Abstract
Background: Calcium hydroxylapatite is one of the most well-studied dermal fillers worldwide and has been extensively used for the correction of moderate-to-severe facial lines and folds and to replenish lost volume. Objectives: To mark the milestone of 10 years of use in the aesthetic field, this review will consider the evolution of calcium hydroxylapatite in aesthetic medicine, provide a detailed injection protocol for a global facial approach, and examine how the unique properties of calcium hydroxylapatite provide it with an important place in today’s market. Methods: This article is an up-to-date review of calcium hydroxylapatite in aesthetic medicine along with procedures for its use, including a detailed injection protocol for a global facial approach by three expert injectors. Conclusion: Calcium hydroxylapatite is a very effective agent for many areas of facial soft tissue augmentation and is associated with a high and well-established safety profile. Calcium hydroxylapatite combines high elasticity and viscosity with an ability to induce long-term collagen formation making it an ideal agent for a global facial approach.
Aesthetic medicine has advanced greatly in the past decade in terms of our understanding of facial anatomy; the cumulative effects of the aging process; and how dermal fillers may be used to repair, reduce, and even reverse these changes. Initially, aesthetic practitioners were “chasing lines and wrinkles,” based on experience with bovine collagen injections beginning in the early 1980s. We now appreciate that a natural and more youthful appearance is dependent on reversing the cumulative effect of age-related changes both on the surface and in the subsurface tissues. For surface aging, restoration of textural and pigmentary alterations is of paramount importance; for the subsurface, restoring lost volume and shape is the key to the more youthful proportions desired by our patients. This focus on facial shape and volume to restore balance, symmetry, and the proportions of youth has led to the development and worldwide clinical use of an ever-expanding list of dermal fillers for treatment of facial aging.
Dermal fillers as a category of implantable medical devices, consist of a wide array of products that differ significantly in their chemical composition, mechanism of action, duration, safety, and interaction with host tissues. Many different methods of categorization have been proposed, based in part on these differing characteristics; however, no single, universally agreed upon system exists to date. Of the proposed classification systems, one based on primary mechanism of action (MOA) first proposed by Werschler and Narurkar has been widely used.1 In this approach, dermal fillers are placed into categories of either collagen biostimulation or replacement volume as a primary MOA.
In this schema, Radiesse® (calcium hydroxylapatite; CaHA, Merz Pharmaceuticals GmbH, Frankfurt, Germany) is a unique product because it provides both replacement volume and collagen biostimulation as a primary MOA. In addition, CaHA is biodegradable and reabsorbed naturally by the host’s metabolic processes. This biostimulatory MOA, with ultimate reabsorption, results in a performance profile that is unique to Radiesse.
CaHA is a highly effective agent for many areas of facial soft-tissue augmentation and is associated with a well-established safety profile.2 The year 2013 marked a decade of Radiesse technology, which first received EU approval in 2003 for plastic and reconstructive surgery, including deep dermal and subdermal soft tissue augmentation of the facial area. In the intervening years, the range of uses for CaHA has evolved alongside developments in the field of aesthetic medicine from a surface-oriented two-dimensional approach, concentrating on removal of facial lines and folds, to a three-dimensional approach that also addresses both soft and hard tissue volume loss in both the face and the hands.3
With the popularity of dermal fillers demonstrated by increasing numbers of treated patients, public awareness and acceptance of nonsurgical enhancement has greatly increased the treatment options available. Along with botulinum toxin injections and energy-based devices, fillers are the mainstay of most medical aesthetic clinics. With increasing patient demand and the increased availability of aesthetic providers, private practices have become more competitive. Patient retention is now a major objective of most aesthetic businesses. Patient satisfaction is a key element for patient retention and requires a portfolio of safe and effective products. Long-term clinical experience, clinical research, peer-reviewed publications and regulatory approvals have combined to demonstrate the safety and efficacy of CaHA. The product has been evolved to meet the demands of a continuum of aesthetic care in terms of enhancement of youthful patients (ages 25-35); early prevention, rejuvenation, and volume restoration for patients in the middle decades of life (35-55); and for the delay and maintenance as part of restoration for mature (55-75+) patients as well.
In this tenth anniversary year, the authors consider the historical milestones of CaHA in aesthetic medicine, propose a protocol for a global facial approach using CaHA, and look at how its unique properties provide it with a place in today’s market and keep it at the forefront of modern aesthetic treatments. Throughout this publication, reference is made to labeled and off-label indications, techniques, and dilution protocols performed by experts in the field of aesthetic medicine. The reader is reminded that some of these are not approved by regulatory authorities and are not endorsed by Merz Pharmaceuticals GmbH.
MECHANISM OF ACTION
Radiesse is a biodegradable filler consisting of 30% synthetic CaHA microspheres (diameter of 25-45μm) suspended in a 70% aqueous carboxymethylcellulose gel carrier.4 The soluble carrier gel evenly distributes the Radiesse CaHA microspheres providing 1:1 correction and gradually dissipates leaving the microspheres at the injection site where they induce neocollagenesis by fibroblast activation.5-7 In a recent clinical histomorphological study, Radiesse significantly stimulated collagen production over nine months of follow-up [Yana Alexandrovna Yutskovskaya, presented at AMEC, October 12, 2013 and 1st eastern AMWC June 2013]. In this manner, Radiesse provides both immediate (replacement volume) and long-lasting (collagen biostimulation) volume enhancement.
