Abstract
Background:
Biologic agents have revolutionized the management of psoriasis but at a higher cost compared with “traditional” agents. Cost must be considered when evaluating management options for psoriasis.
Objective:
To estimate the annual cost of treatment of psoriasis using biologic agents and assess the trend over the past decade.
Methods:
The cost of annual treatment paradigms for etanercept, adalimumab, and ustekinumab was estimated using the average wholesale price. Trends were assessed by calculating the percentage change in annual cost compared with the previous year. A sales-based cost of drugs was estimated using gross US sales of each drug and an estimate of the total number of patients treated based on prescription data.
Results:
The cost of one year of induction and maintenance treatment was highest for ustekinumab ($53,909), followed by etanercept ($46,395), and adalimumab ($39,041). The sales-based cost of drugs was greatest for ustekinumab ($25,012), then adalimumab ($6,786) and etanercept ($6,629). Sales-based cost increased at an average of 20% per year.
Conclusion:
The cost of biologic treatments for psoriasis has been increasing. Cost considerations in the management of psoriasis are likely to increase given the limited healthcare resources that are available.
Keywords: cost analysis, etanercept, adalimumab, ustekinumab, psoriasis
Introduction
Psoriasis is a chronic autoimmune skin condition that affects ≈8 million Americans [1]. Approximately 10% of affected Americans have moderate-to-severe psoriasis that necessitates use of phototherapy or systemic medications, yet many patients remain undertreated [2,3]. According to a survey undertaken by the National Psoriasis Foundation in 2001, 40% of respondents felt frustrated with the ineffectiveness of therapies, and 32% felt that treatment was not sufficiently aggressive [4].
Biologic medications (“biologics”) have revolutionized the management of psoriasis. Biologic agents target specific steps in the immune pathways that lead to psoriasis [5]. Biologics are not metabolized by the cytochrome P450 system, so drug-interaction problems are limited [6]. In addition, compliance with treatment using biologics, though not perfect, is higher than that observed with other psoriasis medications (e.g. topical agents, phototherapy) [6]. Biologics are expensive, but the cost is offset by: significant reductions in the number of hospital stays; use of other systemic therapies; improved Psoriasis Area and Severity Index (PASI) outcomes; and increased satisfaction by patients [7,8].
There are two main categories of biologic treatments for psoriasis: tumor necrosis factor (TNF)-α inhibitors (e.g. etanercept, adalimumab) and interleukin-12/23 inhibitors (e.g. ustekinumab). Cost analyses of these drugs have yielded estimates from $7,993 a year to $48,000 a year [9–11]. Given these high sums, cost is an important consideration for physicians and patients before choosing a biologic agent. Here, we provide an updated estimate of the annual cost of etanercept, adalimumab, and ustekinumab, and review the literature regarding cost analyses pertinent to these drugs. We also discuss the growing role of cost-effectiveness in treatment guidelines.
Methods
Annual drug costs
Annual drug costs were estimated for etanercept, adalimumab, and ustekinumab. The cost of each drug was estimated according to the average wholesale price (AWP) from the Red Book Drug Topics, 2014 [12]. All costs were calculated in US dollars. Additional costs, such as hospitalizations, physician visits, laboratory tests, and adverse events, were not included in this cost analysis. The cost of etanercept was based on a recommended initiation treatment regimen of 50 mg twice weekly for 3 months, followed by 50 mg once weekly [13]. For adalimumab, the cost estimate consisted of an 80-mg loading dose, followed by 40 mg every other week beginning one week after the initial dose [14]. For ustekinumab, recommended treatment doses differ depending on patient weight (≤100 kg or >100 kg). Consequently, a patient weight of 80 kg was assumed. The annual cost estimate included a dose of 45 mg at 0- and 4-weeks, and 45 mg every 12 weeks thereafter [15].
A trend of drug costs from 2004 to 2014 was calculated using the AWP/unit specific to each year listed in Red Book Drug Topics for each drug. Annual cost was then estimated using the same treatment paradigm as outlined above. In addition, the percentage change in annual cost was calculated, and compared with overall annual inflation and annual healthcare inflation.
Estimation of “sales-based cost”
Wholesale prices may not provide an accurate measure of what payers are paying for biologics because prices may be modified by contract issues. To estimate cost after accounting for drug discounting, a sales-based estimate of cost for a biologic drug for psoriasis was calculated using the following formula:
Gross sales in the US were appraised through the 2013 Securities and Exchange Commission 10K Annual Reports for Amgen and Abbvie, the producers of etanercept and adalimumab, respectively [16,17]. Sales of ustekinumab were monitored through annual sales and earnings reports for Johnson and Johnson [18].
