Abstract
Objective
Recent evidence suggests G-protein–coupled receptor-2–interacting protein-1 (GIT1) overexpression in several human metastatic tumors, including breast, lung, and prostate. Tumor metastasis is associated with an increase in angiogenesis. We have showed previously that GIT1 is required for postnatal angiogenesis during lung development. However, the functional role of GIT1 in pathological angiogenesis during tumor growth is unknown.
Approach and Results
In the present study, we show inhibition of angiogenesis in matrigel implants as well as reduced tumor angiogenesis and melanoma tumor growth in GIT1-knockout mice. We demonstrate that this is a result of impaired directional migration of GIT1-depleted endothelial cells toward a vascular endothelial growth factor gradient. Cortactin-mediated lamellipodia formation in the leading edge is critical for directional migration. We observed a significant reduction in cortactin localization and lamellipodia formation in the leading edge of GIT1-depleted endothelial cells. We specifically identified that the Spa homology domain (aa 250–420) of GIT1 is required for GIT1–cortactin complex localization to the leading edge. The mechanisms involved extracellular signal-regulated kinases 1 and 2–mediated Cortactin-S405 phosphorylation and activation of Rac1/Cdc42. Finally, using gain of function studies, we show that a constitutively active mutant of cortactin restored directional migration of GIT1-depleted cells.
Conclusion
Our data demonstrated that a GIT1–cortactin association through GIT1-Spa homology domain is required for cortactin localization to the leading edge and is essential for endothelial cell directional migration and tumor angiogenesis.
Keywords: cortactin, endothelial cells, G-protein–coupled receptor kinase interacting protein-1, tumor angiogenesis
Angiogenesis, the formation of new blood vessels from existing ones, is critical for tissue development, tissue repair, as well as many diseases including diabetic retinopathy and tumor growth.1 Directional migration of endothelial cells (EC) toward a gradient of vascular endothelial growth factor (VEGF) determines vascular formation, which is a hallmark of tumor angiogenesis.1–3 This process involves 3 highly coordinated and regulated steps: sensing the stimuli, cytoskeleton rearrangement, and lastly movement of the cell. Lamellipodia are the critical structures for cell directional migration and they are regions of very rapid actin remodeling, which is mainly mediated by the cortical actin remodeling protein, cortactin.4 Cortactin has emerged as a key signaling protein in many cellular processes, including cell adhesion, migration, angiogenesis, and tumor invasion.5,6 Serine 405 phosphorylation by extracellular signal–regulated kinases 1 and 2 (ERK1/2) in the SRC homology 3 domain of cortactin is required for cortical actin cytoskeleton remodeling and cell migration.7–9 The work of Weed et al10 found that active Rac1/Cdc42 induced cortactin-mediated lamellipodia formation. Recent work also revealed the involvement of Cdc42-associated kinase 1 in inducing cortactin activation to promote cortical actin remodeling via Arp2/3-nWiskott -Aldrich syndrome protein complex.11
G-protein–coupled receptor kinase–interacting protein-1 (GIT1) is a multidomain protein involved in diverse cellular processes, including cell adhesion, migration,12 and permeability.13 GIT1, being a scaffold protein, interacts with other proteins, which can affect its cellular localization and activity. The full-length GIT1 protein has an N-terminal ADP-ribosylation factor GTPase-activating protein domain, 3 ankyrin repeats, a Spa2-homology domain (SHD), a synaptic localizing domain and a paxillin-binding site.14 We previously showed that GIT1 is important for ERK1/2 activation in response to multiple stimuli including VEGF and thrombin in EC.15,16 GIT1 is also important in regulating Rac1 and Cdc42 through its p21-associated kinase (PAK)–interacting exchange factor (PIX)–PAK binding in neurons.14,17 Our laboratory recently revealed the crucial role of GIT1 in postnatal angiogenesis during lung vasculature development.12,16 Several data, including Genecard analysis of human tumors, suggested an overexpression of GIT1 in several metastatic tumors, including breast, lung, melanoma, and prostate cancer.18,19 Based on these findings, we hypothesize that GIT1 is essential for tumor angiogenesis by regulating EC directional migration via cortactin-dependent lamellipodia formation through Rac1/Cdc42 and ERK1/2 pathways.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
GIT1 Is Required for Angiogenesis in Matrigel Plugs
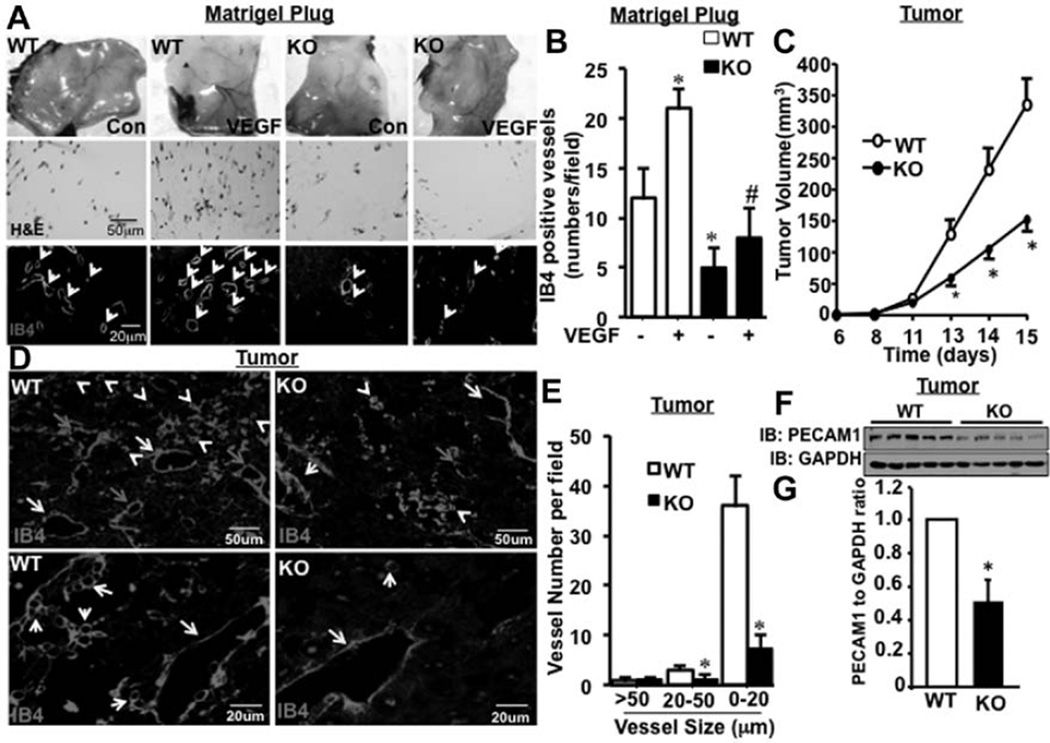
Matrigel plug assay is a commonly used angiogenesis model, which resembles pathological angiogenesis during tumor growth.20 Plugs placed onto GIT1-knockout (KO) mice clearly exhibited fewer vessels compared with GIT1-wild type (WT) after 7 days of incubation (Figure 1A). Also, hematoxylin and eosin staining showed fewer EC in the matrigel plugs of GIT1-KO implants compared with GIT1-WT (Figure 1A). Isolectin B4 staining revealed a 45% increase in vessel density in VEGF-treated matrigel plugs of GIT1-WT mice compared with vehicle-treated controls (Figure 1A and 1B). Significantly, we observed a 68% reduction in vessel density in plugs of GIT1-KO mice compared with GIT1-WT controls treated with VEGF (Figure 1B). Together, these data suggest that GIT1 is required for VEGF-mediated angiogenesis in matrigel implants.
