are preferentially taken up by tumor cells relative to uncharged particles. Researchers have been addressing this dichotomy by designing nanoparticles that shed their neutral coatings to display a charged interior upon entering the tumor environment based on pH or enzymatic activity [54–56].
Beyond charge, nanoparticle surfaces can be engineered to display targeting ligands [5, 7, 57] including peptides [58], antibodies [59], aptamers [60] and small molecules [61]. Receptors that are expressed at high levels on certain tumor cells can serve as targets that drive the binding and intracellular delivery of nanoparticles [6]. For small nanoparticles, targeting has proven useful in anchoring nanoparticles in the tumor environment, resulting in increased accumulation over time [62]. Recently, nanoparticles decorated with ligands expressing a CendR domain have been shown to activate the neuropilin-1 receptor and subsequently increase tissue penetration by initiating a trancellular, active transport pathway [63]. The effect can be made specific to tumor tissue by adding a targeting domain to the peptide. Receptors overexpressed on angiogenic endothelial cells have been used as vascular ‘zip codes’ to direct and capture circulating nanoparticles and concentrate them in tumor environments [6], or can serve as an antiangiogenic target towards the normalization of the vascular bed [3, 64]. Nanoparticles have also been engineered to target the extracellular matrix (ECM), either to improve the retention of nanoparticles [65] or degrade the ECM to improve drug permeation in tumors [66]. Finally, nanoparticles can be engineered to target T cells and enhance immune responses towards tumors [14, 67].
One other design benefit that nanoparticles can offer is the capacity to shield materials that would otherwise be toxic or degraded in the body. The therapeutic cargo may then be carried to the tumor where it will be released or activated prior to local application, thereby minimizing systemic side effects while improving effectiveness despite low dosages. Cargos transported by nanoparticles include: chemotherapies [8, 9] and siRNA for knockdown of gene expression in tumor cells [13, 68], antiangiogenic agents [64, 69], agents that are disruptive to the ECM [70], imaging agents [1, 10], or adjuvants to activate the immune system [14].
The ability of nanoparticles to react to their environment is directly connected to their material composition [71]. Nanoparticles can be engineered to release cargos in an open-loop manner at a predetermined rate based on material erosion, or cargo diffusion through the nanoparticle matrix or pores [72–74]. To increase the level of control, materials can release their cargo or change their physical properties in response to local stimuli in tumor microenvironments, such as pH or enzymatic activity [55, 75–77]. Energy sensitive materials can be activated by global signals such as magnetic fields [78–80], sound [81] or light [11, 82, 83], which can also power them to move, emit light or heat, or to release a cargo.
Overall, the ability of nanoparticles to sense, move and act in the body is determined by their design and interactions with the environment. Engineering the behavior of nanoparticles has become increasingly possible, thanks to a deepening understanding of biology and nanoparticle transport, and the impressive toolbox of nanoparticle modifiers available to bioengineers.
Collective behaviors
In other fields, engineering collective behavior is necessary to achieve a desired system outcome. Engineering the collective behavior of nanoparticles may similarly lead to a better system outcome. Most nanoparticle systems implicitly cooperate, and each nanoparticle is designed to optimize its individual functionality [84]. The collective impact of the nanoparticles as treatment or imaging agents is assumed to be the sum of the independent nanoparticle effects. Understanding the system-level behavior of implicit cooperators may add insight that improves predictions. Emphasis could be placed on studying whether the nanoparticles can collectively distribute throughout a tumor environment or accumulate at effective levels in, or around, targeted cells [4]. Similarly, combination therapies aimed at preventing resistance can be composed of different types of nanoparticles that independently target cells in the tumor [85, 86].
Nanoparticles that physically interact have a more direct means of cooperation. These systems typically self-assemble or disassemble to modify their kinetics or collectively transport combined treatment and imaging agents to tumors. Rapidly diffusing imaging agents are able to anchor in tumors by binding to previously injected gold nanoparticles that have had time to accumulate there due to the EPR effect [29]. Similarly, small (10 nm) gold nanoparticles engineered to release conjugated doxorubicin in acidic tumor environments, can subsequently self-assemble to form larger gold aggregates that are then used for photothermal therapy [28, 87]. In vitro experiments show how nanoparticles that self-assemble in response to enzymatic activity may be able to perform logic computations towards the diagnosis of tumor state [88]. Larger nanoparticles (100 nm) are able to disassemble into smaller nanoparticles once inside the tumor environment, in response to enzymatic activity, thereby improving their circulation time, accumulation in the tumor, and ability to penetrate deep in the tissue [31]. Other multi-stage nanoparticles including nested nanoparticles, mother ships, and nanocells are able to overcome transport barriers through the release of nano-based components in tumor environments [64, 89, 90].
