Abstract
Purpose
To describe daily walking stride rate patterns of young children with cerebral palsy (CP) as compared to a typically developing youth (TDY) cohort relative to age and functional level.
Method
A cross-sectional comparison cohort study compared 209 youth with CP with 368 TDY aged 2–13 years. Youth with CP had Gross Motor Function Classification System (GMFCS) levels I–III with 60% bilateral involvement and 79% spastic. Five days of StepWatch data were averaged and classified into low, moderate and high stride rates. Group differences were examined by t-test and analysis of variance.
Results
Children with CP walk significantly less each day than TDY (F = 245, p ≤ 0.001) and differ by GMFCS (F = 1.51, p < 0.001). TDY walk a similar number of strides in low and moderate stride rates each day while youth with CP do not. TDY attained high stride rates (>60 strides/min) for 8 min/d with levels I–III at 4.0, 3.2 and 0.53 min/d, respectively.
Conclusions
The relative relationship of walking intensity levels within total daily stride activity differs for youth with CP as compared to TDY. The influence of functional walking ability on walking stride activity levels and intensity does not appear to differ significantly across age groups.
Keywords: Cerebral palsy, stride rates, walking activity
Background
Cerebral palsy (CP) is a common disabling condition presenting in childhood that influences individuals across the life span with a reported prevalence of 3.3–3.8/1000 in the United States [1]. The condition is defined as a group of disorders of movement and postural development, which results in limitation of physical activity such as walking. These disorders are attributed to non-progressive disturbances to the developing fetal and infant brain. Ambulatory children with CP have limitations in walk/run/jump skills, are physically less active and walk fewer steps each day. A recent review of habitual physical activity reported that young persons with CP participate in 13–53% less habitual physical activity in daily life than their peers regardless of age or functional level [2]. These activity limitations appear to restrict day-to-day participation in mobility, education, social relations and mobility throughout the life span [3–5].
Strides (steps) taken per minute (or cadence) is a common temporal-spatial parameter of gait. It is related to intensity and can be employed to describe patterns of community-based walking activity by tracking time spent or number of strides taken in ranges of increasing strides rates [6]. Recent work has examined strides per minute to describe intensity of ambulation in laboratory studies of youth [7–10]. Graser et al. in 2009 documented in typically developing youth (TDY) aged 10–12 years that walking at 120 steps/min or greater was associated with moderate to vigorous activity [7]. Similarly, self-selected walking speeds for short distances in 10-year olds was documented at 126.14 ± 10.29 steps/min [11]. Using a waist-mounted accelerometer (Actigraph), Barreira et al. published the first accelerometer data of free-living US children and youth in 2012 [6]. They examined stride rate patterns and peak rates for typically developing US children and adolescents by age and body mass. For monitor-wearing time, this population-based sample of TDY averaged approximately 4 h/d of inactivity, with approximately 9 h/d at rates of 1–59 steps/min and only about 3 min/d at rates >120 steps/min. Values for all peak walking indicators were lower for youth classified as obese versus those with normal weight by body mass index.
A longitudinal study of children with CP documented that overall gross motor function peaks at approximately 7 years of age and declines through adolescence [12]. In 2007, the community-based walking activity in 81 adolescents with CP between 10 and 14 years of age was examined with the ankle worn StepWatch (SW) accelerometer [13]. As compared to a cohort of typically developing children, youth with CP took significantly fewer steps per day and spent less time walking and engaging in activities of high intensity. Stevens et al. in 2012 also examined the daily walking levels (SW) of youth with CP between the ages of 4 and 18 years to an age-matched cohort [14]. Their work confirmed that older children with CP take significantly fewer steps/day, were more inactive with lower stride rates than TDY as well as younger children with CP. Walking activity levels of youth with CP have also been documented to be lower during weekend versus weekdays and influenced by age, topography of motor impairment and functional motor classification [15].
The literature suggests that approximately 75% of ambulatory youth with CP, who lose functional walking skill as they age, will experience this decline before age 25 [16]. Decreases in walking skill and endurance from childhood levels have also been reported by adults with CP [17]. These same adults noted decreased life satisfaction related to the loss of mobility skills. Thus, diminishing functional performance, gait efficiency and activities of daily living are considered secondary complications of this non-progressive disorder as a person with CP ages [18–20]. Despite the stable nature of CP from an etiological perspective, the determinants of this decrease in walking activity are not well understood. Clinically, this decline in mobility is thought to be related to muscular strength and endurance that is unable to accommodate functionally to the change in body mass with growth.
