Abstract
Background
Neuropeptide Y (NPY) is a hypothalamic neuropeptide that plays a prominent role in feeding and energy homeostasis. Expression of the NPY Y1 receptor (Y1R) is highly concentrated in the nucleus accumbens (Acb), a region important in the regulation of palatable feeding. In this study, we performed a number of experiments to investigate the actions of NPY in the Acb.
Methods
First, we determined caloric intake and food choice after bilateral administration of NPY in the Acb in rats on a free-choice diet of saturated fat, 30% sucrose solution, and standard chow and whether this was mediated by the Y1R. Second, we measured the effect of intra-Acb NPY on neuronal activity using in vivo electrophysiology. Third, we examined co-localization of Y1R with enkephalin and dynorphin neurons and the effect of NPY on preproenkephalin messenger RNA levels in the striatum using fluorescent and radioactive in situ hybridization. Finally, using retrograde tracing, we examined whether NPY neurons in the arcuate nucleus projected to the Acb.
Results
In rats on the free-choice, high-fat, high-sugar diet, intra-Acb NPY increased intake of fat, but not sugar or chow, and this was mediated by the Y1R. Intra-Acb NPY reduced neuronal firing, as well as preproenkephalin messenger RNA expression in the striatum. Moreover, Acb enkephalin neurons expressed Y1R and arcuate nucleus NPY neurons projected to the Acb.
Conclusions
NPY reduces neuronal firing in the Acb resulting in increased palatable food intake. Together, our neuroanatomical, pharmacologic, and neuronal activity data support a role and mechanism for intra-Acb NPY-induced fat intake.
Keywords: Accumbens, diet, enkephalin, feeding, neuropeptide, obesity
A major population of neuropeptide Y (NPY)-producing neurons is located in the arcuate nucleus (ARC) of the hypothalamus. Central administration of NPY increases feeding behavior in rodents (1–3) and NPY has been linked to the control of carbohydrate intake (4,5). However, we recently observed that when NPY is administered in the lateral ventricle of rats on a free-choice, high-fat, high-sugar (fcHFHS) diet, it was not sugar but rather saturated fat and chow consumption that increased (6). The link between NPY and carbohydrate intake has been previously studied using administration of NPY into specific hypothalamic regions (5,7). In contrast, we administered NPY into the lateral ventricle, providing contact with not only the hypothalamus but also the corticolimbic areas involved in reward and motivation, such as the nucleus accumbens (Acb), where NPY receptors are localized (8–10). It remains to be explored, however, whether direct action of NPY in the Acb regulates fat consumption.
Reduced neuronal activity in the Acb occurs during feeding, and intra-Acb administration of orexigenic compounds, such as melanin concentrating hormone and muscimol, inhibit Acb neuronal activity (11–14). However, it is not known whether NPY also affects neuronal activity in the Acb. Given that direct NPY administration into other brain areas, including the amygdala and ARC, reduces neuronal activity (15,16), we hypothesized that NPY reduces Acb neuronal activity and that this is associated with increased fat intake.
Opioids have an established role in reward behavior, and opioid-expressing neurons are located within the Acb. Of the opioid-expressing neurons, enkephalin (ENK) neurons are of specific interest as they express dopamine D2 receptors and have been linked to high-fat feeding, i.e., Acb enkephalin gene expression is affected by consumption of the highly palatable Ensure drink (17), which contains fat and sugar, and striatal D2 receptor availability is correlated with high-fat intake (18). Moreover, enkephalin binds to mu-opioid receptors and local Acb administration of the mu-opioid receptor agonist DAMGO specifically increases intake of high-fat foods (19). In turn, the DAMGO-induced increased intake of high-fat foods is NPY Y1 receptor (Y1R) dependent (20). Therefore, the effect of NPY on fat intake may involve changes in Acb enkephalin levels. Given these findings, we hypothesized that Y1Rs are localized on enkephalin neurons and that an intra-Acb NPY injection alters striatal enkephalin levels.
Finally, previous studies have revealed anatomic connections between the ARC and Acb (21,22), as well as the presence of NPY receptors in the Acb (8–10). While a recent study found α-melanocyte-stimulating hormone (α-MSH) projections from the ARC to Acb (23), it has not been determined whether NPY neurons in the ARC project to the Acb.
