Abstract
Although several treatment options are available to reduce hyperglycemia, only about half of individuals with diagnosed diabetes mellitus (DM) achieve recommended glycemic targets. New agents that reduce blood glucose concentrations by novel mechanisms and have acceptable safety profiles are needed to improve glycemic control and reduce the complications associated with type 2 diabetes mellitus (T2DM). The renal sodium-glucose co-transporter 2 (SGLT2) is responsible for reabsorption of most of the glucose filtered by the kidney. Inhibitors of SGLT2 lower blood glucose independent of the secretion and action of insulin by inhibiting renal reabsorption of glucose, thereby promoting the increased urinary excretion of excess glucose. Canagliflozin, dapagliflozin, and empagliflozin are SGLT2 inhibitors approved as treatments for T2DM in the United States, Europe, and other countries. Canagliflozin, dapagliflozin, and empagliflozin increase renal excretion of glucose and improve glycemic parameters in patients with T2DM when used as monotherapy or in combination with other antihyperglycemic agents. Treatment with SGLT2 inhibitors is associated with weight reduction, lowered blood pressure, and a low intrinsic propensity to cause hypoglycemia. Overall, canagliflozin, dapagliflozin, and empagliflozin are well tolerated. Cases of genital infections and, in some studies, urinary tract infections have been more frequent in canagliflozin-, dapagliflozin-, and empagliflozin-treated patients compared with those receiving placebo. Evidence from clinical trials suggests that SGLT2 inhibitors are a promising new treatment option for T2DM.
Keywords: administration, oral; canagliflozin; dapagliflozin; diabetes mellitus, type 2; drug therapy; empagliflozin; antidiabetic agents; sodium-glucose co-transporter 2
Introduction
Type 2 diabetes mellitus (T2DM) accounts for 90% to 95% of all cases of diagnosed diabetes mellitus (DM) in adults [1]. Chronic hyperglycemia is the hallmark of T2DM and is associated with the development of microvascular complications, including retinopathy, neuropathy, and nephropathy [2].
Control of blood glucose is fundamental to T2DM management. The American Diabetes Association/European Association for the Study of Diabetes and the American Association of Clinical Endocrinologists (AACE) recommend glycated hemoglobin (HbA1c) levels <7.0% and ≤6.5%, respectively, for most patients, but that glycemic goals should be individualized for each patient [3,4]. Lifestyle changes such as diet, exercise, and weight loss are typically recommended for patients with T2DM, but most patients require pharmacotherapy to achieve glycemic goals [5].
Despite the availability of several antihyperglycemic agents, only 53% of patients with DM achieve HbA1c <7.0% [6]. Therefore, there is a need for new therapeutic options with innovative mechanisms of action and acceptable safety profiles to improve glycemic control in patients with T2DM.
A new approach to reduce plasma glucose concentrations in patients with T2DM is to inhibit glucose reabsorption by the kidney [7]. Recently, several members of a new class of drugs, sodium-glucose co-transporter 2 (SGLT2) inhibitors, that act via this mechanism have been approved as therapy for T2DM. Here, I discuss the role of the kidney and SGLT2 in glucose homeostasis, and the mechanism of action of SGLT2 inhibitors. Also, I summarize the efficacy and safety of canagliflozin, dapagliflozin, and empagliflozin. These SGLT2 inhibitors have been approved in the United States (US), European Union (EU), and other countries for the treatment of patients with T2DM.
Role of the kidney in glucose homeostasis
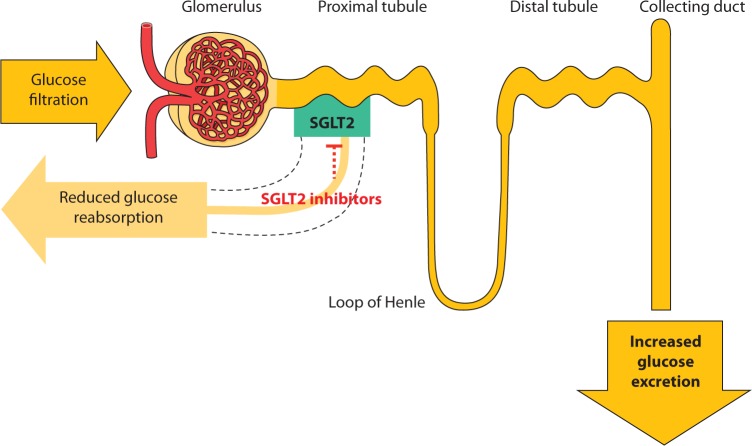
The kidney contributes to glucose homeostasis primarily by glucose reabsorption to the circulation. Glucose is filtered freely by the kidneys. In healthy individuals, almost all of it is reabsorbed by the kidneys [8]. Glucose reabsorption by the kidney is mediated by specific glucose transport proteins, in particular SGLT2, which is responsible for most of the glucose reabsorption by the kidney (Figure 1) [8]. In individuals with T2DM, the renal capacity to reabsorb glucose is increased [9] and contributes to the already increased plasma glucose concentrations. Moreover, the plasma glucose concentration at which renal excretion of glucose occurs (i.e., the threshold) is also elevated in T2DM [9]. The increased threshold and capacity to reabsorb glucose in T2DM may be the result of upregulation of expression of SGLT2 in the proximal tubule [10]. Therefore, inhibition of glucose reabsorption and augmentation of renal excretion of glucose represents a novel mechanism to reduce plasma glucose that is complementary to the mechanisms of current drug classes for T2DM.
Figure 1.
Glucose reabsorption by the kidney. Normally, SGLT2 reabsorbs most of the glucose filtered by the kidney. SGLT2 inhibitors reduce renal reabsorption of glucose, resulting in increased glucose excretion and lowering of plasma glucose concentration.
Inhibition of SGLT2
SGLT2 inhibitor–induced glucose excretion is proportional to the amount of glucose filtered by the kidneys, which is a function of the glomerular filtration rate (GFR) and plasma glucose concentration [11]. This phenomenon results in several important considerations regarding the mechanism of action of SGLT2 inhibitors. First, increased plasma glucose concentration (as observed in DM) leads to increased glucose filtration (dependent on the GFR) and may allow greater excretion of glucose with SGLT2 inhibition. Second, because the mechanism of action of SGLT2 inhibitors is independent of the secretion or action of insulin and diminishes as patients’ plasma glucose concentrations decrease, the intrinsic risk of hypoglycemia with this drug class is low [12]. Furthermore, glucose excretion as a consequence of SGLT2 inhibition results in a loss of calories that leads to a decrease in body weight and fat mass [13,14], an additional benefit that addresses two of the underlying factors in the pathogenesis of T2DM: excessive caloric intake and increased body weight [15]. SGLT2 inhibitors also have a mild diuretic effect [16], with modest lowering of blood pressure (BP) in patients with T2DM [14,17].
Canagliflozin
Canagliflozin (Invokana*) was approved in the US and EU in 2013 for the treatment of T2DM. The recommended starting dose is 100 mg once daily, which can be increased to 300 mg once daily in patients tolerating canagliflozin who have an estimated GFR (eGFR) ≥60 mL/min/1.73 m2 and who require additional glycemic control [18]. A maximum dose of 100 mg is recommended for patients with an eGFR 45 to <60 mL/min/1.73 m2; canagliflozin should not be used in patients with an eGFR <45 mL/min/1.73 m2 [18]. After oral administration of canagliflozin to individuals with T2DM, maximum plasma concentrations were obtained in 1.5 to 2 h, with a mean half-life (t½) of 14 to 16 h, which is consistent with once-daily dosing [19]. Canagliflozin inhibits renal reabsorption of glucose [20] and has some affinity for SGLT1 [21], the major intestinal glucose transporter [22]. At a dose of 300 mg taken before a meal, canagliflozin inhibits SGLT2, but may also inhibit SGLT1 in the intestine and delay postprandial glucose (PPG) absorption [23].
Coadministration of uridine 5′-disphosphate glucuronosyltransferase inducers such as rifampin decreases exposure to canagliflozin, whereas canagliflozin increases exposure to digoxin [18]. Patients taking canagliflozin with digoxin should be monitored appropriately.