How is walking activity (levels and/or intensity) performance information helpful in the clinical management of children with CP? New literature has documented that the relationship of what a child is “able” to perform in a clinic setting and their participation in life is significantly mediated by what they physically “actually” do each day in ambulatory children with CP [21]. These results suggest that participation in day-to-day life maybe positively influenced by interventions focusing on improving what they actually do every day regardless of capacity to perform. Building on these associations, recent findings also suggest that walking activity performance (average strides/day and number of strides, >30 strides/min/d) is significantly associated with the accomplishment of mobility-based life habits (i.e. personal care, mobility in community and recreation) [22].
Thus, in order to inform rehabilitation strategies to potentially enhance walking performance and participation, a better understanding of day-to-day walking activity and intensity in childhood is warranted. This project proposes to examine the walking stride activity levels and intensity patterns of children and youth with CP as compared to a typically developing cohort relative to age and functional level.
Methods
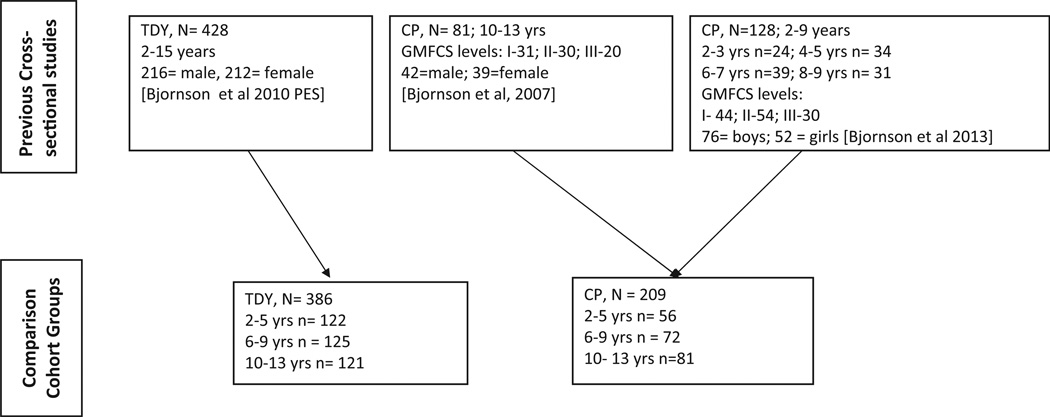
This comparison cohort project is a secondary data analysis of three separate cross-sectional studies, each individually approved by the local institutional review board of a regional children’s hospital in the Pacific Northwest United States (see Figure 1). Data for the typically developing cohort was collected within a cross-sectional descriptive cohort study documenting walking activity with the SW accelerometer in 428 children 2–14 years of age [23]. Walking activity data for youth with CP are from a comparison cohort study of 81 adolescents with CP aged 10–14 years [13] and a project examining the relationship of walking and physical activity to participation in 128 youth with CP aged 2–9 years [22]. Data were collected across the calendar year and between September 2004 and October 2011 with specific recruitment and enrollment strategies documented previously. A subset of 121 participants with (N = 81) and without (N = 30) CP aged 10–14 years, underwent data collection only during the school year [13]. All three projects recruited through direct-approach letters mailed from three specialty care pediatric facilities in the Pacific Northwest United States to potential participants and their families as well as to care providers.
Figure 1.
Participant sources for comparison cohorts of typically developing youth (TDY) and youth with cerebral palsy (CP).
The inclusion criterion for CP group was as follows: (1) 2 to <14 years of age, (2) diagnosis of CP and (3) Gross Motor Function Classification System (GMFCS) levels of I–III. Exclusion criteria included: (1) lower extremity botulinum toxin injections in the last three months, (2) visual or cardiopulmonary impairment limiting physical activity, (3) uncontrolled seizure disorder impacting mobility skills and (4) orthopedic or neurosurgery in the last 6 months. Demographic information was collected by interview of the parent with GMFCS level confirmed by in-person observation of motor skills by the first author during a single research visit.
All data collection occurred during center- or home-based research visits, where participants were instructed to wear the SW device during all of their waking hours except when swimming or bathing for seven consecutive days. This included wearing their current orthotics and/or using assistive devices for mobility as needed. The SW accelerometer is a specifically designed two-dimensional device, which is validated to document when the heel leaves the ground during walking (a step or stride taken) during walking activity within day-to-day life [24]. Comparison to frequently employed pedometers and accuracy to manual counts has confirmed the accuracy and precision of the SW for detecting strides taken [25–27]. The device is designed and validated for individualized calibration of the walking stride patterns by taking into account the height, stride type and activity level. Visually observed strides were counted with a manual counter during a 100+ walking trial and compared to the SW stride counts. Five days (24 h of monitoring) of the seven-day SW data samples were selected for analysis (four week days and one weekend day). Valid SW monitoring data was defined as days with less than three hours of inadequate monitoring (i.e. upside down) or no stride counts that were unexplained (i.e. swimming/bathing) during waking hours (6:00 am to 10:00 pm). Participants wore the SW on their lateral ankle (inside a knit cuff) for seven days. The device was returned to the study team during a home- or center-based research visit or by mail with a postage paid padded envelope.
