Abstract
Background
Allowing patients to measure their blood pressure (BP) at home will be the standard for evaluating the disease state as the process of clinical diagnosis, and it is recognized as having great clinical utility. To measure BP as accurately as possible, innovative techniques have been incorporated into home BP measurement devices.
Objective
The present study aimed to evaluate the performance of the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z), which are equipped with functions to detect irregular pulses and arm movement that lead to inaccurate BP readings.
Methods
A team of three trained medical doctors validated the performance of these devices by comparing the data alternatively obtained from both devices with those from a standard mercury sphygmomanometer.
Results
The magnitude of the difference in BP readings between the tested device and the standard mercury sphygmomanometer in the Omron BP765 and BP760N was within the range of ±3 mmHg (mean) allowed by the American National Standards Institute, Inc/Association for the Advancement of Medical Instrumentation/International Organization for Standardization (ANSI/AAMI/ISO) 81060-2:2009 guidelines.
Conclusion
The Omron BP765 and BP760N were found useful for the self-measurement of BP at home, and their performance fulfilled the requirement of the ANSI/AAMI/ISO 81060-2:2009 guidelines.
Keywords: blood pressure, self-measurement, device, validation, ANSI/AAMI/ISO 81060-2:2009
Introduction
Enabling patients to measure their blood pressure (BP) at home is valuable for the prevention and early detection of hypertension. Since hypertension is a major cause of death that can directly or indirectly influence cardiovascular complications,1 an easy and accurate home BP measurement (HBPM) may help prevent cardiovascular diseases in the future. Recently, HBPM was found to be superior to clinical (casual) BP measurement (CBPM) for the detection and ongoing follow-up of hypertension.2–4 Current guidelines for treating hypertension5–6 claim that HBPM is better than CBPM in clinic patients. Thus, the important clinical role of HBPM has been described in those guidelines, and many devices for HBPM are available on the market worldwide.7–8 To measure BP accurately, the cuff must be wrapped correctly, and proper posture of the patient during the measurement is required. Therefore, home monitoring devices equipped with automated cuff wrapping and a display indicating correct posture have been introduced. In addition, irregular pulses due to arrhythmias and noises caused by arm movements lead to inaccurate BP readings. In the present study, two devices in two separate investigations were validated according to the international protocol of the American National Standards Institute, Inc/Association for the Advancement of Medical Instrumentation/International Organization for Standardization (ANSI/AAMI/ISO) 81060-2:2009 guidelines.9
Methods
Devices
The Omron BP765 (HEM-7311-ZSA; Omron Healthcare Co, Ltd, Kyoto, Japan) is an automatic oscillometric device for BP measurements of the upper arm. The Omron BP765 has a pressure range of 0–299 mmHg and a heart rate range of 40–180 beats/minute. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate are displayed on a liquid crystal digital (LCD) monitor. The inflation uses a fuzzy-logic system controlled by an electric pump. The deflation is by means of an automatic pressure release valve. The dimensions of the device are approximately 183 mm ×99 mm ×230 mm (width × height × depth). The cuff can be used for arm circumferences ranging from 22–42 cm. The Omron BP765 includes memory for 100 measurements for two users. It can also calculate an average value based on the last three measurements taken within 10 minutes. The Omron BP765 can measure BP every minute for three consecutive times, and then the average of those three measurements appears on the display. It detects and displays an irregular heartbeat and body movement during BP measurements.
The Omron BP760N (Hem-7320-Z; Omron Healthcare Co, Ltd) is an automatic oscillometric device for BP measurements of the upper arm. It functions just like the Omron BP765. The device’s dimensions are approximately 124 mm ×90 mm ×161 mm, and the arm cuff can be used for arm circumferences of 22–42 cm. It includes memory for 60 measurements for two users. It can also calculate an average value based on the last three measurements taken within 10 minutes, and it detects and displays an irregular heartbeat and body movements during BP measurements.
Blood pressure measurements
For each experiment, the manufacturer provided standard production device models. The validation team for each device consisted of three observers who were experienced in performing BP measurements. They were trained by the British Hypertension Society’s online program (http://www.bhsoc.org). BP measurements were alternated between the mercury sphygmomanometer and the device. Simultaneous auscultations were performed by two observers using the double stethoscope (Y tube), and the BPs were measured. The two observers were blinded to each other’s readings, and the third observer served as a supervisor who checked the BP readings by the two observers.