A direct estimate of the total number of patients taking each drug was not available. To estimate the total number of patients taking each drug, we used prescription data available on the Bloomberg L.P. database to determine the total number of prescriptions (refills and new) from 2011 to 2013. The total number of prescriptions (refills and new) each year was divided by two based on the assumption that each psoriasis patient receives two prescriptions a year for biologic agents. Information from the database is derived from Symphony Health Solutions (provider of data on prescription audits in the US). It provides information on weekly and monthly retail, non-retail, and mail-order prescription activity.
Results
Expected annual costs for biologic drugs
Estimated annual cost of biologic treatment ranged from $36,038 (adalimumab) to $44,924 (ustekinumab). The cost was higher during the first year (when loading doses are required): $46,395 vs $37,111 for etanercept, $39,041 vs $36,038 for adalimumab, and $53,909 vs $44,924 for ustekinumab (Table 1).
Table 1.
Comparison of annual treatment costs for psoriasis using biologic agents.
| Treatment | Recommended dosing schedulea | Average wholesale priceb (2014 USD) | Initial then maintenance (USD) | Maintenance (USD) |
|---|---|---|---|---|
| Etanercept | Initial: 50 mg twice weekly for 3 months, then 50 mg once weekly | 15.47/mg | 46,395 | 37,111 |
| Adalimumab | Initial: 80 mg single dose Maintenance: 40 mg every other week beginning 1 week after initial dose |
46.92/mg | 39,041 | 36,038 |
| Ustekinumab | Initial: Assuming ≤100 kg: 45 mg at 0- and 4-weeks, then every 12 weeks thereafter | 196.63/mg | 53,909 | 44,924 |
All three drugs increased in cost from 2004 to 2014 (Figure 1). The percentage change in cost for etanercept from 2004 to 2014 was 120%, for adalimumab from 2004 to 2013 was 103%, and for ustekinumab from 2010 to 2014 was 53%. For the five-year interval 2010–2014, the change in cost for etanercept was 48% and for adalimumab was 64%.
Figure 1.
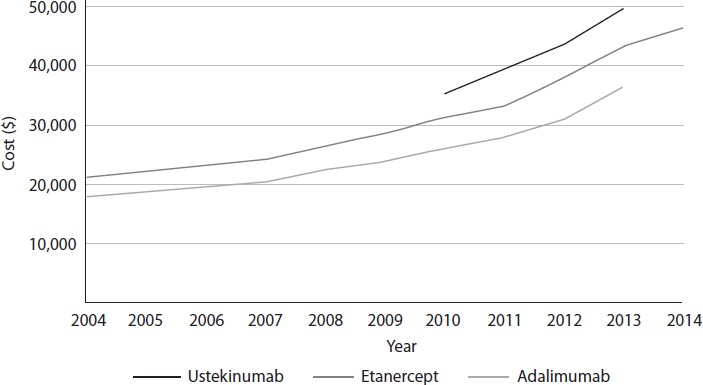
Trends in cost for the first year of treatment from 2004 to 2014.
The average annual rate of increase was of the same order of magnitude for all three drugs: 8.2% for etanercept, 9.2% for adalimumab, and 11.0% for ustekinumab (Figure 2).
Figure 2.
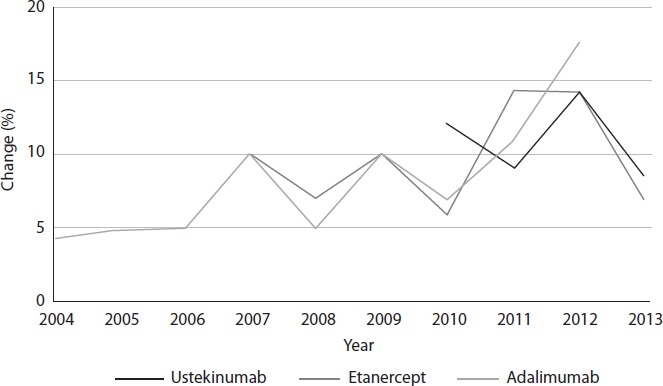
Percent change in annual cost of biologic drugs.
These rates of increase were higher than the overall inflation rate of 1.5% and the overall healthcare inflation rate of 2.5% [19,20].