Statistical analysis
Participant characteristics were summarized using descriptive statistics. Means and standard deviations described continuous variables and frequencies with proportions used to describe categorical variables. We compared continuous variables across groups using Student t test and analysis of variance (ANOVA). Chi-squared test and Fisher’s exact test were employed to compare categorical variables across groups.
Walking stride activity was derived from the average of five days of SW data (24 h) for each participant. Based on examination of the range of stride activity for this sample of TDY and children with CP, we classified walking activity intensity in no activity (0 strides/min), low stride rate (1–30 strides/min), moderate stride rate (31–60 strides/min) and high stride rate (>60 strides/min). Walking activity was quantified with the variables of (1) average time inactive (not walking or 0 strides/min), (2) average peak stride rate (average highest 1-min stride rate), (3) average number of minutes and (4) strides spent per day at low, moderate and high stride rates. We examined between group differences (TDY versus CP) for all walking performance variables using t tests. ANOVA was employed to test for differences across GMFCS levels for the youth with CP. We examined the effect of age within each GMFCS level and tested the age-by-GMFCS interaction with a regression model. A p value less than 0.05 was considered statistically significant.
Results
The study sample comprised a total of 209 children and youth with CP compared to a cohort of 368 typically developing ranging in age from 2.0 to 13.9 years with an average age of 8.2 (SD = 3.4) years (see Table 1). Fifty percent of the TDY cohort was female with the cohort with CP 44% (Fishers Exact; p = 0.12) were female. Gender for the participants with CP ranged from 39 to 49% female (p = 0.86), and the overall study sample was 80% Caucasian. The percent of mothers reporting some college was significantly higher for the participants with CP (p < 0.001). Average calibration accuracy ranged from 90 to 112% with an average of 99.7 (SD = 3.8) with the lowest calibration accuracy for the 2- to 5-year-old participants with CP (mean = 97.8, SD = 2.8). Calibration accuracy for youth with CP (GMFCS levels I–III) were not significantly different by age group (Chi-square; p = 0.36). The majority of the participants had spasticity as their primary movement disorder, and there were significantly more participants with CP who had bilateral involvement (p < 0.001).
Table 1.
Characteristics of participants with cerebral palsy (CP) and typically developing youth (TDY) by age groups of 2–5, 6–9 and 10–13 years.
| Typically developing youth TDY (n) |
% Female |
Mean age years [SD] |
% Caucasian |
SES% some collegea |
% Calibration accuracy [SD] |
GMFCS levels (n) | Movement disorder % spastic |
% Bilateral CPa |
||
|---|---|---|---|---|---|---|---|---|---|---|
| 2–5 yr (122) | 50 | 4.0 [1.2] | 75 | 12 | 99.6 [4.5] | N/A | N/A | N/A | ||
| 6–9 yr (125) | 49 | 8.0 [1.1] | 82 | 10 | 100.5 [3.7] | N/A | N/A | N/A | ||
| 10–13 yr (121) | 50 | 12 [1.2] | 83 | 20 | 100.1 [3.8] | N/A | N/A | N/A | ||
| Total 2–13 yr (368) | 50 | 8.0 [3.4] | 80 | 14 | 100.1 [4.0] | N/A | N/A | N/A | ||
| Cerebral palsy (CP) | ||||||||||
| 2–5 yr (56) | 39 | 4.0 [1.2] | 71 | 27 | 97.8 [2.8] | I – 21 | II – 19 | III – 16 | 73 | 50 |
| 6–9 yr (72) | 42 | 7.9 [1.2] | 90 | 33 | 99.0 [3.0] | I – 23 | II – 35 | III – 14 | 71 | 50 |
| 10–13 yr (81) | 49 | 11.8 [1.3] | 78 | 40 | 99.5 [3.2] | I – 30 | II – 31 | III – 20 | 90 | 77 |
| Total 2–13 yr (209) | 44 | 8.3 [3.4] | 80 | 34 | 98.9 [3.1] | I – 75 | II – 84 | III – 50 | 79 | 60 |
Chi-square – χ2 ≤ 0.001.