To examine the action of NPY in the Acb, we first assessed food intake and preproenkephalin (ppENK) messenger RNA (mRNA) responses to intra-Acb NPY injections in rats on the fcHFHS diet. Second, we measured NPY’s effects on neuronal activity using in vivo electrophysiology. Third, using fluorescent imaging and retrograde tracing, we determined whether Y1Rs are localized on enkephalin and dynorphin neurons and whether NPY neurons in the ARC project to the Acb. We found that intra-Acb NPY stimulates fat intake via the Y1R and is associated with reduced expression of ppENK mRNA and neuronal activity. Moreover, ENK neurons express Y1Rs and NPY neurons in the ARC project to the Acb. These neuroanatomical, pharmacologic, and neuronal activity data support a possible role and mechanism for intra-Acb NPY-induced fat intake.
Methods and Materials
Animals
For behavior and radioactive in situ hybridization, male adult Wistar rats (Charles River, Germany) weighing 270 g to 300 g were used. For electrophysiological experiments and fluorescent in situ hybridization, C57BL/6 mice (Jackson Labs, Bar Harbor, Maine) weighing 25 g to 30 g were used. All animal procedures were performed in accordance with the protocol approved by the Yale Institutional Animal Care and Use Committee and the Committee for Animal Experimentation of the Academic Medical Center of the University of Amsterdam.
Effect of Intra-accumbens NPY and Y1R Antagonist on Food Intake
Rats were housed in a temperature- (21°C to 23°C) and light-controlled room (lights on 0700–1900). One week after arrival, rats were implanted with two cannulae aimed bilaterally at the Acb shell as described in Supplement 1. One week after surgery rats (n = 15) were placed on an fcHFHS diet and were able to choose from the following components: a dish of saturated fat (beef tallow [Ossewit/Blanc de Boeuf, Vandemoortele, Belgium), a bottle of 30% sugar water (mixed from commercial grade sugar and tap water), standard chow (Special Diet Services, Essex, United Kingdom), and tap water. After 1 week of fcHFHS diet exposure, .6 μg NPY [minimum effective dose in previous experiments (24,25) and own observations] or vehicle (1 × phosphate buffered saline [PBS]) was administered at the beginning of the light phase (between 1000 and 1100) in a balanced design. NPY was obtained from Bachem, Germany (H6375) and dissolved in 1 × PBS. Before the start of the experiment, all food components (except water) were removed from the cage. Injector cannulae (33-gauge) (Plastics One, Bilaney Consultants GmbH, Düsseldorf, Germany), extending 1 mm below the guide cannulae, were inserted into the guide cannulae, and animals received bilateral infusions of .3 μL fluid per site at a rate of .15 μL/minute via a syringe infusion pump. Injections were confirmed by monitoring fluid movement in the tubing via a small air bubble. After completion of the injection, the injector was left in place for 1 minute to allow for diffusion. Upon completion of all infusions, food was returned to the cages and all individual food components were measured after 2, 5, and 24 hours, and caloric intake (kcal) for each individual food item and total caloric intake were calculated. Total caloric intake was defined as the sum of each individual food component for which the caloric density was defined as follows: chow: 3.31 kcal/g; fat: 9 kcal/g; and sucrose solution: 1.2 kcal/g. The experiment was repeated 3 days later according to a crossover design.
At the end of the experiment, food was removed in the morning (and not returned) and rats received intra-Acb injection of vehicle or .6 μg NPY. One hour later, rats were anesthetized and transcardially perfused with ice-cold saline followed by 4% paraformaldehyde and postfixed for 2 hours. Brains were washed in PBS, cryoprotected in 30% sucrose at 4°C overnight, and subsequently frozen on dry ice and stored at −80°C. Cryostat sections were cut at 35 μm and mounted on Superfrost Plus slides (Fisher, Gerhard Menzel GmbH, Germany). Some slides were stained for Nissl with thionine and checked for cannula placement with inclusion criteria described below. Remaining slides were air-dried and stored at −80°C to be used for radioactive in situ hybridization. The procedure for radioactive in situ hybridization was performed as described previously (26) and described in Supplement 1.