Efficacy
In clinical trials, canagliflozin at 100 mg/d and 300 mg/d significantly reduced HbA1c by 0.37% to 1.16%, and fasting plasma glucose (FPG) by 25 to 43 mg/dL compared with placebo when used as monotherapy or as add-on therapy to commonly used antidiabetic medications (Table 1). Canagliflozin also decreased PPG compared with placebo or comparator [24,25]. The favorable effect on glycemic parameters was accompanied by reductions in body weight of 1.9 to 3.3 kg. Canagliflozin-induced weight reduction was largely the result of a reduction in fat mass [14]. In these clinical trials, placebo- or comparator-corrected reductions in systolic blood pressure (SBP) and diastolic blood pressure (DBP) of up to −7 mmHg and −3 mmHg, respectively, were observed with canagliflozin.
Table 1.
Placebo- or comparator-corrected changes from baseline to study end in HbA1c, FPG, PPG, and body weight with canagliflozin in Phase III trials.
| HbA1c% | FPG, mg/dL | PPG, mg/dL | Body weight, kg or % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canagliflozin, mg/d | Canagliflozin, mg/d | Canagliflozin, mg/d | Canagliflozin, mg/d | ||||||||||
| Trial | Duration, weeks | Baseline | 100 | 300 | Baseline | 100 | 300 | Baseline | 100 | 300 | Baseline | 100 | 300 |
| Monotherapy (NCT01081834) [28] | 26 | 8.00–8.10 | −0.91 | −1.16 | 167–173 | −36 | −43 | 229–254 | −49 | −65 | 86–88 | −1.9 | −2.9 |
| Add-on to MET (NCT01106677) [24] | 26 | 7.9–8.0 | −0.62 | −0.77 | 164–173 | −31 | −40 | 248–261 | −38 | −47 | 85–89 | −2.2 | −2.5 |
| Add-on to MET and SU (NCT01106625) [75] | 26 | 8.1 | −0.71 | −0.92 | 167–173 | −22 | −34 | 279–297 | −27 | −38 | 91–94 | −1.1 | −1.7 |
| Add-on to MET and SU vs sitagliptin add-on to MET and SU (NCT01137812) [25] | 52 | 8.1 | ND | −0.37 | 166–167 | ND | −27 | 289–295 | ND | −18 | 88–90 | ND | −2.4 |
| Add-on to insulin ± other antihyperglycemic agents (NCT01032629) [76] | 18 | 8.2–8.4 | −0.86 | −0.89 | 153–158 | −25 | −31 | ND | ND | ND | 98–102 | −1.9 | −2.8 |
| Add-on to MET + pioglitazone (NCT01106690) [77] | 26 | 7.9–8.0 | −0.62 | −0.76 | 164–168 | −29 | −36 | ND | ND | ND | 93–95 | −2.7% | −3.7% |
| Add-on to MET vs glimepiride add-on to MET(NCT00968812) [14] | 52 | 7.8 | −0.01 | −0.12 | 164–166 | −6 | −9 | ND | ND | ND | 87 | −4.4 | −4.7 |
| Add-on to MET vs sitagliptin add-on to MET (NCT01106677) [24] | 52 | 7.9 | 0 | −0.15 | 169 | −9 | −18 | ND | ND | ND | 87 | −2.4% | −2.9% |
FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; MET, metformin; ND, not determined; PPG, postprandial glucose; SU, sulfonylurea.
When compared with glimepiride as add-on therapy to metformin, canagliflozin 100 mg/d was noninferior to glimepiride and at 300 mg/d was superior to glimepiride in reducing HbA1c after 52 weeks of treatment (Table 1) [14]. The reduction in FPG with canagliflozin was slightly greater than that seen with glimepiride. Body weight decreased with both canagliflozin doses (−3.7 kg [−4.4%] and −4.0 kg [−4.7%]; P<0.0001 for both doses vs glimepiride), whereas there was a small increase (0.7 kg [1.0%]) with glimepiride.
In patients receiving background metformin therapy, canagliflozin 100 mg/d for 52 weeks (26-week placebo-and sitagliptin-controlled period followed by a 26-week sitagliptin-controlled period [placebo group switched to sitagliptin]) was noninferior and 300 mg/d was superior to sitagliptin in reducing HbA1c (Table 1) [24]. At week 26, canagliflozin 100 and 300 mg/d significantly reduced HbA1c compared with placebo (−0.79% and −0.94%, respectively, vs −0.17% for placebo; P<0.001). Both doses of canagliflozin also significantly reduced body weight (P<0.001), FPG (P<0.001), and SBP (difference from sitagliptin, −2.9 and −4.0 mmHg; P<0.001) to a greater extent than that seen with sitagliptin at 52 weeks. In older patients (mean age, 64 years) with T2DM taking antihyperglycemic medications, placebo-corrected changes from baseline to 26 weeks in HbA1c were −0.57% and −0.70% (both P<0.001 vs placebo) and in FPG were −26 and −28 mg/dL (both P<0.001 vs placebo) with canagliflozin 100 and 300 mg/d, respectively. More patients receiving canagliflozin achieved HbA1c <7% (48% and 59%, respectively; P<0.001 vs placebo) than those receiving placebo (28%) [26]. Placebo-corrected mean changes in body weight were −2.1 and −2.7 kg for canagliflozin (P<0.001 for both doses). Compared with placebo, SBP was reduced by 5 and 8 mmHg with canagliflozin (P<0.001 for both doses).
The mechanism of action of SGLT2 inhibitors is dependent on renal filtration of glucose. Hence, the effect of canagliflozin was assessed in patients with chronic kidney disease (CKD) (mean eGFR, 39.4 mL/min/1.73 m2). Mean changes from baseline HbA1c relative to placebo were −0.30% (P<0.05) and −0.40% (P<0.001) with canagliflozin 100 and 300 mg/d, respectively [27], which were less than those seen in patients with normal or near-normal kidney function [24,25]. Changes in FPG appeared greater with canagliflozin than with placebo, but differences were not analyzed statistically. Body weight was reduced with canagliflozin relative to placebo (−1.4 and −1.6 kg for canagliflozin). Both doses of canagliflozin were associated with greater decreases from baseline compared with placebo in SBP (−6.1 and −6.4 vs −0.3 mmHg) and DBP (−2.6 and −3.5 vs −1.4 mmHg).
Safety
In clinical trials, canagliflozin was, in general, well tolerated. Genital infections were more frequent with canagliflozin than with placebo, especially in women (Table 2) [14,24–26,28]. In most studies, osmotic diuresis–related adverse events (AEs; e.g. pollakiuria and polyuria) were increased with canagliflozin compared with placebo [14,24–26,28]. Canagliflozin may cause hyperkalemia, especially in patients with moderate renal impairment (eGFR 45 to <60 mL/min/1.73 m2) and in patients taking drugs that affect potassium excretion, such as potassium-sparing diuretics or inhibitors of the renin–angiotensin–aldosterone system [29]. Volume-related AEs (e.g. postural dizziness and orthostasis) were modestly increased with canagliflozin in some studies [26,28]. Small, acute decreases in the eGFR with canagliflozin have been reported in patients with T2DM and normal renal function [14] and in those with CKD [27]. Events of hypoglycemia were infrequent and occurred similarly with canagliflozin and placebo in most studies (Table 2). Hypoglycemia AEs increased when canagliflozin was added to insulin therapy.
Table 2.
