Abstract
Background
The objective of this study was to determine the association between the use of antiarrhythmic agents and the risk of malignant neoplasm of liver and intrahepatic bile ducts (MNLIHD).
Methods
We used the research database of the Taiwan National Health Insurance Program to conduct a population-based, case-control study. We identified 9944 patients with antiarrhythmic history who were first diagnosed as having MNLIHD between 2005 and 2010. We identified an additional 19,497 patients with antiarrhythmic history in the same period who did not develop MNLIHD and were frequency-matched using age, sex, and index year to form a control group. Five commercially available antiarrhythmic agents, amiodarone, mexiletine, propafenone, quinidine, and procainamide, were analyzed.
Results
The adjusted odds ratio (OR) of MNLIHD was 1.60 (95% confidence interval [CI], 1.45–1.77) for amiodarone users versus nonamiodarone users. In subgroup analysis, amiodarone use was significantly associated with an increased risk of MNLIHD with an adjusted OR of 18.0 (95% CI, 15.7–20.5) for patients with comorbidities compared to an OR of 2.43 (95% CI, 1.92–3.06) for those without comorbidities. After adjustment for age, sex, statins, anti-diabetes medications, non-steroidal antiinflammatory drugs, propafenone use, quinidine use, and comorbidities, the ORs were 1.49, 1.66, and 1.79 for MNLIHD associated with annual mean defined daily doses of ≤30, 31–145, and >145, respectively.
Conclusions
The results of the present study indicated that amiodarone might be associated with the development of MNLIHD in a dose-dependent manner, particularly among patients with comorbidities.
Introduction
The use of antiarrhythmic medication is recommended in routine clinical practice guidelines as the first-line management of atrial fibrillation (AF) [1]. Antiarrhythmic drugs are administered with the aim of reducing the period and incidence of AF, the need for hospitalization, and the incidence of AF-related mortality. However, numerous limitations have been reported for these drugs, including proarrhythmic and noncardiovascular toxicities, and, more frequently, only modest antiarrhythmic efficacy [2]. Despite these limitations, antiarrhythmics are still widely prescribed for the management of symptomatic AF. Several case reports and reviews have addressed the potential adverse effects associated with these agents, including liver toxicity [3–6], hepatic dysfunction [7, 8], steatohepatitis [9], liver injury [10], and intrahepatic cholestatic jaundice [11]. However, few systemic analyses have been conducted in this setting, and little evidence of an association between the use of antiarrhythmic agents and the risk of developing malignant neoplasm of liver and intrahepatic bile ducts (MNLIHD) has been reported.
A large-scale retrospective cohort study conducted in Taiwan revealed a borderline significant increase in cancer mortality among patients assigned to aamiodarone group [12]. Based on a review of the literature, only one relevant longitudinal cohort study has been reported, and no large-scale case-control study to date has addressed the association of MNLIHD with the use of antiarrhythmic drugs in Taiwan. Among MNLIHD, hepatocellular carcinoma (HCC) remained the second leading cause of cancer deaths after lung cancer in Taiwan in 2012, with a mortality rate of 34.9 per 100,000 persons (18.6% of the total cases) [13], and it is therefore critical to address this problem. Accordingly, we conducted a population-based case-control study by using data from the National Health Insurance (NHI) program of Taiwan to evaluate the relationship between the use of antiarrhythmic drugs and the risk of MNLIHD.
Methods
The NHI Program was established in Taiwan in 1995. It has enrolled up to 99% of the Taiwanese population and contracted with 97% of all medical providers [14]. The National Health Research Institutes (NHRI) is responsible for managing the insurance claims data reported to the Bureau of Health Insurance. For research purpose, the NHRI compiles all medical claims in the NHI program and releases the database annually to the public. In the NHI program, insurants who suffer from certain major diseases, such as malignancies or autoimmunine diseases, and those who require transplants, can apply for a catastrophic illness certificate. Application for a catastrophic illness certificate for malignancies requires cytological or pathological evidence supporting the diagnosis. The NHI database of catastrophic illness integrates multiple NHI databases to provide comprehensive utilization and enrollment information for all patients with severe diseases who obtained copayment exemption from the NHI program. Diagnostics were coded with the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM).
