Abstract
Acute inflammation in the lung is essential to health. So too is its resolution. In response to invading microbes, noxious stimuli or tissue injury, an acute inflammatory response is mounted to protect the host. To limit inflammation and prevent collateral injury of healthy, uninvolved tissue, the lung orchestrates the formation of specialized pro-resolving mediators, specifically lipoxins, resolvins, protectins and maresins. These immunoresolvents are agonists for resolution that interact with specific receptors on leukocytes and structural cells to blunt further inflammation and promote catabasis. This process appears to be defective in several common lung diseases that are characterized by excess or chronic inflammation. Here, we review the molecular and cellular effectors of resolution of acute inflammation in the lung.
Keywords: efferocytosis, pro-resolving mediators, polyunsaturated fatty acid, lipoxin, resolvin, protectin, maresin, asthma, acute respiratory distress syndrome, pneumonia
1. Introduction
In response to injury or pathogen, the acute inflammatory response protects the host from systemic infection and restores tissue homeostasis (1). In the lung, acute inflammation can significantly compromise vital gas exchange, so there are several mechanisms in place to regulate the severity and duration of lung inflammation (2). As known to physicians of ancient civilizations, the “cardinal signs” of inflammation include rubor (redness), tumor (swelling), calor (heat), dolor (pain) and in some cases loss of function, which are the phenotypic manifestations of highly regulated molecular and cellular events in most inflamed tissues (1); however, acute inflammation in the lung is most notable for the vascular events (i.e., rubor, tumor and calor). Inflammation is protective and vital to health (2), but when acute inflammation is unrestrained in amplitude or duration it can lead to disease. In most instances, these molecular and cellular events during acute inflammation are successful in limiting the inciting injury or infection and tissue homeostasis is restored. Most important to a healthy response, the acute inflammation completely resolves. Although acute inflammation is generally self-limited, alternate fates include abscess formation, fibrosis or conversion to chronic inflammation. Several common diseases are characterized by chronic inflammation, including asthma and chronic obstructive pulmonary disease (COPD). For centuries held to be a passive process (1), there are now several lines of evidence that support the concept that resolution of inflammation is an active process (3). Several chemical mediators of resolution have been recently identified as has their pro-resolving receptors and cellular mechanisms of action. This review covers recently identified examples of specific biochemical mediators and cellular mechanisms that have critical anti-inflammatory and pro-resolving roles in the resolution of lung inflammation.
2. Resolution of acute inflammation
In health, both the start and finish of acute inflammation proceed in an efficient manner. Tissue injury can result from either delayed engagement of the acute inflammatory response or ineffective resolution. While there are several examples of immunocompromised hosts having increased susceptibility to infection and tissue injury, there are now a growing number of conditions in which defective resolution appears to contribute to pathophysiology (vide infra). Pathologists define tissue resolution as the disappearance of tissue leukocytes and cessation of further neutrophil recruitment (1). The use of experimental models of acute inflammation that naturally resolve (i.e., self-limited return to homeostasis) has led to the identification of new families of mediators that appear in inflammatory exudates during resolution (4–6). By using this experimental window into the termination of self-limited acute inflammation, specific molecules were identified that are detectable either only during resolution or their levels markedly increase during resolution. The time course for some of these resolution-phase mediators is inversely related to neutrophil numbers in the exudates. Using a systems approach with LC-MS/MS-based metabolo-lipidomics the identity of specific resolution-phase molecules was determined and using material generated by total organic synthesis matching studies for the physical and biological properties of the molecules were performed (5, 6).
With the identification of these new pathways and mediators, it became apparent that resolution is an active host program. For example, mediators present during resolution are distinct from initiation of acute inflammation. In addition to the generation of unique resolution phase molecules, pro-resolving receptors have been identified for these local mediators to operate as resolution agonists. Unintended bystander tissue injury can result from either excess acute inflammation or chronic unresolved inflammation (7), both of which have been linked to important lung diseases. For example, excess acute inflammation is present during the acute respiratory distress syndrome leading to life threatening hypoxemia. Asthma and COPD represent extremely common and important airway diseases in global health that are both characterized by chronic, unremitting inflammation. This presentation is organized to review cellular mechanisms for resolution of lung inflammation, new families of specialized pro-resolving mediators (SPM) and their pro-resolving receptors and will be followed by their link to cellular events and the pathophysiology of lung diseases. For detailed recent reviews covering biosynthesis, stereochemical assignments and total organic synthesis, interested readers should refer to references (8, 9).
3. Cellular mechanisms for the resolution of lung inflammation
Efficient restoration of inflamed tissues to their basal state requires that inflammatory cells are effectively cleared and further leukocyte recruitment is abrogated. During this process, tissue neutrophils undergo apoptosis and are recognized and subsequently engulfed by phagocytic macrophages in a non-inflammatory manner (10). Clearance of apoptotic neutrophils also leads to the production of additional mediators that suppress the progression of inflammation and promote repair of damaged tissues (11, 12).
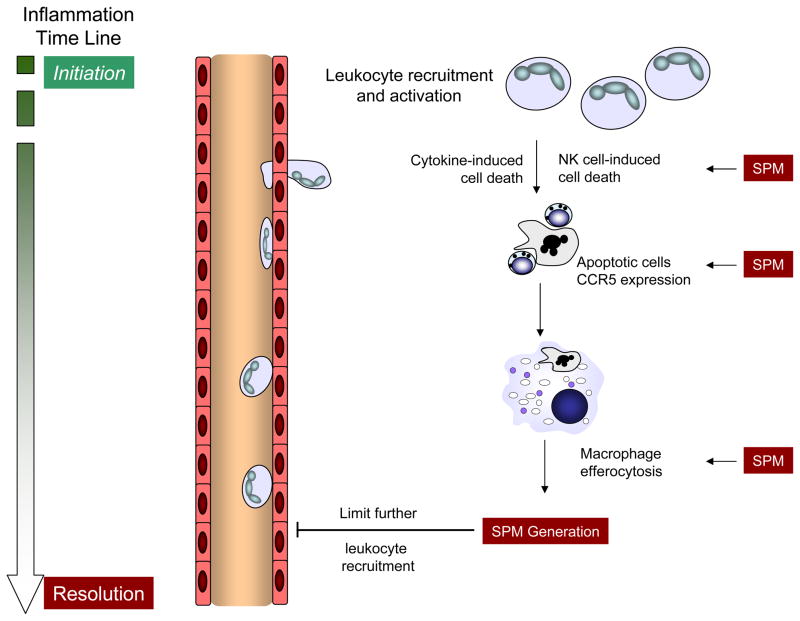
In health, the acute inflammatory response is self-limited. Early tissue edema (minutes-hours) and neutrophil accumulation (hours to days) will decrease with time and lymphocytes, macrophages and other monocytoid cells will traffic to the inflamed tissue (1). While anti-leukocyte actions are commonly considered anti-inflammatory, it is important to view each cell types’ roles in this dynamic process of catabasis. Inhibition of neutrophil transmigration and activation is anti-inflammatory while restitution of barrier integrity (endothelial, epithelial or both), recruitment of monocytoid cells, and promotion of macrophage clearance of apoptotic cells, microbes and tissue debris are all pro-resolving responses orchestrated by SPM. In the lung, restitution of barrier integrity is of heightened importance secondary to concern for alveolar edema leading to hypoxemia. Thus, lung specific pro-resolving events include clearance of edema and transitional matrix, repopulation of the airway epithelia and restoration of pulmonary surfactants. For resolution of adaptive immune responses, allergen-specific or pathogen-specific effector T cells and inflammatory macrophages need to be cleared from the lung. Direct and indirect mechanisms for T cell clearance include natural killer (NK) cell direct cytotoxicity, macrophage engulfment of apoptotic T cells and decrements in pro-inflammatory mediators. SPM induce can T-cell apoptosis (13), their expression of CCR5 for chemokine clearance (14) and NK cell mediated clearance of inflammation in vitro and in vivo (15, 16) (Figure 1).
Figure 1. SPM promote the resolution of tissue inflammation and limit further leukocyte recruitment.
With the initiation of acute inflammation, circulating leukocytes are recruited from the microcirculation to tissues to respond to an invading pathogen, organ injury or a noxious stimulus. To prevent excess inflammation and collateral injury of healthy tissue, there are several mechanisms to restrain the inflammatory response, some of which are illustrated here. With source control, neutrophils (cytokine- or NK cell-induced) and T-cells (activation-induced) undergo apoptosis. SPM increase apoptotic leukocyte expression of CCR5 that serves an important pro-resolving role as a chemokine scavenger. Macrophage efferocytosis of CCR5-expressing apoptotic leukocytes effectively clears the cellular debris and pro-inflammatory chemokines and concomitantly initiates the generation of SPM that also limit further leukocyte recruitment, activation and maturation. These cellular events are pivotal to the termination of acute inflammation.
In addition to these cellular events, inflamed tissues are also notable for the presence of microparticles (MPs) that are remnants of damaged tissues and dying cells. These MPs can serve as “specialized shuttles” to transfer bioactive molecules from dying cells to neighboring living cells. Even apoptotic neutrophils can leave behind MPs with pro-inflammatory properties, so their clearance represents an important component of tissue resolution. Not all MPs are pro-inflammatory. A subset of neutrophil-derived MPs can drive cellular resolution mechanisms (17, 18), and pro-resolving MPs can be modeled with nanoparticles containing pro-resolving mediators as a vehicle for nanomedicine (18). In addition, cellular gene networks selectively regulate microRNAs (miRNAs) during resolution to decrease downstream expression of pro-inflammatory cytokines and chemokines, toll-like receptors and transcription factors (19, 20).