The comparison of walking inactivity, peak stride rate/minute, average strides and min/d at low, moderate and high stride ranges by GMFCS levels and the comparison TDY cohort is displayed in Table 2. The time each day not walking for youth with CP was significantly different by GMFCS level (F = 1.51, p < 0.001). Children with CP across all GMFCS levels spent significantly more time each day not walking as compared to the TDY cohort (F = 245, p ≤ 0.001). Peak stride rate attained within GMFCS levels were significantly different (p < 0.001) except for GMFCS level II to level I (−1.4, p = 0.37). When we added the GMFCS-by-age interaction term in these regression models, none of the coefficients were significant (p = 0.160–0.77); therefore, we only reported results with GMFCS and age main effects. The TDY cohort attained significantly higher peak stride rates (73.1 versus 67, p < 0.001) than the participants with CP.
Table 2.
Summary of time inactive, peak stride/min rate, average number of strides and time spent in low, moderate and high stride rates from five days of StepWatch data.
| Average inactive time min/d (SD) |
Average peak stride/min rate (SD) |
Average number strides Low (SD) |
Average number strides Moderate (SD) |
Average number strides High (SD) |
Time low min/d (SD) |
Time moderate min/d (SD) |
Time high min/d (SD) |
|
|---|---|---|---|---|---|---|---|---|
| TDY (n = 368) | 966 (82) | 73.1 (8.8) | 3992 (757) | 3856 (1826) | 533 (662) | 374 (63) | 91.3 (41.3) | 8.0 (9.7) |
| CP (N = 209) | ||||||||
| GMFCS | ||||||||
| Level I (n = 75) | 1038* (96) | 70 (8.2) | 3436 (887) | 2599 (1426) | 262 (298) | 335 (70) | 48 (25) | 4.0 (4.5) |
| Level II (n = 84) | 1073 (86) | 69 (7.7) | 2977 (861) | 2004 (1039) | 213 (250) | 315 (70) | 48 (25) | 3.0 (3.7) |
| Level III (n = 50) | 1221 (89) | 60 (13.5) | 1347 (840) | 433 (465) | 36 (84) | 208 (82) | 11 (11.5) | 0.53 (1.1) |
SD = standard deviation. Low = 1–30 strides/min, moderate = 31–60 strides/min and high = >60 strides/min.
ANOVA: p < 0.001.
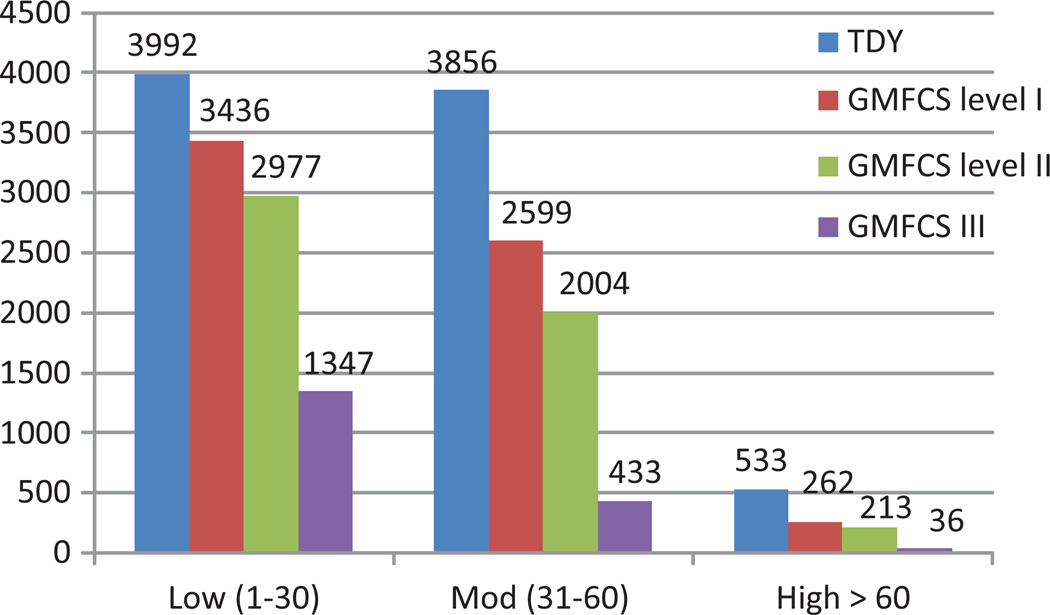
Figure 2 and Table 2 display the average number strides spend in low, moderate and high stride rates for the TDY and youth with CP by GMFCS levels. The average number of strides spent in low and moderate stride ranges decreases significantly with GMFCS level (p = 0.02 to <0.001), and all are significantly lower than the TDY cohort (p < 0.001). Strides completed above 60 strides/min were not significantly different between GMFCS levels I and II, yet all GMFCS levels were significantly lower than the TDY cohort (p < 0.001).