In a separate experiment, we tested whether the Acb NPY Y1 receptor was involved in the intra-Acb NPY-induced increased fat intake by intra-Acb administration of the Y1R antagonist GR 231118 (synonym of LY1229U91, Sigma Aldrich, Zwijndrecht, The Netherlands) or vehicle (in a volume of .2 μL) in the Acb 15 minutes before intra-Acb NPY in rats on the fcHFHS diet (n = 15). These experiments were conducted according to the same protocol (for surgery and cannula placement verification) as described above. We only measured intake of chow, fat, and sugar at one time point, as NPY effects do not show beyond a few hours. The dose of antagonist used (.3 μg) was chosen based on reports published elsewhere (27,28) and on a dose response in a few animals using .3,1, and 3 μg antagonist. Since 1 μg and 3 μg in the Acb decreased overall intake, we chose the .3 μg dose, because it did not significantly affect feeding behavior (data not shown). At the end of the experiment, rats were killed by decapitation and brains were removed, frozen on dry ice, and stored at −80°C Cryostat sections were stained for Nissl with thionine and checked for cannula placement. Data of rats were only included when we verified that they had unilateral or bilateral cannula placements in the Acb shell between bregma 1.0 and 2.20 (Paxinos and Watson 4th ed. 1998) (29,30). Based on these criteria, five animals were excluded from analysis from experiment 1, and one animal was excluded from experiment 2 (with Y1R antagonist). Furthermore, for experiment 2, data from three more animals were excluded from analysis due to loss of the cannula or sickness behavior after injection, which turned out to be associated with infection around the cannula.
Fluorescent In Situ Hybridization
Fluorescent in situ hybridization was performed as described previously (31) and in Supplement 1. Probes for double in situ hybridization were prepared using an in vitro transcription kit with digoxigenin-labeled uridine 58-triphosphate (Roche, Basel, Switzerland) for making the Y1R probe or fluorescein-labeled uridine 58-triphosphate (Molecular Probes, Eugene, Oregon) for making the ppENK or prodynorphin probe (information on probes is provided in Supplement 1).
In Vivo Electrophysiology
Four well-habituated C57BL/6 mice (25 g to 30 g) were implanted with a cannula (Plastics One) and arrays of microwire electrodes (Tucker-Davis Technologies, Alachua, Florida) using aseptic stereotaxic methods described in detail previously (12) and in Supplement 1. The arrays consisted of 16 Teflon-coated, 50 μm stainless steel wires arranged in an 8 × 2 configuration, with each electrode spaced by ~250 μm. The in vitro impedance of the electrodes was 100 kΩ to 300 kΩ. After a recovery period of 5 to 7 days, animals were acclimated to recording procedures (i.e., headsets and cables were attached to the implants) for 1 day. Drug infusions were performed as follows. Animals were lightly anesthetized with halothane via a nose cone during the experiment, and recording headstages were plugged in. After initial recording, data acquisition was paused and animals were infused with either artificial cerebrospinal fluid (aCSF) (sodium chloride 147 mmol/L, calcium chloride 1.3 mmol/L, magnesium chloride .9 mmol/L, potassium chloride 4.0 mmol/L) or NPY (.6 μg). Infusions were made through 33-gauge cannulae (Plastics One) that protruded .2 mm from the tip of the guide cannulae. Injectors were inserted into the guide cannulae and .3 μL of infusion fluid was delivered per site at a rate of .15 μL/minute via a syringe infusion pump (KDS Scientific, Holliston, Massachusetts). Fluid was infused via .38-mm diameter polyethylene tubing that connected the injector to a 5 μL Hamilton syringe (Roche). Injections were confirmed by monitoring movement of fluid in the tubing via a small air bubble. After injection was complete, the injector was left in place for 2 minutes to allow for diffusion. Infusions took place between 1200 and 1400. Recording data were collected for 1 hour after infusions.
Neuronal ensemble recordings were made using a Many Neuron Acquisition Program (Tucker-Davis Technologies). Putative single neuron units were identified online using an oscilloscope and an audio monitor. The Tucker-Davis Technologies offline sorter was used to analyze the signals offline and to remove artifacts due to cable noise and behavioral devices (pump, click stimulus). Principal component analysis and waveform shape were used for spike sorting. Single units were identified as having 1) consistent waveform shape, 2) separable clusters in principal component analysis space, 3) average amplitude estimated at least three times larger than background activity, and 4) a consistent refractory period of at least 2 milliseconds in interspike interval histograms. Those units identified online as potential single units that did not meet these criteria offline were not included in this analysis. Datasets were previewed using OpenSorter (Tucker-Davis Technologies) and subsequently analyzed using custom routines for MATLAB (MathWorks, Natick, Massachusetts). For each well-isolated neuron, postinfusion firing rates were normalized to mean preinfusion firing rates (in the 10 minutes immediately preceding drug infusion) and binned (60-second bins). Activity was then compared between neurons recorded in NPY and aCSF conditions.