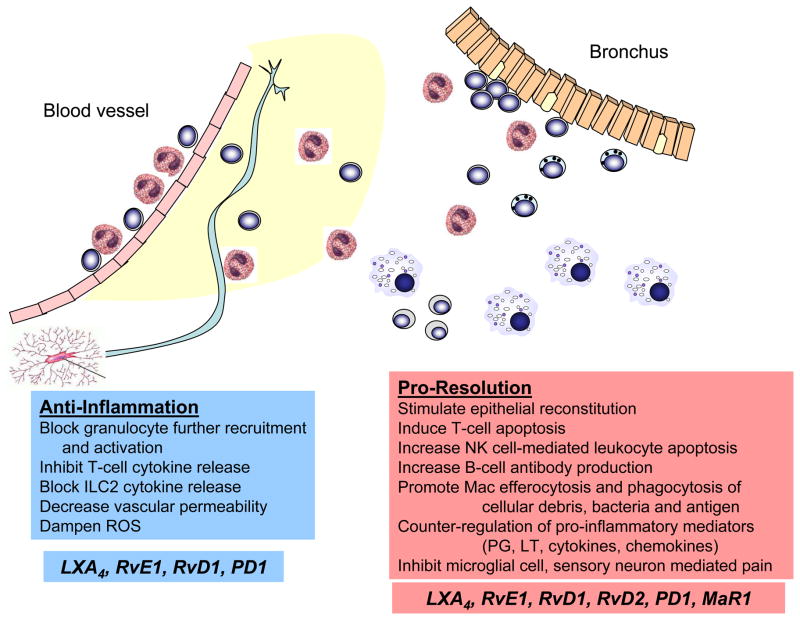
“Anti-inflammatory” and “pro-resolving” do not have the same meaning. These terms have important differences in their definitions. Anti-inflammatory actions decrease granulocyte recruitment and activation, resulting in a predisposition to infection (Figure 2). Mediators with anti-inflammatory properties lead to decreased leukocyte:endothelial cell interactions, platelet aggregation, vascular permeability and leukocyte generation of reactive oxygen species. In sharp contrast, pro-resolving actions activate tissue resident cells to decrease vascular and tissue inflammation to restore organ function – a process termed catabasis to represent a return from the battle of inflammation (Figure 2). Mediators with pro-resolving properties stimulate endothelial nitric oxide and prostacyclin release, mucosal epithelial expression of anti-microbial peptides and macrophage phagocytosis, including efferocytosis, microbial clearance, removal of cellular debris and noxious stimuli (e.g., antigen) and their cellular efflux to lymph nodes. These “pro-resolving” actions increase tissue host defense either directly or indirectly via restoration of tissue homeostasis. Thus, anti-inflammation is not synonymous with resolution.
Figure 2. Anti-inflammation and pro-resolution are not synonymous.
In response to allergic inflammation in the lung, SPM display both anti-inflammatory and pro-resolving actions. The terms – anti-inflammatory and pro-resolving – do not have identical meaning. Anti-inflammation can lead to immunosuppression that increases the host’s susceptibility to infection. Pro-resolution enhances host defense, in part by catabasis; returning inflamed tissue to homeostasis. For SPM, anti-inflammatory properties include blocking granulocyte further recruitment and activation, inhibition of T-cell and ILC2 cytokine release, decreasing vascular permeability and dampening reactive oxygen species generation. Pro-resolving activities include stimulating epithelial reconstitution, inducing T-cell apoptosis, increasing NK cell-mediated leukocyte apoptosis, augmenting B cell differentiation and antibody generation, promoting macrophage efferocytosis and phagocytosis, counter-regulating pro-inflammatory mediators and decreasing CNS microglial cell and peripheral sensory neuron activation.
Cellular indices of resolution have been developed and used to define resolution in quantitative terms. These indices include: Tmax, time point of maximum neutrophil infiltration (Ψmax); T50, time necessary to achieve 50% reduction in neutrophil number (Ψ50) from Ψmax; resolution interval (Ri = T50 -Tmax), time interval between Tmax and T50 (11). These resolution indices enable quantitative comparisons of potential perturbations of natural resolution mechanisms and have helped to identify interventions that are “resolution toxic” as well as interventions that facilitate resolution (16, 21–23). Pharmacological studies of structure-activity relationships and dose responses can use the resolution indices to differentiate the impact of potential therapeutics on pro-inflammatory (Tmax, Ψmax) and pro-resolving events (Ri). With establishment of these metrics, human Phase I-II clinical trials now demonstrate efficacy of a resolvin analog (http://clinicaltrials.gov. Entry Identifier: NCT00799552) and have helped prepare for a Phase III clinical trial.
4. Polyunsaturated fatty acid-derived pro-resolving mediators: immunoresolvents
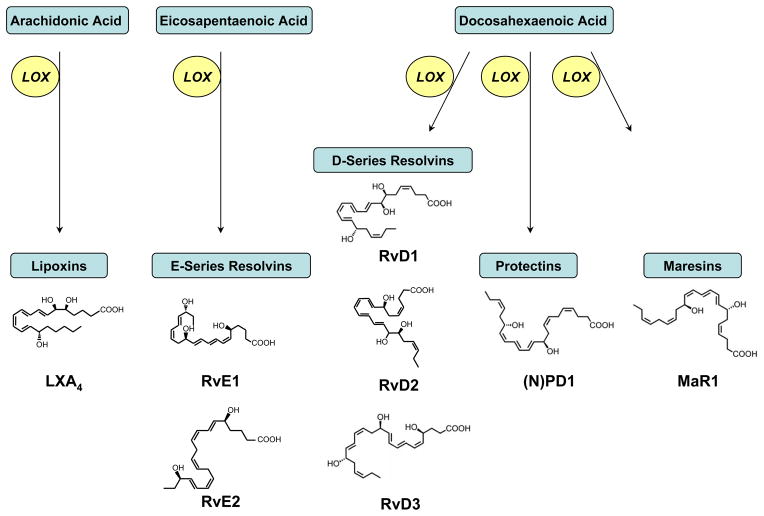
Essential polyunsaturated fatty acids (PUFA) can serve important roles in regulating acute inflammation as substrates for enzymatic conversion to potent PUFA derived mediators (24) (Figure 3). Upon cell activation, PUFAs are rapidly released from cell membranes and enzymatically converted to structurally and functionally distinct lipid mediators. When arachidonic acid (C20:4) is metabolized to prostaglandins (PGs) and cysteinyl leukotrienes (cysLTs) vascular permeability increases (24, 25). In addition, prostaglandins can regulate fever and inflammatory pain. When leukotriene (LT) B4 is generated from C20:4 at sites of inflammation, leukocytes are recruited and activated (24). In contrast to PGs, cysLTs and LTB4, C20:4 can also be converted to lipoxins that decrease LT-mediated pro-phlogistic actions (26). Moreover, lipoxins are the lead family of specialized pro-resolving mediators (SPM) as these interesting molecules carry both anti-inflammatory and pro-resolving bioactions to stop acute inflammation (reviewed in (27)). Present in low abundance during the initiation of acute inflammation, lipoxin levels increase substantially during resolution (22, 28). Disruption of lipoxin formation or lipoxin receptor availability is resolution “toxic” and delays the timely restoration of tissue homeostasis (21, 29–31).
Figure 3. Biosynthesis of specialized pro-resolving mediators (SPM) from polyunsaturated fatty acids.
Specialized pro-resolving mediators (SPM) are enzymatically derived from host essential fatty acids, including arachidonic acid (C20:4n-6), eicosapentaenoic acid (C20:5n-3) and docosahexaenoic acid (C22:6n-3). In a lipoxygenase (LOX) dependent manner, these polyunsaturated fatty acids are converted to families of SPM as indicated. SPM are stereoselective and representative structures of family members with the complete stereochemical assignment established are shown. For additional details, see reference (9).
Additional pro-resolving lipid mediators are increased in resolving exudates that also have anti-inflammatory and pro-resolving actions (reviewed in (32)). To identify SPMs, inflammatory exudates were sampled during resolution and compared to samples from inflammation initiation and control healthy tissues. Sample extracts were prepared and analyzed by liquid chromatography with tandem mass spectrometry (LC-MS/MS) using a systems biology approach. Mammalian experimental models with self-limited and contained exudates, such as the murine dorsal air pouch, were used for SPM discovery (5, 6). The time course for the spatially-contained exudates cellular trafficking and clearance was determined as the foundation for biochemical determination of SPM that were present at the time when neutrophil numbers were decreasing and mononuclear cell numbers (lymphocytes, monocytes, macrophages) were increasing. Molecules that were identified by lipidomics to be unique or increased during the resolution phase were screened for bioactivities. In combination with the potential SPMs, their biosynthetic precursors from PUFAs and further metabolites could be identified and proteomic detection of potential resolvers (enzymes, receptors, non-lipid mediators) could be ascertained. With this systems approach, it was also possible to establish local and temporal SPM biosynthesis (11). For example, upon initiation of inflammation with TNF-α, there was a typical acute-phase response characterized by rapid neutrophil infiltration preceded by local generation of both PGs and LTs. In this setting, conversion of C20:4 to bioactive mediators is highly regulated, and the profiles of lipid mediators switches with time from early pro-inflammatory eicosanoids (i.e., PGs and LTs) to later pro-resolving eicosanoids (i.e., lipoxins) (28). During this time frame, there is an induction of 15-LOX expression that contributes to the formation of select SPM (28) (Figure 3).
In addition to membrane release of arachidonic acid, some biosynthetic intermediates, such as 15-HETE, can also be deacylated from membrane phospholipids for subsequent conversion to lipoxins (33). Some PUFAs, such as the omega-3 fatty acids DHA and EPA that are also SPM precursors, can be carried via plasma edema into the inflammatory milieu and made available for enzymatic conversion to SPM by cells present in the exudates and surrounding inflamed tissues (34). In addition to lipoxins, SPM derived from omega-3 PUFAs are present in resolving exudates with catabatic actions, including resolvins, protections and maresins (reviewed in (35))(Figure 3). As with lipoxins, defects in these SPM pathways can undermine the natural resolution process and contribute to the pathobiology of chronic inflammatory diseases (21, 31, 36–39). These can involve defective receptors, biosynthetic enzymes, intracellular signaling or potential deficiencies in essential PUFA precursors that are required in the diet.
Specialized pro-resolving mediators often carry both anti-inflammatory and pro-resolving bioactivities, serving as “stop” signals for neutrophil activation and “go” signals for macrophage efferocytosis. An example of this distinction is PGE2 that can have anti-inflammatory properties in certain settings via stimulation of cAMP, but is not pro-resolving since it does not enhance macrophage uptake and clearance of apoptotic cells (40). Of note, there are pharmacological inhibitors of COXs as well as certain LOXs that are used clinically because of their capacity to decrease some of the cellular and tissue level events of the inflammatory reaction (e.g. edema formation, neutrophil recruitment, and pain); however, they can adversely impact endogenous pro-resolving circuits (21, 29, 37). In contrast to most non-steroidal anti-inflammatory drugs, aspirin as well as corticosteroids can in some circumstances work synergistically with endogenous pro-resolution pathways (41).