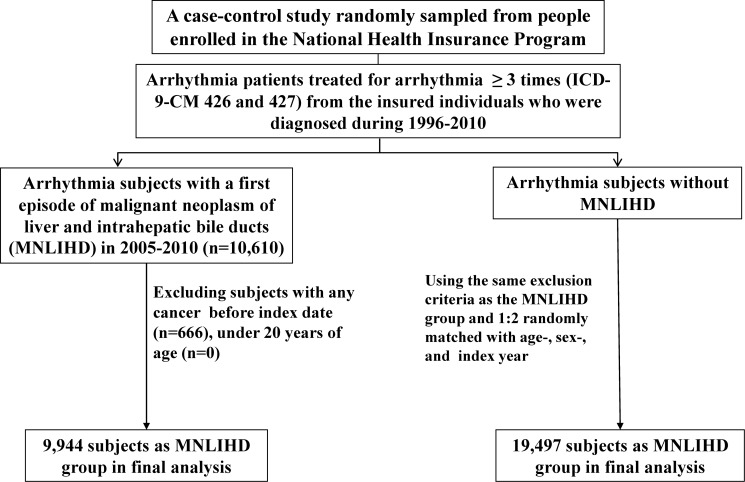
Fig. 1 illustrated the sampling scheme in the study. From the Registry of Catastrophic Illness and NHI program databases, we identified arrhythmia (ICD-9-CM codes 426–427) patients newly diagnosed with MNLIHD (ICD-9-CM code 155) in the period from 2005 to 2010 as a case group. Patients who were younger than 20 years of age were excluded. The date of application for MNLIHD was defined as the index date. For each of the MNLIHD patients, we randomly selected 2 arrhythmia patients without MNLIHD from the same period, using the same exclusion criteria, and frequency-matched the case group by using sex and age (5 y a group). A total of 9944 patients with MNLIHD and 19,497 patients without MNLIHD were included in this study.
Figure 1. Flow chart of participant recruitment in this study.
We considered major comorbidities and medications as covariates, such as diabetes (ICD-9-CM code 250), chronic liver disease and cirrhosis (ICD-9-CM codes 571), hepatitis B virus (HBV) infection (ICD-9-CM codes V02.61, 070.20, 070.22, 070.30, 070.32), hepatitis C virus (HCV) infection (ICD-9-CM codes V02.62, 070.41, 070.44, 070.51, 070.54), alcoholism (ICD-9-CM codes 291, 303, 305.00, 305.01, 305.02, 305.03, 790.3,V11.3), medications of statins, anti-diabetics, and non-steroidal antiinflammatory drugs (NSAIDs) at baseline.
Five commercially available antiarrhythmic drugs in Taiwan were analyzed: amiodarone, mexiletine, propafenone, quinidine, and procainamide. Medication use records were retrieved from ambulatory and inpatient claims data. According to total supply in days and quantity of amiodarone, we calculated the annual mean defined daily dose (DDD) of amiodarone for amiodarone users (ATC C01BD01). For amiodarone, the annual mean DDD was partitioned into 3 levels based on dose tertile.
Sex; age (20–39 y, 40–64y, 65–74y, ≥75 y); medication history of amiodarone, mexiletine, propafenone, quinidine, and procainamide; and comorbidities were compared between the MNLIHD patients and control patients by using a chi-square test. We used a t test for continuous variables. A univariate and multivariate logistic regression model was used to calculate the odds ratio (OR) and 95% confidence interval (CI) for factors associated with the risk of MNLIHD. Only confound variables found significantly in the univariable model were further included in the multivariable model. The multivariate analysis was performed to adjust for possible confounders (including medication of propafenone, quinidine, and comorbidities of diabetes, chronic liver disease and cirrhosis, HBV infection, HCV infection, and alcoholism, and medications of statins, anti-diabetics, and NSAIDs). All analyses were performed using SAS statistical software for Windows (Version 9.2; SAS Institute, Inc., Cary, NC, USA), and the significance level was set at.05.
Ethics Statement
The NHIRD encrypts patient personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claim information, including patients’ sex, dates of birth, medical services utilized, and prescriptions. Patient consent is not required for accessing the NHIRD. This study was approved by the Institutional Review Board of China Medical University (CMU-REC-101–012). Our IRB specifically waived the consent requirement.
Results
The baseline characteristic profiles of the patients are summarized in Table 1. A total of 29,441 arrhythmia patients aged ≥20 years were enrolled in this study. Of the MNLIHD cases, 60.5% were men and 72.8% were ≥65 years of age (Table 1). The mean ages of the MNLIHD patients and non- MNLIHD control patients were 70.6 (±10.9) and 70.3 (±11.2) years, respectively. The proportion of medication history of amiodarone, propafenone, and quinidine was significantly higher for the MNLIHD patients than for the non- MNLIHD control patients. MNLIHD patients were more likely than control patients to exhibit baseline comorbidities (30.7% for diabetes, 91.5% for chronic liver disease and cirrhosis, 34.0% for HBV infection, 42.9% for HCV infection, and 3.63% for alcoholism) and history of medications (40.8% for anti-diabetics and 99.1% for NSAIDs). Compared with the MNLIHD group, the control group was more likely taking statin (31.4% vs 21.0%).