Figure 2.
Average number of strides spent at low, moderate and high stride rates for typically developing youth (TDY) and youth with cerebral palsy by Gross Motor Function Classification level (GMFCS 1–3). Low = 1–30 strides/min, moderate = 31–60 strides/min and high = >60 strides/min.
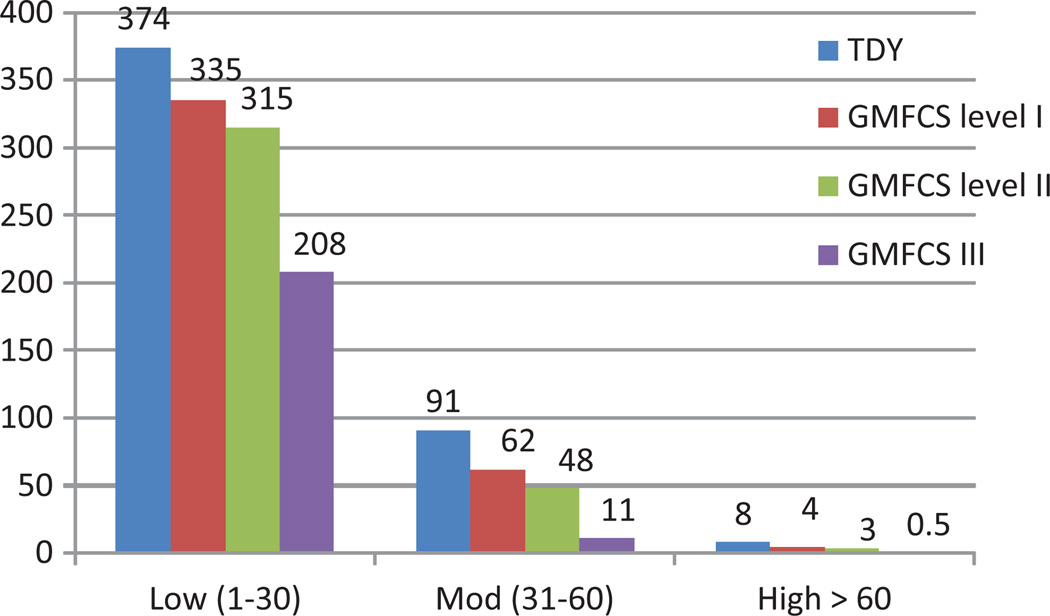
Time spent at low, moderate and high stride rates by functional levels is represented in Figure 3 and Table 2. Typically developing children in this sample spent on an average 6 h/d walking at a rate of 1–30 strides/min, while those at GMFCS level I spent on an average 39 min less, level II approximately 1 h less and level III spends 3 less hours (see Table 2). Time spent in the moderate stride range 31–60 strides/min, for the TDY cohort was approximately 1.5 h, with participants at GMFCS level I just over one hour and dropping to about 11 min for children at GMFCS level III. High stride rates were attained by the TDY cohort for 8 min/d with GMFCS level I at half that duration (4.0 min), Level II at 3.2 min and level III just under 1 min. Kids in older age group tend to spend significantly less time in activities with stride rates >60 strides/min than younger kids.
Figure 3.
Average number of min/d spent at low, moderate and high stride rates for typically developing youth (TDY) and youth with cerebral palsy by Gross Motor Function Classification level (GMFCS 1–3). Low = 1–30 strides/min, moderate = 31–60 strides/min and high = >60 strides/min. One day (24 h) = 1440 min.
Discussion
This project documents walking stride rate patterns generated from a convenience sample of TDY and two cohorts of youth with CP ages of 2–13 years. Walking activity was derived from a summation of minutes and average number of strides spent across stride rates bands (low, moderate and high) based on five days of monitoring with the SW accelerometer. The methods employed have potential for clinical translation through documentation of longitudinal day-to-day walking activity. Walking stride rates and patterns can be employed to examine the influence of rehabilitation strategies (i.e. orthotics, assistive devices, treadmill training, injection therapies and orthopedic or neurological surgery) on walking performance in youth with CP.