SPM are active in the picogram to nanogram dose range to control inflammation, limit tissue damage, shorten resolution intervals, promote healing, and alleviate pain in experimental models of inflammation and resolution. The SPM are part of a larger resolution program that includes several cytokines (e.g. TGFβ, IL-10) that accumulate in resolving exudates (11, 19); microRNA changes (19); glucocorticoids and the glucocorticoid-induced annexin A1 protein, which also regulates inflammatory responses (reviewed in (42)); and the transcription factor NF-κB, which can display anti-inflammatory properties (43). Induction of leukocyte apoptosis and stimulation of macrophage efferocytosis also promotes resolution (13, 14). Inhibitors of cyclin-dependent kinases can pharmacologically serve this purpose (44) as does annexin A1 peptides (42).
A paradigm shift in treating inflammation
When exogenous SPM are administered during experimental inflammation, they exert their protective actions in low nanogram amounts (32). The capacity of these molecules to “jump start” aspects of resolution suggests their intriguing pharmacological roles as potential biotemplates for the design of new therapeutics (32), some of which are the subject of on-going clinical research (45) (see (46)). The activation of endogenous resolution mechanisms represents a paradigm-shifting departure from current inhibition pharmacology (i.e. via small molecule inhibition or antagonism of pro-inflammatory pathways).
To determine which of the resolution phase molecules detected might serve as immunoresolvents, standard criteria were used. First, structure activity relationships were constructed to determine the presence of stereoselectivity and abundance consistent with a molecule’s potency (47). SPM retrograde synthesis was performed for matching studies of the physical properties and recapitulation of in vivo biosynthesis (as in (48, 49)). SPM matching and identification was validated with LC-MS/MS criteria for identification: 1) retention time that matches by coelution authentic compound, 2) diagnostic UV chromophore that matches the authentic compound (i.e., λmax and band shape), and 3) mass spectrometry fragmentation pattern with ≥ 6 diagnostic ions. Regarding SPM abundance, the Serum Metabolome Project has reported levels of lipoxins, resolvins, and protectins in healthy human serum in pM to nM amount (e.g. LXA4, ~1.4 nM; RvD1, ~50 pM; RvE1, ~0.5 nM)(50). These values are in the concentration response range for SPM bioactivity and similar to those reported earlier for RvE1 (51) and for RvE2 (52, 53) in human peripheral blood samples from healthy donors (54). Information on individual SPM follows:
Lipoxins
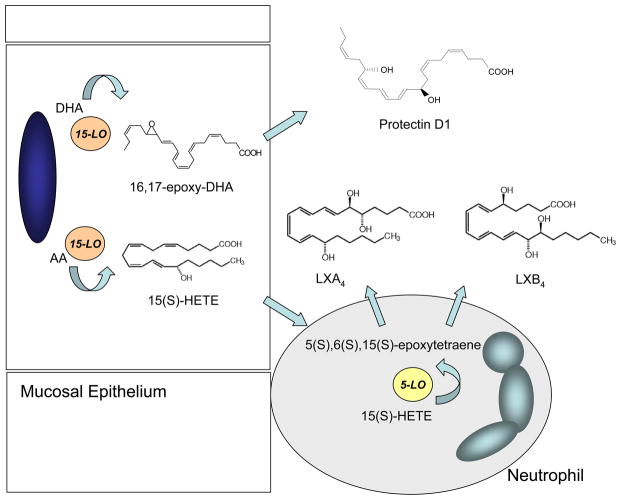
During multicellular host responses in inflammation, leukocytes come in close proximity to tissue resident cells. Together, the cells collaborate to generate new products that neither cell type alone produce in significant amounts. This transcellular biosynthesis is exemplified by lipoxins, which are lipoxygenase (LOX) interaction products from arachidonic acid (AA; C20:4) (24) (Figure 4). In response to airway inflammation, epithelial 15-LOX-derived 15S-hydroxy-eicosatetraenoic acid (15(S)-HETE) can be transformed to neutrophil 5-LOX to an unstable epoxytetraene intermediate that is converted by enzymatic hydrolysis to LXA4 and LXB4 (reviewed in (4)). This biosynthetic route has been established in upper and lower airways and other mucosal sites (55–57). In the vasculature, LXA4 and LXB4 biosynthesis can occur via a second biosynthetic route between platelets and neutrophils. Platelet 12-LOX acts as a LX synthase to convert leukotriene A4 (LTA4) to LXs (58, 59).
Figure 4. Transcellular biosynthesis of SPM occurs via airway neutrophil-epithelium interactions.
Transcellular biosynthesis of SPM provides a collaborative opportunity for two cell types to generate mediators together that neither cell type alone can efficiently produce. For example, arachidonic acid (AA) can be released from cell membranes for conversion by 15-LOX to 15S-HETE that is transferred to neutrophils for subsequent transformation by 5-LOX to an unstable epoxytetraene intermediate that hydrolases can convert to LXA4 and LXB4. Depicted as unidirectional, lipoxin biosynthesis can proceed bidirectionally with neutrophil 5-LOX conversion of AA to leukotriene A4 followed by release and transformation to lipoxins by epithelial 15-LOX. Protectin D1 does not require cell-cell interactions for its generation by 15-LOX catalyzed conversion of DHA via an epoxide-containing intermediate.
Although less abundant, LXA4 and LXB4 can also be generated by single cell types. This route of formation generally requires expression of both 5-LOX and 15-LOX, and there are now examples of cell types with both 5-LOX and 15-LOX activity, including eosinophils, resolution phase neutrophils and macrophages (28, 57, 59). In addition, 15(S)-HETE can be acylated into cell membrane phospholipids for release upon cell activation and subsequent conversion by cells expressing 5-LOX to LXs (33). Thus, single cells can contribute to tissue LX abundance (60, 61).
Aspirin-triggered lipoxins
Aspirin is the lead non-steroidal anti-inflammatory drug and unlike other agents in this class also displays several other protective actions (62). Some of these beneficial actions have been attributed to the formation of aspirin-triggered lipid mediators (reviewed in (32)). Aspirin-triggered lipoxins (ATL) were the first aspirin-triggered mediators to be identified (63). Aspirin irreversibly acetylates a serine in the COX catalytic site to block PG formation. Of interest for COX-2, this covalent modification does not completely inhibit the enzyme activity. Rather, acetylated-COX-2 transforms AA into 15(R)-HETE, which is a substrate for neutrophil 5-LOX conversion to 15(R)-epi-LXA4 and 15(R)-epi-LXB4 (63). The stereochemistry at carbon-15 is consistent between 15(R)-HETE and 15(R)-epi-LXs and distinct from the 15-LOX catalyzed 15(S)-HETE, LXA4 and LXB4. This biochemical activity for aspirin is unique amongst the class of non-steroidal anti-inflammatory drugs. It is noteworthy that some cytochrome P450 enzymes can directly convert AA to 15(R)-HETE, so aspirin is not required for epi-LX biosynthesis. To this end, 15-epi-LXs have also been detected in human subjects not on aspirin (64) and are increased with aspirin ingestion (65). In addition, 15-epi-LXs can mediate the local anti-inflammatory actions of low-dose aspirin in healthy individuals (66). Recently, statins were identified as another pharmacological trigger for 15-epi-LX generation (67), including in the lung (68), but via a distinct biochemical pathway.
LXA4, LXB4 and their 15-epimers are generated at sites of vascular inflammation and down regulate neutrophil transmigration, vascular leakage, pro-inflammatory cytokine release and function and inflammatory pain signals (reviewed in (3, 69)). In addition, these SPM promote resolution by increasing the uptake and removal of apoptotic neutrophils by macrophages and have anti-fibrotic properties (26, 70–73).
Resolvins
In addition to AA (C20:4n-6), the essential n-3 PUFAs are also available at sites of inflammation for enzymatic transformation to bioactive lipid mediators. In health, the total fatty acid pool in whole blood has eicosapentaenoic acid (EPA) (~0.5 to 2.8% of total fatty acids) and docosahexaenoic acid (DHA) (~1.3 to 5.0%)(74–78). Dietary EPA and DHA have health beneficial effects with several reports of antithrombotic, immunoregulatory, and anti-inflammatory properties (79, 80), and these n-3 PUFA have been recognized as essential dietary constituents since 1929 (81). During resolution in a self-limited experimental model of acute inflammation in vivo, LC-MS/MS based profiling revealed that EPA and DHA are enzymatically converted into specific bioactive products with protective anti-inflammatory and pro-resolving actions, leading to their naming as E-series resolvins and D-series resolvins, respectively.
E-series resolvins
EPA (C20:5) can be enzymatically transformed to E-series resolvins that serve as SPM. Similar to 15-epi-LXs biosynthesis, the acetylation of COX-2 is a pivotal event in E-series resolvin biosynthesis. During vascular inflammation, endothelial cell COX-2 is available for acetylation by aspirin and then converts EPA into 18R-hydroxy-eicosapentaenoic acid (18R-HEPE). This intermediate is subsequently transformed by activated leukocytes to an unstable intermediate (5S(6)-epoxy-18R-HEPE) that is hydrolyzed to RvE1. The complete stereochemistry of RvE1 is 5S,12R,18R-trihydoxy-6Z,8E,10E,14Z,16E-EPA (51). Both 18R-HEPE and 18S-HEPE isomers are present in plasma from healthy human subjects, and are increased with aspirin ingestion (82). The 18S-HEPE can also be converted to epimeric RvE1 and RvE2 by 5-LOX and LTA4 hydrolase (82). In the absence of aspirin, RvE1 biosynthesis can be initiated via cytochrome P450 oxygenation of EPA (83). Structure-activity relationships have established the stereoselective actions of E-series resolvins (51). RvE1 and RvE2 (5S,18R-dihydroxy-6E,8Z,11Z,14Z,16E-eicosapentaenoic acid) are generated from a common precursor 5S-hydroperoxy,18-hydroxy-EPE via 5-LOX with two parallel stereospecific pathways (52, 53, 84). RvE2 is present in resolving exudates and human whole blood (52, 53, 84). The third member of the E-series resolvins is resolvin E3 (17R,18S-dihydroxy-5Z,8Z,11Z,13E,15E-eicosapentaenoic acid). There is also a natural 18R stereoisomer of RvE3 (85). As demonstrated in mice, this molecule is generated via the actions of 12/15-LOX so has a distinct route of biosynthesis from RvE1 and RvE2 (86). This biochemical distinction has an immunological correlate. Because 5-LOX (neutrophils) and 12/15-LOX (mouse eosinophils) activity are often segregated into distinct types of granulocytes, RvE1 and RvE2 are predominantly generated by neutrophils (5-LOX), while in murine systems eosinophils (12/15-LOX) participate in the production of RvE3. Mouse and human cells are not orthogonal in their LX activities, in particular eosinophils and macrophages, so roles for RvE3 in human systems are still under investigation.