Subject selection
All subjects were either outpatients recruited from the department of cardiology or were volunteer employees from the Kansai Medical University Hospital (Osaka, Japan). This study was approved by the institutional review board, and written informed consent was obtained from each subject. To participate, subjects were required to be ≥20 years old with an arm circumference of 22–42 cm and able to provide their informed consent. Subjects with arrhythmias, those who moved their arms during the BP measurements, and those with a DBP that was unclear during phase V of the Korotkoff sounds were excluded from the study. In accordance with the ANSI/AAMI/ISO81060-2:2009, subjects were recruited to ensure that sex, age, arm circumference, and BP readings fulfilled the participation conditions.
Procedure
The subjects were seated in a quiet room with a comfortable room temperature, and they were instructed to avoid talking during the procedure. The BP measurements started after a 10-minute rest.
The subjects sat in a chair with their legs uncrossed and their feet flat on the floor. The chair had a supportive back as well as elbow and forearm rests.
Each subject’s arm circumference was measured, and the cuff size was adapted. All BP measurements were performed on the subject’s left arm at the level of the heart.
The devices were validated according to the same-arm, sequential method of the ANSI/AAMI/ISO 81060-2:2009 guidelines. The starting order of the auscultation and use of the device was alternated between the subjects based on the ISO81060-2:2009 requirements.
Analysis
Data analysis was performed according to the ISO81060-2:2009 requirements. Each experiment analyzed criteria 1 and 2.
For criterion 1, the number of device readings and auscultations were counted separately. This sequence was carried out alternately with test device measurements and auscultation. Measurement number of auscultation and test device measurements was the same number. Same measurement number of device readings and auscultation was paired, and this pair was analyzed. All the data for the differences were calculated as the mean ± standard deviation (SD).
For criterion 2, the mean number of device readings and auscultations were calculated for each subject. It compared the differences between average of reference readings on each subject and average of test device readings in each subject. All the subjects’ data were calculated as the mean ± SD.
The data were expressed as the mean ± SD, and the minimum and maximum values with the ranges were calculated.
Results
Omron BP765 (HEM-7311-ZSA)
We screened 99 subjects for this experiment. After excluding 14 subjects according to the criteria specified in the Subject selection section, 39 men (46%) and 46 women (54%) were included fulfilling the subjects requirements of ISO81060-2:2009.
The mean age of subjects was 49±12.6 years old (range, 21–78 years old). The mean arm circumference was 32.3±5.8 cm (range, 22.2–41.9 cm). The percentages of subjects were 44% (criteria ≥40%), 31% (≥20%), 56% (≥40%), and 22% (≥20%) in the arm circumference range of 22.0–32.0 cm, 22.0–27.0 cm, 32.1–42.0 cm, and 37.1–42.0 cm, respectively.
Using the standard mercury sphygmomanometer, the mean values of the 255 measurements were 130±9.7 (range, 84–188) for SBP and 83±13.6 (range, 55–122) mmHg for DBP.
The percentages for high SBP (≥160 mmHg), medium SBP (≥140 mmHg), and low SBP (≤100 mmHg) were 5% (criteria; ≥5%), 34% (criteria; ≥20%), and 7% (criteria; ≥5%), respectively. The percentages of high DBP (≥100 mmHg), medium DBP (≥85 mmHg), and low DBP (≤60 mmHg) were 11% (criteria; ≥5%), 45% (criteria; ≥20%) and 6% (criteria; ≥5%), respectively.
The differences between the two observers were 0±1.3 mmHg and 0±1.3 mmHg for SBP and DBP, respectively. The mean differences between the two observers and the Omron BP765 were 2±5.5 mmHg (range, −13 to 19 mmHg) for SBP and 2±5.1 mmHg (range, −14 to 16 mmHg) for DBP according to criterion 1. These data fulfilled the ISO81060-2:2009 requirements of ≤5±≤8 mmHg. The mean differences between the two observers and the Omron BP765 were 1±4.4 mmHg (range, −9 to 12 mmHg) for SBP and 2±4.4 mmHg (range, −11 to 16 mmHg) for DBP according to criterion 2. Thereby, the SD for SBP is calculated to be less than 6.8 mmHg, and for DBP 6.6 mmHg by criterion l. These results are in accordance with the ISO81060-2:2009 requirements for criteria 1 and 2. Therefore, the Omron BP765 fulfills the validation criteria of the ISO81060-2:2009 requirements.
Figure 1A and B show the differences in the SBP and DBP readings in relation to the mean differences between the BP765 readings and mercury sphygmomanometer measurements.
Figure 1.