In contrast to collective behaviors mediated by direct interactions between nanoparticles, many swarm systems found in nature communicate by modifying the environment. This concept is called stigmergy [19]. Ants deposit and sense chemical signals to form trails to sources of food [20]. Termites are able to build complex structures by modifying and locally sensing their physical environment [27]. In a similar way, nanoparticles have been designed to modify their physical environment or deposit chemical signals. Gold nanorods that accumulate in a tumor, upon heating with NIR light to sub-lethal temperatures, can improve perfusion of angiogenic vessels and in some cases upregulate receptors used in targeting, which in turn improves the delivery of a second wave of nanoparticles, such as liposomes and magnetic nanoworms, to tumors for treatment and imaging purposes [33, 82]. Similarly, nanoparticles that aim to normalize the vascular bed, or degrade the extracellular matrix can improve the transport of secondary nanoparticles [3, 66].
Nanoparticles can also communicate through the environment by depositing a signal according to two possible modes of action. In the first, nanoparticles are able to release either a cargo or energy, which can directly interact with other nanoparticles. This approach offers an important advantage: nanoparticle communication can be engineered to be orthogonal to the host system. As an example, gold nanorods activated through NIR light emit heat in tumors to trigger the release of chemotherapeutics contained in thermally sensitive drug carriers [91]. Unfortunately, the scales at which nanoparticles function make it difficult for a chemical cargo to encounter a second nanoparticle in the environment. In contrast, biological systems have built-in mechanisms to amplify signals, coercing biological cascades can result in longer-range communication systems. Gold nanorods heated through NIR light can cause a clotting cascade in tumors [34]. This biological cascade serves as a signal to communicate the location of the tumor to circulating nanoparticles loaded with chemotherapeutics. To achieve this outcome, the receiving nanoparticle, here a liposome or nanoworm, is targeted to a byproduct of the coagulation cascade (the transglutaminase FXIII). This communication strategy leads to a 40-fold increase in the amount of chemotherapeutic delivered to the tumor when compared to a non-communicating system [34].
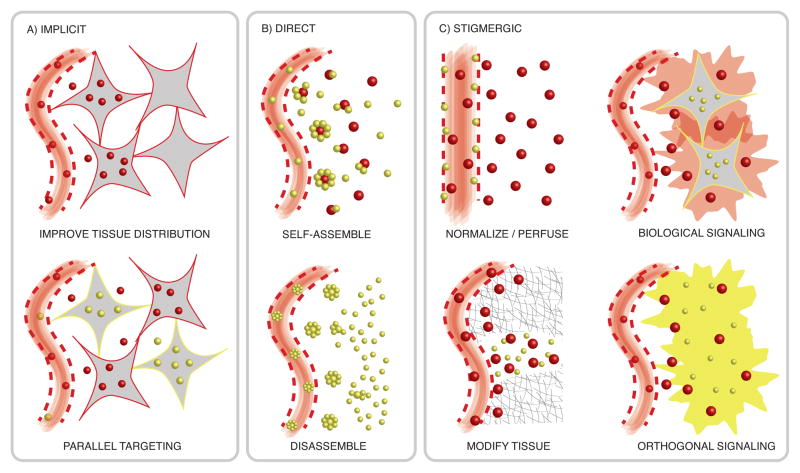
Nanoparticles can cooperate implicitly, directly through self-assembly and disassembly, or through stigmergy (Figure 2). These behaviors have been useful to improve nanoparticle transport, accumulation, and distribution in tumor tissues towards treatment and diagnostic applications.
Figure 2.
Mechanisms of Cooperation in Nanosystems. A) Implicit cooperation: Each nanoparticle works individually to improve the overall tissue distribution (top) or work as a combination therapy (bottom). B) Direct cooperation: Physical interactions enable nanoparticles to self-assemble (top) and disassemble (bottom) towards improved nanoparticle transport and retention in tumors. C) Stigmergic cooperation: Nanoparticles interact through the environment by either modifying the environment to improve the transport of secondary nanoparticles (left), or by depositing a signal (right). Signaling nanoparticles can emit energy and chemicals, or coerce a biological response from the tumor environment, that can be received by a secondary nanoparticle.