Figure 1.
G-protein–coupled receptor-2–interacting protein (GIT1) is required for tumor angiogenesis. A, Matrigel (250 µL) containing vehicle control or vascular endothelial growth factor (VEGF; 50 ng/mL) was injected subcutaneously on the ventral side of the mouse in the groin area. Plugs were isolated 7 days post injection. Cross sections were stained with either hematoxylin and eosin (H&E) or endothelial cell (EC)–specific marker isolectin B4 (IB4) to locate the vessels. B, Vessel density was analyzed by counting the number of IB4-stained vessels per field. *Vs control, #vs control treated with VEGF (n=8; * and #P<0.05). C, Melanoma tumor cells were injected into the thigh muscle of GIT1-wild type (WT; n=5) and GIT1-knockout (KO; n=5) mice. Mean thigh diameters were determined and increased tumor volume was calculated. D and E, Tumors from GIT1-WT and GIT1-KO mice were harvested at day 15 after injection. Tumor tissue sections were stained with IB4 and images were acquired in ×10 and ×40 objective. The number of vessels in 3 different diameter groups (>50 [yellow arrow], >20–≤50 [green arrow], and ≤20 µm [white arrow]) was counted manually using Image Pro Plus software. *Vs WT (n=5; *P<0.05). F and G, Platelet endothelial cell adhesion molecule-1 (PECAM1) expression in tumor samples was detected by Western blot. Quantification of relative expression of PECAM1 normalized to GAPDH. *Vs WT (n=5; *P<0.05).
Melanoma Tumor Growth and Tumor Angiogenesis Are Reduced in GIT1-KO Mice
Melanoma tumor cells secrete high quantities of VEGF.21 VEGF-induced directional migration of EC is critical for angiogenesis to support tumor growth. Therefore, we chose this tumor model to study the effect of GIT1 on tumor angiogenesis and growth. An equivalent number of tumor cells (B16-F0 cell line, a melanoma spontaneously arising in C57BL/6 mice) which secrets VEGF, were injected intramuscular into GIT1-WT and GIT1-KO mice (2–3 months) and the thigh diameters were measured over time ≤15 days. The tumor volume in GIT1-WT and GIT1-KO mice was similar ≤12 days post injection. However, tumor volume in GIT1-KO was significantly reduced after 13 days compared with WT (Figure 1C; 45% at 15 days). Isolectin B4 staining showed significant reduction (83% and 61%) in vessels of smaller size (0–20 and 20–50 µm, respectively) in GIT1-KO mice (Figure 1D and 1E), whereas no difference was observed in vessels >50 µm between GIT1-WT and GIT1-KO mice (Figure 1D and 1E). Western blots revealed a 54% decrease in platelet endothelial cell adhesion molecule-1 expression in GIT1-KO versus GIT1-WT at 15 days (Figure 1F and 1G). These data demonstrate that GIT1 is an important regulator of tumor angiogenesis and tumor growth.
GIT1 Is Required for EC Directional Migration Toward VEGF Gradient
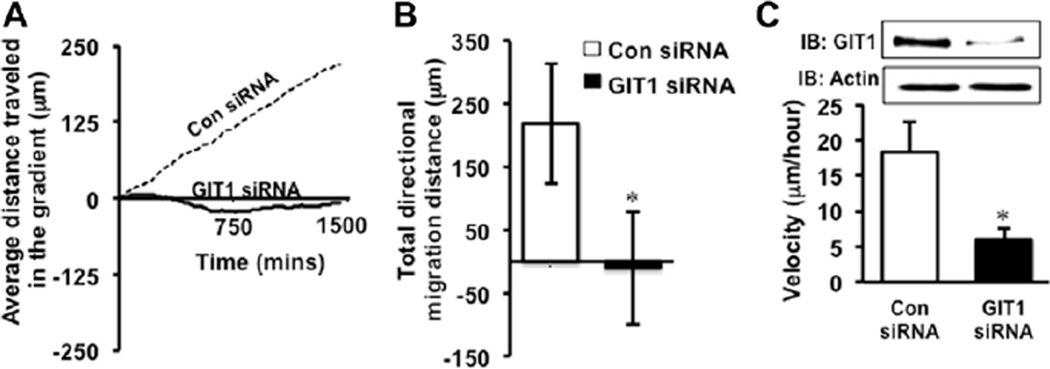
Angiogenesis requires directional migration of EC toward a stimuli.1 As we observed reduced angiogenesis in matrigel plugs containing VEGF and in a melanoma tumor model in the KO mouse compared with WT, we anticipated that the defect in tumor angiogenesis in GIT1-KO mice could be because of altered directional migration of GIT1-KO EC toward VEGF. Therefore, we performed a Dunn’s chamber experiment using control and GIT1-specific small interfering RNA (siRNA)–transfected human umbilical vein EC (HUVEC). HUVEC treated with control siRNA migrated directionally in response to a VEGF gradient (Figure 2A and 2B; Movies I and II in the online-only Data Supplement). In contrast, cells treated with GIT1 siRNA showed significantly decreased migration toward the gradient (Figure 2A and 2B; Movie III in the online-only Data Supplement). There was also a significant decrease in velocity of GIT1 siRNA–treated cells versus control siRNA–treated cells (6 versus 18 µm/h; Figure 2C). Western blot analysis of GIT1 siRNA–transfected HUVEC revealed a 90% decrease in GIT1 protein compared with control siRNA–transfected control (Figure 2C, inset). These data demonstrate that GIT1 plays a significant role in directional migration of EC toward a VEGF gradient.
Figure 2.
G-protein–coupled receptor-2–interacting protein (GIT1) is required for vascular endothelial growth factor (VEGF)–induced directional migration of endothelial cells (EC). A, Human umbilical vein endothelial cell (HUVEC) cultured on fibronectin-coated cover glasses was transfected with either scrambled control (Con) or GIT1-specific small interfering RNA (siRNA) for 24 hours, loaded on Dunn’s chamber with a VEGF (50 ng/mL) gradient and placed into a live cell imaging chamber. Cells were imaged for 24 hours at 10-minute intervals. Videos obtained from live cell imaging experiments were analyzed using Image Pro Plus software. A total of 33 cells from 3 different sets of experiments were tracked from the 2 groups. Trend line showing the average distance traveled by Con and GIT1 siRNA–transfected HUVEC relative to the VEGF gradient. B, Total distance traveled by each cell after 24 hours toward the VEGF gradient from A. *Vs control siRNA (n=3; *P<0.05). C, Cell velocity was also measured using Image Pro Plus software. GIT1 depletion by GIT1 siRNA in HUVEC was demonstrated by Western blot. *Vs control siRNA (n=3; *P<0.05).