Adverse events, including genital infections and urinary tract infections and hypoglycemia,* with canagliflozin in Phase III trials.
| Patients, % | |||||
|---|---|---|---|---|---|
| Canagliflozin, mg/d | |||||
| Trial | Duration, weeks | AE | Placebo or control | 100 | 300 |
| Monotherapy (NCT01081834) [28] | 26 | ≥1 AE | 53 | 61 | 60 |
| Genital | 2 | 6 | 7 | ||
| Urinary | 4 | 7 | 5 | ||
| Hypoglycemia | 3 45 | 4 | 3 | ||
| Add-on to MET and SU (NCT01106625) [75] | 26 | ≥1 AE | 50 | 56 | |
| Genital | 0 | 3 | 3 | ||
| Urinary | 3 | 3 | 4 | ||
| Hypoglycemia | 10 | 22 | 27 | ||
| Add-on to MET and SU vs sitagliptin add-on to MET and SU (NCT01137812) [25] | 52 | ≥1 AE | 78 | ND | 77 |
| Genital | 2 | ND | 12 | ||
| Urinary | 6 | ND | 4 | ||
| Hypoglycemia | 41 | ND | 43 | ||
| Add-on to insulin ± other antihyperglycemic agents (NCT01032629) [76] | 18 | ≥1 AE | 53 | 62 | 67 |
| Genital | 3 | 12 | 14 | ||
| Urinary | 1 | 5 | 4 | ||
| Hypoglycemia | 25 | 42 | 43 | ||
| Add-on to MET + pioglitazone (NCT01106690) [77] | 52 | ≥1 AE | 77 | 70 | 76 |
| Genital | 3 | 8 | 12 | ||
| Urinary | 8 | 5 | 8 | ||
| Hypoglycemia | 6 | 4 | 6 | ||
| Add-on to MET vs glimepiride add-on to MET (NCT00968812) [14] | 52 | ≥1 AE | 69 | 64 | 69 |
| Genital | 2 | 9 | 11 | ||
| Urinary | 5 | 6 | 6 | ||
| Hypoglycemia | 34 | 6 | 5 | ||
| Add-on to MET vs sitagliptin add-on to MET (NCT01106677) [24] | 52 | ≥1 AE | 67 | 72 | 63 |
| Genital | 1 | 8 | 7 | ||
| Urinary | 7 | 8 | 5 | ||
| Hypoglycemia | 3 | 7 | 7 | ||
Documented hypoglycemia defined by fingerstick or plasma glucose ≤70 mg/dL, irrespective of symptoms and episodes of severe hypoglycemia necessitating assistance or resulting in seizures or loss of consciousness.
AE, adverse event; MET, metformin; ND, not determined; SU, sulfonylurea.
Canagliflozin increased low-density lipoprotein cholesterol (LDL-C) by 2% to 12% compared with placebo or comparator and high-density lipoprotein cholesterol (HDL-C) by 1% to 9%. Modest and variable reductions in triglycerides were noted [14,24–26,28]. In a pool of four placebo-controlled trials, canagliflozin increased LDL-C relative to placebo by 4.5% and 8.0% at 100 and 300 mg/d, respectively [18].
Dapagliflozin
Dapagliflozin (Farxiga†), a highly selective inhibitor of SGLT2 [21], was approved for the treatment of T2DM in the EU and other countries in 2012 and in the US in 2014. In the US, the recommended starting dose is 5 mg once daily, which can be increased to 10 mg once daily in patients tolerating dapagliflozin and who require additional glycemic control [30]. In the EU, the recommended starting dose is 10 mg [31]. Kidney function should be assessed before initiating dapagliflozin, and it should not be used in patients with an eGFR <60 mL/min/1.73 m2. Also, dapagliflozin should be discontinued if the eGFR persistently falls below 60 mL/min/1.73 m2 [30].
Dapagliflozin is absorbed rapidly after oral administration [32], with a mean t½ of ≈14 h [33], which is consistent with once-daily dosing. A high-fat meal does not affect overall systemic exposure to dapagliflozin, thus allowing for administration with or without food [34].
Coadministration of dapagliflozin with metformin, glimepiride, pioglitazone, or sitagliptin had no effect on the maximum plasma concentration or area under the plasma concentration compared with time curve of dapagliflozin. In addition, dapagliflozin did not affect the pharmacokinetics of the coadministered drugs [35]. Similarly, no meaningful drug–drug interactions were noted between dapagliflozin and simvastatin, valsartan, warfarin, or digoxin [36].
Efficacy
In 24-week Phase III clinical trials, dapagliflozin reduced mean HbA1c at the approved doses of 5 and 10 mg/d (0.40%–0.54% and 0.50%–0.68%, respectively) compared with placebo in patients with T2DM when used as monotherapy or as add-on therapy to metformin, glimepiride, pioglitazone, sitagliptin, or insulin (Table 3). In these trials, dapagliflozin reduced FPG by 15 to 28 mg/dL compared with placebo. In most of these placebo-controlled trials, dapagliflozin significantly reduced body weight by ≤2.0 kg compared with placebo. As with canagliflozin, the reduction in body weight with dapagliflozin appeared to be largely the result of a reduction in fat mass [13]. Effects of dapagliflozin on glycemic parameters and body weight were maintained for >2 years [37–39].
Table 3.
Placebo- or comparator-corrected changes from baseline to study end in HbA1c, FPG, PPG, and body weight with dapagliflozin in Phase III trials.
| HbA1c,% | FPG, mg/dL | PPG, mg/dL | Body weight, kg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dapagliflozin, mg/d | Dapagliflozin, mg/d | Dapagliflozin, mg/d | Dapagliflozin, mg/d | ||||||||||
| Trial | Duration, weeks | Baseline | 5 | 10 | Baseline | 5 | 10 | Baseline | 5 | 10 | Baseline | 5 | 10 |
| Monotherapy (NCT00528372) [47] | 24 | 7.84–8.01 | −0.54 | −0.66 | 160–167 | −20 | −25 | ND | ND | ND | 88–94 | −0.6 | −1.0 |
| Add-on to MET (NCT00528879) [46] | 24 | 7.92–8.17 | −0.40 | −0.54 | 156–169 | −15 | −17 | ND | ND | ND | 85–88 | −2.1 | −2.0 |
| Add-on to glimepiride (NCT00680745) [48] | 24 | 8.07–8.15 | −0.49 | −0.68 | 172–174 | −19 | −26 | 324–330 | −43 | −49 | 81 | −0.8 | −1.5 |
| Add-on to pioglitazone (NCT00683878) [49] | 24 | 8.34–8.40 | −0.40 | −0.55 | 161–169 | −19 | −24 | 285–308 | −51 | −53 | 85–88 | −1.5 | −1.8 |
| Add-on to sitagliptin ± MET (NCT00984867) [57] | 24 | 7.9–8.0 | ND | −0.50 | 162–163 | ND | −28 | 226–228 | ND | −43 | 89–91 | ND | −1.9 |
| Add-on to insulin (NCT00673231) [50] | 24 | 8.47–8.62 | −0.49 | −0.57 | 171–185 | −20 | −20 | ND | ND | ND | 93–95 | −1.4 | −2.0 |
| Add-on to MET vs glipizide add-on to MET (NCT00660907) [40] | 52 | 7.69–7.74 | ND | 0 | 162–164 | ND | −4 | ND | ND | ND | 88 | ND | −4.7 |
| Add-on to MET + SU (NCT01392677) [78] | 24 | 8.08–8.24 | ND | −0.69 | 167–180 | ND | −33 | ND | ND | ND | 89–90 | ND | −2.1 |
| Dapagliflozin 5 mg combined with MET XR as initial therapy (NCT00643851) [41] | 24 | 9.14–9.21 | −0.70 | ND | 193–197 | −27 | ND | ND | ND | ND | 84–86 | −1.4 | ND |
| Dapagliflozin 10 mg combined with MET XR as initial therapy (NCT00859898) [41] | 24 | 9.03–9.10 | ND | −0.54 | 189–190 | ND | −26 | ND | ND | ND | 87–89 | ND | −2.0 |
FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; MET, metformin; ND, not determined; PPG, postprandial glucose; SU, sulfonylurea; XR, extended release.
In one study, dapagliflozin 10 mg/d was compared with the sulfonylurea glipizide as add-on therapy to metformin in patients with T2DM that was inadequately controlled with metformin [40]. The adjusted mean change from baseline in HbA1c at 52 weeks between dapagliflozin and glipizide treatment groups was identical (−0.52%; Table 3). Both drugs reduced FPG to a similar extent. Dapagliflozin reduced mean body weight by 3.2 kg (P<0.0001 vs glipizide), whereas glipizide increased body weight by 1.4 kg.
Two randomized, 24-week, active-control trials examined the effects of dapagliflozin 5 and 10 mg/d combined with metformin XR (most patients received 2000 mg/d) as initial therapy in treatment-naïve patients with T2DM [41]. Dapagliflozin 5 and 10 mg/d plus metformin XR were more effective than either drug alone in reducing mean HbA1c (P<0.0001; Table 3). Similarly, dapagliflozin 5 and 10 mg/d plus metformin XR were more effective in reducing mean FPG than either drug alone (P<0.0001). Combinations were also more effective in reducing body weight than metformin alone (P<0.0001). In a prespecified comparison, dapagliflozin 10 mg was noninferior to metformin XR in reducing HbA1c and superior to metformin XR in reducing FPG and body weight.