Table 1. Baseline Characteristics between Malignant Neoplasm of Liver and Intrahepatic Bile Ducts (MNLIHD) and Non-MNLIHD Group.
| Non-MNLIHD N = 19,497 | MNLIHD N = 9944 | ||||
|---|---|---|---|---|---|
| N | % | N | % | P value* | |
| Gender | .20 | ||||
| Women | 7848 | 40.3 | 3926 | 39.5 | |
| Men |
11649 |
59.8 |
6018 |
60.5 |
|
| Age group (year) | .79 | ||||
| 20–39 | 154 | 0.79 | 77 | 0.77 | |
| 40–64 | 5262 | 27.0 | 2631 | 26.5 | |
| 65–74 | 6812 | 34.9 | 3486 | 35.1 | |
| ≥75 | 7269 | 37.3 | 3750 | 37.7 | |
| Mean (SD) (year) *
|
70.3 |
11.2 |
70.6 |
10.9 |
.02 |
| Medications | |||||
| Amiodarone | 2060 | 10.6 | 1445 | 14.5 | <.001 |
| Mexiletine | 1350 | 6.92 | 736 | 7.40 | .13 |
| Propafenone | 1218 | 6.25 | 707 | 7.11 | <.001 |
| Quinidine | 105 | 0.54 | 92 | 0.93 | <.001 |
| Procainamide | 119 | 0.61 | 59 | 0.59 | .86 |
| Statins | 6113 | 31.4 | 2092 | 21.0 | <0.001 |
| Anti-diabetics | 5249 | 26.9 | 4058 | 40.8 | <0.001 |
| NSAIDs |
19173 |
98.3 |
9858 |
99.1 |
<0.001 |
| Baseline comorbidities | |||||
| Diabetes | 4118 | 21.1 | 3055 | 30.7 | <.001 |
| Chronic liver disease and cirrhosis | 10478 | 53.7 | 9101 | 91.5 | <.001 |
| HBV infection | 730 | 3.74 | 3382 | 34.0 | <.001 |
| HCV infection | 593 | 3.04 | 4262 | 42.9 | <.001 |
| Alcoholism | 304 | 1.56 | 361 | 3.63 | <.001 |
*Chi-square test and t test comparing subjects with and without MNLIHD. Data are presented as the number of subjects in each group, with percentages given in parentheses.
Table 2 lists the crude and adjusted ORs of MNLIHD risk by arrhythmic drugs use and comorbidities. In the univariate logistic analysis, among the antiarrhythmic drugs we observed only amiodarone that was risk factors of MNLIHD (P<.001). Compared with non-amiodarone users, after adjusting for age, sex, use of antiarrhythmic drugs (propafenone and quinidine), and comorbidities, the OR of MNLIHD risk was 1.60-fold (95% CI, 1.45–1.77) higher for amiodarone users. Based on the multivariate analysis, chronic liver disease and cirrhosis (adjusted OR = 3.73, 95% CI, 3.42–4.07), HBV infection (adjusted OR = 15.2, 95% CI, 13.8–16.7), HCV infection (adjusted OR = 20.4, 95% CI, 18.5–22.5), alcoholism (adjusted OR = 2.04, 95% CI, 1.66–2.50), and anti-diabetics (adjusted OR = 2.03, 95% CI, 1.85–2.23) were associated with an increased risk of MNLIHD. However, patients with taking statins (adjusted OR = 0.62, 95% CI = 0.58–0.67) demonstrated a significant association with decreased MNLIHD risk.
Table 2. Odds Ratios (OR) and 95% Confidence Intervals (CI) of MNLIHD Associated with Amiodarone and Covariates.
|
Crude
|
Adjusted
†
|
|||
|---|---|---|---|---|
| Variable | OR | (95%CI) | OR | (95%CI) |
| Medications | ||||
| Amiodarone | 1.44 | (1.34, 1.55)*** | 1.60 | (1.45, 1.77)*** |
| Mexiletine | 1.07 | (0.98, 1.18) | - | - |
| Propafenone | 1.15 | (1.04, 1.26)*** | 0.97 | (0.85, 1.11) |
| Quinidine | 1.73 | (1.30, 2.28)*** | 1.32 | (0.91, 1.93) |
| Procainamide | 0.97 | (0.71, 1.33) | - | - |
| Statins | 0.58 | (0.55, 0.62)*** | 0.62 | (0.58, 0.67)*** |
| Anti-diabetics | 1.87 | (1.78,1.96) | 2.03 | (1.85, 2.23)*** |
| NSAIDs |
1.94 |
(1.53, 2.46)***
|
1.26 |
(0.92, 1.73) |
| Baseline comorbidities | ||||
| Diabetes | 1.66 | (1.57, 1.75)*** | 1.05 | (0.95, 1.16) |
| Chronic liver disease and cirrhosis | 9.29 | (8.61, 10.0)*** | 3.73 | (3.42, 4.07)*** |
| HBV infection | 13.3 | (12.2, 14.4)*** | 15.2 | (13.8, 16.7)*** |
| HCV infection | 23.9 | (21.8, 26.2)*** | 20.4 | (18.5, 22.5)*** |
| Alcoholism | 2.38 | (2.04, 2.78)*** | 2.04 | (1.66, 2.50)*** |
†Adjusted for statins, anti-diabetics, NSAIDS, antiarrhythmic drugs of propafenone, quinidine, comorbidities of diabetes, chronic liver disease, cirrhosis, HBV infection, HCV infection, and alcoholism