Fibroblasts are found in all connective tissues and the Radiesse CaHA microspheres are thought to elicit their activation and subsequent collagen production regardless of whether Radiesse is injected intra-dermally or at the level of the dermal-subdermal junction. Animal studies have shown that this new collagen growth occurs as early as four weeks post-injection and continues for at least 12 months.6 A recently performed clinical study has confirmed the longevity of collagen stimulation by Radiesse [Yana Alexandrovna Yutskovskaya, presented at AMEC, October 12, 2013 and 1st eastern AMWC June 2013]. The immediate volume correction as well as stimulation of long-term deposition of new collagen surrounding the microspheres contributes to an average duration of effect of 12 to 18 months, though some results have been noted 24 months post-injection.8,9
As progenitor cells for osteogenesis do not exist in soft tissue, no calcification or osteogenesis has been reported in extensive literature describing the use of CaHA in a variety of soft tissue applications. CaHA is a completely biodegradable soft tissue filler. Over time, the CaHA microspheres are broken down into calcium and phosphate ions by the phagocytes.10 Further evidence for the biodegradable nature of CaHA is provided by a study in which computed tomographic (CT) imaging studies were conducted pre- and post-treatment with CaHA in 58 individuals with HIV-associated lipoatrophy or pronounced nasolabial folds.11 In the CT scans taken immediately after injection, CaHA was clearly visible, whereas 12 months after the initial injection, only residual amounts of CaHA could be observed, ft is important to note that in many individuals, the aesthetic effects remained evident at 12 months even though little CaHA was visible.
PHYSICAL CHARACTERISTICS
The main physical characteristics that determine the ability of injectable fillers to provide volume and lift are complex viscosity (η*), elasticity (G′), and cohesivity.
Viscosity. Viscosity (η*) measures the ability of a filler to resist the shearing forces that it is subjected to within a tissue after injection. Radiesse has one of the highest viscosities when compared with other dermal fillers.12 This allows it to remain in the place where it is injected and not to migrate into the surrounding tissue.
Elasticity. Elasticity is a measure of a filler’s stiffness and its ability to resist deformation under an applied pressure that is external to the filler, e.g. when a filler is extruded through a needle during injection and after injection when the filler is subjected to movements of the facial muscles. The higher the G′ the less it deforms under pressure and the more lift it can provide. Radiesse has a high G′ providing it with greater lifting capacity than many other dermal fillers.12
Cohesivity. The lifting capacity of a dermal filler is determined by two material properties: gel hardness (elasticity) and the cohesivity of the gel. Radiesse has been developed for optimal lift with a balance between high elasticity and cohesivity.
PRODUCT MILESTONES
Regulatory approvals. Dermal fillers are classified as medical devices by most regulatory agencies (including the United States Food and Drug Administration [FDA]) as their primary intended action is mechanical (“filling effect”).
Radiesse received a Conformité Européenne (CE) certification mark (medical device class 3) for plastic and reconstructive surgery, including deep dermal and subdermal soft tissue augmentation of the facial area in 2003. ft may be injected into the deep dermis, the subcutaneous tissue, or supraperiosteally depending on the area of the face being treated. The European label includes, but is not limited to, the nasolabial folds, marionette lines, cheek hollows, cheekbone, jawline, oral commissures, chin, temple, bridge of the nose, and the hands. In 2006, Radiesse received FDA approval for the correction of moderate-to-severe facial wrinkles and folds, such as nasolabial folds, and/or the correction of the signs of facial fat loss in people with human immunodeficiency virus.
Pivotal trials. The clinical use of Radiesse has paralleled developments in the field of aesthetic medicine, which began with a focus on individual facial lines and folds. The pivotal clinical trial that led to the FDA approval of the product for aesthetic correction of nasolabial folds was a multicenter study of 117 subjects with symmetric moderate-to-deep nasolabial folds.13 Using a randomized, split-face design, subjects were injected with Radiesse on one side of the face and human collagen (Cosmoplast; Allergan, Irvine, California) on the other. At the six-month evaluation, Radiesse was rated as superior to collagen in 79 percent of folds (P<0.0001) and on average required half the amount of material required for the collagen-treated folds (P<0.0001). An extension of the above study offered re-treatment with Radiesse between 6 and 12 months in both folds to balance asymmetry.9 A total of 102 of the initial 117 subjects were enrolled and evaluated at intervals up to 39 months after the last injection of Radiesse. Of the original 102 subjects, three were lost to follow-up between the two- and three-year time points. At least 30 months after the last Radiesse treatment, 40 percent of the folds evaluated remained improved. There were no long-term or delayed-onset adverse events in patients followed for up to 39 months, including no reports of nodules, foreign body granulomas, or infections.
A second pivotal trial evaluated Radiesse for the treatment of facial lipoatrophy in 100 patients with human immunodeficiency virus receiving highly active antiretroviral therapy. In this open-label study, all subjects reported significant improvement at 12 months and 91 percent reported significant improvement at 18 months. Quality-of-life data collected at 12 months also revealed that 100 percent of subjects had found that Radiesse treatment had been beneficial.14 Since these trials were conducted, Radiesse has been progressively used for a variety of aesthetic facial and hand indications.15-17
Mixing with lidocaine. In July 2009, a protocol for mixing Radiesse with lidocaine to produce a final lidocaine concentration of 0.3% was approved by the FDA. This was associated with an improvement in patient comfort during the injection process and an increase in patient satisfaction.18 In Europe, this protocol has not yet been approved by health authorities.
Distribution milestone. In 2013, Radiesse reached a distribution milestone with more than five million syringes shipped for a variety of aesthetic indications without any safety concerns. The product is now available in over 50 countries and more than 3,000 patients have received it in controlled clinical studies.
PATIENT ASSESSMENT
Patient assessment is one of the most important aspects of a physician’s role as only by making a proper diagnosis can the appropriate treatment be planned. Assessment of an individual for optimal treatment should include an evaluation of the signs of aging and proportions, harmony, and balance of the face. By combining their knowledge of facial anatomy and the aging process, physicians can assess patient suitability and determine the choice of product that will ultimately provide the results that each individual patient desires.
Adults of all ages are selecting soft-tissue augmentation, either as a precursor to or as a substitute for surgery. Patients in different age groups have diverse treatment needs ranging from the correction of fine lines and wrinkles in younger patients to volume restoration in older patients. CaHA is appropriate for a broad range of aesthetic procedures with patients’ individual needs dictating the treatment approach.