In older patients (mean age, 63–64 years) with T2DM and cardiovascular disease [42] or T2DM and cardiovascular disease and hypertension [43], changes from baseline compared with placebo at 24 weeks in HbA1c (−0.3% vs 0.1% with placebo [P<0.001] and −0.4% vs 0.1% [P<0.01], respectively), body weight (−2.5 vs −0.6 kg [P<0.001] and −2.6% vs −0.3% [P<0.01]), and SBP (−2.7 vs 0.3 mmHg [P<0.001] and −3.0 vs −1.0 mmHg [P<0.01]) were greater with dapagliflozin than with placebo.
In addition, more patients in the dapagliflozin groups than in the placebo groups (10.0% vs 1.9% with placebo [P<0.001] and 11.7% vs 0.9% [P<0.0001]) achieved a three-item coprimary end point of reduction of HbA1c ≥0.5%, reduction of body weight ≥3%, and reduction of SBP ≥3 mmHg. These findings suggest that dapagliflozin improves glycemic control and body weight in older patients with advanced T2DM and cardiovascular disease.
The effect of dapagliflozin on HbA1c was assessed in patients with T2DM and moderate renal impairment (>90% of patients had an eGFR of 30–59 mL/min/1.73 m2) [44]. After 24 weeks of treatment, mean decreases from baseline in HbA1c were similar for placebo (−0.32%) and dapagliflozin 5 and 10 mg (−0.41% and −0.44%, respectively). FPG increased with placebo (8.4 mg/dL) but decreased with dapagliflozin (−5.2 and −0.6 mg/dL). Mean changes from baseline in body weight for dapagliflozin were −1.3 and −1.7 kg compared with +0.7 kg for placebo. A pharmacodynamic study in patients with T2DM also suggested that dapagliflozin may have reduced efficacy in patients with moderate (creatinine clearance, 30–50 mL/min) to severe (<30 mL/min) renal impairment [45]. Dapagliflozin is not recommended for use in patients with an eGFR <60 mL/min/1.73 m2 and is contraindicated in patients with an eGFR <30 mL/min/1.73 m2 [44].
BP was assessed as a safety or exploratory end point in the aforementioned Phase III trials. Dapagliflozin treatment resulted in placebo-corrected reductions in SBP of ≤5 mmHg and in DBP of ≤4 mmHg [46–50]. Despite these reductions in BP, no substantial increases in orthostatic hypotension events were reported with dapagliflozin in these studies [46,47,50].
Two double-blind, randomized, placebo-controlled, 12-week studies assessed the effects of dapagliflozin 10 mg/d on seated SBP in patients with T2DM and hypertension and inadequate glycemic (HbA1c, 7.0%–10.5%) and BP (seated SBP/DBP, 140–164/85–104 mmHg) control despite receiving glucose-lowering drugs and an angiotensin-converting enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB) [51] or an ACEI/ARB plus a second antihypertensive drug [52]. In one study, dapagliflozin significantly reduced HbA1c (difference vs placebo, −0.46%; P<0.0001) and seated SBP (−3 mmHg, P=0.001) [51]. Similar responses to dapagliflozin (difference vs placebo: HbA1c, −0.61% [P<0.0001]; seated SBP, −4 mmHg [P=0.0002]) were observed in the other study [52]. There were no marked effects of dapagliflozin on serum electrolytes or on the GFR. These studies showed that dapagliflozin consistently lowered BP in patients with T2DM and hypertension, and improved glycemic parameters in patients with inadequate glycemic control.
Safety
In Phase III trials, AEs reported with dapagliflozin were, in general, mild to moderate and, except for infections of the genital tract and urinary tract, were reported at a similar frequency as with placebo. In patients with T2DM and cardiovascular disease and hypertension, AEs were balanced across dapagliflozin and placebo groups [43]. The most common AEs in most studies were genital and urinary tract infections, headache, diarrhea, nasopharyngitis, and back pain [46–50].
Increased urinary excretion of glucose may increase the risk for genital and urinary tract infections [53]. Therefore, in the dapagliflozin clinical development program, signs and symptoms suggestive of such infections were defined prospectively, and patients were actively questioned about these symptoms at all study visits throughout the trials [46]. Across the trials, signs, symptoms, and other reports suggestive of genital infections and, in some trials, urinary tract infections, were more frequent in dapagliflozin-treated groups compared with placebo groups (Table 4). Events were of mild to moderate intensity. Infections were responsive to standard care, did not typically require interruption of dapagliflozin therapy, and rarely resulted in discontinuation of the studies [46–48].
Table 4.
Adverse events, including genital infections and urinary tract infections and hypoglycemia,* with dapagliflozin in Phase III trials.
| Patients, % | |||||
|---|---|---|---|---|---|
| Dapagliflozin, mg/d | |||||
| Trial | Duration, weeks | AE | Placebo or control | 5 | 10 |
| Monotherapy (NCT00528372) [47] | 24 | ≥1 AE | 60 | 58 | 69 |
| Genital | 1 | 8 | 13 | ||
| Urinary | 4 | 13 | 6 | ||
| Hypoglycemia | 3 | 0 | 3 | ||
| Add-on to MET (NCT00528879) [46] | 24 | ≥1 AE | 64 | 69 | 73 |
| Genital | 5 | 13 | 9 | ||
| Urinary | 8 | 7 | 8 | ||
| Hypoglycemia | 3 | 4 | 4 | ||
| Add-on to glimepiride (NCT00680745) [48] | 24 | ≥1 AE | 47 | 48 | 50 |
| Genital | 1 | 6 | 7 | ||
| Urinary | 6 | 7 | 5 | ||
| Hypoglycemia | 5 | 7 | 8 | ||
| Add-on to pioglitazone (NCT00683878) [49] | 24 | ≥1 AE | 67 | 68 | 71 |
| Genital | 3 | 9 | 9 | ||
| Urinary | 8 | 9 | 5 | ||
| Hypoglycemia | 1 | 2 | 0 | ||
| Add-on to sitagliptin ± MET (NCT00984867) [57] | 24 | ≥1 AE | 48 | ND | 53 |
| Genital | <1 | ND | 8 | ||
| Urinary | 4 | ND | 5 | ||
| Hypoglycemia | 3 | ND | 2 | ||
| Add-on to insulin (NCT00673231) [50] | 24 | ≥1 AE | 73 | 72 | 74 |
| Genital | 3 | 10 | 11 | ||
| Urinary | 5 | 11 | 10 | ||
| Hypoglycemia | 52 | 56 | 54 | ||
| Add-on to MET vs glipizide add-on to MET (NCT00660907) [40] | 52 | ≥1 AE | 78 | ND | 78 |
| Genital | 3 | ND | 12 | ||
| Urinary | 6 | ND | 11 | ||
| Hypoglycemia | 41 | ND | 4 | ||
| Add-on to MET + SU (NCT01392677) [78] | 24 | ≥1 AE | 51 | ND | 49 |
| Genital | 0 | ND | 6 | ||
| Urinary | 6 | ND | 6 | ||
| Hypoglycemia | 4 | ND | 13 | ||
| Dapagliflozin 5 mg combined with MET XR as initial therapy (NCT00643851) [41] | 24 | ≥1 AE | 59 | 69 | ND |
| Genital | 2 | 7 | ND | ||
| Urinary | 8 | 8 | ND | ||
| Hypoglycemia | 0 | 3 | ND | ||
| Dapagliflozin 10 mg combined with MET XR as initial therapy (NCT00859898) [41] | 24 | ≥1 AE | 57 | ND | 60 |
| Genital | 2 | ND | 9 | ||
| Urinary | 4 | ND | 8 | ||
| Hypoglycemia | 3 | ND | 3 | ||
Hypoglycemia defined as symptoms suggestive of hypoglycemia or asymptomatic/symptomatic with plasma glucose <63 mg/dL.
AE, adverse event; MET, metformin; ND, not determined; SU, sulfonylurea; XR, extended release.
In data pooled from 12 randomized trials (12- to 24-week duration), more patients treated with dapagliflozin (2.5, 5, or 10 mg/d) had diagnosed cases of genital infections (4%–6%) than did patients receiving placebo (1%) [54]. Most cases were mild to moderate and responded to standard care. In these trials, percentages of patients reporting diagnosed cases of urinary tract infections were slightly higher with dapagliflozin (4%–6%) than with placebo (4%) [55]. Most cases were mild to moderate and did not recur during the trial duration or result in interruption or discontinuation of dapagliflozin therapy.