*P<.05
**P<.01
***P<.001.
Table 3 illustrates the interaction effect of antiarrhythmic drugs and comorbidities on MNLIHD. Compared with non-amiodarone users without any comorbidities, patients with comorbidities were associated with an increased risk of MNLIHD (adjusted OR = 12.6, 95% CI, 11.3–14.1), followed by patients using amiodarone (adjusted OR = 2.43, 95% CI, 1.92–3.06) and amiodarone users with comorbidities (adjusted OR = 18.0, 95% CI, 15.7–20.5; interaction P <.001). Interactions between amiodarone and HBV (adjusted OR = 18.6, 95% CI = 14.4–24.0; interaction P = .30) and HCV (adjusted OR = 25.9, 95% CI = 20.1–33.5; interaction P = .02) were also associated with increased MNLIHD risk.
Table 3. Interaction Effect between Medications and Comorbidities on Risk of MNLIHD.
| Variable | OR(95% CI) † | P value & | |
|---|---|---|---|
| Amiodarone | Comorbidity ‡ | <.001 | |
| No | No | As a reference | |
| No | Yes | 12.6 (11.3, 14.1)*** | |
| Yes | No | 2.43 (1.92, 3.06)*** | |
| Yes |
Yes |
18.0 (15.7, 20.5)*** |
|
| Amiodarone | HBV | 0.30 | |
| No | No | As a reference | |
| No | Yes | 14.0(12.7, 15.3)*** | |
| Yes | No | 1.48(1.36, 1.61)*** | |
| Yes |
Yes |
18.6(14.4, 24.0)*** |
|
| Amiodarone | HCV | 0.02 | |
| No | No | As a reference | |
| No | Yes | 23.6(21.4, 26.0)*** | |
| Yes | No | 1.52(1.39, 1.66)*** | |
| Yes |
Yes |
25.9(20.1, 33.5)*** |
|
| Mexiletine | Comorbidity ‡ | .29 | |
| No | No | As a reference | |
| No | Yes | 11.3 (10.2, 12.9)*** | |
| Yes | No | 0.88 (0.58, 1.32) | |
| Yes |
Yes |
12.1 (10.5, 13.9)*** |
|
| Propafenone | Comorbidity ‡ | .93 | |
| No | No | As a reference | |
| No | Yes | 11.4 (10.4, 12.7)*** | |
| Yes | No | 1.18 (0.82, 1.70) | |
| Yes |
Yes |
13.5 (11.7, 15.6)*** |
|
| Quinidine | Comorbidity ‡ | .72 | |
| No | No | As a reference | |
| No | Yes | 11.4 (10.4, 12.6)*** | |
| Yes | No | 1.96 (0.69, 5.62) | |
| Yes |
Yes |
17.9 (12.9, 25.0)*** |
|
| Procainamide | Comorbidity ‡ | .48 | |
| No | No | As a reference | |
| No | Yes | 11.4 (10.3, 12.6)*** | |
| Yes | No | 0.62 (0.15, 2.55) | |
| Yes | Yes | 12.2 (8.43, 17.6)*** |
†Adjusted for statins, anti-diabetics, and NSAIDS.
& P value for interaction.
‡Patients with any of the comorbidities (diabetes, chronic liver disease, cirrhosis, HBV infection, HCV infection, and alcoholism) were classified as the comorbidity group.
Furthermore, when estimating risk of MNLIHD based on cumulative DDD for amiodarone use, the amiodarone users were also associated with a higher risk of MNLIHD (Table 4). Compared with the non-amiodarone users, the MNLIHD risk was higher in patients who were administered >145 annual mean DDD of amiodarone (adjusted OR = 1.79, 95% CI, 1.50–2.15), followed by those administered 31–145 annual mean DDD of amiodarone (adjusted OR = 1.66, 95% CI, 1.38–1.99), and those administered ≤30 annual mean DDD of amiodarone (adjusted OR = 1.49, 95% CI, 1.30–1.70). Furthermore, we analyzed the association between MNLIHD and the time differences among the last amiodarone use and index date. Compared with non-amiodarone users, MNLIHD patients with amiodarone use within one year prior to index date were at the greatest risk (adjusted OR = 2.34, 95% CI, 2.05–2.67) and two year prior to index date of developing MNLIHD (adjusted OR = 1.22, 95% CI, 1.06–1.42) (Table 5).