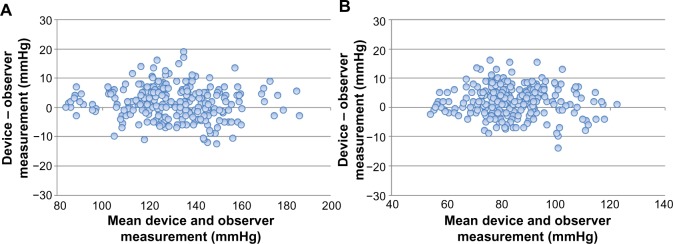
Bland–Altman plots for the differences between the Omron BP765 readings and the observer measurements for systolic blood pressure (A) and diastolic blood pressure (B).
Omron BP760N (HEM-7320-Z)
We screened 105 subjects for this experiment. In the final analysis, we included 85 subjects, 40 men (47%) and 45 women (53%). The mean age of subjects was 48±11.1 years old (range, 22–79 years old). The mean arm circumference was 32.0±5.0 cm (range, 23.5–42.0 cm).
The percentages of subjects were 46%, 28%, 54%, and 22% in the arm circumference ranges of 22.0–32.0 cm, 22.0–27.0 cm, 32.1–42.0 cm, and 37.1–42.0 cm, respectively.
Using the standard mercury sphygmomanometer, the mean values for the 255 measurements were 127±22.8 mmHg (range, 81–177 mmHg) for SBP and 82±14.5 mmHg (range, 48–121 mmHg) for DBP. The percentages of high SBP (≥160 mmHg), medium SBP (≥140 mmHg), and low SBP (≤100 mmHg) were 11%, 26%, and 16%, respectively. The percentages of high DBP (≥100 mmHg), medium DBP (≥85 mmHg), and low DBP (≤60 mmHg) were 11%, 40%, and 7%, respectively.
The differences between the two observers were 0±1.3 mmHg and 0±1.4 mmHg for SBP and DBP, respectively. The mean differences between the observers and the Omron BP760N was −1±5.3 mmHg (range, −14 to 17 mmHg) for SBP and 0±5.0 mmHg (range, −15 to 15 mmHg) for DBP according to criterion 1. The mean differences between the two observers and the Omron BP760N were −1±4.1 mmHg (range, −13 to 13 mmHg) for SBP and 0±4.3 mmHg (range, −15 to 14 mmHg) for DBP according to criterion 2. Thereby, allowance of SD for SBP was less than 6.9 mmHg and for DBP, less than 7.0 mmHg according to the criteria. These results are in accordance with the ISO81060-2:2009 requirements for criteria 1 and 2. Therefore, the Omron BP760N fulfills the validation criteria of the ISO81060-2:2009 requirements.
The difference between the device readings and the mean BP value of the Omron BP760N and the differences between the two observers for all 255 points for SBP and DBP are shown in Figure 2A and B, respectively.
Figure 2.
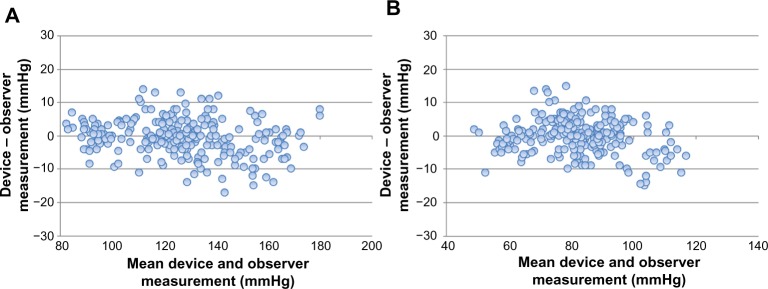
Bland–Altman plots for the differences between the Omron BP760N readings and the observer measurements for systolic blood pressure (A) and diastolic blood pressure (B).
Discussion
The Omron BP765 and BP760N fulfilled the validation criteria of the ANSI/AAMI/ISO81060-2:2009 guidelines and the subject selection (ie, sex, age, arm circumference, and BP level ranges) adequately satisfied the protocol for validating both devices. The mean differences between the two observers were <1 mmHg for SBP and DBP in both of the experiments. The most important point was the differences in the readings between the test device and the sphygmomanometer, which were +1.5 mmHg (mean difference) and +2.2mmHg (mean difference) for SBP and DBP, respectively according to criteria 1 and 2 for the Omron BP 765, which was similar for the Omron BP 760N (−0.6 and −0.1 mmHg for SBP and DBP, respectively). However, the SD was significantly greater because we measured the BP in the same arm by alternating between the test device and sphygmomanometer. The BP changes every moment due to fluctuation of the autonomic nervous system, which controls BP;10 thus, a different timing of the BP measurement, even within a short period, can result in significant difference in BP. This is why the validation guidelines9,11,12 were proposed, and these two devices passed the ANSI/AAMI/ISO81060-2:2009 guidelines.