Analyses of the eGFR from 12 placebo-controlled, randomized trials of ≤24 weeks and from five trials of ≤102 weeks found that the eGFR decreased at week 1, returned to baseline by week 24, and remained stable thereafter to week 102 in patients treated with dapagliflozin 5 or 10 mg/d [56]. It has been suggested that the acute reductions in eGFR with dapagliflozin may reflect reversible hemodynamic effects rather than permanent changes in renal function [16].
Events of hypoglycemia were infrequent and occurred at a similar rate with dapagliflozin as with placebo in the dapagliflozin monotherapy study [47], add-on to metformin studies [13,46], add-on to pioglitazone study [49], and add-on to sitagliptin study (Table 4) [49,57]. The proportion of patients reporting hypoglycemic episodes was higher when dapagliflozin was added to a sulfonylurea (7%–8% vs 5% with placebo) or insulin (54%–56% vs 52% with placebo) [48,50]. In the add-on to insulin study, the proportion of patients with a major hypoglycemic event was low and similar across placebo (1.0%) and dapagliflozin (0.9%–1.5%) groups. In the study in which dapagliflozin 10 mg/d was compared with glipizide as add-on therapy to metformin, the number of patients reporting hypoglycemic episodes was tenfold higher with glipizide (41%) than with dapagliflozin (4%) [40].
Dapagliflozin had variable effects on plasma lipids. Changes in LDL-C (−0.5% to +9.5%), HDL-C (+2.1% to +9.3%), and triglycerides (−0.9% to −10.6%) were noted in clinical trials [17]. In a pool of 13 placebo-controlled studies, the mean percentage change from baseline for total cholesterol after 24 weeks of treatment with dapagliflozin 10 mg was 2.5% compared with 0% for placebo and for LDL-C was −1.0% vs 2.9% [44].
In the clinical development program for dapagliflozin, an imbalance in bladder cancer was observed with dapagliflozin. Newly diagnosed bladder cancer was reported in 10/6045 patients (0.17%) treated with dapagliflozin and 1/3512 patients (0.03%) treated with placebo or comparator [58]. After excluding patients exposed to dapagliflozin for <1 year at the time of the diagnosis of bladder cancer, there were four cases of bladder cancer with dapagliflozin and no cases with placebo or comparator. Risk factors for bladder cancer and hematuria (a potential indicator of preexisting tumors) [59] at baseline were balanced between treatment groups. There were insufficient data to determine whether the cases of bladder cancer were related to dapagliflozin or whether dapagliflozin had an effect on preexisting bladder tumors. The incidence of bladder cancer with dapagliflozin is being monitored during an ongoing cardiovascular outcomes trial as well as through continued postmarketing surveillance [58]. Dapagliflozin should not be used in patients with active bladder cancer, and should be used with caution in those with a history of bladder cancer [30].
Empagliflozin
Empagliflozin (Jardiance‡) was approved in the EU and US in 2014 [60]. The recommended starting dose is 10 mg/d, which can be increased to 25 mg/day [61]. Empagliflozin should not be used in patients with an eGFR <45 mL/min/1.73 m2, and renal function should be monitored more frequently (at least yearly according to EU prescribing information) in patients with an eGFR <60 mL/min/1.73 m2 [61,62]. In patients with T2DM, empagliflozin was absorbed rapidly with a mean t½ of 10 h–19 h [63]. In healthy volunteers, empagliflozin pharmacokinetics were similar with and without coadministration of metformin, glimepiride, pioglitazone, sitagliptin, linagliptin, warfarin, verapamil, ramipril, simvastatin, hydrochlorothiazide, and torasemide [64].
Efficacy
In Phase III clinical trials, placebo-corrected mean changes from baseline in HbA1c for empagliflozin 10 and 25 mg/d were −0.74% to −0.85% if used as monotherapy [65] and −0.38% to −0.64% if used as add-on therapy (Table 5) [66–69]. Empagliflozin also significantly reduced FPG (placebo-corrected change from baseline, −12 to −36 mg/dL) (Table 5) and 2-h PPG after a mixed-meal test (placebo-corrected change from baseline, −33 to −52 mg/dL) [66,67]. Placebo-corrected mean change in body weight with empagliflozin ranged from −1.6 to −2.5 kg (Table 5). Across these clinical trials, empagliflozin consistently reduced SBP (−2.9 to −5.2 vs 7 to −2.9 mmHg for placebo) and DBP (−1.0 to −2.5 vs 0.3 to −2.2 mmHg for placebo) to a greater extent than placebo.
Table 5.
Placebo- or comparator-corrected changes from baseline to study end in HBA1c, FPG, PPG, and body weight with empagliflozin in Phase III trials.
| HbA1c,% | FPG,mg/clL | PPG, mg/dL | Body weight, kg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Empagliflozin, mg/d | Empagliflozin, mg/d | Empagliflozin, mg/d | Empagliflozin, mg/d | ||||||||||
| Trial | Duration, weeks | Baseline | 10 | 25 | Baseline | 10 | 25 | Baseline | 10 | 25 | Baseline | 10 | 25 |
| Monotherapy (NCT01177813) [65] | 24 | 7.86–7.91 | −0.74 | −0.85 | 153–155 | −31 | −36 | ND | ND | ND | 78 | −1.9 | −2.2 |
| Add-on to MET (NCT01159600) [66] | 24 | 7.86–7.94 | −0.57 | −0.64 | 149–156 | −26 | −29 | 252–264 | −52 | −50 | 80–82 | −1.6 | −2.0 |
| Add-on to MET + SU (NCT01159600) [67] | 24 | 8.07–8.15 | −0.64 | −0.59 | 151–156 | −29 | −29 | 280–298 | −33 | −34 | 76–78 | −1.8 | −2.0 |
| Add-on to pioglitazone ± MET(NCT01289990)[68] | 24 | 8.06–8.16 | −0.48 | −0.61 | 152 | −23 | −28 | ND | ND | ND | 78–79 | −2.0 | −1.8 |
| Add-on to insulin ± MET in obese patients (NCT01306214)[69] | 18 52 | 8.29–8.39 | −0.44 −0.38 |
−0.52 −0.46 |
150–159 | −21 −12 |
−28 −14 |
ND | ND | ND | 96–97 | −1.3 −2.4 |
−1.9 −2.5 |
| Add-on to MET vs glimepiride add-on to MET (NCT01167881) [70] | 52 104 |
7.92 – |
ND ND |
−0.07 −0.11 |
150 – |
ND ND |
−11 −12 |
251–253 252–253 |
ND ND |
−13 −14 |
83 – |
ND ND |
−4.8 −4.5 |
FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; MET, metformin; ND, not determined; PPG, postprandial glucose; SU, sulfonylurea.
In a study comparing the effects of empagliflozin (25 mg/d) compared with glimepiride (1–4 mg/d) as add-on therapy to metformin [70], empagliflozin was noninferior to glimepiride (P<0.0001) with respect to the change in HbA1c after 52 and 104 weeks of treatment (Table 5) and was superior to glimepiride at 104 weeks (difference vs glimepiride, −0.11%). Reduction in FPG was significantly (P<0.0001) greater with empagliflozin compared with glimepiride at 52 and 104 weeks (Table 5). Patients receiving empagliflozin had a reduction from baseline in weight at 52 weeks (−3.2 kg) that was maintained at 104 weeks (−3.1 kg). Weight gains with glimepiride were reported at these two time points (1.6 and 1.3 kg, respectively). Almost 90% of the weight loss with empagliflozin appeared to be the result of a reduction in fat mass (abdominal visceral and subcutaneous adipose tissue) [70]. Empagliflozin reduced SBP (−3.6 and −3.1 mmHg) and DBP (−1.9 and −1.8 mmHg) at 52 and 104 weeks, whereas SBP (+2.2 and +2.5 mmHg) and DBP (+0.9 and +0.9 mmHg) were increased with glimepiride at these time points.