Children with CP appear to demonstrate increasing levels of non-walking activity similar to TDY as they age. This may be a function of increase in leg length with somatic growth (thus needing to take fewer strides) and/or changing opportunities for walking activity as they progress in school. Previous work has documented that walking activity in TDY decreases with age even with adjustment for leg length [23]. The TDY stride activity presented in this paper are consistent with the decreasing steps/minute documented with age via a waist-mounted device in a US population-based sample by Barreira et al. in 2012 [6]. The results of this project suggest that TDY appear to walk a similar number of strides in low and moderate stride rate activities. In contrast, the 2012 Tudor-Locke analysis suggests that TDY decrease their steps/d in the moderate rate as compared to the low stride rate range (low = 2197–2444 steps/min; moderate = 934–1523 steps/min) [6]. It should be noted that the work of Barreira et al. reports “steps” taken (counting both legs), while this paper reports “strides” of one leg. This may be a function of differing devices and/or sensitivity to actual steps taken (Actigraph versus SW), attachment sites (waist versus ankle) and wearing time (of active monitor time versus 24 h) [6,15]. Differing from the TDY cohort, youth with CP in this analysis, appear to take fewer strides in the moderate stride range than the low range. This decreasing ability to reach and/or attain higher stride rates is consistent with the clinical presentation of youth with CP who have difficulty keeping up with peers in situations that require the ability to bust or ramp up walking intensity (i.e. soccer, T-ball and playground play).
Stride activity levels for youth with CP aged 2–13 years as a group are lower than the TDY cohort and have differing patterns of stride rate activity by each GMFCS level. This is consistent with earlier work by the primary author who examined the older subset of adolescents (10–13 years) [13,28]. Interaction analysis suggests that functional walking level (GMFCS) appears to override the influence of age in youth with CP for average number of strides taken and/or time spent in the three stride rate bands examined for youth at GMFCS levels II and III. Age and GMFCS level appear to influence high stride rate activity for youth at GMFCS level I. These results are in contrast to data reported by Steven et al. examining walking activity in 27 youth aged 4–18 years with and without CP with the SW [14], where there was a documented significant influence of age. Stevens et al., who employed a dichotomous age analysis presumably due to sample size, did not analyze relative to functional level (GMFCS) and participants with CP were primarily at GMFCS level I.
Relative to levels of physical activity intensity, Graser et al. have suggested that rates >120 steps/min can be considered moderate to vigorous physical activity (MPVA) [7]. Greater than 120 steps/min is comparable to the SW > 60 stride/min as the SW only counts the strides/steps of one leg. Walking thus as part of overall physical activity has potential to enhance levels of physical activity and health. This TDY cohort on an average demonstrated approximately 8.0 min/d at that level, which is over three times greater than the 2.5 min/d documented by Barreira et al. in an analysis of the US NHANES walking activity data 2005–2006 for youth 6–19 years of age [6]. Yet, well below the recommended 60 min/d of MVPA recommended for optimal health benefits for TDY [29], these level of walking activity supports the premise that few children are reaching these recommended cut points. This difference in time at MPVA, again, maybe due to differing monitoring devices and their relative sensitivity to steps taken during walking. For the youth with CP in this project, their overall average time at MPVA (high stride rates) across GMFCS levels was similar (2.5 min/d) to the average levels reported by Barreira et al.
Limitation should be noted with interpretation of these results. (1) This was secondary analysis of data, which was not all collected within the same time frame. Thus, influences of varying environmental and personal factors may bias the results. (2) This was a cross-sectional analysis of a convenience sample matched by age ranges only from one geographical area. Longitudinal tracking of walking stride activity and patterns would be helpful to understand how these patterns change as children with and without disability age. Replication of these methods on adolescents followed into adulthood would help clarify factors influencing the documented decline in functional mobility reported by adults with CP. (3) A larger sample would confirm the differences noted between GMFCS levels as the standard deviations for some variables and functional levels were relatively wide. (4) The study sample appears biased toward Caucasian youth from higher socioeconomic levels (mother’s education) compared to recent demographic characteristics of CP prevalence estimates in the United States [1]. Thus, future work needs to exam these findings within a population-based sample with broader racial and socioeconomic status.
What are the clinical or rehabilitation implications of this work? First, these results suggest that youth with CP regardless of GMFCS level and TDY are demonstrating maximal walking activity levels that are far below those recommended for overall health. Thus, both populations need to be encouraged to walk and/or be more active more each day. The monitoring methods presented have potential to inform interventions specifically for walking optimization as well as overall health. For example, this device may be used to document the effect of a particular orthotic prescription and/or surgical intervention on day-to-day walking performance.