Sales-based cost of drugs
Our sales-based cost of drugs was lower than published wholesale cost rates, which may reflect the effects of contracting (as was anticipated; Table 2). Sales-based cost in 2013 was greatest for ustekinumab ($25,012), followed by adalimumab and etanercept, which were priced similarly at $6,786 and $6,629, respectively (Table 2). The percent change in price from 2011 to 2013 was 17–23% for etanercept and adalimumab, and 11–39% for ustekinumab.
Table 2.
Estimated sales-based cost of biologic drugs [gross US sales ($)/total number of patients treated*].
| 2013 | % Change | 2012 | % Change | 2011 | |
|---|---|---|---|---|---|
| Etanercept | |||||
| Sales-based cost | 6,629 | 18 | 5,603 | 17 | 4,756 |
| US sales (million)a | 4,256 | 3,967 | 3,458 | ||
| Total prescriptions dispensedb | 1.286 million | 1.416 million | 1.454 million | ||
|
| |||||
| Adalimumab | |||||
| Sales-based cost | 6,786 | 19 | 5,662 | 23 | 4,600 |
| US sales (million)c | 5,236 | 4,377 | 3,426 | ||
| Total prescriptions dispensedb | 1.543 million | 1.546 million | 1.489 million | ||
|
| |||||
| Ustekinumab | |||||
| Sales-based cost | 28,161 | 11 | 25,390 | 39 | 18,247 |
| US sales (million)d | 957 | 627 | 443 | ||
| Total prescriptions dispensedb | 67,966 | 49,390 | 48,555 | ||
Amgen. 2013 Annual Report and 10-K, 2013 [16].
Bloomberg L.P. (2014) Retail Scripts/Institution Sales. [Retrieved 17 April 2014 from Bloomberg database].
AbbVie. 2013 Annual Report on Form 10-K and 2014 Proxy Statement, 2013 [17].
Johnson J. Sales and Earnings, 2014 [18].
Total number of patients treated estimated by dividing the total number of prescriptions dispensed by two (assuming patients receive two prescriptions per year).
Discussion
The cost of one year of induction and maintenance treatment was highest for ustekinumab ($53,909), followed by etanercept ($46,395), and adalimumab ($39,041). These estimates are considerably higher compared with previous estimates: $7,993 to $48,731 per year for etanercept, $19,000 to $37,000 for adalimumab, and $15,243 to $34,951 for ustekinumab [1,9–11, 21–24]. These differences may be attributable to: differences in treatment paradigms; variations in valuation methodology; increases in the AWP of drugs over time. For example, some studies included the cost of laboratory tests and office visits [1,9,10,21,23], and used the wholesale acquisition price for drug costs [25,26]. The AWP is typically set 20–25% above the wholesale acquisition cost or list price [27]. These studies were conducted several years ago and our calculations suggest that the AWP increased over time, especially for ustekinumab (>50% over five years). Hence, it is likely that our higher estimate is also due to a rising AWP.
Inflation could be another potential cause for the higher costs estimated in this analysis. However, our calculated increases in cost clearly outstrip the increase in Consumer Price Index-Urban for overall and healthcare-specific inflation – a finding that echoes the work of Beyer et al. [22]. Other factors that may be responsible (at least in part) for rising costs include: increasing costs for research and development; costs of subject recruitment; increasing competition in the market for drugs with similar efficacies; increasing safety regulation (which translates into longer and more complicated clinical trials) [28]. Biologic agents are also more complicated to produce compared with more “traditional” therapies [22].
Compared with biologic treatment of other systemic immune-mediated diseases, the cost of biologic therapy for psoriasis is similar or slightly lower. For example, certolizumab pegol for rheumatoid arthritis costs $79,750 a year based on the AWP at a maintenance regimen of 200 mg every other week [12]. Natalizumab is used in a regimen for Crohn’s disease of 300 mg every 4 weeks, and costs $67,370 a year based on wholesale prices [12].
Balancing efficacy and cost in guidelines
Given the economic climate, it is prudent to consider cost-effectiveness when establishing treatment guidelines. The UK National Institute for Health and Clinical Excellence (NICE) incorporates efficacy and cost-effectiveness in the recommendations in its guidelines [29]. These guidelines suggest that methotrexate should be the first agent used in individuals who meet the criteria for systemic therapy. There is no recommendation as to which biologic agent should be administered first. However, the guidelines recommend use of biologics including ustekinumab, etanercept, or adalimumab if the psoriasis is severe (PASI ≥10 and Dermatology Life Quality Index (DLQI) >10) and has not responded to systemic therapies including cyclosporine, methotrexate, and psoralen combined with ultraviolet A, or if the patient has a contraindication to these therapies (e.g. pregnancy, leukopenia) [29,30].