GIT1 Is Required for Cortactin Localization to the Leading Edge of EC In Vitro
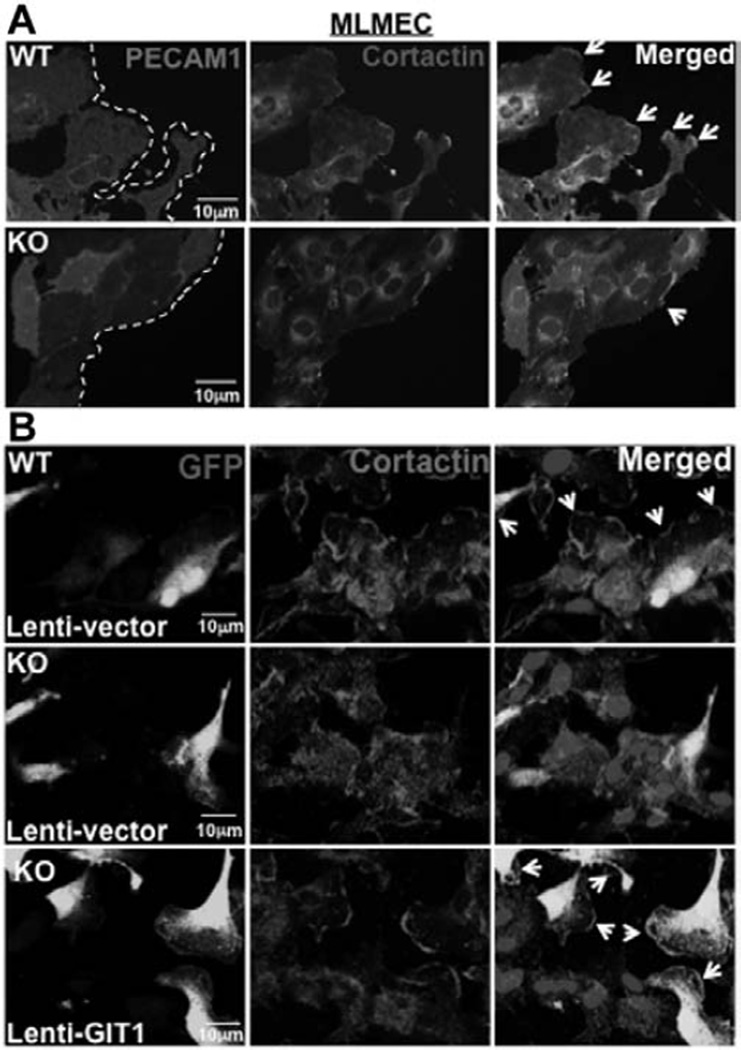
Cortactin-mediated lamellipodia formation in the leading edge is important for directional migration.7 We next performed wound healing assay using mouse lung microvascular EC (MLMEC). There was a 57% decrease in cortactin localization to the leading edge in GIT1-KO MLMEC compared with GIT1-WT MLMEC near the wound (Figure 3A; n=3; P<0.05).
Figure 3.
G-protein–coupled receptor-2–interacting protein (GIT1) localizes cortactin to the leading edge of endothelial cells (EC) in vitro and in vivo. A, Wounds were created in monolayers of GIT1-wild type (WT) and knockout (KO) mouse lung microvascular EC (MLMEC) and healing proceeded for 6 hours. Cells were then fixed and stained for platelet endothelial cell adhesion molecule-1 (PECAM1) and cortactin (n=3). B, GIT1 was overexpressed in GIT1-KO MLMEC using a lentiviral vector. After 48 hours of infection, cell migration in a wound healing assay was determined. Cells were fixed and stained for cortactin. Infected cells were identified by green fluorescent protein (GFP) expression (n=3).
To confirm these data, we determined cortactin localization in GIT1 siRNA–treated HUVEC. Control siRNA–treated cells exhibited VEGF-stimulated actin filament reorganization and showed dramatically increased cortactin in lamellipodia like structures that colocalized with actin at the leading edge (Figure IA in the online-only Data Supplement). Both lamellipodia formation and cortactin localization were decreased markedly in HUVEC transfected with GIT1 siRNA; 61% reduction in unstimulated cells and 73% in VEGF-stimulated cells (Figure IA and IB in the online-only Data Supplement). As actin filament reorganization was also altered significantly (Figure IA in the online-only Data Supplement), the colocalization of cortactin and actin was markedly reduced in GIT1-depleted HUVEC (Figure IA in the online-only Data Supplement). There was a significant 68% decrease in sprout length (Figure IIA–IIC in the online-only Data Supplement) and 84% reduction of cortactin localization in the leading edge of GIT1-KO aortas compared with WT control (Figue IID–IIJ in the online-only Data Supplement).
To confirm that cortactin mislocalization in GIT1-KO EC is directly attributable to the loss of GIT1, we performed a rescue experiment by overexpressing GIT1 in GIT1-KO MLMEC using a GIT1-expressing cytomegalovirus promoter–based lentiviral expression vector in which GIT1 is expressed on an internal ribosome entry site-containing bicistronic mRNA that also expresses enhanced green fluorescent protein. We detected green fluorescent protein expression in ≈60% of GIT1-KO MLMEC infected with either vector control or GIT1-containing lentivirus (Figure 3B). GIT1-WT MLMEC showed high level of cortactin in leading edge, whereas GIT1-KO MLMEC infected with control lentivirus had only 33% of the cortactin in the leading edge. However, overexpression of GIT1 in GIT1-KO MLMEC restored cortactin localization to the leading edge of migrating EC to 94% (n=3; P<0.05). Expression of GIT1 in GIT1-KO MLMEC was confirmed by Western blot (data not shown). These results show that GIT1 is required for cortactin localization to the leading edge of EC during directional migration.
SHD of GIT1 Is Required for GIT1–Cortactin Association and Membrane Localization to Induce EC Migration
To characterize the mechanism by which GIT1 regulates cortactin localization in EC, we used an in vitro wound healing assay using HUVEC. VEGF (10 ng/mL, for 6 hours) stimulated a 2.2-fold increase in colocalization of GIT1 and cortactin at the leading edge (Figure IIIA and IIIB in the online-only Data Supplement). Immunoprecipitation of GIT1 from HUVEC stimulated with VEGF (10 ng/mL, 15 minutes) showed a 6-fold increase in GIT1 and cortactin association (Figure IIIC in the online-only Data Supplement). Furthermore a 4-fold increase in the level of phosphorylated S405-cortactin in the GIT1–cortactin complex was observed on VEGF stimulation (Figure IIIC in the online-only Data Supplement). To identify the cellular compartment of GIT1–cortactin association, we performed cell fractionation and found that GIT1–cortactin association maximally occurs in the membrane of EC (Figure IIID in the online-only Data Supplement).