The relative relationships between the number of strides in low, moderate and high strides rates differ for children with CP as compared to TDY in this sample as measured by the SW. While TDY youth walk a relatively similar number of strides in low and moderate stride rates, youth with CP do not. Could this limitation in the ability to attain moderate stride intensity, regardless of total number of strides taken each day, be a potential area of focus for rehabilitation strategies for youth with CP? The newly documented association of higher walking activity and intensity to enhanced levels of accomplishment of mobility-based life habits suggests the need to explore walking activity and/or intensity-based interventions to enhance the life experience of children with CP [22].
Research is warranted to examine whether rehabilitation strategies can increase daily stride rates and/or walking intensity. Rehabilitation strategies may focus on opportunities and/or interventions to enhance moderate or high stride rate activities. Short-burst interval treadmill training would be an example of such a therapeutic modality, which is currently being tested by this study team. It is unknown whether the ability to enhance moderate or high stride rates in walking will differ by GMFCS level. Based on clinical observations, youth at GMFCS level I may have the greatest potential to enhance the higher intensity walking stride rates than those at level III. It is possible that interventions may not increase overall number of strides taken each day but result in a shift from lower to higher stride rates. Future work should explore how daily stride levels and/or intensity may inform and focus interventions aimed at enhancing habitual walking activity and the day-to-day lives of ambulatory children with CP.
Implications for Rehabilitation.
Limitation in the ability to attain moderate stride-rate intensity, regardless of total number of strides taken each day for ambulatory youth with CP, is a potential area of focus for intervention.
Understanding of stride activity levels and intensity in youth with CP may be employed to focus rehabilitation strategies to enhance habitual walking activity.
Community-based stride rate data has potential as an effectiveness outcome for rehabilitation strategies focused on walking (i.e. orthopedic surgery, orthoses and injections therapies).
Acknowledgments
This work was supported by funding from NIH K23 HD060764 and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1RR025014, Rehabilitation Science Training Grant (T32 HD07424) through the Department of Rehabilitation Medicine at the University of Washington, NIH NRSA award through NINDS (F31-NS048740) and by a Hester McLaws Nursing Scholarship. Staheli Endowment Fund, Clinical Steering Committee Research Award, Department of Orthopedic Surgery, Seattle Children’s Hospital, University of Washington School of Medicine-Medical Student Research Training Program, Seattle, Washington and CTSA 1 UL1 RR025014-01 (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of interest
None of the investigators have significant financial interest in this research, products, or its sponsors.
References
- 1.Yeargin-Allsopp M, Van Naarden K, Dorenberg NS, et al. Prevalence of cerebral paly in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 2.Carlon SL, Taylor NF, Dodd KJ, Shields N. Differences in habitual physical activity levels of young people with cerebral palsy and their typically developing peers: a systematic review. Disabil Rehabil. 2013;35:647–655. doi: 10.3109/09638288.2012.715721. [DOI] [PubMed] [Google Scholar]
- 3.Colver AF. The definition and classification of cerebral palsy. Dev Med Child Neurol. 2007;49:15–16. doi: 10.1111/j.1469-8749.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- 4.Gage JR. Gait analysis in cerebral palsy. London: MacKeith Press; 1991. [Google Scholar]
- 5.Sutherland DH, Sussman MD. The diplegic child. Chicago: American Academy of Orthopedic Surgeons; 1992. Outcome assessment in cerebral palsy: has walking improved? pp. 133–144. [Google Scholar]
- 6.Barreira TV, Katzmarzyk PT, Johnson WD, Tudor-Locke C. Cadence patterns and peak cadence in US children and adolescents: NHANES, 2005–2006. Med Sci Sports Exerc. 2012;44:1721–1727. doi: 10.1249/MSS.0b013e318254f2a3. [DOI] [PubMed] [Google Scholar]
- 7.Graser SV, Pangrazi RP, Vincent WJ. Step it up: activity intensity using pedometers. J Phys Educ Recreat Dance. 2009;80:22–24. [Google Scholar]
- 8.Jago R, Watson K, Baranowski T, et al. Pedometer reliability, validity and daily activity targets among 10- to 15-year-old boys. J Sports Sci. 2006;24:241–251. doi: 10.1080/02640410500141661. [DOI] [PubMed] [Google Scholar]