At the end of the experiment, mice were perfused transcardially with ice-cold saline followed by 4% paraformaldehyde. After fixation, brains were cryoprotected in 30% sucrose at 4°C overnight. Microtome sections were cut at 40 μm along the horizontal axis and mounted on Superfrost Plus slides (Fisher). Electrode and cannulae placement in horizontal sections were verified microscopically using the mouse brain atlas from Paxinos and Franklin (2004).
Cholera Toxin B Tracing
One week after arrival, six male Wistar rats (Charles River) weighing 270 g to 300 g received two cannulae: one aimed at the right Acb shell for infusion of the cholera toxin B (CTB) conjugated to alexa-555 (C-22843, Invitrogen, Bleiswijk, The Netherlands) tracer and one cannula in the left lateral ventricle for the infusion of colchicine to block neuronal transport (C9754, Sigma-Aldrich, Zwijndrecht, The Netherlands). Surgical procedures were the same as for the rat study described above. For the lateral ventricle, a permanent 22-gauge stainless steel guide cannula was used with coordinates .8 mm posterior from bregma, 1.4 mm lateral from the midline, and 4.5 mm below the surface of the brain. In contrast to CTB, fluorophore-labeled CTB cannot be applied by iontophoresis, so we used pressure injection (21). Rats were injected unilaterally with 100 nL to 150 nL 1% CTB into the Acb shell. The injection needle was left in place and fixed with dental cement to the skull to minimize leakage from the tract (21). Twelve days later, rats were deeply anesthetized and 100 μg colchicine in 5 μL PBS was injected in the lateral ventricle. Twenty-four to 36 hours later, rats were transcardially perfused with saline, followed by a solution of 4% paraformaldehyde in .1 mol/L PBS (pH 7.4) at 4°C. Brains were removed and kept in a 30% sucrose solution dissolved in PBS for 2 hours. Subsequently, the brains were washed briefly with PBS, frozen on dry ice, and stored at −80°C until further processing for fluorescent immunohistochemistry. The procedure for the fluorescent immunohistochemistry was performed as described previously (32) and in Supplement 1.
Statistical Analysis
For the effects of NPY on food intake and on enkephalin mRNA, t tests were performed. The data on NPY in combination with the Y1R antagonist were tested with a two-way repeated-measures analysis of variance (ANOVA) (for paired comparison) and were followed with Sidak’s multiple comparisons tests. Data obtained with electrophysiology were analyzed with a repeated-measures ANOVA, followed by Tukey’s post hoc tests where appropriate. For all analyses, significance was assigned at the p ≤ .05 level. All data are presented as mean ± SEM.
Results
Effect of NPY and Y1R Antagonist in Nucleus Accumbens on Food Intake
Local administration of NPY significantly increased fat intake after 2 hours (Figure 1A) but not after 5 hours and 24 hours (Figure S1 in Supplement 1). Intra-Acb NPY did not affect intake of the chow or sugar component after 2 hours (Figure 1A) or after 5 hours and 24 hours (Figure S1 in Supplement 1). Subsequently, the effect of pretreatment with the selective Y1R antagonist GR 231118 on NPY-induced fat intake was tested. Again, when infused in the Acb, NPY specifically increased fat intake (Figure S2 in Supplement 1). Interestingly, when cannulae were placed bilaterally in the shell of the Acb (which was the case in 5 out of 11 animals), pretreatment with Y1R antagonist completely prevented the NPY-induced increase in fat intake (interaction pretreatment with Y1 antagonist × NPY, F1,20 = 6.3; p = .02); a trend for NPY effect (F1,20 = 3.5; p = .08); and no effect of the Y1 antagonist (F1,20 = 1.5; p = .24) (Figure 1B).
Figure 1.