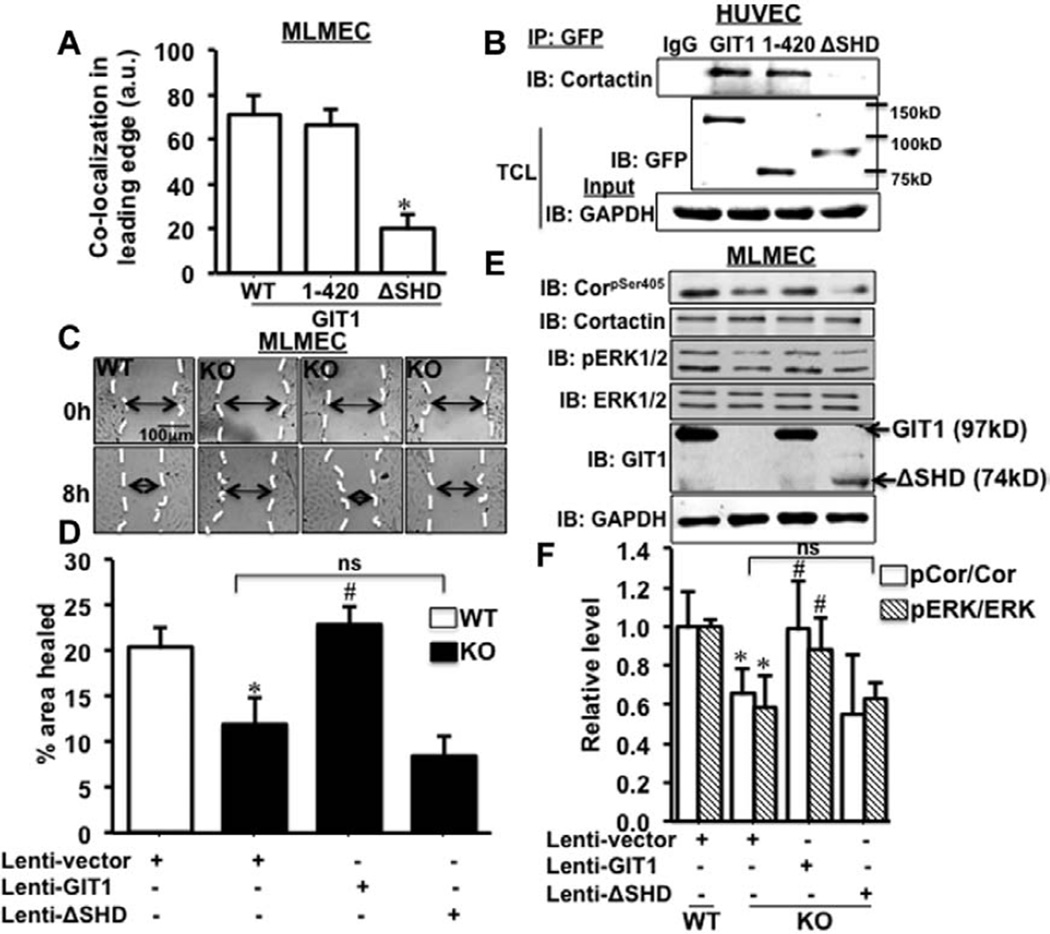
To identify the specific domain of GIT1 responsible for association with cortactin, we transfected GIT1-KO MLMEC with GIT1 deletion mutants that lacked predicted binding domains. Extensive colocalization of GIT1 mutant (green) and cortactin (red) in the cell periphery was observed in MLMEC transfected with green fluorescent protein-GIT1 and green fluorescent protein-GIT1 (1–420; Figure IV in the online-only Data Supplement; Figure 4A). In contrast, GIT1 lacking the functional SHD (del250–420) showed a 71% decrease in colocalization at the cell periphery (Figure IV in the online-only Data Supplement; Figure 4A). Coimmunoprecipitation analysis of HUVEC transfected with the mutants demonstrated that full-length GIT1 and GIT1 (1–420) were associated with cortactin, whereas GIT1(del250–420) that lacks the SHD was not (Figure 4B).
Figure 4.
Spa homology domain (SHD) of G-protein–coupled receptor-2–interacting protein (GIT1) is important for GIT1–cortactin association. A, Green fluorescent protein (GFP)-tagged wild type (WT)-GIT1 and 2 deletion mutants (1–420) GIT1 and (ΔSHD) GIT1 were expressed in GIT1-knockout (KO) mouse lung microvascular EC (MLMEC). Cells were fixed and stained for cortactin (red). Level of colocalization in the cell periphery specifically in the leading edge was analyzed using Image Pro Plus software. *Vs WT (n=3; *P<0.05). B, GFP-tagged GIT1 and the deletion mutants were overexpressed in human umbilical vein endothelial cells (HUVEC) and immunoprecipitated with GFP antibody and complexes probed for cortactin. Expression of GFP-tagged GIT1 and the deletion mutants were confirmed by blotting total cell lysates with GFP antibody. GAPDH was the loading control (n=3). C, GIT1 and (ΔSHD) GIT1 were overexpressed in GIT1-KO MLMEC using lentiviral vectors and the cells used in a wound healing assay. GIT1-WT MLMEC served as control. D, Percentage of wound healing was analyzed by measuring the area of wound at 0 and 8 hours of incubation. *Vs Lenti vector–treated WT; #vs Lenti vector–treated KO (n=3; * and #P<0.05). E, Cells used in wound healing assay were lysed and extracts probed by Western blot to detect pS405-cortactin and phosphorylated extracellular signal–regulated kinases 1 and 2 (pERK1/2) level in GIT1 and (ΔSHD) GIT1 overexpressed GIT1-KO MLMEC. The expression of GIT1 and (ΔSHD) GIT1, which contains the N-terminal of GIT1 was detected using GIT1 antibody (detects the N-terminal of GIT1). F, Analysis of Western blot was performed using ImageJ software. *Vs Lenti vector–treated WT; #vs Lenti vector–treated KO (n=3; * and #P<0.05). ns indicates not significant.
Consistent with our previous finding in GIT1-depleted HUVEC,18 GIT1-KO MLMEC showed a 49% reduction in wound healing compared with GIT1-WT MLMEC (Figure 4C and 4D). Lentivirus-mediated overexpression of full-length GIT1 in GIT1-KO MLMEC completely restored EC migration to 100%, whereas GIT1(del250–420) overexpression had no effect (Figure 4C and 4D). These data demonstrate that GIT1-SHD mediates GIT1 and cortactin association, which is necessary for EC migration by localizing cortactin to the leading edge.