Systems nanotechnology
A systems approach could help engineer collective behaviors for nanoparticles (Figure 3) [92]. Currently, most nanoparticle systems are engineered based on the desired functionalities of the individual nanoparticles. Designing cooperative behaviors requires bioengineers to initially define the behavior that should be displayed by the nanoparticle collective within their environment of action. Examples include: “accumulate in all tumor cells at lethal levels, assuming sites of extravasation are no further than 200um from the cells” [16]. The challenge is then to understand which individual nanoparticle designs could give rise to this collective behavior.
Figure 3.
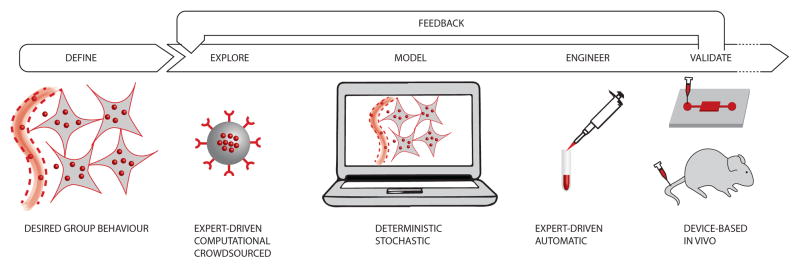
Systems Approach Towards Nanomedicine. A systems approach could help design the individual nanoparticles that would give rise to desired cooperative behaviors. This requires advances in computational exploration, modeling and the fast prototyping of experiments before validation in vivo.
Currently, the exploration of nanoparticle designs is conducted by experts with a deep knowledge of the intricacies of cancer nanomedicine. This paradigm is ideal when the collective behavior resulting from a given nanoparticle design and its iterations are well understood. When new designs are needed, or more complex collective behaviors are envisaged, automatic exploration tools built around machine learning and crowdsourcing can help. Mathematical optimization was used to explore formulations, in terms of binding kinetics and diffusion, which would enable targeted nanoparticles to penetrate deep into tumor tissues [16]. Regression analysis was used to explore the impact of nanoparticle structure on their ability to deliver siRNAs to tumor cells [93]. When the search space is too large, the power of the crowd can enable human guided exploration of nanoparticle designs. Crowdsourcing has been shown in the past to find solutions to complex scientific problems that were intractable for computers, despite their impressive processing power [94]. NanoDoc (http://nanodoc.org), for example, provides an online tool to crowdsource the design of nanoparticles. Using an iterative approach, players design nanoparticle treatments, inject them into a virtual tumor, and continuously improve their design until they are able to achieve a desired collective behavior.
Once provided with a nanoparticle design, computer simulations and mathematical models can help predict their collective behavior. Analytical and Monte Carlo simulations have been used to predict the impact of multivalency of targeting moieties on the super-selectivity of nanoparticles to cancer cells [95]. Modeling the kinetics of nanoparticle populations in tumors has been demonstrated by several research groups using stochastic and deterministic approaches [16–18, 36, 96]. The models describe how nanoparticles extravasate, diffuse through the interstitial space, and bind to, or are internalized by cancer cells. Ideally, iterations between nanoparticle design and the resulting simulated behavior give rise to general guidelines that can be applied to nanosystems without the need to iterate at length experimentally.
After developing plans for an idealized nanoparticle, these designs must then be engineered. Most often, this step requires deep expertise, based on the state-of-the-art in the field. However, automatic tools can help combine nanoparticle features, such as size, coating and cargo in a deterministic fashion [37]. Particle replication in non-wetting templates (PRINT) technology offers an easy to use platform for the design and fabrication of monodisperse particles from a wide range of matrices with complete control over the physicochemical properties of the particle [38]. Combinatorial approaches in designing and synthesizing polymeric systems enable high-throughput synthesis and screening of nanoparticles with desired drug loading, retention in circulation and targeting. For this purpose, a number of polymer libraries have been designed [97–99]. Recent work on DNA self-assembly also enables the rapid prototyping of nanoscale structures [100–103].