Treatment guidelines set by the British Association of Dermatology in 2009 recommend use of TNF-α inhibitors before ustekinumab because there had been limited exposure by patients to ustekinumab at the time the guidelines were written. However, since then, favorable five-year safety data have been reported [31]. In contrast, treatment guidelines from the American Academy of Dermatology in 2011 for moderate-to-severe psoriasis do not specify the sequence by which biologics should be prescribed.
A review of cost-effectiveness studies on biologic agents in psoriasis yielded inconsistent results for the most cost-effective agent (Supplemental Table 1), probably because different patient cohorts and agents were studied [9–11,21,23,25,26,32–35]. Conclusions drawn from cost-effectiveness models should be interpreted with caution because they rely on data drawn from idealized conditions for randomized controlled trials, a study period of 12 weeks, and drug prices that may not reflect the contracted price of the drug paid by insurers.
Our cost analysis had several limitations. First, other direct costs, such as physician visits, laboratory tests, and treatment for adverse events, were not included. However, they are likely to have been minimal compared with the cost of biologic drugs. Indirect costs (including comorbidities and increased mortality with severe psoriasis) are also important considerations. However, we could not measure these costs, so they were not included in this therapeutics-specific analysis. Second, in our estimate of the sales-based cost according to the total number of patients treated with biologic drugs, we assumed that patients fill two prescriptions each year, but this “ballpark estimate” may not reflect true prescribing patterns. Lastly, our estimate of sales-based cost does not reflect the specific contracts and prices of the insurers of individual patients. Thus, if choosing a therapy for a specific patient, then estimates of which drug is the most expensive may not apply because we do not know that contracted drug-purchasing rates of the insurer. We did not have access to insurance databases in this study, but using data from these sources may be helpful in future studies.
Psoriasis is a chronic, long-term condition, so awareness of treatment costs is important. Our cost analysis demonstrates that the annual cost of drugs is expensive and continues to increase each year. New oral therapies on the market may provide less costly alternatives. For example, apremilast, an inhibitor of phosphodiesterase-4 approved by the Food and Drug Administration in March 2014 for psoriatic arthritis, costs $27,375 a year based on wholesale pricing of a dose of 30 mg twice a day. However, with a lower efficacy rate, it may not be more cost-effective [10]. Tofacitinib, an inhibitor of JAK-kinase – if used for psoriasis at 5 mg twice a day – would cost $32,000 a year based on wholesale prices [12]. It is hoped that “biosimilars” will lower the cost of biologic agents. However, biosimilars are anticipated to cost only 20–30% less than branded biologics – a less drastic reduction in cost compared to generics which, at least in the past, lowered prices ≤90% and captured a large share of the market [36]. As a significantly low-cost, highly effective, safe alternative to biologics for patients who have failed conventional therapy is not on the horizon, costs will continue to play a part in the choice of therapy for severe psoriasis.
Supplemental Table 1.
Summary of cost-effectiveness analyses of biologic agents for psoriasis based on US pricing.
| Author, year, reference | Number of trials | Cost methodology | Efficacy methodology | Most cost-effective biologic |
|---|---|---|---|---|
| Hankin et al., 2005a [1] | 16 studies (1966–2004) | Annual cost (AWP, treatment administration, adverse-event monitoring and treatment, reimbursement rate from Medicare) | PASI% between 6 weeks and 14 weeks | Infliximab 5 mg/kg at weeks 0, 2, and 6 |
| Menter et al., 2005 [34] | 3 RCTs | 18 months of treatment (AWP, office fees, injection fees, costs due to adverse events, laboratory monitoring) | PASI-75 at 18 months | Etanercept 50 mg twice weekly ×12 weeks, then 50 mg weekly |