GIT1 Is Required for VEGF-Induced Phosphorylation of Cortactin-S405 (pS405) by ERK1/2 That Promotes Cortactin Localization to Lamellipodia
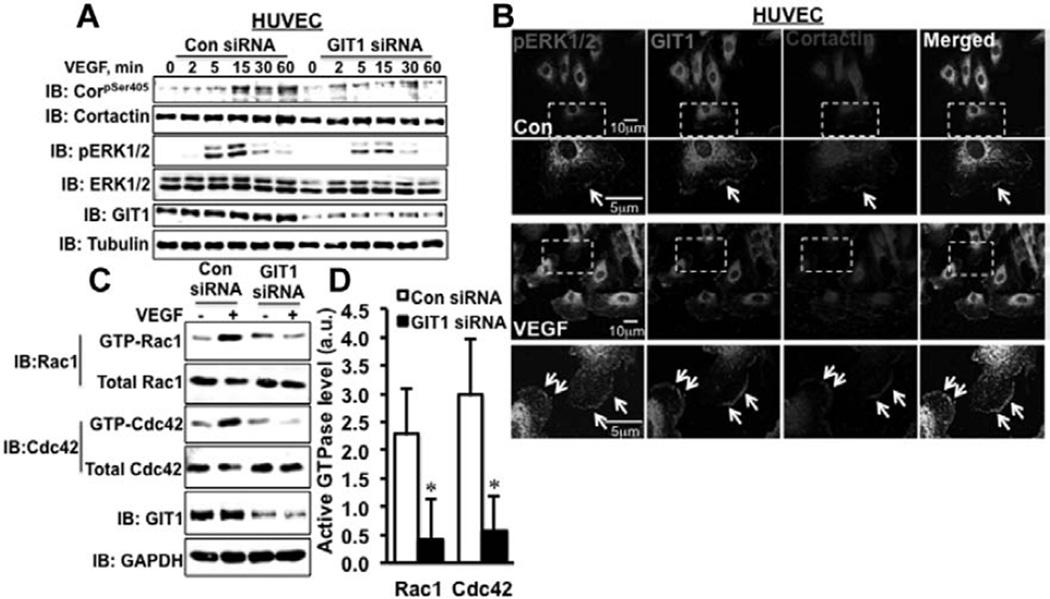
ERK1/2-mediated phosphorylation of cortactin-S405 is required for lamellipodia formation and cell migration.22 In HUVEC stimulated with VEGF, fetal bovine serum, or EGF, there was an 8-fold increase in pS405-cortactin with a peak at 15 minutes (Figure VA and VB in the online-only Data Supplement). In HUVEC transfected with GIT1 siRNA, there was a 66% decrease in ERK1/2 and 70% decrease in S405-cortactin phosphorylation relative to control siRNA in response to VEGF (Figure 5A; Figure VC and VD in the online-only Data Supplement; n=3; P<0.05). There was no significant change in VEGF-induced phosphorylation of cortactin Y421 on GIT1 depletion (required for actin filament reorganization and stress fiber formation) after GIT1 depletion (Figure VE and VF in the online-only Data Supplement).
Figure 5.
G-protein–coupled receptor-2–interacting protein (GIT1) is required for extracellular signal–regulated kinases 1 and 2 (ERK1/2) and Rac1/Cdc42-mediated activation and localization of cortactin. A, Human umbilical vein endothelial cells (HUVEC) were transfected with either scrambled control (Con) or GIT1-specific small interfering RNA (siRNA) for 36 hours and then serum starved for 2 hours. Cells were stimulated with vascular endothelial growth factor (VEGF; 10 ng/mL) and pS405-cortactin and pERK1/2 were measured by Western blot (n=3). B, Wounds were created in the monolayer of HUVEC cultured on fibronectin-coated cover glasses and serum starved overnight. Next, cells were treated with VEGF (10 ng/mL) and incubated for 6 hours. Cells were fixed and triple stained for pERK1/2 (green), GIT1 (red) and cortactin (blue). As shown by arrowhead, GIT1–ERK1/2–cortactin complex formation was detected by appearance of purple (colocalization) in the leading edge (n=3). C, GIT1-depleted HUVECs were serum starved for 2 hours followed by stimulating with VEGF (10 ng/mL) for 30 minutes. Cells were lysed and equal amounts of protein (400 µg) were incubated with 20 µg p21-associated kinase (PAK) 1-p21 binding domain (PBD) agarose beads for 60 minutes at 4°C. Active Rac1/Cdc42 (GTP bound Rac1/Cdc42) was precipitated with PAK1-PBD agarose beads and the amount of active Rac1/Cdc42 was measured by Western blotting. D, Quantification by densitometric analysis using ImageJ software and the active Rac1/Cdc42 were normalized with the total Rac1/Cdc42 in whole cell lysates. *Vs control siRNA (n=3; *P<0.05).
Furthermore, in GIT1-KO MLMEC there was a 37% and 43% reduction of S405-cortactin and ERK1/2 phosphorylation, respectively, compared with GIT1-WT MLMEC (Figure 4E and 4F). Lentiviral re-expression of GIT1 in KO MLMEC almost completely recovered S405-cortactin (98%) and ERK1/2 phosphorylation (89%) to the level of GIT1-WT control (Figure 4E and 4F). However, overexpression of GIT1 (del250–420) did not restore S405-cortactin and ERK1/2 phosphorylation (Figure 4E and 4F). Together these data support the role of GIT1-SHD in promoting S405-cortactin and ERK1/2 phosphorylation that is required for EC directional migration.
Treatment of HUVEC with the ERK1/2 inhibitor PD98059 caused a decrease in EC migration, a 42% reduction in cortactin at the leading edge of migrating EC and a concomitant reduction in lamellipodia formation (Figure VIA and VII in the online-only Data Supplement). Treatment of HUVEC with PD98059 completely blocked VEGF-induced S405-cortactin phosphorylation, suggesting that ERK1/2 is the key mediator of S405 phosphorylation of cortactin (Figure VIJ and VIK in the online-only Data Supplement). These data demonstrated that blocking ERK1/2 activation either by GIT1 depletion or PD98059 treatment inhibited S405-cortactin phosphorylation, which is essential for lamellipodia formation.
VEGF Promotes the Formation of GIT1–pERK1/2–Cortactin Complex in the Leading Edge of EC
Previously, we showed EGF-dependent increase in GIT1 association with ERK1/2 in focal adhesion.23 To determine colocalization of GIT1, pERK1/2, and cortactin at the leading edge of migrating EC, we performed triple staining in HUVEC. VEGF significantly increased the GIT1–pERK1/2–cortactin colocalization compared with untreated controls (Figure 5B; n=3). Furthermore, in the wound healing assay, there was a significant decrease in colocalization of pERK1/2 and cortactin in HUVEC treated with GIT1 siRNA versus control siRNA (30 versus 94 pixel number; Figure VIIA and VIIB in the online-only Data Supplement). In addition, coimmunoprecipitation studies demonstrated that pERK1/2, GIT1, and cortactin coprecipitated (10 ng/mL VEGF; 15 minutes; Figure VIIC in the online-only Data Supplement; n=3). These data demonstrated that GIT1, ERK1/2, and cortactin form a complex that is necessary for phosphorylation of cortactin and its localization to the leading edge of migrating EC.