D-series resolvins
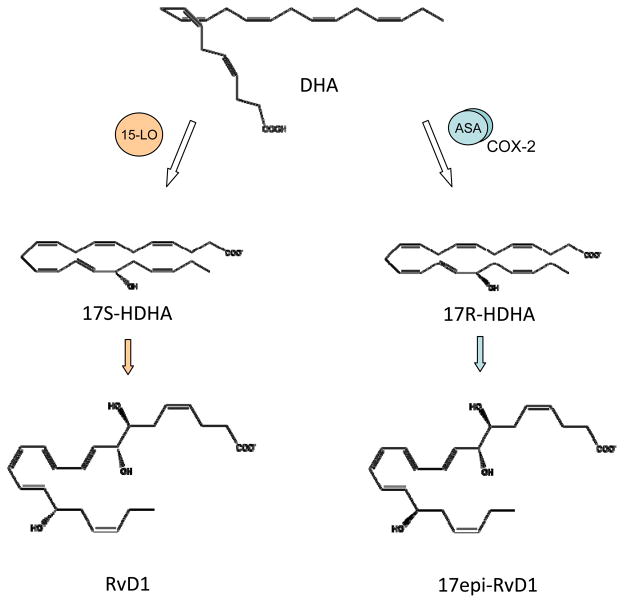
As with EPA, the other major n-3 fatty acid DHA (C22:6) is enzymatically transformed to SPM during inflammation. Similar to E-series resolvins, the D-series resolvins are generated via transcellular biosynthesis. Aspirin-acetylated COX-2 in vascular endothelial cells converts DHA to 17R-hydroxy-docosahexaenoic acid (17R-HDHA)(Figure 5), which can be transformed by neutrophil LOX into two series of di- and trihydroxy products; one initiated via oxygenation at carbon 7 and the other at carbon 4 (6). In addition to the aspirin-triggered 17R-hydroxy-D-series resolvins, their epimers with a 17S-hydroxy group (Figure 5) are present in resolving exudates and can be generated by isolated human cells in the absence of aspirin (6, 87). The stereochemistry of some of the D-series resolvins have been established: 17S-RvD1 is 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid, 17R-RvD1 is 7S,8R,17R-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid (48), RvD2 is 7S, 16R, 17S-trihydroxy-4Z, 8E, 10Z, 12E, 14E, 19Z-docosahexaenoic acid (88), and RvD3 is 4S, 11R, 17S-trihydroxy-5Z, 7E, 9E, 13Z, 15E, 19Z-docosahexaenoic acid (89). There are additional members of this family have been detected (RvD4-RvD6). Each of these additional D-series resolvins has distinct chemical structures and potentially additional bioactions that are currently under investigation (90). These mediators display stereoselective and cell-type specific actions and, like RvE1, D-series resolvins are present in healthy whole blood and increased with dietary n-3 supplementation (50, 54). Cytochrome P450 enzymes can also initiate D-series resolvin biosynthesis by converting DHA to 17R-hydroxy-DHA, in an aspirin-independent manner, for subsequent transformation to 17R-RvD1 (i.e., AT-RvD1).
Figure 5. Aspirin-triggered biosynthesis of epimer resolvins.
Aspirin (ASA) acetylated cyclooxygenase-2 (COX-2) is not catalytically inactive. Rather, aspirin-acetylated COX-2 can convert DHA to 17R-HDHA that can be transferred to leukocytes for subsequent transformation by 5-LOX to 17-epi-resolvins, including 17-epi-RvD1. These aspirin-triggered resolvins are epimers of the 15-LOX-derived 17S D-series resolvins.
Protectins
In addition to D-series resolvins, DHA can also be converted to a family of SPM named protectins (PD) (or neuroprotectins (NPD) when generated in neural tissues). These molecules were trivially named based on their protective bioactivities for immune functions and neural tissues and their distinct structures. The complete stereochemistry of Protectin D1 (PD1) is 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid and this molecule carries stereoselective actions (87, 91). There are several detectable isomers of PD1 in inflammatory exudates, but these possess lower bioactivity than PD1, including 10S,17S-diHDHA, 4S,17S-diHDHA, 7S,17S-diHDHA, and 22-hydroxy-10,17S-docosatriene (a putative inactivation product of PD1) (6, 87). PD1 biosynthesis proceeds in an LOX-dependent manner via a C16(17)-epoxide intermediate (87, 91) (Figure 4). There are aspirin-triggered protectins in which acetylated COX-2 leads to 17R-PD1 biosynthesis from DHA (92) with the complete stereochemistry of 10R,17R-dihydroxy-4Z,7Z,11E,13E,15Z,19Z-docosahexaenoic acid (17R-PD1). In keeping with their assignment as SPM, these protectins decrease neutrophil transmigration and enhance macrophage efferocytosis to decrease neutrophil tissue accumulation in vivo (21, 93).
Maresins
A third family of DHA-derived mediators has more recently been elucidated. The maresins are structurally distinct molecules in the DHA metabolome. Macrophages are a principal source of maresins (49). These cells play essential roles in catabasis (94) and contribute to the generation of bioactive mediators to orchestrate inflammatory responses, including inflammation resolution. Macrophage biosynthesis of SPM serve as autacoids for their pro-resolving and homeostatic functions. Macrophage phagocytosis of apoptotic neutrophils initiates SPM production (12, 21), as well as 14-hydroxy-docosahexaenoic acid (14-HDHA) (49) that can be converted to maresin (macrophage mediator in resolving inflammation) 1 (first in the family) (MaR1) (49). The complete stereochemical assignment of MaR1is established as 7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid (95). MaR1 biosynthesis proceeds via a novel 13,14-epoxide intermediate (96). Regarding its biological functions, MaR1 has distinct and separate actions on neutrophils compared with mononuclear cells. To expedite resolution of murine peritonitis, MaR1 limits further neutrophil accumulation and stimulates macrophage efferocytosis (49). In addition, MaR1 has a direct action on tissue regeneration in planaria, supporting the evolutionary conservation of this interesting molecule (97). MaR1 matching studies for its physical and biological properties with authentic material from total organic synthesis has confirmed the findings with biogenic material (97).
5. SPM Receptors
ALX/FPR2 receptors
The ALX/FPR2 receptor can interact with and transmit intracellular signals from LXA4 and 15-epi-LXA4 as well as RvD1 and AT-RvD1 (20, 98–100). By screening cDNA clones from differentiated HL60 cells, the formyl peptide receptor like-1 receptor was first identified as the putative LXA4 G-Protein coupled receptor (99). Based on its high affinity binding for LXA4, this receptor was named ALX/FPR2 by an international nomenclature committee (101). LXA4 binding to ALX/FPR2 is stereoselective, specific and reversible with a Kd ~ 0.5 nM (102). RvD1 also directly binds to human neutrophils and monocytes with high affinity (Kd ~ 0.17 nM) (100). RvD1 binding is displaced by LXA4 (~ 60%) and screening assays identified significant bioactivity for RvD1 in cells over expressing ALX/FPR2 receptors, but not several related GPCRs (e.g. BLT1, BLT2, CB1, GPR-1, FPR, and CMKLR-1) (100).
In addition to LXA4 and RvD1, the corticosteroid-induced protein annexin A1 and its N-terminal peptides can activate ALX/FPR2 (41), representing an evolutionary convergence for ALX/FPR2 for counter-regulatory immune actions. Several human leukocyte classes and tissue resident cells express ALX/FPR2 (98). This receptor is also expressed in several other mammalian species with additional orthologues in mice (103) and rats (104). Myeloid-targeted transgenic expression of human ALX/FPR2 decreases neutrophil accumulation during zymosan-induced peritonitis (105) and eosinophil accumulation in allergic airways inflammation (106). In conceptual alignment, ALX/FPR2 deficient mice respond to inflammatory challenge with increased acute inflammation and delayed resolution (30). As demonstrated earlier in mice, using a self-limited experimental blister model in healthy human subjects, leukocyte ALX/FPR2 expression was found to control both the magnitude and duration of acute inflammation (107).
ALX/FPR2 receptors are broadly expressed in human cells and tissues, but their expression is regulated by several local factors, including inflammatory mediators. Transcription factors and epigenetic mechanisms that regulate ALX/FPR2 promoter activity have been determined (108). Of interest, LXA4 increases ALX/FPR2 expression by activating its promoter in a positive feedback mechanism. This mechanism is relevant to the pathogenesis of chronic lung inflammation in severe asthma, as both LXA4 generation and granulocyte expression of ALX/FPR2 are decreased (reviewed in (109)). Regarding its translational importance to human disease, it is also noteworthy that a single nucleotide polymorphism in the ALX/FPR2 promoter was recently described that decreases its activity and is associated with increased cardiovascular risk (108). As an aside, some LXs can also interact with the cysteinyl LT receptor 1 (CysLT1), with equal affinity as LTD4, to block the actions of pro-phlogistic CysLT actions (110). In addition, LXA4 can serve as an allosteric inducer of the cannabinoid receptor CB1 (111).
CMKLR1 receptors
The chemokine receptor-like 1 (CMKLR1) receptors were originally defined as receptors for the peptide mediator chemerin. In addition to chemerin, RvE1 also binds to CMKLR1 receptors (51). Using CMKLR1 transfected cells, RvE1 binds in a stereoselective manner with high affinity (Kd ~ 11 nM). As anticipated, RvE1 binding to CMKLR1 is displaced by chemerin, in particular a synthetic peptide fragment (YHSFFFPGQFAFS) of chemerin (112). Several tissues express CMKLR1 receptors, including brain, kidney, cardiovascular, gastrointestinal, and myeloid tissues (51). Cellular CMKLR1 expression is most notable in innate immune cells (i.e., NK cells, ILCs, macrophages, dendritic cells, and epithelial cells (113–115)), and CMKLR1 knockout mice display increased lung inflammation following LPS challenge (116).
A second GPCR for RvE1 has been identified (51, 117). In addition to CMKLR1, RvE1 also specifically binds to the LTB4 receptor 1 (BLT1) on human neutrophils with high affinity with a Kd of ~50 nM. Of note, RvE1 binding to neutrophils is not displaced by chemerin, supporting the interaction of RvE1 with receptors in addition to CMKLR1 (117). The bioactions of RvE2 are also cell-type specific for leukocytes (52, 53). RvE2 binds to human neutrophils with high affinity (Kd ~ 25 nM) and may share receptors, in part, with RvE1 (52).