The efficacy and safety of empagliflozin compared with placebo added to existing antidiabetic drugs were assessed in patients with T2DM and stage-II (eGFR ≥60 to <90 mL/min/1.73 m2), stage-III (≥30 to <60 mL/min/1.73 m2), or stage-IV (eGFR ≥15 to <30 mL/min/1.73 m2) CKD [71]. In patients with stage-II CKD, empagliflozin (10 and 25 mg/d) significantly reduced HbA1c from baseline at 24 weeks compared with placebo (difference vs placebo −0.52% and −0.68%, P<0.0001 for both). Both doses of empagliflozin also produced significant placebo-corrected reductions in FPG (−20 and −24 mg/dL), body weight (−1.4 and −2.0 kg), SBP (−3.6 and −5.1 mmHg), and DBP (−2.5 and −3.3 mmHg). Changes in glycemic measures, body weight, and BP were sustained at week 52. As expected, based on the mechanism of action of empagliflozin, the changes in glycemic and metabolic measures in patients with stage-III and -IV CKD were less than those in patients with stage-II CKD. In patients with stage-III CKD, empagliflozin (25 mg/d only) significantly reduced HbA1c (difference vs placebo, −0.42%), FPG (−20 mg/dL), body weight −0.9 kg), SBP (−4.3 mmHg), and DBP (−1.9 mmHg) at week 24. These changes were sustained at 52 weeks of treatment. In patients with stage-IV CKD, empagliflozin (25 mg/d) had little or no effect on HbA1c and FPG compared with placebo after 24 and 52 weeks of treatment. However, greater reductions in body weight (−1.4 vs −0.1 kg), SBP (−7.4 vs 1.2 mmHg), and DBP (−2.4 vs 0.7 mmHg) occurred in patients receiving empagliflozin for 24 weeks than in those receiving placebo; these effects were sustained for 52 weeks.
Safety
Empagliflozin was, in general, well tolerated, with similar incidences of AEs in empagliflozin-treated groups and placebo groups (Table 6). As was observed with canagliflozin and dapagliflozin, genital infections were more common with empagliflozin than with placebo (Table 6). In most studies, hypoglycemic events were rare and similar across treatment groups. Hypoglycemia was more frequent with empagliflozin than with placebo when added to a sulfonylurea [67].
Table 6.
Adverse events, including genital infections and urinary tract infections and hypoglycemia,* with empagliflozin in Phase III trials.
| Patients, % | |||||
|---|---|---|---|---|---|
| Empagliflozin, mg/d | |||||
| Trial | Duration, weeks | AE | Placebo or control | 10 | 25 |
| Monotherapy (NCT01177813) [65] | 24 | ≥1 AE | 61 | 55 | 61 |
| Genital | 0 | 3 | 4 | ||
| Urinary | 5 | 7 | 5 | ||
| Hypoglycemia | <1 59 | <1 | <1 | ||
| Add-on to MET (NCT01159600) [66] | 24 | ≥1 AE | 57 | 50 | |
| Genital | 0 | 4 | 5 | ||
| Urinary | 5 | 5 | 6 | ||
| Hypoglycemia | <1 | 2 | 1 | ||
| Add-on to MET + SU (NCT01159600) [67] | 24 | ≥1 AE | 63 | 68 | 64 |
| Genital | 1 | 3 | 2 | ||
| Urinary | 8 | 10 | 8 | ||
| Hypoglycemia | 8 | 16 | 12 | ||
| Add-on to pioglitazone ± MET (NCT01289990) [68] | 24 | ≥1 AE | 73 | 67 | 71 |
| Genital | 2 | 9 | 4 | ||
| Urinary | 16 | 17 | 12 | ||
| Hypoglycemia | 2 | 1 | 2 | ||
| Add-on to insulin ± MET in obese patients (NCT01306214) [69] | 52 | ≥1 AE | 90 | 86 | 85 |
| Genital | 2 | 4 | 10 | ||
| Urinary | 15 | 16 | 15 | ||
| Hypoglycemia | 58 | 51 | 58 | ||
| Add-on to MET vs glimepiride add-on to MET (NCT01167881) [70] | 104 | ≥1 AE | 86 | ND | 86 |
| Genital | 2 | ND | 12 | ||
| Urinary | 13 | ND | 14 | ||
| Hypoglycemia | 25 | ND | 4 | ||
Hypoglycemia defined by plasma glucose ≤70 mg/dL and/or requiring assistance. AE, adverse event; MET, metformin; ND, not determined; SU, sulfonylurea.
The overall incidence of AEs was similar with placebo and empagliflozin in patients with stage-II or -III CKD [71]. The proportion of patients with ≥1 AE was higher with empagliflozin (92%) than with placebo (84%) in patients with stage-IV CKD. Hypoglycemia (38% vs 32%) as well as urinary tract infections (19% vs 8%) and genital infections (3% vs 0%) were more common with empagliflozin than with placebo in patients with stage-IV CKD. Small decreases in the eGFR were observed with empagliflozin in patients with stage-II, -III, or -IV CKD. The eGFR returned to baseline three weeks after treatment completion in all CKD groups.
Most studies reported a small increase in HDL-C and no change in triglycerides with empagliflozin compared with placebo [65–68,70]. Small increases [66,70] or no change in LDL-C were noted with empagliflozin [65,67–69].
Implications for healthcare providers
The integration of lifestyle management, appropriate self-care, and medication adherence is vital to the successful addition of SGLT2 therapies into the antidiabetic regimen. The American Association of Diabetes Educators recognizes seven self-care behaviors that are critical in DM self-management: (i) monitoring, (ii) taking medication, (iii) being active, (iv) eating a healthy diet, (v) problem solving, (vi) healthy coping, and (vii) reducing risk factors [6]. Healthcare providers can play a pivotal part in improving adherence to therapy and support the efforts of other members of the healthcare team in helping patients with T2DM attain and maintain glycemic goals [72–74].
SGLT2 inhibitors are a new class of agents and most patients may not know the difference between classes. Hence, it is important that healthcare providers explain the unique mechanism of action of these drugs and how to identify, take, and store these medications. Healthcare providers should inform patients about the risk of developing genital infections and urinary tract infections. As the number of patients taking SGLT2 inhibitors increases, healthcare providers will play a very important part in helping patients maintain medication adherence and approach their HbA1c goals.
Summary
Canagliflozin, dapagliflozin, and empagliflozin are medications from the novel SGLT2 inhibitor class that offer a unique treatment option for T2DM that is independent of the secretion or action of insulin. The AACE recommends that SGLT2 inhibitors be considered as a monotherapy option in patients for whom metformin is contraindicated or not tolerated [4]. In addition, SGLT2 inhibitors may be an option as add-on therapy to metformin or another first-line agent as part of dual or triple therapy. Canagliflozin, dapagliflozin, and empagliflozin reduce HbA1c, FPG, and PPG (Table 7) in patients across various stages of T2DM by promoting removal of excess glucose via increased urinary excretion of glucose. In addition, they are complementary to existing antihyperglycemic agents and improve glycemic control in elderly patients and in patients inadequately controlled with metformin, sulfonylurea, thiazolidinedione, dipeptidyl peptidase-4 inhibitors, or insulin or who are treatment naïve. The weight loss associated with SGLT2 inhibitors may be a benefit to overweight and obese patients with T2DM, and may attenuate the weight gains characteristic of some other therapeutic options. SGLT2 inhibitors also cause modest reductions in BP, probably as a result of weight loss and diuretic action. Increases in HDL-C and minor changes in triglycerides were noted in most clinical trials. However, increases in LDL-C were also noted and need to be monitored and treated. Canagliflozin, dapagliflozin, and empagliflozin were, in general, well tolerated in clinical trials and, as expected based on their mechanism of action, the frequency of hypoglycemia was low except if taken with insulin or an insulin secretagogue. There were increased episodes of genital infections and, in some trials, urinary tract infections, with these agents. These infections were, in general, mild to moderate and resolved with standard treatment. These SGLT2 inhibitors should not be used in patients with impaired renal function (eGFR: <45 mL/min/1.73 m2 for canagliflozin and empagliflozin, <60 mL/min/1.73 m2 for dapagliflozin).
Table 7.
Summary of effects of SGLT2 inhibitors.
| Parameter | Response |
|---|---|
| HbA1c | ⬇ |
| FPG | ⬇ |
| PPG | ⬇ |
| Body weight | ⬇ |
| Blood pressure | ⬇ |
| Lipids | |
| LDL-C | ⬆ |
| HDL-C | ⬆ |
| Triglycerides | ⬇⬆ or ⬌ |
| Infections | |
| Genital | ⬆ |
| Urinary tract | ⬆ or ⬌ |
FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PPG, postprandial glucose; SGLT2, sodium-glucose co-transporter 2.