INJECTION PROTOCOLS FOR A GLOBAL FACIAL APPROACH
As well as the development of lines and wrinkles, it is now known that facial volume loss, via a combination of tissue laxity (collagen and elastic tissue breakdown), bone resorption (osteopenia of the facial skeleton), and lipoatrophy, all play an important role in the appearance of aging.19-22 With this knowledge, the focus of facial aesthetic treatments has changed from a two-dimensional approach concentrating on removing facial lines and improving skin tone and texture to a three-dimensional approach that also addresses facial volume loss. The correction of volume loss is performed with an emphasis on the restoration of facial symmetry, balance, and proportion. Radiesse is well suited to this approach as a result of its high elasticity and viscosity, which provide it with an impressive lifting capacity when molded and contoured into place. It is also unique among fillers in that the aesthetic results are not just derived from the volume of product injected, but also from the long-term stimulation of the patient’s own collagen production,5 leading to a longer duration of effect. These physical properties of Radiesse provide it with great versatility and make it suitable for most aspects of facial augmentation—line filling, skin tightening, lifting, contouring, and volumizing.
Studies of saccadic eye movements have revealed the features that people look at when studying a face. Interestingly, different cultures appear to focus on different face information. For example, Caucasian observers fixate on the eye region and the mouth, whereas East Asian observers fixate more on the central region to extract information from faces.23 During this scanning of another person’s face, we subconsciously register many different aspects of that person’s anatomy that provide us with quite a precise estimate of that person’s age. As physicians, we need to address all of the details that are being scanned in these initial observations to provide our patients with optimal treatment results. To restore a natural more youthful look, a global facial approach is often required to restore the subtle variations of side-to-side symmetry, upper-mid-lower face balance, and proportion. Although this article is restricted to the consideration of CaHA, facial aging is a complex process and a combination of nonsurgical technologies is typically used to minimize hollows, lines, and wrinkles; recontour features; and improve the skin’s overall tone. It may be stated that the youthful face has more highlights than lowlights, more convexity than concavity, more reflections than shadows, and more tone than texture. When undertaking a global facial approach, a multi-level technique generally provides the best results—deep volume restoration at the supraperiosteal level, subcutaneous contour reconstruction, and superficial dermal reduction of skin laxity (the latter is not an approved indication for Radiesse). The use of a volumizing filler, such as CaHA can immediately restore volume as well as fill and correct specific creases and hollows. It is ideal for use in all areas of the face except the glabella, periorbital area, and lips. The amount injected will vary depending on the site and extent of the restoration or augmentation desired, but in all cases, the correction ratio for Radiesse is approximately 1:1 (i.e., overcorrection should be avoided).
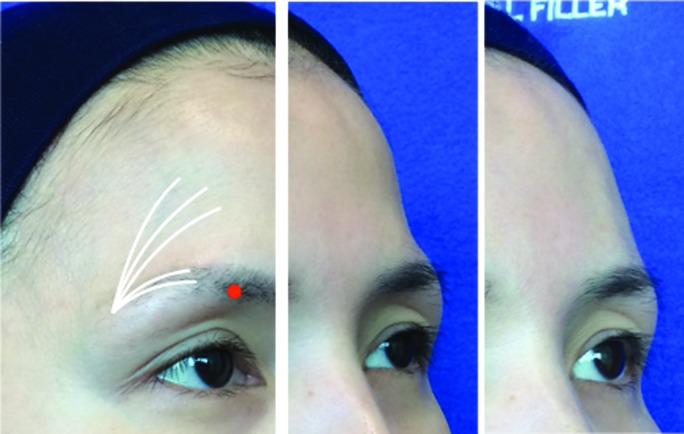
A detailed injection protocol for a global facial approach is provided in Table 1. In the upper face, sunken temples and the area above the eye brows (frontal concavity) can be significant contributors to an aged look. Volume restoration with CaHA can return a youthful appearance to these areas and even raise the outer brow (Figures 1 and 2). The outer edge of the eyebrows can sometimes droop with age. The addition of a small amount of Radiesse to this area provides a very subtle lift to the brow by restoring volume to a tiny fat pad located directly underneath the eyebrow (Figure 3). This technique may be especially effective when used in combination with botulinum toxin to relax the muscles that pull the brow down (lateral brow depressors).
TABLE 1.
Radiesse injection protocol for a global face approach
| TREATMENT AREA | BENEFITS | INJECTION PROTOCOL | SAFETY CONSIDERATIONS |
|---|---|---|---|
| Frontal concavity (parallel and superior to eyebrows) | Reduces skeletonized appearance, restores youthful frontal convexity and contributes to brow lift | Mark frontal concavity prior to injection. Inject only at the subgaleal/ supraperiosteal plane |
Facial nerve, supraorbital/supratrochleal nerve and arteries. Cannula technique is advised starting from temporal crest |
| Temporal concavity | Restores oval of the face, reduces skeletonized appearance and contributes to lift of lateral brow | Submuscular/supraperiosteal/subdermal injection protocols | Submuscular injection protocol: for Radiesse, aspiration before injection is not a reliable test to avoid intravascular injection as the filler inside the needle will be too viscous to allow blood to enter the syringe. Therefore, multiple boluses are injected in the submuscular/supraperiosteal plane. With the needle tip gently touching the periosteum, the chance of intra-arterial injection is minimized. Injector should remain on periosteum during entire injection. Slow bolus injection minimizes risk of retrograde arterial migration of product and low volume of bolus (0.1-0.2mL per bolus) minimizes risk of necrosis as only a small area could be blocked and will allow for collateral perfusion. Subdermal injection protocol: venous network, superficial temporal artery. Advisable to use blunt cannulas. Injectors often choose to dilute according to the FDA dilution protocol (0.3mL lidocaine for a 1.5mL Radiesse syringe) |