| Miller et al., 2006a [9] | 16 studies | Annual cost (treatment administration, adverse-event monitoring and treatment) | PASI% (treatment period not specified) | Infliximab 5 mg/kg |
| Pearce et al., 2006a [10] | 13 RCTs (1998–2004) | 12 weeks of treatment (AWP, physician visits, laboratory tests, Medicare fee for schedule of infusions) | PASI-75 after 12 weeks | Infliximab 5 mg/kg |
| Nelson et al., 2008 [21] | 11 RCTs (2003–2007) | 12 weeks of treatment (AWP, physician visits, laboratory testing, Medicare fee for schedule of infusions) | PASI-75, DLQI after 12 weeks | Etanercept 25 mg once weekly (DLQI MID) Infliximab 3 mg/kg (PASI-75) |
| Hankin et al., 2010a [26] | 22 RCTs (1966–2008) | Annual cost (WAC, adverse event monitoring and treatment, Medicare fee for schedule of infusions) | PASI-75, PGA 0/1 after 6–14 weeks of treatment | Infliximab 5 mg/kg at weeks 0, 2, 6, then every 8 weeks |
| Staidle et al., 2011a [11] | 22 RCTs (2001–2011) | Annual cost (AWP, office visits, laboratory tests, monitoring procedures) | PASI-75, DLQI MID after 12 weeks of treatment | Infliximab 5 mg/kg every 8 weeks (PASI and DLQI) |
| Anis et al., 2011 [32] | 22 RCTs | 10–16 weeks of treatment (AWP, treatment administration, monitoring, laboratory tests) | PASI between 10–16 weeks | Adalimumab 40 mg every other week (QALY) |
| Martin et al., 2011 [25] | ACCEPT trial (ustekinumab, etanercept) | 16 weeks of treatment (WAC) | PASI-75 after 12 weeks | Ustekinumab (45 mg or 90 mg depending on weight) |
| Villacorta et al., 2013 [35] | ACCEPT trial (ustekinumab, etanercept) | 3 years of treatment (Medicare Part B average sales price, treatment of adverse events, physician visits) | PASI after 12 weeks | Ustekinumab 45 mg ($150,000 threshold per QALY) |
| Ahn et al., 2013 [23] | 27 RCTs (1995–2012) | 12 weeks of treatment (AWP, physician visits, laboratory tests, Medicare fee for schedules of IV procedures) | PASI-75, DLQI after 12 weeks | Infliximab 3 mg/kg (PASI-75 and DLQI) |
| Chi et al., 2014 [33] | 13 RCTs (2005–2012) | 6 months of treatment (AWP) | PASI-75 and PGA 0/1 after 6 months | Adalimumab 80 mg loading dose, then 40 mg every other week (PASI-75 and PGA 0/1) |
Study included non-biologic agents (i.e. phototherapy, cyclosporine, methotrexate, acitretin).
RCT, randomized controlled trial; PASI, Psoriasis Area and Severity Index; DLQI MID, Dermatology Life Quality Index Minimally Important Difference; PGA 0/1, Physician Global Assessment clear/minimal; ACCEPT, Active Comparator (CNTO1275/Enbrel) Psoriasis Trial; AWP, average wholesale price; WAC, wholesale acquisition cost.
Abbreviations
- ACCEPT
Active Comparator (CNTO1275/Enbrel) Psoriasis Trial
- AWP
average wholesale price
- DLQI MID
Dermatology Life Quality Index Minimally Important Difference
- PASI
Psoriasis Area and Severity Index
- PGA 0/1
Physician Global Assessment clear/minimal
- TNF
tumor necrosis factor
- WAC
wholesale acquisition costs
Footnotes
Contributions
- SRF: Conception and design of the study, data interpretation, manuscript revision for important intellectual content.
- JC: Acquisition, analyses and interpretation of data, preparation and revision of the manuscript for important intellectual content.
Potential conflicts of interest
The International Committee of Medical Journal Editors’ (ICMJE) Potential Conflicts of Interests forms for the authors are summarized below. The original form is available for download at: http://www.drugsincontext.com/wp-content/uploads/2014/11/dic.212266-COI.pdf
Dr. Feldman is a consultant and speaker for Galderma, Connetics, Abbott Laboratories, Warner Chilcott, Centocor, Amgen, Photomedex, Genentech, BiogenIdec, and Bristol Myers Squibb. Dr. Feldman has received grants from Galderma, Connetics, Astellas, Abbott Laboratories, Warner Chilcott, Centocor, Amgen, Photomedex, Genentech, BiogenIdec, Coria, Pharmaderm, Ortho Pharmaceuticals, Aventis Pharmaceuticals, Roche Dermatology, 3M, Bristol Myers Squibb, Stiefel, GlaxoSmithKline, and Novartis. Dr. Feldman has received stock options from Photomedex. Ms. Cheng has no conflicts of interest to disclose.
Funding declaration
None to declare.