Table 4. Odds Ratio (OR) and 95% Confidence Intervals (CI) of MNLIHD Associated with Annual Mean Daily Defined Dose (DDD) Use of Amiodarone.
|
|
Case/control N
|
Crude OR (95% CI)
|
Adjusted OR
†
(95% CI)
|
|---|---|---|---|
| Non-use of Amiodarone | 8499/17437 | 1.00 (reference) | 1.00 (reference) |
| Amiodarone | |||
| ≤30 DDD | 714/1729 | 1.44 (1.31, 1.59)*** | 1.49 (1.30, 1.70)*** |
| 31–145 DDD | 367/897 | 1.42 (1.24, 1.63)*** | 1.66 (1.38, 1.99)*** |
| >145 DDD | 364/879 | 1.45 (1.27, 1.66)*** | 1.79 (1.50, 2.15)*** |
| P for trend | <.001 |
†Adjusted for statins, anti-diabetics, NSAIDS, antiarrhythmic drugs of propafenone, quinidine, comorbidities of diabetes, chronic liver disease, cirrhosis, HBV infection, HCV infection, and alcoholism
*P<.05
**P<.01
***P<.001.
Table 5. Odds Ratios (ORs) for MNLIHD between Amiodarone and non- Amiodarone group.
|
|
Non-MNLIHD
|
MNLIHD
|
|
|
||
|---|---|---|---|---|---|---|
| Time for Amiodarone use | Amiodarone No. | % | Amiodarone No. | % | OR † | (95% CI) |
| No use | 17437 | 95.2 | 8499 | 91.6 | 1.00 | (reference) |
| Within one year prior to index | 872 | 4.76 | 783 | 8.44 | 2.34 | (2.05, 2.67)*** |
| Within two year prior to index | 978 | 5.31 | 564 | 6.22 | 1.22 | (1.06, 1.42)** |
†Adjusted for statins, anti-diabetics, NSAIDS, antiarrhythmic drugs of propafenone, quinidine, comorbidities of diabetes, chronic liver disease, cirrhosis, HBV infection, HCV infection, and alcoholism
**P<.01
***P<.001.
Discussion
We conducted a comprehensive population-based case-control study, using NHI databases to investigate the association of antiarrhythmic drug use with the risk of MNLIHD in a group of 29,441 arrhythmia patients. The MNLIHD group (9944 patients) and non- MNLIHD group (19,497 patients) were adjusted for baseline comorbidities, comprising diabetes, chronic liver disease, cirrhosis, HBV infection, HCV infection, and alcoholism, all of which might also be risk factors for MNLIHD. After adjustment for age, sex, statins, anti-diabetics, NSAIDS, propafenone use, and quinidine use, amiodarone users were still more likely to be diagnosed with MNLIHD (OR = 1.60, P<.001). The risk of developing MNLIHD was increased further for amiodarone users exhibiting comorbidities (adjusted OR = 18.0, P<.001) compared with those without comorbidities (OR = 2.43, P <.001), and the risk was also higher for patients who did not use amiodarone but exhibited comorbidities (OR = 12.6, P <.001). Among HBV and HCV patients, amiodarone also markedly exhibited an significant increment of MNLIHD risk. In addition, we observed a dose-dependent effect for the amiodarone-associated risk of MNLIHD, whereby patients with an annual mean DDD of ≤30 exhibited an OR of 1.49, those with an annual mean DDD of 31–145 exhibited an OR of 1.66, and those with an annual mean DDD of >145 exhibited an OR of 1.79 (all P <.001).
Oral antiarrhythmic agents are often classified according to their molecular targets, which are sodium channels (Class I), β-adrenergic receptors (Class II), potassium channels (Class III), and calcium channels (Class IV). Antiarrhythmics used in Taiwan include amiodarone, mexiletine, propafenone, quinidine, and procainamide, all of which have shared, multiple aforementioned targets [2, 15]. Although few case reports have described liver toxicity arising from the use of some antiarrhythmics, amiodarone has been extensively studied and has been demonstrated to induce chronic liver injury and cirrhosis in patient subgroups [5, 8, 9]. Primary HCC is the fifth leading cause of cancer deaths worldwide, and the second leading cause of cancer deaths in Taiwan [13, 16]. People diagnosed with cirrhosis are at particular risk of developing HCC [17]. Based on our results without adjustment for baseline comorbidities, treatment with amiodarone, propafenone, and quinidine, were all significantly associated with MNLIHD (P <.001); however, after adjusting for age, sex, statins, anti-diabetics, NSAIDS, propafenone use, quinidine use, and comorbidities, amiodarone use was the only significant risk factor for MNLIHD among the aforementioned antiarrhythmic drugs (OR = 1.60, P <.001).