Testing the collective behavior of nanoparticles is made challenging by the fact that the environment plays such an important role in the rise of emergent behaviors. In vivo experiments are an ideal testbed, provided that high-resolution imaging can be performed during the course of the experiment. Multimodal studies of tumor pathophysiology and nanoparticle dynamics can be performed with the help of intravital microscopy in window chambers [104, 105]. When in vivo experiments are not feasible, micro-devices, capable of capturing essential features of the tumor environment that are necessary for the emergence of collective nanoparticle behaviors can find utility. A tumor-on-a-chip system, in which a tumor-like spheroid is placed in a microfluidic channel, enables real-time analysis of nanoparticle kinetics at physiological flow conditions [106]. Recent technologies, including 3D printing, scaffolding, and cell patterning, layering and self-assembly, have enabled construction of complex 3D-human tissues, including blood and lymph capillary networks, and could be used as a testbed for cooperative nanosystems in vitro [39, 107, 108].
A key grand challenge is the integration of all the technologies needed to provide a full pipeline - from problem definition to clinical translation.
Concluding remarks and future perspectives
Nanoparticle behaviors result from their individual designs and interactions with the environment. The control of a nanoparticle is enabled by changing its shape, charge, material, coating and cargo. The behavior of trillions of nanoparticles in vivo determines their success as treatment and diagnostic agents. The field could therefore benefit from an increased understanding and systematic approaches to engineering the collective behavior of nanoparticles. Examples of self-organization in nature show that emergent behaviors arise when simple agents interact directly, or through the environment, and can lead to modular, scalable, and efficient strategies that outperform the capabilities of individual agents. Such concepts are already being used in other fields of engineering, such as swarm robotics.
Designing individual nanoparticles towards a desired cooperative strategy is challenging and could benefit from a systems approach. Such an endeavor would build on advances in computational exploration, simulation, high-throughput design of nanoparticles and the engineering of experimental testbeds in vitro and in vivo. This approach is now made possible thanks to advances in nanoparticle design and a deeper understanding of biology, which have given rise to the first instances of cooperative nanoparticles that can communicate tumor locations, improve transport, and self-assemble or disassemble. The next frontier is to learn from these examples to design biomedical systems that can perform complex tasks such as optimization, computation, decision-making, construction, self-assembly and collective motion. Strategies of cooperation could go beyond nanoparticles to integrate engineered biological systems including synthetic bacteria and cells of the immune system towards biomedical applications.
Highlights.
Nanoparticle designs and interactions in the body determine their individual behavior.
The collective behavior of nanoparticles can be exploited to improve performance
Bio-inspired mechanisms of cooperation include self-assembly and communication.
Systems approach builds on new nanomaterials, biological insight, and computation.
Acknowledgments
The authors are grateful to Dr. Fleming for her help in reviewing this manuscript. Dr. Hauert and Dr. Bhatia acknowledge support from the Human Frontier Science Program. Dr. Bhatia is an HHMI investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bao G, et al. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu Rev Biomed Eng. 2013;15:253–282. doi: 10.1146/annurev-bioeng-071812-152409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis ME, et al. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 2010;28:181–188. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliver Rev. 2012;64:206–212. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Ruoslahti E, et al. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010;188:759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanapathipillai M, et al. Nanoparticle targeting of anti-cancer drugs that alter intracellular signaling or influence the tumor microenvironment. Adv Drug Deliver Rev. 2014 doi: 10.1016/j.addr.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Cho K, et al. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 9.Wang AZ, et al. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 10.Ryu JH, et al. Tumor-targeting multi-functional nanoparticles for theragnosis: new paradigm for cancer therapy. Adv Drug Deliver Rev. 2012;64:1447–1458. doi: 10.1016/j.addr.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Melancon M, et al. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc Chem Res. 2011;44:947–956. doi: 10.1021/ar200022e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pissuwan D, et al. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006;24:62–67. doi: 10.1016/j.tibtech.