DRV1/GPR32 receptors
In addition to ALX/FPR2 receptors, RvD1 binds to GPR32, but not several related GPCRs (e.g. BLT1, BLT2, CB1, GPR-1, FPR, and CMKLR-1) (100). RvD1 and its aspirin-triggered epimer (AT-RvD1) activate GPR32 receptors with similar potencies and EC50 (20). Because RvD1 and AT-RvD1 directly activate GPR32 receptors, it has been renamed as the RvD1 receptor (DRV1) per IUPAC recommendations (118). RvD1 interactions at DRV1 are concentration-dependent. Low RvD1 concentrations (~1 nM) block neutrophil-endothelial cell interactions in a DRV1 dependent manner, whereas high concentrations (≥10 nM) are ALX/FPR2 receptor-specific (119). Human DRV1/GPR32 is expressed in peripheral blood leukocytes and vascular tissues (100). Because human neutrophils rapidly mobilize ALX/FPR2, but not DRV1/GPR32, from secretory granules to cell membranes, RvD1’s pro-resolving actions are linked to ALX/FPR2 signaling – findings confirmed with ALX/FPR2 receptor knockout mice (119). Together, these findings support a role for RvD1 interactions with DRV1/GPR32 receptors during physiological responses in homeostasis and with ALX/FPR2 receptors to regulate inflammatory responses. Of note, RvD5 can also activate DRV1/GPR32 receptors (90).
Protectin D1 receptors
The molecular identification of PD1 receptors has not been established; however, PD1’s bioactions are stereoselective and cell type specific, suggesting the presence of one or more PD1 receptors. (Neuro)Protectin D1 ((N)PD1) binds to both tissue resident and immune cells with a Kd of ~ 30 pmol/mg of cell protein. (N)PD1 free acid had a higher affinity than its methyl ester (~74%), and (N)PD1 binding is displaced by unlabelled homoligand (90–100%), but not structurally related molecules (i.e., 17S-HDHA, LXA4, RvE1). For human neutrophils, there appear to be high and low affinity binding sites (Kd ~ 25 nM and ~ 200 nM) (120).
6. SPM display cell type specific actions
Because acute inflammation is essential for host defense and survival, there are multiple pathways to rapidly engage innate immunity and ignite acute inflammation. It stands to reason that it is equally vital to control and resolve this inflammation to protect essential tissues from collateral and unnecessary injury. The existence of overlapping cellular pro-resolving actions for SPM and conservation of signaling pathways emphasize this point. In health, the acute inflammatory response resolves in a highly orchestrated manner. With maturation of the inflammatory reaction, leukocytes become apoptotic (e.g., cytokine-induced cell death of neutrophils, activation induced cell death of T cells) and SPM induce CCR5 expression as a scavenger for residual pro-inflammatory chemokines (Figure 1). In catabasis, macrophage engulfment of these apoptotic leukocytes clears tissues of both leukocytes and chemokines. Efferocytosis also leads to SPM generation as a positive feedback mechanism and to limit further granulocyte recruitment and activation. In addition to these general pro-resolving events, each SPM possesses additional cell type and tissue specific activities (reviewed in (121)). Moreover, the sites for SPM biosynthesis, distribution of SPM receptors and their signal transduction underlie the selectivity and specificity of SPM regulation of inflammation resolution.
Investigators have developed criteria to define SPM that are based on these molecules cell type specific actions (4). SPM criteria include (1) generation during resolution, (2) inhibition of granulocyte tissue infiltration and activation, (3) induction of macrophage phagocytic activity for apoptotic cells and/or microbes, (4) clearance of leukocytes from mucosal surfaces and (5) enhanced anti-microbial actions. Molecules that fulfill these criteria are assigned to the SPM class – a new genus of endogenous mediators.
SPM influence cell shape change in a cell type specific manner. SPM limit neutrophil diapedesis in vitro and in vivo (34, 52, 122, 123). In contrast, SPM initiate macrophage shape change for efferocytosis and phagocytic clearance of microbes (21, 124). Additional distinct cellular bioactivities include increased macrophage phagocytosis and anti-inflammatory (IL-10) production and decreased pro-inflammatory cytokine release; decreased neutrophil adhesion and reactive oxygen species generation; increased endothelial nitric oxide and prostacyclin generation and decreased expression of adhesion receptors and pro-inflammatory cytokines; and decreased dendritic cell migration and IL-12 production. Recently, 17-HDHA and resolvin D1, but not protectin D1, were demonstrated to strongly increase activated human B cell IgM and IgG production (125). The increased antibody production was secondary to increased B cell differentiation toward a CD27(+)CD38(+) Ab-secreting cell phenotype. B cell proliferation was not affected. These findings support an adjuvant type impact of some SPM that may be critical to host defense. Sensory neurons and microglial cells regulate inflammatory responses and pain and are also targets for SPM (126)(Figure 1).
These SPM actions are triggered by specific receptors that initiate pro-resolving signaling. In human macrophages, ALX/FPR2 initiates the rapid activation of small GTPases and cytoskeletal protein redistribution (127, 128). CMKLR1 receptors in macrophages regulate Akt signaling and activation of proteins for phagocytosis (124). RvD1 and RvE1 decrease leukocyte adhesion to endothelial cells by decreasing CD11b surface expression (100, 123). The impact of SPM on leukocyte shape and migration is evident in single cells using microfluidic devices (34, 129, 130).
One intracellular signaling mechanism for SPM regulation of cellular responses in human neutrophils is via receptor-mediated inhibition of protein kinase C – βII (PKCβII) phosphorylation and activation of polyisoprenyl diphosphate phosphatase 1 (PDP1), a pivotal phosphatase for polyisoprenyl diphosphate remodeling (131). Polyisoprenyl phosphates are present in resting cell membranes. In neutrophils, soluble stimuli rapidly, but transiently, convert presqualene diphosphate (PSDP) into its monophosphate form presqualene monophosphate (PSMP) (132). PSDP is a potent inhibitor of phosphoinositol 3-kinase and phospholipase D, properties not shared by PSMP (133, 134). The phosphatase responsible for the rapid conversion of PSDP to PSMP was recently identified as PDP1 (135, 136). Both PSDP and PDP1 are present in human neutrophils (132, 136), and when neutrophils are activated by soluble pro-inflammatory stimuli, there is an increase in phospho-PKCβII (131) that can phosphorylate PDP1 and lead to PSDP conversion to PSMP, facilitating NADPH oxidase assembly and other functional responses (131). In contrast, the SPM 15-epi-LXA4 interacts with ALX/FPR2 receptors to block agonist-triggered PKCβII phosphorylation and subsequent PDP1 activation, leading to increased availability of PSDP that restrains cellular pro-inflammatory responses (131).
In addition to phospho-regulation of signaling proteins and polyisoprenyl phosphate remodeling, SPM signaling induces a specific miRNA signature to regulate gene expression. During the resolution of acute peritonitis in mice, miR-21, miR-146b, miR-208a, and miR-219 are significantly induced. miR-21 promotes the expression of the anti-inflammatory cytokine IL-10, and RvD1 induces miR-21, as well as miR-146b, miR-208a, and miR-219 (19). RvD1 induction of miR-146 targets the TNF-α-NF-κB axis, a key regulatory pathway for inflammation, and its induction of miR-208a downregulates CD14, CD40 ligand, prostacyclin receptor, thromboxane A2 receptor, and programmed cell death 4 (PDCD4), a translational repressor of IL-10, leading to increased anti-inflammatory IL-10 (19). RvD1 induction of miR-219 regulates CD14 and 5-LOX, a key biosynthetic enzyme for both leukotrienes and SPM.
7. SPM and lung inflammation in human disease
Several common lung diseases are characterized by chronic inflammation, including asthma and COPD. Why the lung inflammation in these conditions fails to resolve remains a mystery. This puzzle is particularly vexing in COPD where some individuals experience inflammation for years after tobacco cessation. The last decade’s discovery of resolution mediators and mechanisms is shining new light on the pathobiology of these important chronic inflammatory diseases and will be the focus of this section. At present, much more information is available on the lipoxins than other SPM.
Lipoxins are present in the respiratory tracts of patients with asthma (137–139), yet lipoxin generation is decreased in aspirin-exacerbated respiratory disease (140) and other forms of severe or uncontrolled asthma (39, 139). Decreased LX production in uncontrolled asthma is attributable, in part, to dysregulated expression of LX biosynthetic genes (reviewed in (109)). Decreased formation of LXs in uncontrolled asthma has now been identified in geographically dispersed populations of adults and children from several countries, including the U.S., Poland, France, Turkey and China. Expression of the LXA4 receptor ALX/FPR2 is also dysregulated in asthma (141). In contrast to LXs, leukotriene generation is increased in severe asthma (39). CysLTs are the most potent bronchoconstricting molecules and their actions are blocked by lipoxins (110). Not surprisingly, peripheral blood levels of LXA4 and the ratio of LXA4/CysLTs positively correlate with lung function (FEV1 percent predicted values) (39, 137), and inhaled LXA4 can protect asthmatic subjects from CysLT-initiated bronchoprovocation challenge in asthmatic individuals (142).
In addition to AA (C20:4), the airway mucosa is enriched with DHA (C22:6) (143) and mucosal levels of DHA are decreased in asthma and cystic fibrosis (143). Both 17S-HDHA (17S-hydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid) and PD1 are detectable in exhaled breath condensates from healthy human subjects (144). Similar to LXs in uncontrolled asthma, PD1 levels also decrease during acute exacerbations of asthma (144). Using a murine model of allergic airways responses, PD1 (2 – 200 ng) given intravenously prior to aeroallergen challenge markedly suppresses airway hyperreactivity to methacholine, mucous metaplasia, lung eosinophil accumulation and pro-inflammatory cytokine and lipid mediator release (144). Moreover, PD1 also mediates pro-resolving actions on established pulmonary inflammation when given after allergen challenge, leading to significantly expedited resolution of allergic airway inflammation (144). Because PD1 mediates bronchoprotective actions in this murine experimental model of allergic asthma in vivo, a decreased capacity to generate PD1 would be predicted to lead to a resolution defect for airway inflammation in these unstable asthmatic individuals (144). In addition to asthma, defective LX generation has also been described in the chronically inflamed airways of patients suffering from cystic fibrosis and interstitial lung disease (38, 145). Together, these results indicate that SPM are generated during airway inflammatory responses and decreased SPM levels contribute to asthma pathobiology.