- 9.Jago R, Fox K, Page A, et al. Physical activity and sedentary behaviour typologies of 10–11 year olds. Int J Behav Nutr Phys Act. 2010;7:59–69. doi: 10.1186/1479-5868-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graser SV, Groves A, Prusak KA, Pennington TR. Pedometer steps-per-minute, moderate intensity, and individual differences in 12- to 14-year-old youth. J Phys Act Health. 2011;8:22–24. doi: 10.1123/jpah.8.2.272. [DOI] [PubMed] [Google Scholar]
- 11.Dusing SC, Thorpe DE. A normative sample of temporal and spatial gait parameters in children using the GAITRite® electronic walk-way. Gait Posture. 2007;25:135–139. doi: 10.1016/j.gaitpost.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Hanna SE, Rosenbaum PL, Bartlett DJ, et al. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol. 2009;51:295–302. doi: 10.1111/j.1469-8749.2008.03196.x. [DOI] [PubMed] [Google Scholar]
- 13.Bjornson KF, Belza B, Kartin D, et al. Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys Ther. 2007;87:248–257. doi: 10.2522/ptj.20060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens SL, Holbrook EA, Fuller DK, Morgan DW. Influence of age on step activity patterns in children with cerebral palsy and typically developing children. Arch Phys Med Rehabil. 2010;91:1891–1896. doi: 10.1016/j.apmr.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Wely L, Becher JG, Balemans ACJ, Dallmeijer AJ. Ambulatory activity of children with cerebral palsy: which characteristics are important? Dev Med Child Neurol. 2012;54:436–442. doi: 10.1111/j.1469-8749.2012.04251.x. [DOI] [PubMed] [Google Scholar]
- 16.Murphy KP, Molnar GE, Lankasky K. Medical and functional staus of adults with cerebral palsy. Dev Med Child Neurol. 1995;37:1075–1084. doi: 10.1111/j.1469-8749.1995.tb11968.x. [DOI] [PubMed] [Google Scholar]
- 17.Jahnsen R, Villien L, Egeland T, et al. Locomotion skills in adults with cerebral palsy. Clin Rehabil. 2004;18:309–316. doi: 10.1191/0269215504cr735oa. [DOI] [PubMed] [Google Scholar]
- 18.Maher CA, Williams MT, Olds T, Lane AE. Physical and sedentary activity in adolescents with cerebral palsy. Dev Med Child Neurol. 2007;49:450–457. doi: 10.1111/j.1469-8749.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 19.Novacheck T, Gage J. Orthopedic management of spasticity in cerebral palsy. Childs Nerv Syst. 2007;23:1015–1031. doi: 10.1007/s00381-007-0378-6. [DOI] [PubMed] [Google Scholar]
- 20.Bottos M, Gericke C. Ambulatory capacity in cerebral palsy: prognostic criteria and consequences for intervention. Dev Med Child Neurol. 2003;45:786–790. doi: 10.1017/s0012162203001452. [DOI] [PubMed] [Google Scholar]
- 21.Bjornson K, Zhou C, Stevenson RD, Christakis D. Capacity to participation in cerebral palsy: evidence of an indirect path via performance. Arch Phys Med Rehabil. 2013 doi: 10.1016/j.apmr.2013.06.020. [Epub ahead of print]. http://dx.doi.org/10.1016/j.apmr.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjornson K, Zhou C, Stevenson RD, Christakis D. Relationship of stride activity to participation in mobility-based life habits among children with cerebral palsy. Arch Phys Med Rehabil. 2013 doi: 10.1016/j.apmr.2013.10.022. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjornson K, Song K, Lisle J, et al. Measurement of walking activity throughout childhood: influence of leg length. Pediatr Exerc Sci. 2010;22:581–595. doi: 10.1123/pes.22.4.581. [DOI] [PubMed] [Google Scholar]
- 24.OrthoCare Innovations. StepWatch Function. [last accessed 11 Oct 2013];2013 Available from: http://orthocareinnovations.com/pages/stepwatch_trade. [Google Scholar]
- 25.Bjornson KF, Yung D, Jacques K, et al. Accuracy and precision of the StepWatch in stride counting and oxygen consumption. J Pediatr Rehabil Med. 2012;5:7–14. doi: 10.3233/PRM-2011-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster RC, Lanningham-Foster LM, Manohar C, et al. Precision and accuracy of ankle-worn acclerometer-based pedometer in step counting and energy expenditure. Prev Med. 2005;41:778–783. doi: 10.1016/j.ypmed.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Mitre N, Lanningham-Foster L, Foster R, Levine JA. Pedometer accuracy in children: can we recommend them for our obese population? Pediatrics. 2009;123:e127–e131. doi: 10.1542/peds.2008-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjornson KF, Song K, Zhou C, et al. Walking stride rate patterns in children and youth. Pediatr Phys Ther. 2011;23:354–363. doi: 10.1097/PEP.0b013e3182352201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Dept of Health and Human Services. Physical Activity Guidelines for Americans: Fact Sheet for Professionals. [last accessed 2 Jun 2013];2012 Available from: http://www.health.gov/paguidelines/factsheetprof.aspx.