| Brow lift | Lifts brow, reduces lateral hooding | Submuscular/supraperiosteal or subcutaneous/dermal injection at the level of the lateral brow to the peak/middle of the brow. Needle or cannula technique. Our technique is only for supraperiosteal placement, as vascular entry/cannulation is too much of a risk in this area. | Venous plexus, supraorbital artery and nerve, intraorbital area. Avoid masculinization of the female brow: limit volume. A cannula is advised to avoid intravascular injection. If a needle is used, inject with: 1) low volume, 2) low pressure, 3) retrograde in an area where there are no large arteries—the dermal/subdermal plane of the area from the peak to the tail of the brow; more medially from the peak, direct arterial connections to the intra-orbital area are present (supraorbital artery, supratrochlear artery). Also avoid deep injection to prevent intra-arterial injection. Advocate no more than 0.3mL total volume to brow in a single injection sitting |
| Zygomatic area | Restores V-shape of the face, restores youthful cheek convexity, lifts sagging soft tissues, reduces lower lid lag, and reduces tear trough (without treating the latter). | Multilevel approach: Needle or cannula technique. Cannula: 2-point technique: Zygomatic arch entry point and medial zygoma entry point Above alar-tragal line: Inject only at the periosteal level. Below alar-tragal line: Inject more “superficially” (meaning dermal/subdermal junction) |
Infraorbital foramen; lymphatic drainage, infraorbital fat compartments, malar edema. Proper patient selection is necessary, for example, care should be taken when treating patients with fat bags. Assess for any asymmetry of the zygomatic arches in each patient. Augmentation of the midface in individuals with herniated fat pads may help to reduce the appearance of fat pads. However, care should be taken not to inject into the Arcus Marginalis, as this may result in protrusion of the fat, and development of “white bangers” as Radiesse aggregates and becomes visible through the skin. Note: Avoid injection of product directly into a fat pad, as fat pads are quite vascular. Advocate injection only over periosteum and lightly mold when complete |
| Mandibular augmentation | Restores mandibular definition, reduces jowl, pre-jowl sulcus, and marionette lines | 2-point cannula technique. Mandibular angle: Placement at dermal/subdermal junction for skin tightening and redefining mandible. Point (depot) technique at the mandibular angle may help to define the lateral jaw, especially in older patients and those with poorly defined definition from face to neck, and contribute to curvilinear mandibular sweep. Pre-jowl sulcus entry point: Multilevel technique (dermal/ subdermal junction plus supraperiosteal placement). Supraperiosteal placement to augment masseter projection |
Mandibular angle: Facial arteries; avoid placing product into jowl, as that might aggravate jowling. Parotid gland Pre-jowl sulcus entry point: Mental foramen, avoid placing product into mucosa and muscle as intramuscular injection can lead to nodule formation Pre-jowl sulcus (PJS) injection
Angle of jaw (AOJ) injection
|
| Mentum augmentation | Reduces marionette lines, restores convexity of mentum, balances the face with Steiner’s line (joins soft tissue pogonion to the midpoint between subnasal and nasal tip; the lips should touch this line) | Multi-level approach: submuscular at the periosteum of the mentum and dermal/subdermal junction. 1-point cannula technique | Submental artery. Avoid injecting in the oral mucosa, so supraperiosteal injection only when injecting submuscularly. Typical injection is subdermal. Care must be taken when injecting to periosteum at this level, given the product may show or surface to the lower gingival sulcus immediately following injection (physician thought he/she was lower than the gingival sulcus and was not). Total volume injected during a single treatment session is often around 0.5mL per side |
Figure 1.
Frontal concavity. The danger point (supraorbital foramen) is marked and should be avoided as there is a direct access to the intraorbital area.38 The cannula entry point is chosen at the temporal crest, lateral to the frontal concavity. The cannula is advanced through the muscle, through the galea aponeurotica and advanced in the glide plane between the galea and the periosteum of the frontal bone. Radiesse is deposited in retrograde linear threads.
Figure 2.
Sunken temples. The danger zone is marked in red: Intravascular injection into the superficial temporal artery should be avoided while injecting with a needle. At the point of maximum depression, the needle is placed perpendicular to the skin and advanced slowly until contact with the periosteum is felt. Slow injection of small amounts of Radiesse will maximize safety. There is evidence of small arteries running over the periosteum so there is a theoretical risk of intra-arterial injection. Slow injection reduces the risk of retrograde displacement and embolization of intraorbital (including retinal) arteries. Low volume injection minimizes necrotic area. Due to supraperiosteal placement, the temporal muscle, which is connected by loose connective tissue, is hydrodissected from the periosteum.
Figure 3.
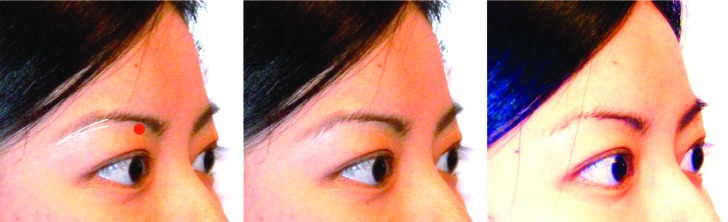
Brow lift. The danger zone (supra orbital foramen) is marked in red. The entrance for the cannula is chosen at the tail of the brow. A multi-level approach is used with one or two retrograde linear threads at the dermal-subdermal junction and two additional retrograde linear threads at the periosteal plane on the orbital rim. With female patients in particular, care should be taken not to overcorrect here to avoid masculinization (max 0.15-0.2mL for the lateral brow).
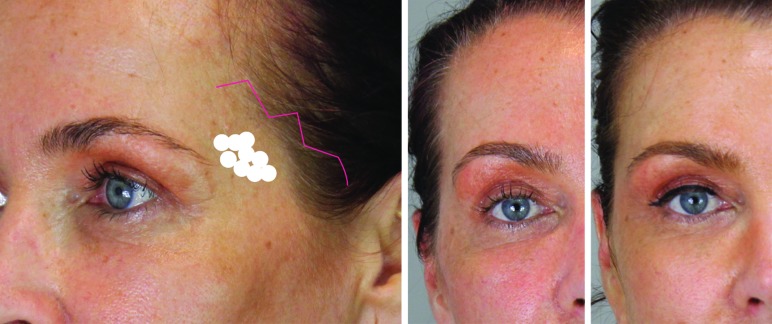
In the midface, restoring volume to the cheekbone and the sunken area beneath the cheekbone (submalar hollowing) returns the face to a younger heart shape (triangle of youth) and is a subtle way of making a patient look and feel younger (Figure 4). The effect is to lift sagging soft tissues, reduce lower eyelid lag, and reduce the tear trough without the need for treating the tear trough area. Adding volume to the cheekbones (midface) also provides lift to the cheek and jowls (lower face). Above the alar-tragal line (Hinderer’s line), CaHA should be injected supraperiosteally, while below the alar-tragal line, injection should be at least deep dermally to the junction with the subcutis.