Resolvin E1 and RvD1 have potent anti-inflammatory and pro-resolving actions in a number of murine models of inflammation and are bioactive in very low concentrations (picomolar to nanomolar) in vivo and in vitro (5). RvE1 and RvD1 dampen the development and promote the resolution of allergic airway responses in a murine experimental model of asthma (16, 22, 146–148). RvE1 decreases eosinophil and lymphocyte accumulation and airway mucous metaplasia and improves airway hyper-responsiveness to inhaled methacholine (22). During resolution of allergic inflammation, RvE1 rapidly decreased IL-6, IL-17 and IL-23 in the lung while increasing IFN-γ and LXA4 formation (22). RvE1-mediated inhibition of IL-17 was additive with LX-mediated decreases in this cytokine; however, only RvE1 and not LXA4 regulated IL-23 and IFNγ levels (22). These findings support the presence of independent pro-resolving signalling circuits for RvE1 and LXA4 that converge on the regulation of IL-17 to hasten catabasis.
Distinct from the pathologically sustained immune responses in asthma, murine experimental models of allergic airway responses are self-limited in what were healthy mice prior to manipulation. To determine natural resolution mechanisms, the study of this model’s late resolution phase has uncovered an unanticipated pro-resolving role for NK cells (16). NK cell phenotype and trafficking to draining mediastinal lymph nodes is temporally regulated during resolution in a CXCL9-CXCR3 (ligand-receptor) dependent manner (16). Depletion of NK cells delays resolution, including responses to RvE1 as NK cells express CMKLR1 receptors (16). These findings indicate new functions for NK cells in promoting resolution of adaptive immune responses and suggest that NK cells are targets for SPM for clearance of eosinophils and activated T cells from inflamed lung. RvD1 and AT-RvD1 also display pro-resolving roles in the murine model of allergic airway responses (148). Of interest, AT-RvD1 was more stable with increased bioactivity in vivo than RvD1. AT-RvD1 potently regulated NF-κB and macrophage phagocytic clearance of allergen during resolution (148). No published reports are currently available for RvE1 or RvD1 in human asthma.
To translate these murine findings to human asthma, roles for NK cells and the related innate lymphoid cell (ILC) family members type 2 ILCs (ILC2s) in asthma were recently determined (15). NK cells and type 2 ILCs were identified in peripheral blood by FACS from healthy and asthmatic subjects. NK cells were highly activated in severe asthma, related to eosinophilia and when incubated with autologous eosinophils or neutrophils induced their apoptosis (15). In addition, peripheral blood ILC2s were identified and isolated by FACS. ILC2s released IL-13 in response to receptor-mediated stimuli, including IL-25, IL-33 and PGD2. Both NK cells and ILC2s expressed ALX/FPR2 and CMKLR1 receptors, and LXA4 increased NK cell-mediated eosinophil apoptosis and decreased ILC2 production of IL-13 (15). These findings assign critical roles to ILCs in asthma pathobiology and identify ILCs as SPM targets to decrease early (ILC2) and late (NK cell) asthmatic airway inflammatory responses.
Distinct from the dysregulated AA (C20:4) metabolism in severe asthma that favors LTs and under-produces LXs, these families of eicosanoids are coordinately biosynthesized in COPD (31, 139). Of interest, the chronic and corticosteroid-refractory inflammation in COPD after smoking cessation is linked to inhibition of LX signaling at ALX/FPR2 receptors (31). In addition to ALX/FPR2 counter-regulatory ligands LXA4, RvD1, their epimers and Annexin A1 peptides, these interesting receptors can also interact with ligands that initiate a pro-inflammatory response. The acute phase reactant protein serum amyloid A (SAA) stimulates lung epithelial IL-8 release and neutrophil activation in an ALX/FPR2-dependent manner (31, 149). SAA is a pro-inflammatory mediator in the lung that is markedly increased in COPD exacerbations. It is produced locally by pulmonary macrophages, in response to diverse innate stimuli (31). Both LXA4 and 15-epi-LXA4 can inhibit SAA actions at ALX/FPR2 via allosteric interaction, but the very high levels of SAA in COPD overwhelm this counter-regulatory mechanism. In this manner, SAA can commandeer naturally pro-resolving signaling circuits for pro-inflammatory responses. In addition, corticosteroids, the most commonly used anti-inflammatory drug for airway mucosal inflammation, increases SAA expression and production. Together, these findings support the notion that 15-epi-LXA4, if present in large enough amounts, can ameliorate the steroid-refractory chronic inflammation from SAA over-production in COPD and its exacerbations. Since bioactive stable analogs of 15-epi-LXA4 have been prepared and proven efficacious in murine models of lung inflammation (150) and human infantile eczema (45), these translational findings suggest a clinical indication for LX stable analogs in COPD.
8. Summary and Conclusions
In health, acute inflammatory responses in the lung are a near daily response that swiftly resolve. Most respiratory pathogens and respirable noxious stimuli elicit an acute inflammatory response that resolves. This immune response is vital for host defense, but it is equally important that the lung’s inflammatory response resolves in a timely manner. If unrestrained, ARDS can develop. If acute inflammation becomes chronic, then asthma or COPD can develop. Healthy resolution of inflammation is an active process that programs specific signals and cellular mechanisms to control the intensity and duration of acute inflammation. SPM, which include the tissue dependent production of lipoxins, resolvins, protectins and maresins, comprise an essential component of the host’s resolution program that is governed by local mediators. SPM are rapidly formed and rapidly inactivated to serve as local autacoids to influence cellular responses. Lipoxins were the first members of the SPM family to be discovered. This class of mediator now comprised of several new families enzymatically derived from n-3 PUFA, including E-and D-series resolvins, (neuro)protectins, and maresins. SPM bioactions are stereospecific, receptor-mediated, and potent (pico- to nanogram amounts). SPM actions are also cell type specific, including some redundant actions for key cell types, such as neutrophils, macrophages and endothelial cells. In health, resolution proceeds in a highly orchestrated manner; however, defects in the resolution program can lead to chronic inflammation, which has been linked to the pathobiology of human lung disease. Resolution defects can result from decreased SPM formation, inhibition of SPM sites of action and perhaps genetic variation in the proteins that command the biosynthesis and actions of SPM. Identification of the mediators and mechanisms underlying resolution is opening up new fields of study, new insights into pathobiology and potential new pro-resolving therapeutic approaches to diseases characterized by excess or chronic inflammation.
Summary Points.
General
-
1
Resolution of acute, self-limited inflammation is an active process with recently identified specific cellular and molecular events.
-
2
Specialized pro-resolving mediators are enzymatically derived from essential polyunsaturated fatty acids, including AA as well as the omega-3 fatty acids EPA and DHA (from diet) that are precursors to lipoxins, resolvins, protectins and maresins.
-
3
Pro-resolving mediators interact with specific receptors to regulate cellular and tissue responses.
-
4
Criteria for pro-resolving mediators include their cessation of granulocyte recruitment and activation, counter-regulation of pro-inflammatory mediators (prostaglandins, leukotrienes, cytokines, chemokines) and stimulation of macrophage efferocytosis and phagocytosis of bacteria.
Lung-Specific
-
5
Innate lymphoid cells are a recently identified target for pro-resolving mediators to regulate lung inflammation.
-
6
Uncontrolled asthma is associated with a defect in pro-resolving mediator generation.
-
7
Chronic inflammation in COPD can result from over-production of serum amyloid A that interacts with ALX/FPR2 receptors to directly promote inflammation and inhibit pro-resolving mediator signaling.
Acknowledgments
The authors wish to acknowledge the many important contributions of our coauthors and collaborators. This work was funded in part by the U.S. National Institutes of Health grants HL068669, P50HL107166, U10HL109172, U01HL108712 and P01GM095467.
Glossary
Acronyms
- ATL
aspirin-triggered lipoxin
- AT-PD1
aspirin-triggered protectin D1 (10R, 17R-dihydroxy-docosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid)
- AT-RvD1
aspirin-triggered-resolvin D1 (7S, 8R, 17R-trihydroxy-docosa-4Z, 9E, 11E, 13Z, 15E, 19Z-hexaenoic acid)
- AT-RvD2
aspirin-triggered-resolvin D2 (7S, 16R, 17R-trihydroxy-docosa-4Z, 8E, 10Z, 12E, 14E, 19Z-hexaenoic acid)
- AT-RvD3
aspirin-triggered-resolvin D3 (4S, 11R, 17R-trihydroxy-docosa-5E, 7E, 9E, 13Z, 15E, 19Z-hexaenoic acid)
- AT-RvD4
aspirin-triggered-resolvin D4. (4S, 5R, 17R-trihydroxy-docosa-6E, 8E, 10Z, 13Z, 15E, 19Z-hexaenoic acid)
- COX-2
cyclooxygenase-2
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- GPCR
G protein-coupled receptor
- LC-UV-MS/MS
liquid chromatography-ultraviolet spectrometry-tandem mass spectrometry
- LOX
lipoxygenase
- LTB4
leukotriene B4 (5S, 12R-dihydroxy-eicosa-6Z, 8E, 10E, 14Z-tetraenoic acid)
- LXA4
lipoxin A4 (5S, 6R, 15S-trihydroxy-eicosa-7E, 9E, 11Z, 13E-tetraenoic acid)
- MaR1
maresin 1 (7R,14S-dihydroxy-4Z,8E,10E,12Z,16Z,19Z-docosahexaenoic acid)
- NF-κB
nuclear factor kappa B
- PD1
protectin D1 (10R, 17S-dihydroxy-4Z, 7Z, 11E, 13E, 15Z, 19Z-docosahexaenoic acid)
- PUFA
polyunsaturated fatty acid
- RvD1
resolvin D1 (7S, 8R, 17S-trihydroxy-4Z, 9E, 11E, 13Z, 15E, 19Z-docosahexaenoic acid)
- RvD2
resolvin D2 (7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid)
- RvD3
resolvin D3 (4S, 11R, 17S-trihydroxy-5Z, 7E, 9E, 13Z, 15E, 19Z-docosahexaenoic acid)
- RvD4
resolvin D4 (4S, 5, 17S-trihydroxy-6E, 8E, 10Z, 13Z, 15E, 19Z-docosahexaenoic acid)
- RvE1
resolvin E1 (5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid)
- RvE2
resolvin E2 (5S,18R-dihydroxy-6E,8Z,11Z,14Z,16E-eicosapentaenoic acid)
- RvE3
resolvin E3 (17R,18S-dihydroxy-5Z,8Z,11Z,13E,15E-eicosapentaenoic acid)
Key Terms
- Anti-inflammation
Inhibition of the cardinal signs of inflammation; can be immunosuppressive; Anti-inflammation is not the same as pro-resolution
- Catabasis
the return from the battle field of acute inflammation to a normal state; the process of terminating the local acute inflammatory response and stimulating resolution with the reversion of the tissue from pathology to homeostasis.