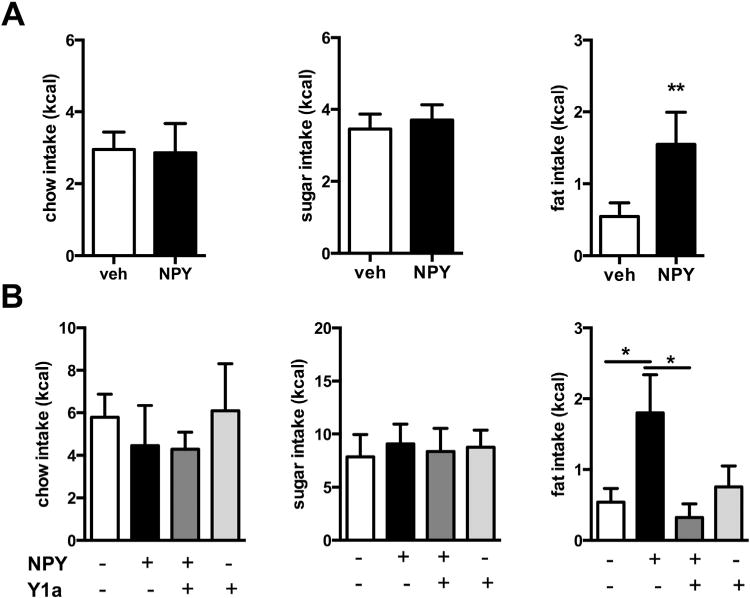
Effect of intra-nucleus accumbens (Acb) neuropeptide Y (NPY) in rats on intake of chow, sugar, and fat components of the free-choice, high-fat, high-sugar diet. (A) NPY in the Acb significantly increased fat intake and not sugar or chow intake; total intake tended to be increased (vehicle [veh] 6.5 ± .8 vs. NPY 8.7 ± 1.1; p = .1) (n = 10). (B) The increase in fat intake was prevented by pretreatment with Y1 receptor antagonist (Y1a) GR 231118, injected bilaterally into the shell of Acb, whereas the Y1 receptor antagonist did not affect fat intake (or chow and sugar) alone (n = 5). Data are presented as mean ± SEM. A significant effect is depicted by *p < .05 and **p < .01.
NPY Reduces Cell Firing in the Nucleus Accumbens In Vivo
To evaluate the effects of NPY on neuronal activity in vivo, neuronal firing data were collected during intra-Acb infusions of aCSF or NPY with microwire recording electrodes implanted in the Acb (Figure 2A,B). Multiunit recordings performed under anesthesia revealed a significant reduction in firing rates of Acb neurons treated with .6 μg NPY (n = 29) and a significant interaction between time and treatment, p = .0010, two-way ANOVA F39,1482 = 1.874 (Figure 2C,D). Firing in 11/29 (37.9%) units was suppressed 50% below from baseline. Another 9/29 (31.0%) units were suppressed 25% below from baseline. The remaining 9 units were either unchanged (6 [20.7%] of 29) or increased (3 [10.3%] of 29) relative to preinfusion firing rates. This effect lasted 25 minutes, starting within 5 minutes after infusion (Figure 2C).
Figure 2.
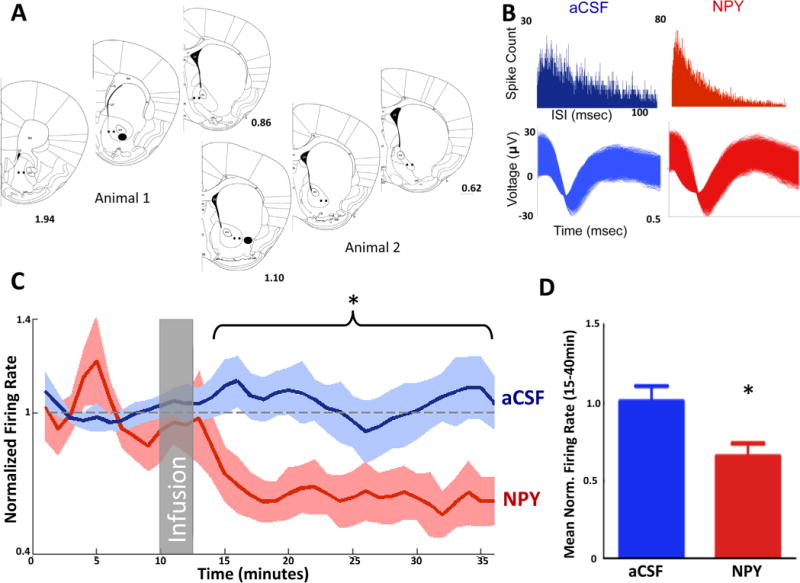
Neuropeptide Y (NPY) in the nucleus accumbens decreases neuronal activity in vivo. (A) Location of electrodes (black spots) and cannulae (filled black circles) projected on a mouse brain atlas section (51). (B) Interspike interval (ISI) histograms and waveforms from a single unit in artificial cerebrospinal fluid (aCSF) infusion sessions (left) and the same neuron in NPY infusion sessions (right). (C, D) Average neuronal activity for NPY sessions and aCSF. Gray box represents time of infusion. NPY significantly reduces firing rate from 15 minutes to 40 minutes. * p < .05. Norm., normalized.