Figure 4.
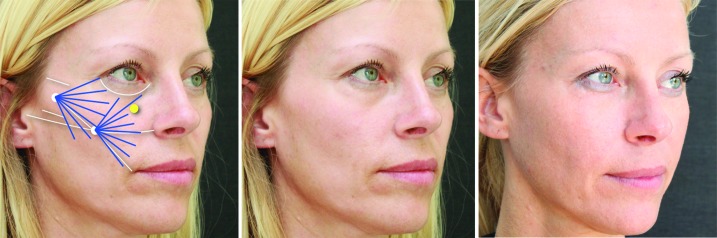
Zygomatic area. The danger zone (infra-orbital foramen) is marked in yellow and the marking at the infra-orbital rim serves as a reminder of the cranial limit of this area. For the augmentation of the cheek, a two-point cannula technique is advised. As a general rule of thumb, the injections cranial to the alar-tragal line should be placed at the supraperiosteal level, whereas the injections below this line should be placed at the level of the dermal-subdermal junction. The first entry point is found between the superior and inferior demarcation of the zygomatic arch, immediately in front of the hair line. From this point, a vector line can be drawn to the oral commissure. At the intersection of the vector line and the alar-tragal line, the second entry point can be found. Left: Before with markings; middle: Before; right: After 1.5mL Radiesse in the cheeks.
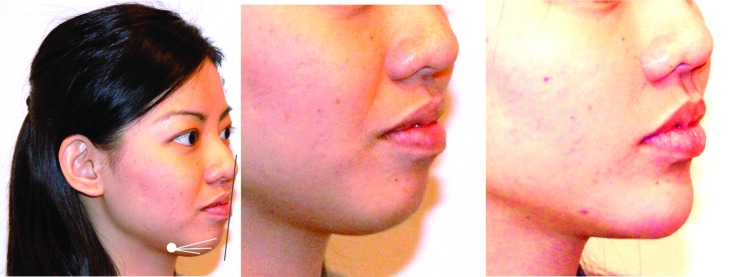
In the lower face, the jawline loses its curvilinear (oval) shape and smooth line with age. Recommended insertion points for recontouring the jawline and disguising the jowls are at the mandibular angle and prejowl sulcus, and CaHA should be placed at the dermal–hypodermal junction or deep dermis (Figure 5). Augmentation of the mandible just supraperiosteally below the masseter (mandibular angle) is effective in both men and women, where loss of volume of the masseter and mandible can be simultaneously addressed. The use of CaHA for mentum augmentation afters the projection of the chin and reduces marionette lines, providing noticeable improvements to an individual’s appearance (Figure 6). At the mentum, a multi-level approach is also recommended, where supraperiosteal placement is combined with dermal/subdermal placement.
Figure 5.
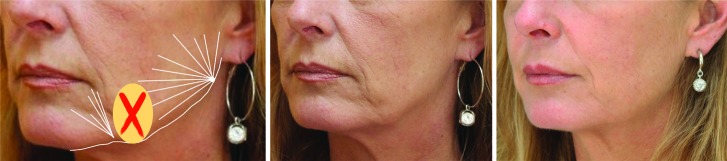
Mandibular augmentation. As bone resorption is most apparent at the dorsum of the mandible, at the mandibular angle, the cannula entry point for mandibular augmentation should be placed at the dorsum of the mandibular angle.22 From there, the cannula should be advanced at the level of the dermal-subdermal junction to the pre-auricular area, from where a fanning technique can be used with diluted Radiesse for easier spreading of the product (in this case 0.8mL lidocaine per 1.5mL Radiesse). To enhance definition of the mandible, the first and last thread can be made with more volume (e.g., 0.2–0.3mL). Care should be taken to avoid placing Radiesse into the jowl fat compartment, as this can aggravate the aged appearance. To improve the mandibular area further, Radiesse can be placed in the marionette lines and mental crease via the pre-jowl sulcus entry point as shown.
Left: Before with markings; middle: Before; right: After 1.5mL Radiesse in the jawline plus marionette lines.
Figure 6.
Mentum augmentation. For augmentation of the mentum, a multi-level approach is generally used. In this case, a cannula was inserted at the pre-jowl sulcus entry point and advanced in both the supraperiosteal layer, as well as the junction between the dermis and subcutis. Augmentation was continued until the patient had a good Steiner Line.39 Left: Before with markings; middle: Before; right: After 1.5mL Radiesse in the mentum.
A set of consensus recommendations on the use of CaHA for jawline rejuvenation has recently been published, which reinforces the approach described above.24 The recommendations use a validated jawline scale for defining loss of oval facial contours to provide clinicians with step by step guidance on jawline rejuvenation with CaHA, including advice on type of anesthesia, injection techniques, volume for injection, and use in combination with other procedures.
NEEDLE OR CANNULA
Dermal fillers may be injected using either a hypodermic needle or blunt-tip microcannula. CaHA is generally injected with a 30mm-long 27 gauge or 19mm-long 28 gauge inner diameter needle or a 25 or 27 gauge variable length microcannula. Needles have the advantage of extreme precision of movement, the possibility of deep intradermal injection, and a requirement for smaller injection volumes. Disadvantages of needles include pain, bruising, and nerve/vessel laceration. Cannulas cause less trauma, pain, and bruising and allow a large area to be treated at the chosen injection depth.