- Efferocytosis
The process of removing dying or dead cells by phagocytosis, most commonly performed by macrophages.
- Exudate
A fluid that has exuded out of the vasculature into inflamed or injured tissues; composition is variable but generally includes plasma proteins and lipids and, in some cases, cells from the circulation, including red blood cells, platelets and white blood cells.
- Homeostasis
The active process of regulating the body’s internal environment to maintain a stable, relatively constant condition; maintenance of healthy function
- Immunoresolvents
Agents that stimulate resolution
- Pro-resolution
Activation of endogenous resolution mechanisms; differs from anti-inflammation by the additional enhancement of anti-microbial host defense and clearance of cellular debris and apoptotic leukocytes
Footnotes
Disclosures
B.D.L. and C.N.S. are inventors on patents assigned to Brigham and Women’s Hospital, some of which (Rvs) are licensed to Resolvyx Pharmaceuticals. C.N.S. was scientific founder of Resolvyx Pharmaceuticals and owns founder stock in the company. The interests of B.D.L. and C.N.S. were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Contributor Information
Bruce D. Levy, Email: blevy@partners.org, Pulmonary and Critical Care Medicine, Department of Internal Medicine, Brigham and Women’s Hospital, Harvard Medical School, Harvard Institutes of Medicine Bldg. (HIM855), 77 Avenue Louis Pasteur, Boston, MA, USA 02115, Telephone/Fax: 617-525-5407/617-525-5413
Charles N. Serhan, Email: cnserhan@zeus.bwh.harvard.edu, Center for Experimental Therapeutics and Reperfusion Injury, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Harvard Institutes of Medicine Bldg. (HIM829), 77 Avenue Louis Pasteur, Boston, MA, USA 02115, Telephone/Fax: 617-525-5001/617-525-5017
References
- 1.Majno G, Joris I. Cells, Tissues and Disease: Principles of General Pathology. Cambridge: Blackwell Science; 1996. [Google Scholar]
- 2.Ward PA. Acute and Chronic Inflammation. In: Serhan CN, Ward PA, Gilroy DW, editors. Fundamentals of Inflammation. Cambridge: Cambridge University Press; 2010. pp. 1–16. [Google Scholar]
- 3.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB Journal. 2007;21:325–32. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. Journal of Experimental Medicine. 2000;192:1197–204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. Journal of Experimental Medicine. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–72. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta. 2010:1260–73. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–43. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–55. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 12.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006:38376–84. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 13.Ariel A, Li P-L, Wang W, Tang W-X, Fredman G, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005:280. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 14.Ariel A, Fredman G, Sun Y-P, Kantarci A, Van Dyke TE, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–16. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Science Translational Medicine. 2013;5:1–11. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J Immunol. 2011;186:6129–35. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008:2512–9. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 18.Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011:5543–7. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB Journal. 2011;25:544–60. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 Receptor Stereoselectivity and Regulation of Inflammation and Proresolving MicroRNAs. American Journal of Pathology. 2012 doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–9. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro-Xavier RA, Newson J, Silveira VL, Farrow SN, Gilroy DW, Bystrom J. A new strategy for the identification of novel molecules with targeted proresolution of inflammation properties. J Immunol. 2010;184:1516–25. doi: 10.4049/jimmunol.0902866. [DOI] [PubMed] [Google Scholar]
- 24.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987:1171–6. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 25.Flower RJ. Prostaglandins, bioassay and inflammation. Br J Pharmacol. 2006;147(Suppl 1):S182–92. doi: 10.1038/sj.bjp.0706506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:5335–9. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nature Immunology. 2001;2:612–9. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 29.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. Journal of Immunology. 2005;174:5033–9. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 30.Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, et al. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. Journal of Immunology. 2010;184:2611–9. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, et al. Serum amyloid A opposes lipoxin A4 to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:935–40. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–37. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 33.Brezinski ME, Serhan CN. Selective incorporation of (15S)-hydroxyeicosatetraenoic acid in phosphatidylinositol of human neutrophils: agonist-induced deacylation and transformation of stored hydroxyeicosanoids. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:6248–52. doi: 10.1073/pnas.87.16.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, et al. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. Journal of Immunology. 2008;181:8677–87. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB Journal. 2011;25:1441–8. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol. 2010:6418–26. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 38.Karp CL, Flick LM, Park KW, Softic S, Greer TM, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nature Immunology. 2004;5:388–92. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 39.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, et al. Diminished lipoxin biosynthesis in severe asthma. American Journal of Respiratory & Critical Care Medicine. 2005;172:824–30. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunkel SL, Ogawa H, Conran PB, Ward PA, Zurier RB. Suppression of acute and chronic inflammation by orally administered prostaglandins. Arthritis Rheum. 1981;24:1151–8. doi: 10.1002/art.1780240906. [DOI] [PubMed] [Google Scholar]
- 41.Perretti M, Chiang N, La M, Fierro IM, Marullo S, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nature Medicine. 2002;8:1296–302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perretti M, Dalli J. Exploiting the Annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br J Pharmacol. 2009;158:936–46. doi: 10.1111/j.1476-5381.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–7. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 44.Leitch AE, Lucas CD, Marwick JA, Duffin R, Haslett C, Rossi AG. Cyclin-dependent kinases 7 and 9 specifically regulate neutrophil transcription and their inhibition drives apoptosis to promote resolution of inflammation. Cell Death Differ. 2012 doi: 10.1038/cdd.2012.80. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. British Journal of Dermatology. 2013;168:172–8. doi: 10.1111/j.1365-2133.2012.11177.x. [DOI] [PubMed] [Google Scholar]
- 46.ClinicalTrials.gov Identifier: NCT00799552 http://clinicaltrials.gov.
- 47.Serhan CN, Haeggstrom JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokines. FASEB J. 1996;10:1147–58. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 48.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, et al. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–34. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 49.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. Journal of Experimental Medicine. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, et al. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. Journal of Experimental Medicine. 2005;201:713–22. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin E2 formation and impact in inflammation resolution. Journal of Immunology. 2012;188:4527–34. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chemistry & Biology. 2006;13:1193–202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clinical Chemistry. 2012;58:1476–84. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 55.Edenius C, Kumlin M, Bjork T, Anggard A, Lindgren JA. Lipoxin formation in human nasal polyps and bronchial tissue. FEBS Lett. 1990:25–8. doi: 10.1016/0014-5793(90)80440-t. [DOI] [PubMed] [Google Scholar]
- 56.Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alpha-induced IL-8 release. J Exp Med. 1998;187:1285–94. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levy BD, Romano M, Chapman HA, Reilly JJ, Drazen J, Serhan CN. Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins. Journal of Clinical Investigation. 1993;92:1572–9. doi: 10.1172/JCI116738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romano M, Serhan CN. Lipoxin generation by permeabilized human platelets. Biochemistry. 1992;31:8269–77. doi: 10.1021/bi00150a021. [DOI] [PubMed] [Google Scholar]
- 59.Serhan CN, Sheppard KA. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J Clin Invest. 1990;85:772–80. doi: 10.1172/JCI114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiore S, Serhan CN. Formation of lipoxins and leukotrienes during receptor-mediated interactions of human platelets and recombinant human granulocyte/macrophage colony-stimulating factor-primed neutrophils. Journal of Experimental Medicine. 1990;172:1451–7. doi: 10.1084/jem.172.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayadas TN, Mendrick DL, Brady HR, Tang T, Papayianni A, et al. Acute passive anti-glomerular basement membrane nephritis in P-selectin-deficient mice. Kidney International. 1996;49:1342–9. doi: 10.1038/ki.1996.190. [DOI] [PubMed] [Google Scholar]
- 62.Weissmann G. Aspirin. Scientific American. 1991;264:84–90. doi: 10.1038/scientificamerican0191-84. [DOI] [PubMed] [Google Scholar]
- 63.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995:9475–9. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Claria J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Molecular Medicine. 1996;2:583–96. [PMC free article] [PubMed] [Google Scholar]
- 65.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004:15178–83. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. Journal of Immunology. 2009;183:2089–96. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 67.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Nishi SP, et al. Augmentation of myocardial production of 15-epi-lipoxin-a4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–35. doi: 10.1161/CIRCULATIONAHA.106.629907. Epub 2006 Aug 14. [DOI] [PubMed] [Google Scholar]
- 68.Planaguma A, Pfeffer MA, Rubin G, Croze R, Uddin M, et al. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal immunology. 2010;3:270–9. doi: 10.1038/mi.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spite M, Serhan CN. Lipid signatures of unstable atheromas: fossils or a step toward personalized lipidomics-metabolomics? Circ Cardiovasc Genet. 2011;4:215–7. doi: 10.1161/CIRCGENETICS.111.960344. [DOI] [PubMed] [Google Scholar]
- 70.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. Journal of Immunology. 2000;164:1663–7. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 71.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem. 1997:272. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 72.Serhan CN, Hamberg M, Samuelsson B. Trihydroxytetraenes: a novel series of compounds formed from arachidonic acid in human leukocytes. Biochem Biophys Res Commun. 1984;118:943–9. doi: 10.1016/0006-291x(84)91486-4. [DOI] [PubMed] [Google Scholar]
- 73.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–26. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gong J, Rosner B, Rees DG, Berson EL, Weigel-DiFranco CA, Schaefer EJ. Plasma docosahexaenoic acid levels in various genetic forms of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1992;33:2596–602. [PubMed] [Google Scholar]
- 75.Newcomer LM, King IB, Wicklund KG, Stanford JL. The association of fatty acids with prostate cancer risk. Prostate. 2001;47:262–8. doi: 10.1002/pros.1070. [DOI] [PubMed] [Google Scholar]
- 76.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–8. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 77.Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am J Clin Nutr. 2004;79:674–81. doi: 10.1093/ajcn/79.4.674. [DOI] [PubMed] [Google Scholar]