NPY Y1R Co-localizes with Enkephalin and Dynorphin Positive Neurons in Nucleus Accumbens
Since NPY receptors are present in the Acb (10), we investigated whether Y1R co-localizes with enkephalin and dynorphin neurons by performing double-labeled fluorescent in situ hybridization. Co-localization of Y1R on enkephalin and dynorphin neurons was found throughout the striatum and Acb; about 59% of Y1R co-localized with enkephalin neurons (Figure 3A) and about 18% with dynorphin neurons (Figure S3 in Supplement 1) as revealed by confocal analysis.
Figure 3.
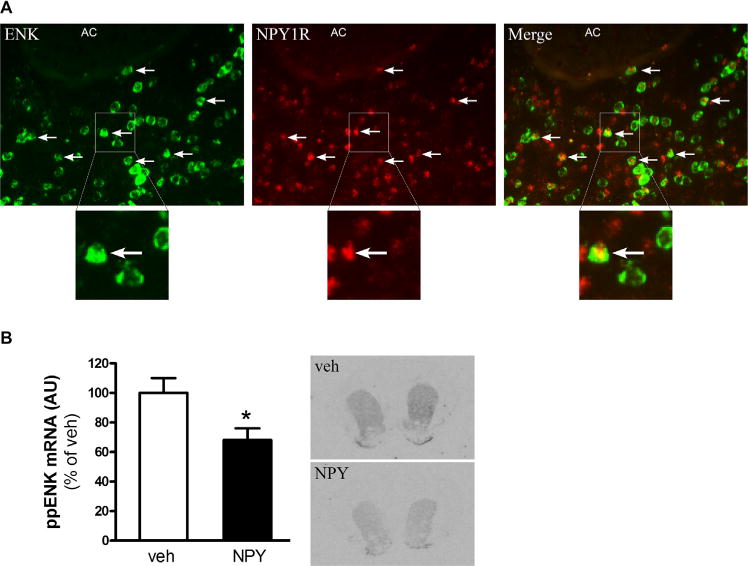
Neuropeptide Y (NPY) Y1 receptor (NPY1R) is expressed on enkephalin (ENK) neurons in the nucleus accumbens (Acb) and intra-Acb NPY downregulates preproenkephalin (ppENK) messenger RNA (mRNA) levels. (A) Expression pattern of Y1R and ENK in the Acb. Horizontal arrows indicate co-localization. (B) ppENK gene expression levels in the ventral striatum and representative images after intra-Acb injection of NPY. Values are means ± SEM of seven to nine rats per group. *p < .05 NPY vs. vehicle (veh). AC, anterior commissure.
NPY Administration in the Nucleus Accumbens Decreases ppENK mRNA Expression
Striatal enkephalin, as opposed to dynorphin, has been described to be affected by changes in energy balance (33). Additionally, we found that Y1R is mainly located on enkephalin neurons. Therefore, we investigated the effect of NPY on enkephalin mRNA levels. We quantitatively measured ppENK mRNA levels using radioactive in situ hybridization and found that 1 hour after intra-Acb NPY administration, ppENK mRNA expression was significantly downregulated in both the ventral striatum (Acb) as well as the dorsal striatum (Figure 3B) (p < .05).
Subset of Arcuate NPY Neurons Project to Nucleus Accumbens
To investigate the origin of NPY neurons projecting to the Acb, the CTB tracer was administered in the Acb and co-localization with NPY antibody was determined. The tracer was mostly concentrated at the border of the medial shell and core, positioned dorsomedially to the anterior commissure (Figure 4A). Anterograde CTB tracing was found in the medial part of the subcommissural ventral pallidum and lateral hypothalamus confirming previous findings (34–36). In this study, our main focus was to investigate afferent projections to the Acb and consequently to determine which of these projections co-label with NPY. Retrograde transport of CTB tracer was examined by serial sectioning and immunostaining in the neocortex, thalamus, hypothalamus, amygdaloid nuclei, ventral pallidum, and subiculum.
Figure 4.