When using a microcannula, the authors suggest first making a stab incision with a needle that is 1 to 2 gauges thicker than the cannula. The cannula should then be inserted while stretching the skin to open the needle aperture. The cannula should be twisted and rotated until any resistance has passed. When the cannula is through the dermis the skin in front of the cannula should be pinched in order to go deeper, or the skin should be stretched in the direction of the cannula for superficial injections (one finger in front and one finger behind the cannula).
DILUTION
The FDA has approved a protocol for mixing Radiesse with lidocaine. This makes the experience more pleasant for patients, which will keep them motivated to return for additional treatments. In addition, the treatment site is anesthetized as the injection process progresses, which eliminates distortion of the treatment site from local anesthetic.
An additional benefit of the mixing protocol is that CaHA with 0.3% lidocaine is easier to extrude from the needle than CaHA alone. A rheometric study of several hyaluronic acid (HA) fillers and Radiesse found that Radiesse alone had the highest G′ and viscosity, followed by Radiesse diluted with 0.3% lidocaine, Perlane® (Galderma) and Restylane® (Galderma) in the medium group, and the Juvederm® (Allergan) product family in the low G′ and viscosity group.12 When mixing with lidocaine, the viscoelastic properties of the gel change, at least temporarily, for any dermal filler. Sundaram et al12 showed that when mixing with 20% by volume lidocaine (as per the FDA-approval study18), the viscosity and elasticity of Radiesse are slightly reduced. It is therefore important to refer to the FDA-approved protocol for mixing Radiesse and lidocaine as this represents the optimal protocol for preserving the viscoelastic properties of the product.
The addition of lidocaine adjusts the cohesiveness of CaHA to make it suitable as a layering rather than volumizing filler while also allowing the use of smaller needles. Some expert injectors use different volumes and dilutions of CaHA to perform distinct roles when augmenting the face. For example, undiluted CaHA may be injected at a deep level just above the periosteum to replenish volume loss, a slightly diluted CaHA may be used to restore contours and proportions at the subcutaneous level, and an even more diluted version may be injected into the dermis and subdermis to reduce skin laxity and provide the patient with a smoother, more radiant complexion. The above can also be performed as individual treatments, depending on the patient’s needs and desires. Some injectors use lidocaine for the initial dilution to reduce discomfort with further dilutions achieved by adding normal saline solution. A common choice of many injectors is to use lidocaine premixed with adrenaline. The advantage of this approach is that the vasoconstriction triggered by adrenalin reduces post-treatment edema.
It should be noted that while dilution of CaHA is a method of titrating its viscosity and expanding its use to applications that require more superficial placement and greater product spread (e.g., fine lines, dorsum of the hand), the technique is not approved for this purpose and its use in this manner must therefore be regarded as off-label.
SAFETY
Radiesse has been extensively used for the correction of moderate-to-severe facial lines and folds and to replenish lost volume. The Radiesse CaHA microspheres are smooth in shape and identical in composition to a mineral component of human bone and teeth and are thus inert and nonantigenic. In the pivotal clinical trials that led to FDA approval of Radiesse for aesthetic correction, adverse events were generally mild (bruising, edema, erythema, pain, and pruritis) and short in duration.13,14 In the trial comparing Radiesse- and collagen-treated patients, there was no significant difference in adverse event rates or duration.13 There have been no reports of granuloma formation in studies injecting Radiesse for aesthetic use.9,16,25-28 Rare incidences of untoward foreign body reactions with CaHA have been described in only a handful of case reports in over 10 years of clinical use29-31 and in none of these cases could any definite conclusions be drawn as to whether the events were related to Radiesse or other circumstances (i.e., treatment with other, permanent fillers in the past, improper tissue placement, medical history, etc.).32,33 Indeed, the literature frequently describes case reports of dermal filler complications without adequate proof that the alleged material was actually injected.34 Adverse events that do occur with CaHA are usually temporary and injection-related (e.g., bruising and edema). As with all dermal fillers, most adverse events are avoidable with proper planning and technique.
DISCUSSION
Radiesse is a very effective agent for many areas of facial soft tissue augmentation and is associated with a high and well-established safety profile.32 It is currently the only resorbable filler on the market that immediately replenishes lost volume while at the same time stimulating the production of the skin’s natural collagen for long-lasting results. Treatment effects have been clinically proven to last a year or more in many patients. Only one other filler has been shown to stimulate new collagen formation, but the polymethylmethacrylate (PMMA) microspheres of ArteFill® (Suneva Medical) are permanent and not resorbed.
The unique characteristics of Radiesse (high elasticity and viscosity, combined with its ability to induce long-term collagen formation) provide it with great versatility, and its use has evolved in parallel with developments in the aesthetic field from an agent for simple line filling to one that can be used for a global facial approach. As a result, Radiesse is appropriate for treating patients at any stage of the aging process. To date, more than five million syringes have been shipped for a variety of aesthetic indications. Radiesse is approved for aesthetic use in more than 50 countries, including the United States, where the FDA has the most rigorous criteria for filler approvals worldwide.
Off-label uses of drugs and devices are commonly practiced by physicians in consultation with patients in virtually every area of medicine, and the aesthetic field is no exception. Furthermore, what constitutes an off-label use in one country may be an approved indication in another. Expert injectors continue to expand the potential uses for Radiesse with additional as yet off-label indications and techniques for diluting and injecting more superficially. Most recently, this involves hyperdilution of the product so that it can be used very superficially for skin tightening [Yana Alexandrovna Yutskovskaya, unpublished data]. A further off-label indication involves placing the product under the hair, below the galea, to correct bone resorption of the frontal bone and to lift the forehead [Jani van Loghem, unpublished data]. While off-label procedures such as these are not approved by regulatory authorities and are not endorsed by Merz Pharmaceuticals GmbH, in the hands of expert injectors they can be regarded as innovation in medicine and will likely lead to new and better treatments and procedures in the future.