These compact self-measuring BP devices can detect irregular pulses that cause incorrect BP readings. Moreover, they detect noises and wave pulses within the cuff that are caused by arm movements. These novel device functions may help provide accurate BP readings. Since self-BP measurement at home provides useful information not only for the management of BP in hypertensive patients but also for health care professionals,13,14 these devices are useful for everyone, including normotensives, hypertensives, and health care professionals.
Conclusion
Our findings indicated that the Omron BP760N and BP765 are accurate devices for self-measuring BP. In addition, these devices were easy to manipulate when measuring BP, and the displays were easy to read.
Acknowledgments
We are grateful to the volunteers who agreed to have their blood pressure measured for the purpose of validating the Omron BP760N and BP765 and to Omron Healthcare Co, Ltd, for supplying these standard devices used in the present study. This research received no special grant from any funding agency by public, commercial, or not-for-profit organizations but was supported by the Kansai Medical University.
Footnotes
Disclosure
The Omron Healthcare Co, Ltd provided the standard devices, BP760N and BP765, used in the present study. The authors have no other conflicts of interest in the work.
References
- 1.Ikeda N, Saito E, Kondo N, et al. What has made the population of Japan healthy? Lancet. 2011;378(9796):1094–1105. doi: 10.1016/S0140-6736(11)61055-6. [DOI] [PubMed] [Google Scholar]
- 2.Stergiou GS, Ntineri A, Kollias A, Ohkubo T, Imai Y, Parati G. Blood pressure variability assessed by home measurements: a systematicre view. Hypertens Res. 2014;37(6):565–572. doi: 10.1038/hr.2014.2. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep. 2013;15(11):413. doi: 10.1007/s11886-013-0413-z. [DOI] [PubMed] [Google Scholar]
- 4.Uhlig K, Patel K, Ip S, Kitsios GC, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159(3):185–194. doi: 10.7326/0003-4819-159-3-201308060-00008. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence (NICE) Hypertension: management of hypertension in adults in primary care. NICE clinical guideline 34. London: National Institute for Health and Care Excellence; 2006. [Accessed July 23, 2014]. Available from: http://www.nice.org.uk/CG34. [Google Scholar]
- 6.Shimamoto K. 110th Scientific Meeting of the Japanese Society of Internal Medicine: Symposium: 3. Social impacts and controversial points of clinical practice guidelines; 1) Overview of guidelines: concepts of making clinical practice guidelines and discussion on their social impacts. (3) Guideline for managements of hypertension-concept and social significance. Nihon Naika Gakkai Zasshi. 2013;102(9):2296–2300. doi: 10.2169/naika.102.2296. Japanese. [DOI] [PubMed] [Google Scholar]
- 7.Bramlage P, Deutsch C, Krüger R, et al. Validation of the custo screen 400 ambulatory blood pressure-monitoring device according to the European Society of Hypertension International Protocol revision 2010. Vasc Health Risk Manag. 2014;10:303–309. doi: 10.2147/VHRM.S63602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang YY, Zeng WF, Zhang L, Li Y, Wang JG. Validation of the AVITA BPM15S wrist blood pressure monitor for home blood pressure monitoring according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2014;19(3):183–186. doi: 10.1097/MBP.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 9.Association for the Advancement of Medical Instrumentation American National Standard: non-invasive sphygmomanometers – part 2: clinical validation of automated measurement type; ANSI/AAMI/ISO. 2009. [Accessed January 1, 2014]. 81060–81062. Available from: http://www.aami.org/subscriptions/cdchart.pdf.
- 10.Grassi G, Bombelli M, Brambilla G, et al. Total cardiovascular risk, blood pressure variability and adrenergic overdrive in hypertension: evidence, mechanisms and clinical implications. Curr Hypertens Rep. 2012;14(4):333–338. doi: 10.1007/s11906-012-0273-8. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien E, Pickering T, Asmar R, et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit. 2002;7(1):3–17. doi: 10.1097/00126097-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien E, Atkins N, Stergiou G, et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15(3):23–38. doi: 10.1097/MBP.0b013e3283360e98. [DOI] [PubMed] [Google Scholar]
- 13.Stergiou GS, Asayama K, Thijs L, et al. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675–682. doi: 10.1161/HYPERTENSIONAHA.113.02741. [DOI] [PubMed] [Google Scholar]
- 14.Bobrie G, Postel-Vinay N, Delonca J, Corvol P, SETHI Investigators Self-measurement and self-titration in hypertension: a pilot telemedicine study. Am J Hypertens. 2007;20(12):1314–1320. doi: 10.1016/j.amjhyper.2007.08.011. [DOI] [PubMed] [Google Scholar]