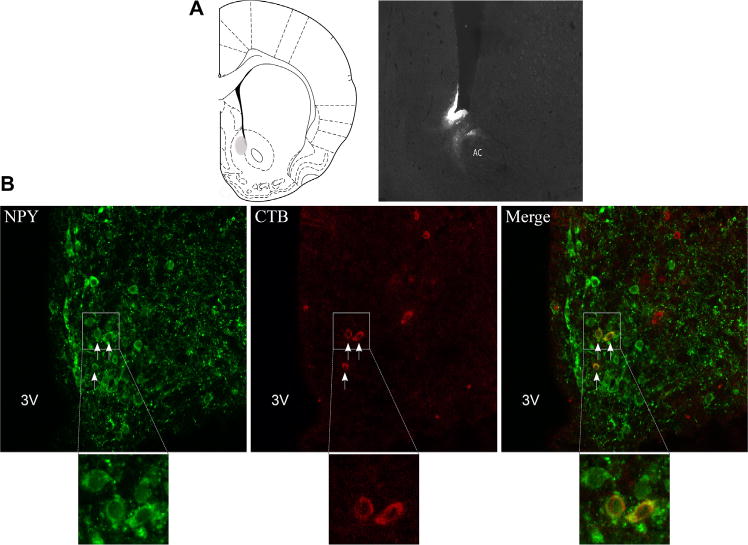
Neuropeptide Y (NPY)-positive neurons in the arcuate nucleus project to the nucleus accumbens. (A) Representative image for cholera toxin B (CTB) injection in the nucleus accumbens. (B) Confocal microscopy shows co-localization of NPY and CTB in the arcuate nucleus of rats. Arrows indicate co-localization. AC, anterior commissure; 3V, third ventricle.
Consistent with previous studies, CTB staining was found in labeled perikarya in the agranular insular cortex, perirhinal cortex, midline thalamic nuclei, and (baso)lateral amygdala (22,35,36). However, in none of these areas did CTB co-localize with NPY staining.
The primary site for synthesis of NPY peptide is the ARC. Consistent with others (21,22), we found CTB staining in the ARC. CTB labeling was present in the ventral medial and ventral lateral parts of the ARC. In the ventral medial ARC, the majority of CTB cells were dually immunostained with NPY (Figure 4B), indicating NPY positive cells projecting from the ARC to Acb. This finding was confirmed in three successfully injected cases. CTB cells in the lateral portion of the ARC, which did not co-localize with NPY, may be α-MSH neurons, as melanocortinergic projections from the ARC to Acb have been shown previously (23).
Discussion
In the present study, we used a combination of behavioral, neuroanatomical, and in vivo electrophysiological measures to investigate the effect of NPY on Acb neuronal activity, neuropeptide expression, and behavioral output. We demonstrated that NPY administration in the Acb in rats on a fcHFHS diet increased fat intake specifically, and this effect was attenuated by pretreatment with a Y1R antagonist in the Acb shell. Furthermore, we identified NPY Y1 receptors on ENK neurons in the Acb and found that NPY administration lowered Acb neuronal activity and levels of striatal ppENK mRNA. Finally, we showed that a subset of ARC NPY neurons project to the Acb. Together with our earlier findings that a fcHFHS diet increased NPY mRNA in the ARC and increased sensitivity for NPY (37,38), these findings provide evidence for a role of NPY in the intake of palatable foods, possibly via inhibition of D2-enkephalin neurons. Moreover, the increased levels of NPY mRNA in the ARC observed in rats on a fcHFHS diet may, via projections to the Acb, be related to the increased motivation and active lever pressing as well as the persistent hyperphagia observed in these rats (37,39).
Consistent with others, we found that intra-Acb NPY did not affect chow (4,25) and sucrose (25) intake. Interestingly, a recent study showed that intra-Acb NPY increased intake of sucrose pellets (40), which implies that intra-Acb NPY may increase sucrose intake in the absence of fat or that the effect is specific to solid and not liquid sugar. NPY has been associated with carbohydrate intake in a number of studies (4,5,41), yet NPY is often administered in the third ventricle or directly into hypothalamic areas. In this study, NPY was directed to the Acb and specifically increased fat intake, suggesting that the effect of NPY on food component consumption may be dependent on the specific brain site. Basal caloric intake in rats on the fcHFHS diet comprises 50% from chow, 30% from sugar, and 20% from fat. Because fat is not the main preferred component under baseline conditions with this diet, we infer that NPY-induced fat intake is not confounded by a pre-existing baseline fat preference.