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Kanasty R, et al. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 14.Moon JJ, et al. Engineering nano- and microparticles to tune immunity. Adv Mater. 2012;24:3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taurin S, et al. Anticancer nanomedicine and tumor vascular permeability; Where is the missing link? J Control Release. 2012;164:265–275. doi: 10.1016/j.jconrel.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Hauert S, et al. A computational framework for identifying design guidelines to increase the penetration of targeted nanoparticles into tumors. Nano Today. 2013;8:566–576. doi: 10.1016/j.nantod.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurber GM, Weissleder R. A systems approach for tumor pharmacokinetics. PLoS ONE. 2011;6:e24696. doi: 10.1371/journal.pone.0024696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittrup KD, et al. Practical theoretic guidance for the design of tumor-targeting agents. Methods Enzymol. 2012;503:255–268. doi: 10.1016/B978-0-12-396962-0.00010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonabeau E, et al. Swarm intelligence: From natural to artificial systems. Oxford University Press; 1999. [Google Scholar]
- 20.Camazine S, et al. Self-Organization in Biological Systems. Princeton University Press; 2003. [Google Scholar]
- 21.Couzin ID. Collective cognition in animal groups. Trends Cogn Sci. 2009;13:36–43. doi: 10.1016/j.tics.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Krause J, et al. Swarm intelligence in animals and humans. Trends Ecol Evol. 2010;25:28–34. doi: 10.1016/j.tree.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Sahin E. Swarm Robotics. Springer; Berlin Heidelberg: 2005. Swarm robotics: From sources of inspiration to domains of application; pp. 10–20. [Google Scholar]
- 24.Winfield AFT, et al. Swarm Robotics. Springer; Berlin Heidelberg: 2005. Towards dependable swarms and a new discipline of swarm engineering; pp. 126–142. [Google Scholar]
- 25.Hauert S, et al. Reynolds flocking in reality with fixed-wing robots: communication range vs. maximum turning rate. IEEE/RSJ International Conference on Intelligent Robots and Systems. 2011:5015–5020. [Google Scholar]
- 26.Kernbach S. Handbook of Collective Robotics: Fundamentals and Challenges. Pan Stanford Publishing; 2013. [Google Scholar]
- 27.Werfel J, et al. Designing collective behavior in a termite-inspired robot construction team. Science. 2014;343:754–758. doi: 10.1126/science.1245842. [DOI] [PubMed] [Google Scholar]
- 28.Nam J, et al. pH-Induced aggregation of gold nanoparticles for photothermal cancer therapy. J Am Chem Soc. 2009;131:13639–13645. doi: 10.1021/ja902062j. [DOI] [PubMed] [Google Scholar]
- 29.Perrault SD, Chan WCW. In vivo assembly of nanoparticle components to improve targeted cancer imaging. Proc Natl Acad Sci U S A. 2010;107:11194–11199. doi: 10.1073/pnas.1001367107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godin B, et al. Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc Chem Res. 2011;44:979–989. doi: 10.1021/ar200077p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong C, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci USA. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong GA, et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol. 2013;31:63–70. doi: 10.1038/nbt.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JH, et al. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc Natl Acad Sci U S A. 2010;107:981–986. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Maltzahn G, et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat Mater. 2011;10:545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phan JH, et al. Convergence of biomarkers, bioinformatics and nanotechnology for individualized cancer treatment. Trends Biotechnol. 2009;27:350–358. doi: 10.1016/j.tibtech.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Florence AT. “Targeting” nanoparticles: the constraints of physical laws and physical barriers. J Control Release. 2012;164:115–124. doi: 10.1016/j.jconrel.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Abeylath SC, et al. Combinatorial-designed multifunctional polymeric nanosystems for tumor-targeted therapeutic delivery. Acc Chem Res. 2011;44:1009–1017. doi: 10.1021/ar2000106. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, et al. Future of the particle replication in nonwetting templates (PRINT) technology. Angew Chem. 2013;52:6580–6589. doi: 10.1002/anie.201209145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vickerman V, et al. Design, Fabrication and Implementation of a Novel Multi Parameter Control Microfluidic Platform for Three-Dimensional Cell Culture and Real-Time Imaging. Lab Chip. 2008;8:1468–1477. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deisboeck TS, Couzin ID. Collective behavior in cancer cell populations. Bioessays. 2009;31:190–197. doi: 10.1002/bies.200800084. [DOI] [PubMed] [Google Scholar]