Conclusion
SGLT2 inhibitors are new insulin-independent agents that, given adequate renal function, improve glycemic control in patients with T2DM not adequately controlled by diet and exercise or by other antidiabetic agents. Canagliflozin, dapagliflozin, and empagliflozin also provide reductions in body weight and BP. The most common adverse effects are genital infections and urinary tract infections. Further studies are being conducted to assess the effects of these drugs on cardiovascular outcomes. SGLT2 inhibitors are a promising new treatment option for T2DM.
Acknowledgments
Editorial support was provided by Richard M Edwards, PhD, and Janet E Matsuura, PhD, from Complete Healthcare Communications, Inc., and was funded by Bristol-Myers Squibb and AstraZeneca.
Abbreviations
- AACE
American Association of Clinical Endocrinologists
- ACEI
angiotensin-converting enzyme inhibitor
- AE
adverse event
- ARB
angiotensin receptor blocker
- BP
blood pressure
- CKD
chronic kidney disease
- DBP
diastolic blood pressure
- DM
diabetes mellitus
- eGFR
estimated glomerular filtration rate
- EU
European Union
- FPG
fasting plasma glucose
- GFR
glomerular filtration rate
- HbA1c
glycated hemoglobin
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- PPG
postprandial glucose
- SBP
systolic blood pressure
- t½
half-life
- T2DM
type 2 diabetes mellitus
- US
United States
Footnotes
Registered trademark of Janssen Pharmaceuticals, Inc., Titusville, NJ, USA
Registered trademark of AstraZeneca, Wilmington, DE, USA
Registered trademark of Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT, USA
Funding declaration
The author reports no competing financial interests. The author did not receive any financial or monetary transfer of value for the writing of this manuscript.
Contributions
The author participated in the preparation, review, editing, and final approval of the manuscript.
Potential conflicts of interest
The International Committee of Medical Journal Editors’ (ICMJE) Potential Conflicts of Interests form for the author is summarized below. The original form is available for download at: http://www.drugsincontext.com/wp-content/uploads/2014/12/dic.212264-COI.pdf
References
- 1.Centers for Disease Control and Prevention National diabetes statistics report: estimates of diabetes and its burden in the United States. 2014. [Last accessed September 23, 2014]. Available at: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
- 2.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. http://dx.doi.org/10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(suppl 1):S14–S80. doi: 10.2337/dc14-S014. http://dx.doi.org/10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 4.Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement–executive summary. Endocr Pract. 2013;19(3):536–57. doi: 10.4158/EP13176.CS. http://dx.doi.org/10.4158/EP13176.CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. http://dx.doi.org/10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36(8):2271–9. doi: 10.2337/dc12-2258. http://dx.doi.org/10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfister M, Whaley JM, Zhang L, List JF. Inhibition of SGLT2: a novel strategy for treatment of type 2 diabetes mellitus. Clin Pharmacol Ther. 2011;89(4):621–5. doi: 10.1038/clpt.2011.16. http://dx.doi.org/10.1038/clpt.2011.16. [DOI] [PubMed] [Google Scholar]
- 8.Mather A, Pollock C. Glucose handling by the kidney. Kidney Int. 2011;79(suppl 120):S1–S6. doi: 10.1038/ki.2010.509. http://dx.doi.org/10.1038/ki.2010.509. [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013;36(10):3169–76. doi: 10.2337/dc13-0387. http://dx.doi.org/10.2337/dc13-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54(12):3427–34. doi: 10.2337/diabetes.54.12.3427. http://dx.doi.org/10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 11.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27(2):136–42. doi: 10.1111/j.1464-5491.2009.02894.x. http://dx.doi.org/10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int. 2011;79(suppl 120):S20–S7. doi: 10.1038/ki.2010.512. http://dx.doi.org/10.1038/ki.2010.512. [DOI] [PubMed] [Google Scholar]
- 13.Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–31. doi: 10.1210/jc.2011-2260. http://dx.doi.org/10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 14.Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–50. doi: 10.1016/S0140-6736(13)60683-2. http://dx.doi.org/10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 15.Eckel RH, Kahn SE, Ferrannini E, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? Diabetes Care. 2011;34(6):1424–30. doi: 10.2337/dc11-0447. http://dx.doi.org/10.2337/dc11-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–62. doi: 10.1111/dom.12127. http://dx.doi.org/10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013;125(3):181–9. doi: 10.3810/pgm.2013.05.2667. http://dx.doi.org/10.3810/pgm.2013.05.2667. [DOI] [PubMed] [Google Scholar]
- 18.Invokana® (canagliflozin) Full Prescribing Information. Janssen Pharmaceuticals; Titusville, NJ: 2013. [Google Scholar]
- 19.Devineni D, Curtin CR, Polidori D, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013;53(6):601–10. doi: 10.1002/jcph.88. http://dx.doi.org/10.1002/jcph.88. [DOI] [PubMed] [Google Scholar]
- 20.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13(7):669–72. doi: 10.1111/j.1463-1326.2011.01406.x. http://dx.doi.org/10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 21.Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14(1):83–90. doi: 10.1111/j.1463-1326.2011.01517.x. http://dx.doi.org/10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 22.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261(1):32–43. doi: 10.1111/j.1365-2796.2006.01746.x. http://dx.doi.org/10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 23.Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36(8):2154–61. doi: 10.2337/dc12-2391. http://dx.doi.org/10.2337/dc12-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582–92. doi: 10.1007/s00125-013-3039-1. http://dx.doi.org/10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36(9):2508–15. doi: 10.2337/dc12-2491. http://dx.doi.org/10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bode B, Stenlof K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract (1995) 2013;41(2):72–84. doi: 10.3810/hp.2013.04.1020. http://dx.doi.org/10.3810/hp.2013.04.1020. [DOI] [PubMed] [Google Scholar]
- 27.Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15(5):463–73. doi: 10.1111/dom.12090. http://dx.doi.org/10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenlof K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15(4):372–82. doi: 10.1111/dom.12054. http://dx.doi.org/10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weir MR, Kline I, Xie J, Edwards R, Usiskin K. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR) Curr Med Res Opin. 2014;30(9):1759–68. doi: 10.1185/03007995.2014.919907. http://dx.doi.org/10.1185/03007995.2014.919907. [DOI] [PubMed] [Google Scholar]
- 30.Farxiga® (dapagliflozin) Full Prescribing Information, Bristol-Myers Squibb and AstraZeneca. Princeton; NJ, and Wilmington, DE, USA: 2014. [Google Scholar]
- 31.Forxiga, summary of product characteristics. [Last accessed September 23, 2014]. Available at: http://www.forxiga.eu/sites/default/files/Forxiga%20Summary%20of%20Product%20CharacteristicsSmPC.pdf.