Su et al. conducted a population-based cohort study to assess the risk of various cancers, including those of the lung, thyroid, skin, and liver, among patients treated with amiodarone, and revealed a borderline significant (standardized incidence ratios, 1.12; 95% CI, 0.99–1.26) increased risk associated with the use of this drug [12]. An analysis of data from a postmarketing surveillance report of the U.S. Food and Drug Administration (FDA) suggested that treatment with amiodarone was associated with the development of lung masses, thyroid cancer, and skin cancer [18]. However, the present population-based, case-control study is the first to report a significant association between the administration of amiodarone and the development of MNLIHD.
Amiodarone, a benzofuran Class III antiarrhythmic agent, was approved by the U.S. FDA in 1985 for the treatment of a wide spectrum of ventricular and supraventricular tachyarrhythmias [2]. Because of its fat-soluble properties and long half-life (53 d), amiodarone can accumulate in the soft tissues during long-term treatment and can thus potentially cause toxicity in several organ systems, including the thyroid gland, lung, skin, and liver [19]. Amiodarone and its N-dealkylated metabolites represent hepatic mitochondrial toxins [20], that can uncouple oxidative phosphorylation, inhibit complex I enzymatic reactions of the electron transport chain, and disrupt fatty acid β-oxidation, all of which are essential biological processes [21–24]. Long-term treatment with amiodarone lasting months or years might cause steatosis. It can evolve into steatohepatitis, a medley of inflammatory process, hepatocellular injury, and fibrosis, often resulting in cirrhosis and HCC [24, 25]. Although previous animal mutagenicity studies have revealed no amiodarone-induced carcinogenicity [26], several case reports have suggested a possible link between amiodarone use and the progression of malignancies in humans [27–32]. Additionally, the structure of amiodarone is similar to that of thyroxine, increasing the risk of both hypothyroidism and hyperthyroidism [33], and patients with thyrotoxicosis and goiter have been reported to have a 3% to 12% increased risk of developing thyroid cancer [34, 35]. In a meta analysis, 4 of 15 randomized controlled trials reported cancer-related deaths and revealed a significant increase in cancer mortality among patients assigned to an amiodarone group compared with a control group (0.8% vs 0.3%) [36]. Similarly, we observed that the presence of MNLIHD was significantly associated with amiodarone treatment, but not with administration of mexiletine, propafenone, quinidine, or procainamide (after adjusting for comorbidities). The lack of association between the administration of these other drugs and MNLIHD might be attributable to the small number of cases and the consequent lack of sufficient statistical power.
Numerous case reports have described the development of cancer following the regular use of amiodarone for 2 to 5 years [32, 37]. It has therefore been suggested that a latency period and the cumulative dose effects of amiodarone might be critical factors in the progression of amiodarone-associated malignancies. This hypothesis was supported by the study of Su et al. [12], in which a significant association was observed among male patients and those with a cumulative DDD (cDDD) of >180 within the first year, and male patients with a cDDD of >180 exhibited a high standardized incidence ratio of 1.46 (P = .008). By using the multivariate Cox regression model, they also confirmed a dose-dependent effect in the high and low cDDD tertiles regarding the incidence of cancer, with an adjusted hazard ratio of 1.98 (P = .006) [12]. In our study, the risk of MNLIHD was observed to be significantly associated with the dose of amiodarone in the multivariate analysis, after adjusting for age, sex, several medications (statins, anti-diabetics, NSAIDS, propafenone, quinidine) and comorbidities.
This study had limitations; first, the NHIRD in Taiwan does not provide patient details that might have constituted risk factors for MNLIHD, including body mass index, lifestyle factors (eg, physical activity, smoking, and alcohol consumption), environmental exposure, and family history of malignancy, and thus, these were not available for analysis. Second, we were unable to contact the patients directly to obtain additional information because of the anonymized nature of this database. The claims data in the NHIRD are also used primarily for administrative billing purposes and are not analyzed for scientific reliability for clinical studies.
Despite these limitations, this study is the first nationwide population-based case-control study of the association between MNLIHD and the use of antiarrhythmic agents. We observed a higher risk of MNLIHD among patients administered with amiodarone, particularly if they received a high DDD of this drug or exhibited comorbidities. This observed dose-dependent effect is consistent with the findings of Su et al. [12] The present study only addressed the amiodarone-related risk of MNLIHD, and larger scale trials and observational studies are required to assess the risk of amiodarone therapy for a wide range of other cancers.
Data Availability
All data and related metadata are deposited in an appropriate public repository: The study population’s data were from Taiwan NHIRD (http://w3.nhri.org.tw/nhird//date_01.html) are maintained by Taiwan National Health Research Institutes (http://nhird.nhri.org.tw/). The National Health Research Institutes (NHRI) is a non-profit foundation established by the government.
Funding Statement
These authors have no support or funding to report.