An important issue, however, is the skillset of individuals performing the procedures, particularly non-physician injectors who may also have a limited knowledge of facial anatomy. Nodule formation can occur after injection of CaHA into the oral mucosa and the lips.2 The injection of CaHA in this highly mobile area may cause the CaHA particles to clump and is not an approved indication nor is it approved by Merz. The nodules occur soon after injection and are a result of accumulated particles and not a granulomatous reaction. They are completely resolved with injection of saline and vigorous massage35 or with the use of fractional carbon dioxide laser therapy.36 Special care should be taken to avoid injection of any filler into a blood vessel. This may occlude the vessel and could cause infarction or embolism leading to ischemia, necrosis, or scarring. Although rare, cases have been reported with a number of dermal fillers including CaHA.37 They are all related to poor injection technique and not filler product.
Physician experience is essential for satisfied patients, and is being addressed by leading pharmaceutical companies in the aesthetic field (e.g., by the unique service of Field Clinical Specialists offered by Merz Pharmaceuticals GmbH). These are medical professionals who conduct intensive individual training of aesthetic physicians to ensure best practice in the aesthetic industry and best outcomes for patients. Radiesse has emerged as a versatile, durable, and safe filler. It has an excellent volumizing effect for a variety of aesthetic indications, and its use is only anticipated to grow as more clinicians and patients gain first-hand experience with it.
Footnotes
DISCLOSURE:Dr. van Loghem is a consultant for Merz Aesthetics. Dr. Yutskovskaya reports no relevant conflicts of interest. Dr. Werschler is on the advisory board and speaks for Merz. He also receives honoraria from Merz.
REFERENCES
- 1.Werschler WP, Narurkar VN. Facial volume restoration: selecting and applying appropriate treatments. Technique poster. Cosrnet Dermatol. 2006;19(Suppl 2):S1. [Google Scholar]
- 2.Tzikas TL. A 52-month summary of results using calcium hydroxylapatite for facial soft tissue augmentation. Dermatol Surg. 2008;34(Suppl 1):S9–S15. doi: 10.1111/j.1524-4725.2008.34237.x. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers JD, Glogau RG, Blitzer A. Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type A hyaluronic acid dermal fillers, and combination therapies—consensus recommendations. Plast Reconstr Surg. 2008;121(5Suppl):5S–30S. doi: 10.1097/PRS.0b013e31816de8d0. [DOI] [PubMed] [Google Scholar]
- 4.Graivier MH, Bass LS, Busso M, et al. Calcium hydroxylapatite (Radiesse) for correction of the mid- and lower face: consensus recommendations. Plast Reconstr Surg. 2007;120(6 Suppl):55S–66S. doi: 10.1097/01.prs.0000285109.34527.b9. [DOI] [PubMed] [Google Scholar]
- 5.Marmur ES, Phelps R, Goldberg DJ. Clinical, histologic, and electron microscopic findings after injection of a calcium hydroxylapatite filler. J Cosrnet Laser Ther. 2004;6:223–226. doi: 10.1080/147641704100003048. [DOI] [PubMed] [Google Scholar]
- 6.Coleman KM, Voigts R, DeVore DP, Termin P. Coleman WP 3rd. Neocollagenesis after injection of calcium hydroxylapatite composition in a canine model. Dermatol Surg. 2008;34(Suppl 1):S53–S55. doi: 10.1111/j.1524-4725.2008.34243.x. [DOI] [PubMed] [Google Scholar]
- 7.Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008;34(Suppl 1):S64–S67. doi: 10.1111/j.1524-4725.2008.34245.x. [DOI] [PubMed] [Google Scholar]
- 8.Jacovella PF. Use of calcium hydroxylapatite (Radiesse®) for facial augmentation. Clin Interv Aging. 2008;3:161–174. doi: 10.2147/cia.s2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bass LS, Smith S, Busso M, McClaren M. Calcium hydroxylapatite (Radiesse) for treatment of nasolabial folds: long-term safety and efficacy results. Aesthet Surg J. 2010;30:235–238. doi: 10.1177/1090820X10366549. [DOI] [PubMed] [Google Scholar]
- 10.Bentkover S. The biology of facial fillers. Facial Plast Surg. 2009;25:73–85. doi: 10.1055/s-0029-1220646. [DOI] [PubMed] [Google Scholar]
- 11.Carruthers A, Liebeskind M, Carruthers J, Forster BB. Radiographic and computed tomographic studies of calcium hydroxylapatite for treatment of HIV-associated facial lipoatrophy and correction of nasolabial folds. Dermatol Surg. 2008;34(Suppl 1):S78–S84. doi: 10.1111/j.1524-4725.2008.34247.x. [DOI] [PubMed] [Google Scholar]
- 12.Sundaram H, Voigts B, Beer K, Meland M. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(Suppl 3):1859–1865. doi: 10.1111/j.1524-4725.2010.01743.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith S, Busso M, McClaren M, Bass LS. A randomized, bilateral, prospective comparison of calcium hydroxylapatite microspheres versus human-based collagen for the correction of nasolabial folds. Dermatol Surg. 2007;33:S112–S121. doi: 10.1111/j.1524-4725.2007.33350.x. [DOI] [PubMed] [Google Scholar]
- 14.Silvers SL, Eviatar JA, Echavez MI, et al. Prospective, open-label, 18-month trial of calcium hydroxylapatite (Radiesse) for facial soft tissue augmentation in patients with human immunodeficiency virus associated lipoatrophy: one year durability. Plast Reconstr Surg. 2006;118(Suppl):34S–45S. doi: 10.1097/01.prs.0000234847.36020.52. [DOI] [PubMed] [Google Scholar]