GIT1 Is Required for VEGF-Induced Activation of Rac1 and Cdc42
Rac1 and Cdc42 are necessary for the activation of cortactin and the induction of lamellipodia formation.10,24 Although GIT1-SHD regulates Rac1 and Cdc42 through its PIX–PAK binding domain in neurons,14,17 its role in Rac1 and Cdc42 activation in VEGF-stimulated EC is unknown. Rac1 and Cdc42 activation in response to VEGF peaked at 30 minutes in HUVEC (data not shown). In HUVECs treated with GIT1 siRNA, VEGF-induced Rac1 and Cdc42 activation was inhibited by 68% and 80%, respectively, compared with control siRNA–treated cells (Figure 5C and 5D). A previous article by Smith et al25 showed that PAK1 activates ERK1/2 in macrophages to regulate lamellipodial stability, which suggests that Rac1/Cdc42 is upstream of ERK1/2. Therefore, we used adenoviral delivery of dominant-negative (DN)-Rac1 and DN-Cdc42 to study their effects on ERK1/2 and cortactin activation. Infection of adeno-DN-Rac1 or adeno-DN-Cdc42 in HUVEC diminished VEGF-induced ERK1/2 activation by 49% and cortactin-S405 phosphorylation by 82% (Figure VIII in the online-only Data Supplement), which suggests that Rac1 and Cdc42 act upstream of ERK1/2. In addition, greater reduction in cortactin phosphorylation compared with ERK1/2 activation implies that Rac1/Cdc42 may also regulate cortactin activation through PAK.26 Overall, these data showed that GIT1 promotes the localization of cortactin to lamellipodia through Rac1/Cdc42 and ERK1/2 pathways.
Expression of Constitutively Active Cortactin Rescues GIT1-Depleted Phenotype In Vitro
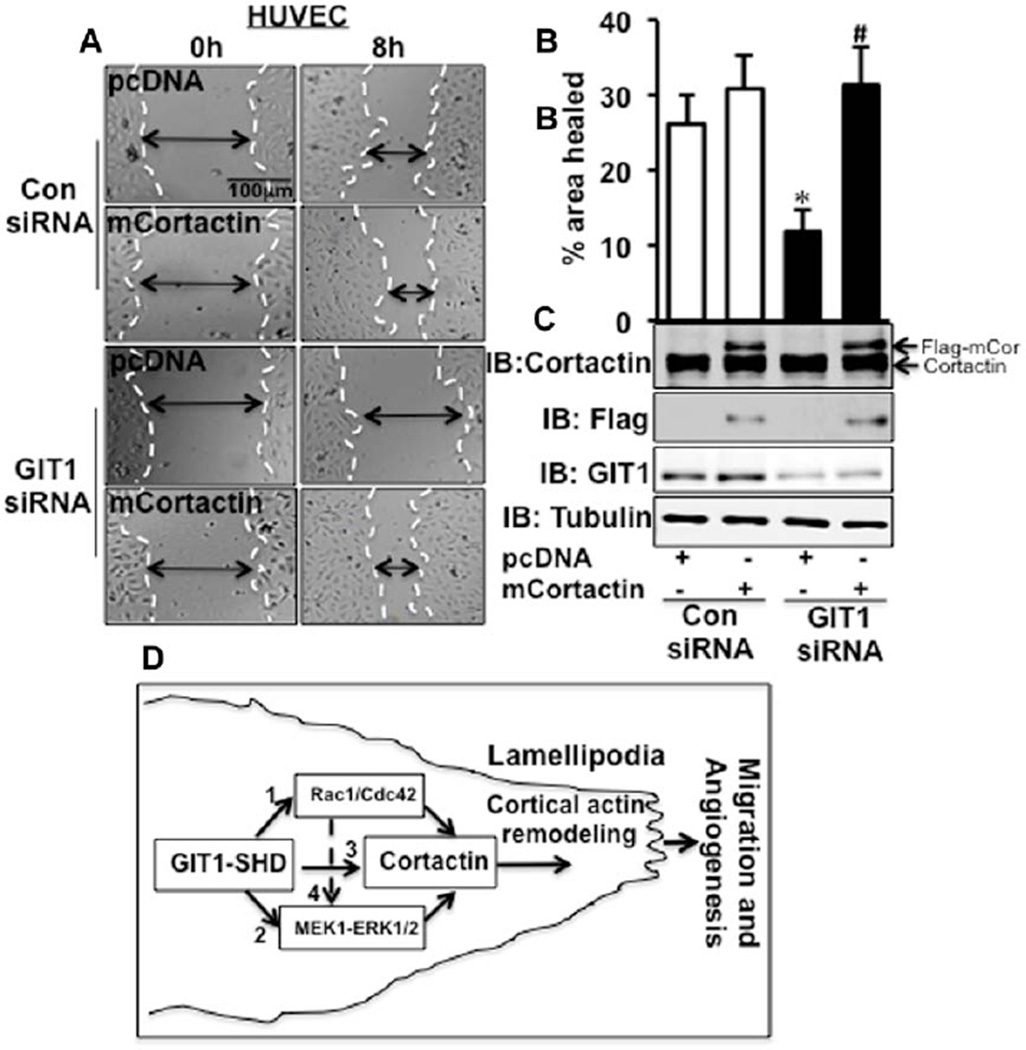
To confirm that the effect of GIT1 on EC migration is pS405-cortactin dependent, we expressed a constitutively active cortactin, cortactinS405D,S418D (mCortactin), in GIT1-depleted HUVEC. After cotransfection of GIT1 siRNA and mCortactin, we performed a wound healing assay. GIT1-depleted HUVEC showed 54.6±8.0% inhibition of wound healing compared with control HUVEC (Figure 6A–6C). Expression of mCortactin in control siRNA did not stimulate HUVEC migration when compared with pcDNA vector control (Figure 6A–6C). However, mCortactin stimulated a 2.6-fold increase in migration of GIT1-depleted cells (Figure 6A–6C). We also confirmed the presence of mCortactin expressing EC in wound edge (Figure IX in the online-only Data Supplement). These data show that pS405-cortactin is necessary for GIT1-dependent regulation of EC migration.
Figure 6.