- 32.Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85(5):520–6. doi: 10.1038/clpt.2008.251. http://dx.doi.org/10.1038/clpt.2008.251. [DOI] [PubMed] [Google Scholar]
- 33.Obermeier M, Yao M, Khanna A, et al. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dispos. 2010;38(3):405–14. doi: 10.1124/dmd.109.029165. http://dx.doi.org/10.1124/dmd.109.029165. [DOI] [PubMed] [Google Scholar]
- 34.Kasichayanula S, Liu X, Zhang W, et al. Effect of a high-fat meal on the pharmacokinetics of dapagliflozin, a selective SGLT2 inhibitor, in healthy subjects. Diabetes Obes Metab. 2011;13(8):770–3. doi: 10.1111/j.1463-1326.2011.01397.x. http://dx.doi.org/10.1111/j.1463-1326.2011.01397.x. [DOI] [PubMed] [Google Scholar]
- 35.Kasichayanula S, Liu X, Shyu WC, et al. Lack of pharmacokinetic interaction between dapagliflozin, a novel sodium-glucose transporter 2 inhibitor, and metformin, pioglitazone, glimepiride or sitagliptin in healthy subjects. Diabetes Obes Metab. 2011;13(1):47–54. doi: 10.1111/j.1463-1326.2010.01314.x. http://dx.doi.org/10.1111/j.1463-1326.2010.01314.x. [DOI] [PubMed] [Google Scholar]
- 36.Kasichayanula S, Chang M, Liu X, et al. Lack of pharmacokinetic interactions between dapagliflozin and simvastatin, valsartan, warfarin, or digoxin. Adv Ther. 2012;29(2):163–77. doi: 10.1007/s12325-011-0098-x. http://dx.doi.org/10.1007/s12325-011-0098-x. [DOI] [PubMed] [Google Scholar]
- 37.Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43. doi: 10.1186/1741-7015-11-43. http://dx.doi.org/10.1186/1741-7015-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolinder J, Ljunggren O, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16(2):159–69. doi: 10.1111/dom.12189. http://dx.doi.org/10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- 39.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over two years. Diabetes Obes Metab. 2014;16(2):124–36. doi: 10.1111/dom.12187. http://dx.doi.org/10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 40.Nauck MA, Del Prato S, Meier JJ, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015–22. doi: 10.2337/dc11-0606. http://dx.doi.org/10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012;66(5):446–56. doi: 10.1111/j.1742-1241.2012.02911.x. http://dx.doi.org/10.1111/j.1742-1241.2012.02911.x. [DOI] [PubMed] [Google Scholar]
- 42.Leiter LA, Cefalu WT, de Bruin TW, Gause-Nilsson I, Sugg JE, Parikh S. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc. 2014;62(7):1252–62. doi: 10.1111/jgs.12881. http://dx.doi.org/10.1111/jgs.12881. [DOI] [PubMed] [Google Scholar]
- 43.Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg JE, Parikh S. Dapagliflozin treatment for type 2 diabetes mellitus patients with comorbid cardiovascular disease and hypertension [abstract] Diabetes. 2012;61(suppl 1):A271. [Google Scholar]
- 44.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–71. doi: 10.1038/ki.2013.356. http://dx.doi.org/10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasichayanula S, Liu X, Pe Benito M, et al. The influence of kidney function on dapagliflozin exposure, metabolism and pharmacodynamics in healthy subjects and in patients with type 2 diabetes mellitus. Br J Clin Pharmacol. 2013;76(3):432–44. doi: 10.1111/bcp.12056. http://dx.doi.org/10.1111/bcp.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9733):2223–33. doi: 10.1016/S0140-6736(10)60407-2. http://dx.doi.org/10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 47.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217–24. doi: 10.2337/dc10-0612. http://dx.doi.org/10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13(10):928–38. doi: 10.1111/j.1463-1326.2011.01434.x. http://dx.doi.org/10.1111/j.1463-1326.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- 49.Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35(7):1473–8. doi: 10.2337/dc11-1693. http://dx.doi.org/10.2337/dc11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilding JPH, Woo V, Soler NG, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156(6):405–15. doi: 10.7326/0003-4819-156-6-201203200-00003. http://dx.doi.org/10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 51.Weber MA, Mansfield TA, Alessi F, Ptaszynska A. Effects of dapagliflozin on blood pressure in diabetic patients with hypertension inadequately controlled by a renin-angiotensin system blocker [abstract] Circulation. 2013;128(suppl 22):A13144. [Google Scholar]
- 52.Weber MA, Mansfield TA, T’joen C, Ptaszynska A. Dapagliflozin for reduction of blood pressure in diabetic patients inadequately controlled with combination antihypertensive regimen [abstract] Circulation. 2013;128(suppl 22):A13165. [Google Scholar]
- 53.Hoepelman AIM, Meiland R, Geerlings SE. Pathogenesis and management of bacterial urinary tract infections in adult patients with diabetes mellitus. Int J Antimicrob Agents. 2003;22(suppl 2):35–43. doi: 10.1016/s0924-8579(03)00234-6. http://dx.doi.org/10.1016/S0924-8579(03)00234-6. [DOI] [PubMed] [Google Scholar]
- 54.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27(5):479–84. doi: 10.1016/j.jdiacomp.2013.04.012. http://dx.doi.org/10.1016/j.jdiacomp.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 55.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27(5):473–8. doi: 10.1016/j.jdiacomp.2013.05.004. http://dx.doi.org/10.1016/j.jdiacomp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Ptaszynska A, Chalamandaris A, Sugg JE, et al. Effect of dapagliflozin on renal function [abstract] Diabetes. 2012;61(suppl 1):A283. [Google Scholar]
- 57.Jabbour SA, Hardy E, Sugg J, Parikh S, Study 10 Group Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37(3):740–50. doi: 10.2337/dc13-0467. http://dx.doi.org/10.2337/dc13-0467. [DOI] [PubMed] [Google Scholar]
- 58.US Food Drug Administration FDA briefing document. Dapagliflozin oral tablets, 5 and 10 mg. Dec 12, 2013. [Last accessed September 22, 2014]. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM378076.pdf.
- 59.Griffiths TR, on behalf of Action on Bladder Cancer Current perspectives in bladder cancer management. Int J Clin Pract. 2013;67(5):435–48. doi: 10.1111/ijcp.12075. http://dx.doi.org/10.1111/ijcp.12075. [DOI] [PubMed] [Google Scholar]
- 60.Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi: 10.7573/dic.212262. http://dx.doi.org/10.7573/dic.212262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jardiance® (empagliflozin) Full Prescribing Information. Boehringer Ingelheim Pharmaceuticals and Eli Lilly and Company; Ingelheim, Germany, and Indianapolis, IN, USA: 2014. [Google Scholar]
- 62.Jardiance® Summary of product characteristics. [Last accessed September 29, 2014]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002677/WC500168592.pdf.
- 63.Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4(2):331–45. doi: 10.1007/s13300-013-0030-2. http://dx.doi.org/10.1007/s13300-013-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scheen AJ. Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet. 2014;53(3):213–25. doi: 10.1007/s40262-013-0126-x. http://dx.doi.org/10.1007/s40262-013-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208–19. doi: 10.1016/S2213-8587(13)70084-6. http://dx.doi.org/10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 66.Häring H-U, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650–9. doi: 10.2337/dc13-2105. http://dx.doi.org/10.2337/dc13-2105. [DOI] [PubMed] [Google Scholar]
- 67.Häring H-U, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396–404. doi: 10.2337/dc12-2673. http://dx.doi.org/10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147–58. doi: 10.1111/dom.12188. http://dx.doi.org/10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 69.Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(7):1815–23. doi: 10.2337/dc13-3055. http://dx.doi.org/10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 70.Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691–700. doi: 10.1016/S2213-8587(14)70120-2. http://dx.doi.org/10.1016/S2213-8587(14)70120-2. [DOI] [PubMed] [Google Scholar]
- 71.Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–84. doi: 10.1016/S2213-8587(13)70208-0. http://dx.doi.org/10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 72.Glasgow RE, Emont S, Miller DC. Assessing delivery of the five ‘As’ for patient-centered counseling. Health Promot Int. 2006;21(3):245–55. doi: 10.1093/heapro/dal017. http://dx.doi.org/10.1093/heapro/dal017. [DOI] [PubMed] [Google Scholar]
- 73.Joy SV. Clinical pearls and strategies to optimize patient outcomes. Diabetes Educ. 2008;34(suppl 3):54S–9S. doi: 10.1177/0145721708319233. http://dx.doi.org/10.1177/0145721708319233. [DOI] [PubMed] [Google Scholar]
- 74.Martin C, Daly A, McWhorter LS, Shwide-Slavin C, Kushion W. The scope of practice, standards of practice, and standards of professional performance for diabetes educators. Diabetes Educ. 2005;31(4):487–8. 490, 492. doi: 10.1177/0145721705279719. http://dx.doi.org/10.1177/0145721705279719. [DOI] [PubMed] [Google Scholar]
- 75.Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67(12):1267–82. doi: 10.1111/ijcp.12322. http://dx.doi.org/10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenstock J, Davies MJ, Dumas R, et al. Effects of canagliflozin added on to basal insulin +/− other antihyperglycemic agents in type 2 diabetes [abstract] Diabetes. 2013;62(suppl 1):A280. [Google Scholar]
- 77.Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16(5):467–77. doi: 10.1111/dom.12273. http://dx.doi.org/10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matthaei S, Rohwedder K, Grohl A, Johnsson E. Dapagliflozin improves glycaemic control and reduces body weight as add-on therapy to metformin plus sulphonylurea; Presented at: European Association for the Study of Diabetes; September 23–27, 2013; Barcelona, Spain. [Google Scholar]