- 15.Jansen DA, Graivier MH. Evaluation of a calcium hydroxylapatite-based implant (Radiesse) for facial soft tissue augmentation. Plast Reconstr Surg. 2006;(118Suppl):22S. doi: 10.1097/01.prs.0000234903.55310.6a. [DOI] [PubMed] [Google Scholar]
- 16.Sadick N, Katz BE, Roy D. A multicenter, 47-month study of safety and efficacy of calcium hydroxylapatite for soft tissue augmentation of nasolabial folds and other areas of the face. Dermatol Surg. 2007;33:S122–S127. doi: 10.1111/j.1524-4725.2007.33351.x. [DOI] [PubMed] [Google Scholar]
- 17.Sadick NS. A 52-week study of safety and efficacy of calcium hydroxylapatite for rejuvenation of the aging hand. J Drugs Dermatol. 2011;10:47–51. [PubMed] [Google Scholar]
- 18.Marmur E, Green L, Busso M. Controlled, randomized study of pain levels in subjects treated with calcium hydroxylapatite premixed with lidocaine for correction of nasolabial folds. Dermatol Surg. 2010;36:309–315. doi: 10.1111/j.1524-4725.2009.01435.x. [DOI] [PubMed] [Google Scholar]
- 19.Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219–2227. doi: 10.1097/01.prs.0000265403.66886.54. [DOI] [PubMed] [Google Scholar]
- 20.Rohrich RJ, Pessa JE. The retaining system of the face: histologic evaluation of the septal boundaries of the subcutaneous fat compartments. Plast Reconstr Surg. 2008;121:1804–1809. doi: 10.1097/PRS.0b013e31816c3c1a. [DOI] [PubMed] [Google Scholar]
- 21.Gierloff M, Stohring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconstr Surg. 2012;129:263–273. doi: 10.1097/PRS.0b013e3182362b96. [DOI] [PubMed] [Google Scholar]
- 22.Shaw RB, Katzel EB, Koltz PF, et al. Aging of the facial skeleton: aesthetic implications and rejuvenation strategies. Plast Reconstr Surg. 2011;127:374. doi: 10.1097/PRS.0b013e3181f95b2d. [DOI] [PubMed] [Google Scholar]
- 23.Blais C, Jack RE, Scheepers C, Fiset D, Caldara R. Culture shapes how we look at faces. PLoS One. 2008;3:e3022. doi: 10.1371/journal.pone.0003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dallra JM, Baspeyras M, Bui P, Cartier H, Charavel MH, Dumas L. Calcium hydroxylapatite for jawline rejuvenation: consensus recommendations. J Cosmet Dermatol. 2014;13:3–14. doi: 10.1111/jocd.12074. [DOI] [PubMed] [Google Scholar]
- 25.Moers-Carpi M, Vogt S, Santos BM, et al. A multicenter, randomized trial comparing calcium hydroxylapatite to two hyaluronic acids for treatment of nasolabial folds. Dermatol Surg. 2007;33(Suppl 2):S144–S151. doi: 10.1111/j.1524-4725.2007.33354.x. [DOI] [PubMed] [Google Scholar]
- 26.Beer K, Yohn M, Cohen JL. Evaluation of injectable CaHA for the treatment of mid-face volume loss. J Drugs Dermatol. 2008;7:359–366. [PubMed] [Google Scholar]
- 27.Siclovan HR, Jomah JA. Injectable calcium hydroxylapatite for correction of nasal bridge deformities. Aesthetic Plast Surg. 2009;33:544–548. doi: 10.1007/s00266-008-9281-0. [DOI] [PubMed] [Google Scholar]
- 28.Rokhsar C, Ciocon DH. Nonsurgical rhinoplasty: an evaluation of injectable calcium hydroxylapatite filler for nasal contouring. Dermatol Surg. 2008;34:944–946. doi: 10.1111/j.1524-4725.2008.34182.x. [DOI] [PubMed] [Google Scholar]
- 29.Sankar V, McGuff HS. Foreign body reaction to calcium hydroxylapatite after lip augmentation. J Am Dent Assoc. 2007;138:1093–1096. doi: 10.14219/jada.archive.2007.0321. [DOI] [PubMed] [Google Scholar]
- 30.Daley T, Damm DD, Haden JA, Kolodychak MT. Oral lesions associated with injected hydroxyapatite cosmetic filler. Oral Surg OralMed Oral Pathol Oral Radiol. 2012;114:107–111. doi: 10.1016/j.oooo.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Moulonguet I, Arnaud E, Bui P, Plantier F. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37–e40. doi: 10.1097/DAD.0b013e3182732324. [DOI] [PubMed] [Google Scholar]
- 32.Pavicic T. Calcium hydroxylapatite filler: an overview of safety and tolerability. J Drugs Dermatol. 2013;12:996–1002. [PubMed] [Google Scholar]
- 33.Werschler WP. Response to “Oral lesions associated with injected hydroxyapatite cosmetic filler.”. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:417–419. doi: 10.1016/j.oooo.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Freshwater MF. Was it really Radiesse or whose algorithm is better? J Plast Reconstr Aesthet Surg. 2014;67:569–570. doi: 10.1016/j.bjps.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Voigts R, DeVore DP, Grazer JM. Dispersion of calcium hydroxylapatite accumulations in the skin: animal studies and clinical practices. Dermatol Surg. 2010;36:798–803. [Google Scholar]
- 36.Reddy KK, Brauer JA, Anolik R, et al. Calcium hydroxylapatite nodule resolution after fractional carbon dioxide laser therapy. Arch Dermatol. 2012;148:634–636. doi: 10.1001/archdermatol.2011.3374. [DOI] [PubMed] [Google Scholar]
- 37.DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J. 2014;34:584–600. doi: 10.1177/1090820X14525035. [DOI] [PubMed] [Google Scholar]
- 38.Pessa JE, Rohrich RJ. Facial Topography. Clinical Anatomy of the Face. St Louis, Mo: Quality Medical Publishing Inc; 2012. [Google Scholar]
- 39.Erbay EF, Caniklioğlu CM, Erbay SK. Soft tissue profile in Anatolian Turkish adults: Part I Evaluation of horizontal lip position using different soft tissue analyses. Am J Orthod Dentofacial Orthop. 2002;121:57–64. doi: 10.1067/mod.2002.119780. [DOI] [PubMed] [Google Scholar]