Constitutively active cortactin reversed G-protein–coupled receptor-2–interacting protein (GIT1) depleted defect in cell migration. A, mCortactin was expressed in GIT1-depleted human umbilical vein endothelial cells (HUVEC). Scratch wounds were performed as described in Methods. Images are representative of 3 individual experiments. B, Percentage of wound healing was analyzed by measuring the area of wound healing after 8 hours. *Vs Con small interfering RNA (siRNA) pcDNA; #vs GIT1 siRNA pcDNA (n=3; # and *P<0.05). C, Western blot analysis of the same cells as used for wound healing to detect flag tagged cortactin expression (n=3). D, Model outlining the role of GIT1 in EC directional migration. Pathway 1, GIT1 activates Rac1/Cdc42 to induce cortactin activation. Pathway 2, On vascular endothelial growth factor (VEGF) stimulation GIT1 binds with mitogen-activated protein kinase kinase (MEK)-1–extracellular signal-regulated kinases 1 and 2 (ERK1/2) and further phosphorylates cortactin. Pathway 3, GIT1 forms a complex with cortactin. Pathway 4, Activated Rac1/Cdc42 also contributes toward ERK1/2-mediated activation of cortactin. Through these mechanisms, which are mainly regulated by GIT1-Spa homology domain (SHD), cortactin is recruited to the membrane to induce lamellipodia formation and directional migration.
Discussion
Major findings of the present study are GIT1 is required for VEGF-induced cortactin activation and localization to leading edge to promote lamellipodia formation, which is necessary for EC directional migration and tumor angiogenesis. Three major mechanisms were identified (see model in Figure 6D): (1) GIT1 increases Rac1/Cdc42 activation through PIX–PAK complex to promote cortactin activation and localization to membrane. (2) GIT1 assembles a complex of mitogen-activated protein kinase kinase-1–ERK1/2 that induces Ser405 cortactin phosphorylation. (3) GIT1 forms a complex with cortactin to promote cortactin membrane localization and lamellipodia formation. In addition, we described that GIT1-mediated activation of Rac1/Cdc42 also contributes to ERK1/2-driven activation of cortactin (pathway 4). Importantly, we identified the SHD of GIT1 as the domain essential for these functions.
Recent evidence suggested that GIT1-mediated activation of mitogen-activated protein kinase kinase-1–ERK1/2 induces growth in human liver and colon cancer.19 Several data, including Genecard analysis of human tumors, suggested increased GIT1 expression in several metastatic tumors, including breast, lung, and prostate.18,19 These data all suggest a crucial role of GIT1 in tumor progression. Long established evidence suggested that tumor metastasis is correlated with the extent of vascular structure and increases in angiogenesis that supports the metastatic potential of tumor cells.27 Our findings reveal that GIT1 is required for tumor angiogenesis to support the growth of tumors. Deletion of GIT1 diminished tumor microvasculature density and prevented tumor growth. We specifically identified that the major reason behind this effect is because of compromised directional migration of GIT1-depleted EC toward a VEGF gradient.
GIT1 promotes the assembly of signaling complexes in focal adhesions where it associates with a complex that includes PAK, PIX, paxillin, and focal adhesion kinase.17 Moreover, on EGF stimulation, GIT1 colocalized with ERK1/2 in focal adhesions that mediated migration of HeLa cells.23 However, the mechanisms by which GIT1 regulates lamellipodia formation and directional migration are still unknown. Cortactin plays a key role in lamellipodia formation and directional migration.8,9 Our data showed that GIT1 associates with cortactin and localizes it to leading edge to promote lamellipodia formation and directed cell migration. Depletion of GIT1 remarkably impaired cortactin activation and localization. ERK1/2 and Rac1/Cdc42 are the major signaling pathways responsible for cortactin activation. We found significant decreases of both ERK1/2 phosphorylation and Rac1/Cdc42 activation by loss of GIT1. In addition, DN-Rac1/DN-Cdc42 showed greater inhibition on Ser405 cortactin phosphorylation than ERK1/2 phosphorylation, which implies the involvement of other downstream target of Rac1/Cdc42 such as PAK on cortactin activation.26
A significant finding of the present study is that the SHD of GIT1 mediates binding and translocation of cortactin to the leading edge. Most importantly, we identified that GIT1-SHD is required for cortactin-S405 phosphorylation, which in turn regulates EC migration. Intriguingly, GIT1 and GIT2 are the only mammalian proteins that contain a SHD. The SHD of GIT1 is responsible for regulating src-mediated phospholipase cγ activation,28 ERK1/2 activation in focal adhesions,29 and the interaction with PIX and PAK complex to regulate small GTPase, such as Rac1 and Cdc42,17 as well as the regulation of GIT1 binding to focal adhesions. We identified another critical function of GIT1-SHD, which associates with cortactin to promote cortactin activation and localization to the leading edge during angiogenesis. We also found that GIT1-mediated localization of cortactin is important for EC directional migration and angiogenesis in matrigel plugs and tumor.
In recent years, several studies indicated the involvement of GIT1 in cancer including its role in cancer cell migration,30 cellular transformation,31 and growth.19 Our present studies together with previous findings imply that GIT1 overexpression in metastatic tumors could be associated with activation of cortactin, which possibly promote tumor angiogenesis as required during tumor metastasis. Finally, as GIT1-SHD is unique to GIT1 and GIT1 is highly overexpressed in several metastatic tumor,18,19 future studies by identifying small molecule inhibitors or peptides to abrogate the functions of GIT1-SHD could enable us to specifically target GIT1 during metastatic tumor progression.
Supplementary Material
Significance.
Recent evidence suggests G-protein–coupled receptor-2–interacting protein (GIT1) overexpression in several human metastatic tumors, including breast, lung, and prostate. Tumor metastasis is associated with increases in angiogenesis. The present study is significant by showing that GIT1 is required for tumor angiogenesis and tumor growth via cortactin-dependent directional migration of endothelial cells. There are 3 major mechanisms: (1) GIT1 increases Rac1/Cdc42 activation through p21-associated kinase-interacting exchange factor–p21-associated kinase complex to promote cortactin activation and localization to membrane. (2) GIT1 assembles a complex of MEK1–extracellular signal-regulated kinases 1 and 2 that induces Ser405 cortactin phosphorylation. (3) GIT1 forms a complex with cortactin to promote cortactin membrane localization and lamellipodia formation. Most importantly, we identified the Spa homology domain of GIT1 as the domain essential for this function. Because the GIT1-Spa homology domain is unique in the mammalian genome, understanding the structural nature of its interaction with multiple signal mediators may enable specific targeting of individual pathways necessary for angiogenesis.
Acknowledgments
We thank Dr Alan S. Mak, Professor of Biochemistry, Department of Biomedical and Molecular Sciences, Queen’s University, Kingston, Ontario, Canada for kindly providing cortactinS405D.S418D construct. We thank Martha Zettel for her help with the tissue sectioning.
Sources of Funding
This work was supported by a grant from the National Institutes of Health to B.C. Berk (HL63462).
Nonstandard Abbreviations and Acronyms
- EC
endothelial cells
- ERK1/2
extracellular signal–regulated kinases 1 and 2
- GIT1
G-protein–coupled receptor kinase–interacting protein-1
- HUVEC
human umbilical vein endothelial cells
- MLMEC
mouse lung microvascular endothelial cells
- PAK
p21-associated kinase
- PIX
PAK-interacting exchange factor
- SHD
Spa homology domain
- siRNA
small interfering RNA
- VEGF
vascular endothelial growth factor
- WT
wild type
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.302689/-/DC1.