References
- 1. Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, et al. (2012) Canadian Cardiovascular Society Atrial Fibrillation Guidelines Committee. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 28:125–136. 10.1016/j.cjca.2012.01.021 [DOI] [PubMed] [Google Scholar]
- 2. Zimetbaum P (2012) Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 125:381–389. 10.1161/CIRCULATIONAHA.111.019927 [DOI] [PubMed] [Google Scholar]
- 3. Farver DK, Lavin MN (1999) Quinine-induced hepatotoxicity.Ann Pharmacother. 33:32–34. 10.1345/aph.18172 [DOI] [PubMed] [Google Scholar]
- 4. Cocozzella D, Curciarello J, Corallini O, Olivera A, Alburquerque MM, et al. (2003) Propafenone hepatotoxicity: report of two new cases. Dig Dis Sci. 48:354–357. 10.1023/A:1021943930424 [DOI] [PubMed] [Google Scholar]
- 5. Babatin M, Lee SS, Pollak PT (2008) Amiodarone hepatotoxicity. CurrVascPharmacol. 6:228–236. [DOI] [PubMed] [Google Scholar]
- 6. Younan LB, Barada KA, Faraj WG, Tawil AN, Jabbour MN, et al. (2013) Propafenone hepatotoxicity: report of a new case and review of the literature. Saudi J Gastroenterol. 19:235–237. 10.4103/1319-3767.118137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higa K, Hirata K, Dan K (1997) Mexiletine-induced severe skin eruption, fever, eosinophilia, atypical lymphocytosis, and liver dysfunction. Pain. 73:97–99. 10.1016/S0304-3959(97)00066-3 [DOI] [PubMed] [Google Scholar]
- 8. Arkun A, Van Deusen SK, Grau T, Birkhahn RH (2010) Hepatic dysfunction and neurotoxicity in a patient receiving long-term low-dose amiodarone therapy. J Emerg Med. 38:337–339. 10.1016/j.jemermed.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 9. Raja K, Thung SN, Fiel MI, Chang C (2009) Drug-induced steatohepatitis leading to cirrhosis: long-term toxicity of amiodarone use. Semin Liver Dis. 29:423–428. 10.1055/s-0029-1240011 [DOI] [PubMed] [Google Scholar]
- 10. Mondardini A, Pasquino P, Bernardi P, Aluffi E, Tartaglino B, et al. (1993) Propafenone-induced liver injury: report of a case and review of the literature. Gastroenterology. 104:1524–1526. [DOI] [PubMed] [Google Scholar]
- 11. Chuang LC, Tunier AP, Akhtar N, Levine SM (1993) Possible case of procainamide-induced intrahepatic cholestatic jaundice. Ann Pharmacother. 27:434–437. [DOI] [PubMed] [Google Scholar]
- 12. Su VY, Hu YW, Chou KT, Ou SM, Lee YC, et al. (2013) Amiodarone and the risk of cancer: a nationwide population-based study. Cancer. 119:1699–1705. 10.1002/cncr.27881 [DOI] [PubMed] [Google Scholar]
- 13. Department of Health. Taiwan: Main causes of death in 2012. Available: http://www.doh.gov.tw. Accessed 2014 April.
- 14. Bureau of National Health Insurance, Department of Health. Bureau of National Health Insurance, Department of Health: Taiwan. Available: http://www.nhi.gov.tw/webdata/AttachFiles/Attach_14258_2_97-ch.pdf. Accessed. 2010 Sep.
- 15. Mason JW (1987) Amiodarone. N Engl J Med. 316: 455–466. 10.1056/NEJM198702193160807 [DOI] [PubMed] [Google Scholar]
- 16. Malek NP, Schmidt S, Huber P, Manns MP, Greten TF (2014) The diagnosis and treatment of hepatocellular carcinoma. DtschArzteblInt. 111:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, et al. (2014) Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 59:1577–1590. 10.1002/hep.26898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration (2004) Medication Guide: AmiodaroneHCl NDA 18–972/S-038/039. Washington, DC: US Food and DrugAdministration; 2004 Available: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/018972s038s039lbl.pdf. [Google Scholar]
- 19. Stelfox HT, Ahmed SB, Fiskio J, Bates DW (2004) Monitoringamiodarone’s toxicities: Recommendations, evidence, and clinical practice. Clin Pharmacol Ther. 75: 110–122. 10.1016/j.clpt.2003.09.010 [DOI] [PubMed] [Google Scholar]
- 20. Zahno A, Brecht K, Morand R, Maseneni S, Torok M, et al. (2011) The role of CYP3A4 in amiodarone-associatedtoxicity on HepG2 cells. Biochem Pharmacol. 81: 432–441. 10.1016/j.bcp.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 21. Morse RM, Valenzuela GA, Greenwald TP, Eulie PJ, Wesley RC, et al. (1988) Amiodarone-induced liver toxicity. AnnIntern Med. 109: 838–840. [DOI] [PubMed] [Google Scholar]