References
- 1.Hankin CS, Feldman SR, Szczotka A, Stinger RC, Fish L, Hankin DL. A cost comparison of treatments of moderate to severe psoriasis. Drug Benefit Trends. 2005;17(5):200–14. [Google Scholar]
- 2.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–9. doi: 10.1046/j.1087-0024.2003.09102.x. http://dx.doi.org/10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 3.Horn EJ, Fox KM, Patel V, Chiou CF, Dann F, Lebwohl M. Are patients with psoriasis undertreated? Results of National Psoriasis Foundation survey. J Am Acad Dermatol. 2007;57(6):957–62. doi: 10.1016/j.jaad.2007.06.042. http://dx.doi.org/10.1016/j.jaad.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280–4. [PubMed] [Google Scholar]
- 5.Lynch M, Kirby B, Warren RB. Treating moderate to severe psoriasis-best use of biologics. Expert Rev Clin Immunol. 2014;10(2):269–79. doi: 10.1586/1744666X.2014.873701. http://dx.doi.org/10.1586/1744666X.2014.873701. [DOI] [PubMed] [Google Scholar]
- 6.Bhosle MJ, Feldman SR, Camacho FT, Timothy Whitmire J, Nahata MC, Balkrishnan R. Medication adherence and health care costs associated with biologics in Medicaid-enrolled patients with psoriasis. J Dermatol Treat. 2006;17(5):294–301. doi: 10.1080/09546630600954594. http://dx.doi.org/10.1080/09546630600954594. [DOI] [PubMed] [Google Scholar]
- 7.Fonia A, Jackson K, Lereun C, Grant DM, Barker JN, Smith CH. A retrospective cohort study of the impact of biologic therapy initiation on medical resource use and costs in patients with moderate to severe psoriasis. Br J Dermatol. 2010;163(4):807–16. doi: 10.1111/j.1365-2133.2010.09944.x. http://dx.doi.org/10.1111/j.1365-2133.2010.09944.x. [DOI] [PubMed] [Google Scholar]
- 8.Driessen R, Bisschops L, Adang E, Evers AW, Van De Kerkhof PC, De Jong EM. The economic impact of high-need psoriasis in daily clinical practice before and after the introduction of biologics. Br J Dermatol. 2010;162(6):1324–99. doi: 10.1111/j.1365-2133.2010.09693.x. [DOI] [PubMed] [Google Scholar]
- 9.Miller DW, Feldman SR. Cost-effectiveness of moderate-to-severe psoriasis treatment. Expert Opin Pharmacother. 2006;7(2):157–67. doi: 10.1517/14656566.7.2.157. http://dx.doi.org/10.1517/14656566.7.2.157. [DOI] [PubMed] [Google Scholar]
- 10.Pearce DJ, Nelson AA, Fleischer Jr AB, Balkrishnan R, Feldman SR. The cost-effectiveness and cost of treatment failures associated with systemic psoriasis therapies. J Dermatol Treat. 2006;17(1):29–37. doi: 10.1080/09546630500504754. http://dx.doi.org/10.1080/09546630500504754. [DOI] [PubMed] [Google Scholar]
- 11.Staidle JP, Dabade TS, Feldman SR. A pharmacoeconomic analysis of severe psoriasis therapy: a review of treatment choices and cost efficiency. Expert Opin Pharmacother. 2011;12(13):2041–54. doi: 10.1517/14656566.2011.590475. http://dx.doi.org/10.1517/14656566.2011.590475. [DOI] [PubMed] [Google Scholar]
- 12.Red Book Drug Topics. 2014. [Last accessed: 6 November 2014]. Available at: http://www.micromedexsolutions.com/micromedex2/librarian.
- 13.Sterry W, Ortonne JP, Kirkham B, et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ. 2010;340:c147. doi: 10.1136/bmj.c147. http://dx.doi.org/10.1136/bmj.c147. [DOI] [PubMed] [Google Scholar]
- 14.FDA Humira (adalimumab) label – FDA. 2011. [Last accessed: 6 November 2014]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125057s0276lbl.pdf.
- 15.FDA [Last accessed: 6 November 2014];Stelara FDA Label. 2009 Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125261lbl.pdf. [Google Scholar]
- 16.Amgen 2013 Annual Report and 10-K. 2013. [Last accessed: 6 November 2014]. Available at: http://investors.amgen.com/phoenix.zhtml?c=61656&p=irol-reportsannual.
- 17.AbbVie 2013 Annual Report on Form 10-K and 2014 Proxy Statement. 2013. [Last accessed: 6 November 2014]. Available at: http://www.abbvieinvestor.com/phoenix.zhtml?c=251551&p=irol-reportsannual.
- 18.Johnson J. Sales and Earnings. 2014. [Last accessed: 6 November 2014]. Available at: http://www.investor.jnj.com/sales-earnings.cfm.
- 19.Bureau of Labor Statistics Consumer Price Index – All Urban Consumers: Medical Care. 2014. [Last accessed: 6 November 2014]. Available at: http://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths.