- 42.Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10:785–794. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, et al. More effective nanomedicines through particle design. Small. 2011;7:1919–1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dreaden EC, et al. Size matters: gold nanoparticles in targeted cancer drug delivery. Ther Deliv. 2012;3:457–478. doi: 10.4155/tde.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kievit FM, Zhang M. Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Adv Mater. 2011;23:217–247. doi: 10.1002/adma.201102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi HS, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Longmire M, et al. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomed. 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeda H, et al. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Delivery Rev. 2012;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Perrault SD, et al. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 51.Venkataraman S, et al. The effects of polymeric nanostructure shape on drug delivery. Adv Drug Deliver Rev. 2011;63:1228–1246. doi: 10.1016/j.addr.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez PL, et al. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris TJ, et al. Protease-triggered unveiling of bioactive nanoparticles. Small. 2008;4:1307–1312. doi: 10.1002/smll.200701319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poon Z, et al. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano. 2011;5:4284–4292. doi: 10.1021/nn200876f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romberg B, et al. Sheddable coatings for long-circulating nanoparticles. Pharm Res. 2008;25:55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamaly N, et al. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearce TR, et al. Peptide targeted lipid nanoparticles for anticancer drug delivery. Adv Mater. 2012;24:3803–3822. 3710. doi: 10.1002/adma.201200832. [DOI] [PubMed] [Google Scholar]
- 59.Julien DC, et al. Utilization of monoclonal antibody-targeted nanomaterials in the treatment of cancer. mAbs. 2011;3:467–478. doi: 10.4161/mabs.3.5.16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang L, et al. Aptamer-conjugated nanomaterials and their applications. Adv Drug Deliver Rev. 2011;63:1361–1370. doi: 10.1016/j.addr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valencia PM, et al. Effects of ligands with different water solubilities on self-assembly and properties of targeted nanoparticles. Biomaterials. 2011;32:6226–6233. doi: 10.1016/j.biomaterials.2011.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Timko BP, et al. Advances in drug delivery. Annu Rev Mater Res. 2011;41:1–20. [Google Scholar]
- 63.Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv Mater. 2012;24:3747–3756. doi: 10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sengupta S, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 65.Ji T, et al. Using functional nanomaterials to target and regulate the tumor microenvironment: diagnostic and therapeutic applications. Adv Mater. 2013;25:3508–3525. doi: 10.1002/adma.201300299. [DOI] [PubMed] [Google Scholar]
- 66.Waite C, Roth C. Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: Current progress and opportunities. Crit Rev Biomed Eng. 2012;40:21–41. doi: 10.1615/critrevbiomedeng.v40.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silva JM, et al. Immune system targeting by biodegradable nanoparticles for cancer vaccines. J Control Release. 2013;168:179–199. doi: 10.1016/j.jconrel.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Hou KK, et al. Mechanisms of Nanoparticle-Mediated siRNA Transfection by Melittin-Derived Peptides. ACS Nano. 2013;7:8605–8615. doi: 10.1021/nn403311c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bocci G, et al. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16:481–492. doi: 10.1007/s10456-013-9334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goodman TT, et al. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int J Nanomedicine. 2007;2:265–274. [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu L, Torchilin VP. Stimulus-responsive nanopreparations for tumor targeting. Integr Biol. 2013;5:96–107. doi: 10.1039/c2ib20135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kost J, Langer R. Responsive polymeric delivery systems. Adv Drug Deliver Rev. 2012;64:327–341. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 73.Kumari A, et al. Biodegradable polymeric nanoparticles based drug delivery systems. Colloid Surf B. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Sailor MJ, Park JH. Hybrid nanoparticles for detection and treatment of cancer. Adv Mater. 2012;24:3779–3802. doi: 10.1002/adma.201200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Danhier F, et al. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 76.Lin K, et al. Nanoparticles that sense thrombin activity as synthetic urinary biomarkers of thrombosis. ACS Nano. 2013:9001–9009. doi: 10.1021/nn403550c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olson ES, et al. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc Natl Acad Sci USA. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cole AJ, et al. Cancer theranostics: the rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011;29:323–332. doi: 10.1016/j.tibtech.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Derfus aM, et al. Remotely triggered release from magnetic nanoparticles. Adv Mater. 2007;19:3932–3936. [Google Scholar]