- 22. Lewis JH, Ranard RC, Caruso A, Jackson LK, Mullick F, et al. (1989) Amiodarone hepatotoxicity:Prevalence and clinicopathologic correlations among 104 patients. Hepatology. 9: 679–685. 10.1002/hep.1840090504 [DOI] [PubMed] [Google Scholar]
- 23. Lewis JH, Mullick F, Ishak KG, Ranard RC, Ragsdale B, et al. (1990) Histopathologic analysis of suspected amiodarone hepatotoxicity. Hum Pathol. 21: 59–67. 10.1016/0046-8177(90)90076-H [DOI] [PubMed] [Google Scholar]
- 24. Dowman JK, Hopkins LJ, Reynolds GM, Nikolaou N, Armstrong MJ, et al. (2014) Development of hepatocellular carcinoma in a murine model of nonalcoholic steatohepatitisinduced by use of a high-fat/fructose diet and sedentary lifestyle. Am J Pathol. 184:1550–61. 10.1016/j.ajpath.2014.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waldhauser KM, Török M, Ha HR, Thomet U, Konrad D, et al. (2006) Hepatocellular toxicity and pharmacologicaleffect of amiodarone and amiodarone derivatives. J Pharmacol Exp Ther. 319: 1413–1423. 10.1124/jpet.106.108993 [DOI] [PubMed] [Google Scholar]
- 26. Brambilla G, Martelli A (2009) Update on genotoxicity and carcinogenicitytesting of 472 marketed pharmaceuticals. Mutat Res. 681:209–229. 10.1016/j.mrrev.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 27. Arnon R, Raz I, Chajek-Shaul T, Berkman N, Fields S, et al. (1988) Amiodarone pulmonary toxicity presenting as a solitary lung mass. Chest. 93:425–427. 10.1378/chest.93.2.425 [DOI] [PubMed] [Google Scholar]
- 28. Piccione W Jr, Faber LP, Rosenberg MS (1989) Amiodarone-inducedpulmonary mass. Ann Thorac Surg. 47:918–919. 10.1016/0003-4975(89)90037-4 [DOI] [PubMed] [Google Scholar]
- 29. Monk BE (1995) Basal cell carcinoma following amiodarone therapy. Br JDermatol. 133:148–149. 10.1111/j.1365-2133.1995.tb02515.x [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez-Garcia JL, Garcia-Nieto JC, Ballesta F, Prieto E, Villanueva MA, et al. (2001) Pulmonary mass and multiple lung nodulesmimicking a lung neoplasm as amiodarone-induced pulmonary toxicity. Eur J Intern Med. 12:372–376. 10.1016/S0953-6205(01)00127-3 [DOI] [PubMed] [Google Scholar]
- 31. Azzam I, Tov N, Elias N, Naschitz JE (2006) Amiodarone toxicity presentingas pulmonary mass and peripheral neuropathy: the continuing diagnostic challenge. Postgrad Med J. 82:73–75. 10.1136/pgmj.2005.040105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maoz KB, Dvash S, Brenner S, Brenner S (2009) Amiodarone-inducedskin pigmentation and multiple basal-cell carcinomas. Int J Dermatol. 48:1398–1400. 10.1111/j.1365-4632.2008.03819.x [DOI] [PubMed] [Google Scholar]
- 33. Inaba H, Suzuki S, Takeda T, Kobayashi S, Akamizu T, et al. (2012) Amiodarone-induced thyrotoxicosis with thyroid papillary cancer inmultinodular goiter: case report. Med Princ Pract. 21:190–192. 10.1159/000333697 [DOI] [PubMed] [Google Scholar]
- 34. Trip MD, Wiersinga W, Plomp TA (1991) Incidence, predictability, andpathogenesis of amiodarone-induced thyrotoxicosis and hypothyroidism. Am J Med. 91:507–511. 10.1016/0002-9343(91)90187-3 [DOI] [PubMed] [Google Scholar]
- 35. Mellemgaard A, From G, Jorgensen T, Johansen C, Olsen JH, et al. (1998) Cancer risk in individuals with benign thyroid disorders. Thyroid. 8:751–754. 10.1089/thy.1998.8.751 [DOI] [PubMed] [Google Scholar]
- 36. Piccini JP, Berger JS, O’Connor CM (2009) Amiodarone for the preventionof sudden cardiac death: a meta-analysis of randomized controlledtrials. Eur Heart J. 30:1245–1253. 10.1093/eurheartj/ehp100 [DOI] [PubMed] [Google Scholar]
- 37. Hall MA, Annas A, Nyman K, Talme T, Emtestam L (2004) Basaliomaafter amiodarone therapy-not only in Britain. Br J Dermatol. 151:932–933. 10.1111/j.1365-2133.2004.06193.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and related metadata are deposited in an appropriate public repository: The study population’s data were from Taiwan NHIRD (http://w3.nhri.org.tw/nhird//date_01.html) are maintained by Taiwan National Health Research Institutes (http://nhird.nhri.org.tw/). The National Health Research Institutes (NHRI) is a non-profit foundation established by the government.