- 20.Bureau Labor Statistics CPI Detailed Report: Data for March 2014. [Last accessed: 6 November 2014]. Available at: http://www.bls.gov/cpi/cpid1403.pdf.
- 21.Nelson AA, Pearce DJ, Fleischer Jr AB, Balkrishnan R, Feldman SR. Cost-effectiveness of biologic treatments for psoriasis based on subjective and objective efficacy measures assessed over a 12-week treatment period. J Am Acad Dermatol. 2008;58(1):125–35. doi: 10.1016/j.jaad.2007.09.018. http://dx.doi.org/10.1016/j.jaad.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Beyer V, Wolverton SE. Recent trends in systemic psoriasis treatment costs. Arch Dermatol. 2010;146(1):46–54. doi: 10.1001/archdermatol.2009.319. http://dx.doi.org/10.1001/archdermatol.2009.319. [DOI] [PubMed] [Google Scholar]
- 23.Ahn CS, Gustafson CJ, Sandoval LF, Davis SA, Feldman SR. Cost effectiveness of biologic therapies for plaque psoriasis. Am J Clin Dermatol. 2013;14(4):315–26. doi: 10.1007/s40257-013-0030-z. http://dx.doi.org/10.1007/s40257-013-0030-z. [DOI] [PubMed] [Google Scholar]
- 24.Schabert V, Korn J, Bilir P, DeKoven M, Harrison DJ, Joseph G. Cost per treated patient for biologics across adult indications using claims data. Value Health. 2013;16(3):A220. http://dx.doi.org/10.1016/j.jval.2013.03.1118. [Google Scholar]
- 25.Martin S, Feldman SR, Augustin M, Szapary P, Schenkel B. Cost per responder analysis of ustekinumab and etanercept for moderate to severe plaque psoriasis. J Dermatol Treat. 2011;22(3):138–43. doi: 10.3109/09546634.2010.542800. http://dx.doi.org/10.3109/09546634.2010.542800. [DOI] [PubMed] [Google Scholar]
- 26.Hankin CS, Bhatia ND, Goldenberg G, et al. A comparison of the clinical effectiveness and cost-effectiveness of treatments for moderate to severe psoriasis. Drug Benefit Trends. 2010;22(1):17–27. [Google Scholar]
- 27.AMCP Task Force on Drug Payment Methodologies AMCP guide to pharmacuetical payment methods, 2013 Update (Version 3.0) [Last accessed: 28 November 2014]. Available at: http://www.amcp.org/Tertiary.aspx?id=16359.
- 28.Tufts Center for the Study of Drug Development Total cost to develop a new prescription drug, including cost of post-approval research, is $897 million, according to Tufts Center for the Study of Drug Development. [Last accessed: 6 November 2014]. Available at: http://www.businesswire.com/news/home/20030513005057/en/Total-Cost-Develop-Prescription-Drug-Including-Cost-.U1l39OZdWkQ.
- 29.National Clinical Guideline Centre Psoriasis: assessment and management of psoriasis. 2012. [Last accessed: 6 November 2014]. Available at: https://www.nice.org.uk/guidance/cg153.
- 30.Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate and psoriasis: 2009 National Psoriasis Foundation consensus conference. J Am Acad Dermatol. 2009;60(5):824–37. doi: 10.1016/j.jaad.2008.11.906. http://dx.doi.org/10.1016/j.jaad.2008.11.906. [DOI] [PubMed] [Google Scholar]
- 31.Pathirana D, Ormerod A, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1–70. doi: 10.1111/j.1468-3083.2009.03389.x. [DOI] [PubMed] [Google Scholar]
- 32.Anis AH, Bansback N, Sizto S, Gupta SR, Willian MK, Feldman SR. Economic evaluation of biologic therapies for the treatment of moderate to severe psoriasis in the United States. J Dermatol Treat. 2011;22(2):65–74. doi: 10.3109/09546630903551258. http://dx.doi.org/10.3109/09546630903551258. [DOI] [PubMed] [Google Scholar]
- 33.Chi C-C, Wang SH. Efficacy and cost-efficacy of biologic therapies for moderate to severe psoriasis: a meta-analysis and cost-efficacy analysis using the intention-to-treat principle. Biomed Res Int. 2014:862851. doi: 10.1155/2014/862851. http://dx.doi.org/10.1155/2014/862851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menter A, Baker T. Cost-efficacy analysis of biological treatments in psoriasis: an 18-month assessment. J Med Econ. 2005;8(14):139–46. http://dx.doi.org/10.3111/200508135146. [Google Scholar]
- 35.Villacorta R, Hay JW, Messali A. Cost effectiveness of moderate to severe psoriasis therapy with etanercept and ustekinumab in the United States. Pharmacoeconomics. 2013;31(9):823–39. doi: 10.1007/s40273-013-0078-x. http://dx.doi.org/10.1007/s40273-013-0078-x. [DOI] [PubMed] [Google Scholar]
- 36.Blackstone E, Fuhr Jr JP. Innovation and competition: will biosimilars succeed? Biotechnol Healthc. 2012;9(1):24–7. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1.