- 80.McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliver Rev. 2008;60:1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mo S, et al. Ultrasound-enhanced drug delivery for cancer. Expert Opin Drug Deliv. 2012;9:1525–1538. doi: 10.1517/17425247.2012.739603. [DOI] [PubMed] [Google Scholar]
- 82.Bagley AF, et al. Plasmonic photothermal heating of intraperitoneal tumors through the use of an implanted near-infrared source. ACS Nano. 2013;7:8089–8097. doi: 10.1021/nn4033757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.von Maltzahn G, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng Z, et al. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eldar-Boock A, et al. Nano-sized polymers and liposomes designed to deliver combination therapy for cancer. Curr Opin Biotechnol. 2013;24:682–689. doi: 10.1016/j.copbio.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 86.Greco F, Vicent MJ. Combination therapy: opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Adv Drug Deliver Rev. 2009;61:1203–1213. doi: 10.1016/j.addr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 87.Nam J, et al. pH-responsive assembly of gold nanoparticles and spatiotemporally concerted drug release for synergistic cancer therapy. ACS Nano. 2013:3388–3402. doi: 10.1021/nn400223a. [DOI] [PubMed] [Google Scholar]
- 88.von Maltzahn G, et al. Nanoparticle self-assembly gated by logical proteolytic triggers. J Am Chem Soc. 2007;129:6064–6065. doi: 10.1021/ja070461l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anglin EJ, et al. Porous silicon in drug delivery devices and materials. Adv Drug Deliver Rev. 2008;60:1266–1277. doi: 10.1016/j.addr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serda RE, et al. Multi-stage delivery nano-particle systems for therapeutic applications. Biochim Biophys Acta. 2011;1810:317–329. doi: 10.1016/j.bbagen.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park JH, et al. Cooperative nanoparticles for tumor detection and photothermally triggered drug delivery. Adv Mater. 2010;22:880–885. doi: 10.1002/adma.200902895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Godin B, et al. An integrated approach for the rational design of nanovectors for biomedical imaging and therapy. Adv Genet. 2010;69:31–64. doi: 10.1016/S0065-2660(10)69009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren Y, et al. Identification and characterization of receptor-specific peptides for siRNA delivery. ACS Nano. 2012;6:8620–8631. doi: 10.1021/nn301975s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cooper S, et al. Predicting protein structures with a multiplayer online game. Nature. 2010;466:756–760. doi: 10.1038/nature09304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martinez-Veracoechea FJ, Frenkel D. Designing super selectivity in multivalent nano-particle binding. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1105351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Florence AT. Reductionism and complexity in nanoparticle-vectored drug targeting. J Control Release. 2012;161:399–402. doi: 10.1016/j.jconrel.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 97.Anderson DG, et al. A combinatorial library of photocrosslinkable and degradable materials. Adv Mater. 2006;18:2614–2618. [Google Scholar]
- 98.Green J, et al. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41 doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valencia PM, et al. Microfluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapy. ACS Nano. 2013;7:10671–10680. doi: 10.1021/nn403370e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Douglas SM, et al. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature. 2009;459:414–418. doi: 10.1038/nature08016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Douglas SM, et al. A logic-gated nanorobot for targeted transport of molecular payloads. Science. 2012;335:831–834. doi: 10.1126/science.1214081. [DOI] [PubMed] [Google Scholar]
- 102.Iinuma R, et al. Polyhedra self-assembled from DNA tripods and characterized with 3D DNA-PAINT. Science. 2014;344:65–69. doi: 10.1126/science.1250944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maune HT, et al. Self-assembly of carbon nanotubes into two-dimensional geometries using DNA origami templates. Nat Nanotechnol. 2010;5:61–66. doi: 10.1038/nnano.2009.311. [DOI] [PubMed] [Google Scholar]
- 104.Amornphimoltham P, et al. Intravital microscopy as a tool to study drug delivery in preclinical studies. Adv Drug Deliver Rev. 2011;63:119–128. doi: 10.1016/j.addr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hak S, et al. Intravital microscopy in window chambers: a unique tool to study tumor angiogenesis and delivery of nanoparticles. Angiogenesis. 2010;13:113–130. doi: 10.1007/s10456-010-9176-y. [DOI] [PubMed] [Google Scholar]
- 106.Albanese A, et al. Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat Commun. 2013;4:2718. doi: 10.1038/ncomms3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lanza R, et al. Principles of tissue engineering. Academic press; 2011. [Google Scholar]
- 108.Miller JS, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]