Disclosures
The provisional patent application of G-protein-coupled receptor-2–interacting protein-1 Spa homology domain acting as a therapeutic target in angiogenesis-related diseases was filed. The authors report no conflicts.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya R, Kwon J, Li X, Wang E, Patra S, Bida JP, Bajzer Z, Claesson-Welsh L, Mukhopadhyay D. Distinct role of PLCbeta3 in VEGF-mediated directional migration and vascular sprouting. J Cell Sci. 2009;122(Pt 7):1025–1034. doi: 10.1242/jcs.041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 4.Ren G, Crampton MS, Yap AS. Cortactin: coordinating adhesion and the actin cytoskeleton at cellular protrusions. Cell Motil Cytoskeleton. 2009;66:865–873. doi: 10.1002/cm.20380. [DOI] [PubMed] [Google Scholar]
- 5.Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton. 2008;65:687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaluza D, Kroll J, Gesierich S, Yao TP, Boon RA, Hergenreider E, Tjwa M, Rössig L, Seto E, Augustin HG, Zeiher AM, Dimmeler S, Urbich C. Class IIb HDAC6 regulates endothelial cell migration and angiogenesis by deacetylation of cortactin. EMBO J. 2011;30:4142–4156. doi: 10.1038/emboj.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly RJ. Cortactin signalling and dynamic actin networks. Biochem J. 2004;382(Pt 1):13–25. doi: 10.1042/BJ20040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol. 2002;12:1270–1278. doi: 10.1016/s0960-9822(02)01035-7. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004;24:5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weed SA, Du Y, Parsons JT. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J Cell Sci. 1998;111(Pt 16):2433–2443. doi: 10.1242/jcs.111.16.2433. [DOI] [PubMed] [Google Scholar]
- 11.Kelley LC, Weed SA. Cortactin is a substrate of activated Cdc42- associated kinase 1 (ACK1) during ligand-induced epidermal growth factor receptor downregulation. PLoS One. 2012;7:e44363. doi: 10.1371/journal.pone.0044363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Taba Y, Pang J, Yin G, Yan C, Berk BC. GIT1 mediates VEGF-induced podosome formation in endothelial cells: critical role for PLCgamma. Arterioscler Thromb Vasc Biol. 2009;29:202–208. doi: 10.1161/ATVBAHA.108.174391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell. 2007;18:2346–2355. doi: 10.1091/mbc.E06-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119(Pt 8):1469–1475. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- 15.van Nieuw Amerongen GP, Natarajan K, Yin G, Hoefen RJ, Osawa M, Haendeler J, Ridley AJ, Fujiwara K, van Hinsbergh VW, Berk BC. GIT1 mediates thrombin signaling in endothelial cells: role in turnover of RhoA-type focal adhesions. Circ Res. 2004;94:1041–1049. doi: 10.1161/01.RES.0000125627.77235.0C. [DOI] [PubMed] [Google Scholar]
- 16.Pang J, Hoefen R, Pryhuber GS, Wang J, Yin G, White RJ, Xu X, O’Dell MR, Mohan A, Michaloski H, Massett MP, Yan C, Berk BC. G-protein-coupled receptor kinase interacting protein-1 is required for pulmonary vascular development. Circulation. 2009;119:1524–1532. doi: 10.1161/CIRCULATIONAHA.108.823997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishiya N, Kiosses WB, Han J, Ginsberg MH. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol. 2005;7:343–352. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]
- 18.Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, Kwei KA, Hernandez-Boussard T, Wang P, Gazdar AF, Minna JD, Pollack JR. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng H, Dara L, Li TW, Zheng Y, Yang H, Tomasi LM, Tomasi I, Giordano P, Mato JM, Lu SC. Methionine adenosyltransferase 2b-git1 interplay activates mek1-erk1/2 to induce growth in human liver and colon cancer. Hepatology. 2013 doi: 10.1002/hep.26258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Negrão R, Costa R, Duarte D, Gomes TT, Azevedo I, Soares R. Different effects of catechin on angiogenesis and inflammation depending on VEGF levels. J Nutr Biochem. 2013;24:435–444. doi: 10.1016/j.jnutbio.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Mehnert JM, McCarthy MM, Jilaveanu L, Flaherty KT, Aziz S, Camp RL, Rimm DL, Kluger HM. Quantitative expression of VEGF, VEGF-R1, VEGF-R2, and VEGF-R3 in melanoma tissue microarrays. Hum Pathol. 2010;41:375–384. doi: 10.1016/j.humpath.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley LC, Hayes KE, Ammer AG, Martin KH, Weed SA. Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence. PLoS One. 2010;5:e13847. doi: 10.1371/journal.pone.0013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang N, Cai W, Yin G, Nagel DJ, Berk BC. GIT1 is a novel MEK1-ERK1/2 scaffold that localizes to focal adhesions. Cell Biol Int. 2010;34:41–47. doi: 10.1042/CBI20090016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidal C, Geny B, Melle J, Jandrot-Perrus M, Fontenay-Roupie M. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the cortical-actin binding protein cortactin. Blood. 2002;100:4462–4469. doi: 10.1182/blood.V100.13.4462. [DOI] [PubMed] [Google Scholar]
- 25.Smith SD, Jaffer ZM, Chernoff J, Ridley AJ. PAK1-mediated activation of ERK1/2 regulates lamellipodial dynamics. J Cell Sci. 2008;121(Pt 22):3729–3736. doi: 10.1242/jcs.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb BA, Zhou S, Eves R, Shen L, Jia L, Mak AS. Phosphorylation of cortactin by p21-activated kinase. Arch Biochem Biophys. 2006;456:183–193. doi: 10.1016/j.abb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 28.Haendeler J, Yin G, Hojo Y, Saito Y, Melaragno M, Yan C, Sharma VK, Heller M, Aebersold R, Berk BC. GIT1 mediates Src-dependent activation of phospholipase Cgamma by angiotensin II and epidermal growth factor. J Biol Chem. 2003;278:49936–49944. doi: 10.1074/jbc.M307317200. [DOI] [PubMed] [Google Scholar]
- 29.Yin G, Haendeler J, Yan C, Berk BC. GIT1 functions as a scaffold for MEK1-extracellular signal-regulated kinase 1 and 2 activation by angiotensin II and epidermal growth factor. Mol Cell Biol. 2004;24:875–885. doi: 10.1128/MCB.24.2.875-885.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huck B, Kemkemer R, Franz-Wachtel M, Macek B, Hausser A, Olayioye MA. GIT1 phosphorylation on serine 46 by PKD3 regulates paxillin trafficking and cellular protrusive activity. J Biol Chem. 2012;287:34604–34613. doi: 10.1074/jbc.M112.374652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo SM, Antonyak MA, Cerione RA. The adaptor protein and Arf GTPase-activating protein Cat-1/Git-1 is required for cellular transformation. J Biol Chem. 2012;287:31462–31470. doi: 10.1074/jbc.M112.353615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.