- 78.Wakai K, Ito Y, Kojima M, Tokudome S, Ozasa K, et al. Intake frequency of fish and serum levels of long-chain n-3 fatty acids: a cross-sectional study within the Japan Collaborative Cohort Study. J Epidemiol. 2005;15:211–8. doi: 10.2188/jea.15.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–50. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 80.Iigo M, Nakagawa T, Ishikawa C, Iwahori Y, Asamoto M, et al. Inhibitory effects of docosahexaenoic acid on colon carcinoma 26 metastasis to the lung. Br J Cancer. 1997:650–5. doi: 10.1038/bjc.1997.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem. 1929:345–67. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 82.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–81. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Serhan CN, Clish CB, Brannon J, Colgan SP, Gronert K, Chiang N. Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: a novel mechanism for NSAID and N-3 PUFA therapeutic actions. J Physiol Pharmacol. 2000;51:643–54. [PubMed] [Google Scholar]
- 84.Ogawa S, Urabe D, Yokokura Y, Arai H, Arita M, Inoue M. Total synthesis and bioactivity of resolvin E2. Org Lett. 2009;11:3602–5. doi: 10.1021/ol901350g. [DOI] [PubMed] [Google Scholar]
- 85.Isobe Y, Arita M, Iwamoto R, Urabe D, Todoroki H, et al. Stereochemical assignment and anti-inflammatory properties of the omega-3 lipid mediator resolvin E3. J Biochem. 2013;153:355–60. doi: 10.1093/jb/mvs151. Epub 2013 Jan 3. [DOI] [PubMed] [Google Scholar]
- 86.Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. Journal of Biological Chemistry. 2012;287:10525–34. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–87. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 88.Spite M, Norling LV, Summers L, Yang R, Cooper D, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–91. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chemistry & Biology. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–8. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–59. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 92.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–17. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 93.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, et al. Novel proresolving aspirin-triggered DHA pathway. Chemistry & Biology. 2011;18:976–87. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007:S9–S17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 95.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB Journal. 2012;26:1755–65. doi: 10.096/fj.11-201442. Epub 2012 Jan 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggström JZ, et al. The novel 13S, 14S-epoxy-maresin is converted by human macrophages to maresin1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB Journal. 2013 doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, et al. Macrophage pro-resolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012:1755–65. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacological Reviews. 2006;58:463–87. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 99.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. Journal of Experimental Medicine. 1994;180:253–60. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1660–5. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–61. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fiore S, Ryeom SW, Weller PF, Serhan CN. Lipoxin recognition sites. Specific binding of labeled lipoxin A4 with human neutrophils. Journal of Biological Chemistry. 1992;267:16168–76. [PubMed] [Google Scholar]
- 103.Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chiang N, Takano T, Arita M, Watanabe S, Serhan CN. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br J Pharmacol. 2003:89–98. doi: 10.1038/sj.bjp.0705220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Devchand PR, Arita M, Hong S, Bannenberg G, Moussignac RL, et al. Human ALX receptor regulates neutrophil recruitment in transgenic mice: roles in inflammation and host defense. FASEB J. 2003:652–9. doi: 10.1096/fj.02-0770com. [DOI] [PubMed] [Google Scholar]
- 106.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–23. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 107.Morris T, Stables M, Colville-Nash P, Newson J, Bellingan G, et al. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8842–7. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simiele F, Recchiuti A, Mattoscio D, De Luca A, Cianci E, et al. Transcriptional regulation of the human FPR2/ALX gene: evidence of a heritable genetic variant that impairs promoter activity. FASEB Journal. 2011;26:1323–33. doi: 10.1096/fj.11-198069. [DOI] [PubMed] [Google Scholar]
- 109.Levy BD, Vachier I, Serhan CN. Resolution of inflammation in asthma. Clinics in Chest Medicine. 2012;33:559–70. doi: 10.1016/j.ccm.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan CN. Selectivity of recombinant human leukotriene D(4), leukotriene B(4), and lipoxin A(4) receptors with aspirin-triggered 15-epi-LXA(4) and regulation of vascular and inflammatory responses. American Journal of Pathology. 2001:3–9. doi: 10.1016/S0002-9440(10)63937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pamplona FA, Ferreira J, Menezes de Lima O, Jr, Duarte FS, Bento AF, et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci U S A. 2012;109:21134–9. doi: 10.1073/pnas.1202906109. Epub 2012 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–85. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB Journal. 2007;21:3162–70. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 114.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, et al. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. Journal of Experimental Medicine. 2008;205:767–75. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Du XY, Leung LL. Proteolytic regulatory mechanism of chemerin bioactivity. Acta Biochimica et Biophysica Sinica. 2009;41:973–9. doi: 10.1093/abbs/gmp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, et al. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. Journal of Immunology. 2009;183:6489–99. doi: 10.4049/jimmunol.0901037. [DOI] [PubMed] [Google Scholar]
- 117.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. Journal of Immunology. 2007;178:3912–7. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 118.Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, et al. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- 119.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocytes recruitment to inflammatory loci: receptor dependent actions. Arterioscler Thromb Vasc Biol. 2012:1970–8. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, et al. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2010;82:27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fredman G, Serhan CN. Specialized pro-resolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem J. 2011:185–97. doi: 10.1042/BJ20110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Butler KL, Ambravaneswaran V, Agrawal N, Bilodeau M, Toner M, et al. Burn injury reduces neutrophil directional migration speed in microfluidic devices. PLoS ONE [Electronic Resource] 2012;5:e11921. doi: 10.1371/journal.pone.0011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008:848–55. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2009;285:3451–61. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. Journal of Immunology. 2012;189:1036–42. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–7. doi: 10.1038/nm.2123. 1p following 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maderna P, Cottell DC, Berlasconi G, Petasis NA, Brady HR, Godson C. Lipoxins induce actin reorganization in monocytes and macrophages but not in neutrophils: differential involvement of rho GTPases. Am J Pathol. 2002:2275–83. doi: 10.1016/S0002-9440(10)61175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reville K, Crean JK, Vivers S, Dransfield I, Godson C. Lipoxin A4 redistributes myosin IIA and Cdc42 in macrophages: implications for phagocytosis of apoptotic leukocytes. J Immunol. 2006;176:1878–88. doi: 10.4049/jimmunol.176.3.1878. [DOI] [PubMed] [Google Scholar]
- 129.Jones CN, Dalli J, Dimisko L, Wong E, Serhan CN, Irimia D. Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20560–5. doi: 10.1073/pnas.1210269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kurihara T, Jones CN, Yu YM, Fischman AJ, Watada S, et al. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB Journal. 2013;27:2270–81. doi: 10.1096/fj.12-219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carlo T, Kalwa H, Levy BD. 15-Epi-lipoxin A4 inhibits human neutrophil superoxide anion generation by regulating polyisoprenyl diphosphate phosphatase 1. FASEB Journal. 2013 doi: 10.1096/fj.12-223982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Levy BD, Petasis NA, Serhan CN. Polyisoprenyl phosphates in intracellular signalling. Nature. 1997;389:985–90. doi: 10.1038/40180. [DOI] [PubMed] [Google Scholar]
- 133.Bonnans C, Fukunaga K, Keledjian R, Petasis NA, Levy BD. Regulation of phosphatidylinositol 3-kinase by polyisoprenyl phosphates in neutrophil-mediated tissue injury. Journal of Experimental Medicine. 2006;203:857–63. doi: 10.1084/jem.20052143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Levy BD, Fokin VV, Clark JM, Wakelam MJ, Petasis NA, Serhan CN. Polyisoprenyl phosphate (PIPP) signaling regulates phospholipase D activity: a ‘stop’ signaling switch for aspirin-triggered lipoxin A4. FASEB Journal. 1999;13:903–11. doi: 10.1096/fasebj.13.8.903. [DOI] [PubMed] [Google Scholar]
- 135.Carlo T, Petasis NA, Levy BD. Activation of polyisoprenyl diphosphate phosphatase 1 remodels cellular presqualene diphosphate. Biochemistry. 2009;48:2997–3004. doi: 10.1021/bi8020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fukunaga K, Arita M, Takahashi M, Morris AJ, Pfeffer M, Levy BD. Identification and functional characterization of a presqualene diphosphate phosphatase. J Biol Chem. 2006;281:9490–7. doi: 10.1074/jbc.M512970200. [DOI] [PubMed] [Google Scholar]
- 137.Kazani S, Planaguma A, Ono E, Bonini M, Zahid M, et al. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. Journal of Allergy and Clinical Immunology. 2013 doi: 10.1016/j.jaci.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee TH, Crea AE, Gant V, Spur BW, Marron BE, et al. Identification of lipoxin A4 and its relationship to the sulfidopeptide leukotrienes C4, D4, and E4 in the bronchoalveolar lavage fluids obtained from patients with selected pulmonary diseases. American Review of Respiratory Disease. 1990;141:1453–8. doi: 10.1164/ajrccm/141.6.1453. [DOI] [PubMed] [Google Scholar]
- 139.Vachier I, Bonnans C, Chavis C, Farce M, Godard P, et al. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005;115:55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 140.Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, et al. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur Respir J. 2000;16:44–9. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- 141.Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. American Journal of Respiratory & Critical Care Medicine. 2008;178:574–82. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. American Review of Respiratory Disease. 1992;145:1281–4. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- 143.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. New England Journal of Medicine. 2004;350:560–9. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 144.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kowal-Bielecka O, Kowal K, Distler O, Rojewska J, Bodzenta-Lukaszyk A, et al. Cyclooxygenase- and lipoxygenase-derived eicosanoids in bronchoalveolar lavage fluid from patients with scleroderma lung disease: an imbalance between proinflammatory and antiinflammatory lipid mediators. Arthritis & Rheumatism. 2005;52:3783–91. doi: 10.1002/art.21432. [DOI] [PubMed] [Google Scholar]
- 146.Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, et al. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367:509–15. doi: 10.1016/j.bbrc.2008.01.012. Epub 2008 Jan 9. [DOI] [PubMed] [Google Scholar]
- 147.Bilal S, Haworth O, Wu L, Weylandt KH, Levy BD, Kang JX. Fat-1 transgenic mice with elevated omega-3 fatty acids are protected from allergic airway responses. Biochimica et Biophysica Acta. 2011;1812:1164–9. doi: 10.1016/j.bbadis.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, et al. Resolvin d1 and aspirin-triggered resolvin d1 promote resolution of allergic airways responses. Journal of Immunology. 2012;189:1983–91. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.El Kebir D, Jozsef L, Khreiss T, Pan W, Petasis NA, et al. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. Journal of Immunology. 2007;179:616–22. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 150.Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, et al. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. 2007;21:3877–84. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]