Summary of cost-effectiveness analyses of biologic agents for psoriasis based on US pricing.
| Author, year, reference | Number of trials | Cost methodology | Efficacy methodology | Most cost-effective biologic |
|---|---|---|---|---|
| Hankin et al., 2005a [1] | 16 studies (1966–2004) | Annual cost (AWP, treatment administration, adverse-event monitoring and treatment, reimbursement rate from Medicare) | PASI% between 6 weeks and 14 weeks | Infliximab 5 mg/kg at weeks 0, 2, and 6 |
| Menter et al., 2005 [34] | 3 RCTs | 18 months of treatment (AWP, office fees, injection fees, costs due to adverse events, laboratory monitoring) | PASI-75 at 18 months | Etanercept 50 mg twice weekly ×12 weeks, then 50 mg weekly |
| Miller et al., 2006a [9] | 16 studies | Annual cost (treatment administration, adverse-event monitoring and treatment) | PASI% (treatment period not specified) | Infliximab 5 mg/kg |
| Pearce et al., 2006a [10] | 13 RCTs (1998–2004) | 12 weeks of treatment (AWP, physician visits, laboratory tests, Medicare fee for schedule of infusions) | PASI-75 after 12 weeks | Infliximab 5 mg/kg |
| Nelson et al., 2008 [21] | 11 RCTs (2003–2007) | 12 weeks of treatment (AWP, physician visits, laboratory testing, Medicare fee for schedule of infusions) | PASI-75, DLQI after 12 weeks | Etanercept 25 mg once weekly (DLQI MID) Infliximab 3 mg/kg (PASI-75) |
| Hankin et al., 2010a [26] | 22 RCTs (1966–2008) | Annual cost (WAC, adverse event monitoring and treatment, Medicare fee for schedule of infusions) | PASI-75, PGA 0/1 after 6–14 weeks of treatment | Infliximab 5 mg/kg at weeks 0, 2, 6, then every 8 weeks |
| Staidle et al., 2011a [11] | 22 RCTs (2001–2011) | Annual cost (AWP, office visits, laboratory tests, monitoring procedures) | PASI-75, DLQI MID after 12 weeks of treatment | Infliximab 5 mg/kg every 8 weeks (PASI and DLQI) |
| Anis et al., 2011 [32] | 22 RCTs | 10–16 weeks of treatment (AWP, treatment administration, monitoring, laboratory tests) | PASI between 10–16 weeks | Adalimumab 40 mg every other week (QALY) |
| Martin et al., 2011 [25] | ACCEPT trial (ustekinumab, etanercept) | 16 weeks of treatment (WAC) | PASI-75 after 12 weeks | Ustekinumab (45 mg or 90 mg depending on weight) |
| Villacorta et al., 2013 [35] | ACCEPT trial (ustekinumab, etanercept) | 3 years of treatment (Medicare Part B average sales price, treatment of adverse events, physician visits) | PASI after 12 weeks | Ustekinumab 45 mg ($150,000 threshold per QALY) |
| Ahn et al., 2013 [23] | 27 RCTs (1995–2012) | 12 weeks of treatment (AWP, physician visits, laboratory tests, Medicare fee for schedules of IV procedures) | PASI-75, DLQI after 12 weeks | Infliximab 3 mg/kg (PASI-75 and DLQI) |
| Chi et al., 2014 [33] | 13 RCTs (2005–2012) | 6 months of treatment (AWP) | PASI-75 and PGA 0/1 after 6 months | Adalimumab 80 mg loading dose, then 40 mg every other week (PASI-75 and PGA 0/1) |
Study included non-biologic agents (i.e. phototherapy, cyclosporine, methotrexate, acitretin).
RCT, randomized controlled trial; PASI, Psoriasis Area and Severity Index; DLQI MID, Dermatology Life Quality Index Minimally Important Difference; PGA 0/1, Physician Global Assessment clear/minimal; ACCEPT, Active Comparator (CNTO1275/Enbrel) Psoriasis Trial; AWP, average wholesale price; WAC, wholesale acquisition cost.