Our findings indicate that NPY inhibits neuronal activity in the Acb. To our knowledge, this is the first study investigating the effects of NPY on Acb neuronal activity. The inhibitory effects in the Acb are inconsistent with NPY’s inhibitory effects in other brain areas, such as the amygdala and ARC (15,16), and coincide with the downregulation of ppENK mRNA expression upon intra-Acb NPY administration, another novel finding in this study. This may suggest that NPY’s feeding effects in the Acb may occur via downregulation of D2-containing ENK neurons. The ability of NPY to reduce neuronal firing in the Acb is consistent with a general model from other pharmacologic and electrophysiologic studies in which reduced Acb neuronal firing stimulates food intake (12,14). Future studies are needed to determine the precise mechanism underlying NPY’s inhibitory actions in the Acb.
Mu-opioid receptor agonists (such as DAMGO) elicit robust feeding responses; however, administration of different doses of Met-enkephalin or a synthetic and protease-resistant analog of enkephalin ([D-Ala2] Met-enkephalin) in different Acb regions did not produce any significant effects on food intake (42). The majority of enkephalin neurons co-express gamma-aminobutyric acid (GABA) (43). Therefore, the NPY-mediated downregulation of ppENK neurons may also represent a reduction of GABAergic output. Reduced Acb GABAergic output has consistently been shown to elicit intense feeding in satiated rats and to increase intake of palatable foods (11,13,14,44,45). This reduced Acb GABAergic output likely affects food intake via the lateral hypothalamus (LH) and medial ventral pallidum (46). The downstream outputs from the LH involve autonomic structures and structures that directly control brainstem pattern generators for the motor actions of eating (47). Therefore, we speculate that the downstream mechanisms of the inhibitory effects of NPY in the Acb (leading to the increase in fat intake) involve reduced GABAergic output to the LH via the medial ventral pallidum.
We demonstrated CTB tracing and NPY staining in brain areas consistent with others (22,48). However, co-localization was only found in the ARC, suggesting that NPY neurons projecting to the Acb predominantly originate from the ARC. Although it remains to be determined whether ARC-derived NPY is the principal mediator in palatable feeding regulation when rats are on a fcHFHS diet, the NPY-ergic ARC projections to the Acb supplement findings describing α-MSH projections from the ARC to the Acb (23) and suggest that afferent signals from ARC to Acb provide input about the general metabolic state of the body. Receptors of nearly all known metabolic hormones, such as leptin, insulin, glucocorticoid, and ghrelin, are present on ARC NPY neurons. It remains to be explored whether the NPY neurons that project to the Acb are also controlled by these peripheral signals.
In conclusion, our data indicate a role for NPY in the Acb in the intake of palatable food, which is likely mediated by inhibition of enkephalin neurons, probably leading to decreased GABAergic output. Future studies are needed to establish a direct role of GABAergic enkephalin neurons in NPY’s effect on fat intake. Our findings confirm and extend research suggesting a role for NPY in the feeding-related neural circuit controlled by the Acb (20,49,50). The feeding effects of the hypothalamic peptide NPY in the Acb further links the homeostatic and hedonic feeding circuits in the control and regulation of palatable feeding.
Supplementary Material
Acknowledgments
This research was supported by the Netherlands Organization for Scientific Research (ZonMw VIDI 91796331); JKvdH was supported by a Fulbright scholarship and by the Foundation “De Drie Lichten” in The Netherlands.
We thank Tim Snel for helping with the body weight and energy intake and Dr. J. M. Chou-Green for English editing.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2014.06.008.
References
- 1.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 2.Stanley BG, Leibowitz SF. Neuropeptide Y: Stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 3.Levine AS, Morley JE. Neuropeptide Y: A potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- 4.Morley JE, Levine AS, Gosnell BA, Kneip J, Grace M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am J Physiol. 1987;252:R599–R609. doi: 10.1152/ajpregu.1987.252.3.R599. [DOI] [PubMed] [Google Scholar]
- 5.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides. 1985;6:1205–1211. doi: 10.1016/0196-9781(85)90452-8. [DOI] [PubMed] [Google Scholar]
- 6.van den Heuvel JK, Eggels L, van Rozen AJ, Luijendijk MC, Fliers E, Kalsbeek A, et al. Neuropeptide Y and leptin sensitivity is dependent on diet composition. J Neuroendocrinol. 2014;26:377–385. doi: 10.1111/jne.12155. [DOI] [PubMed] [Google Scholar]
- 7.Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: Evidence for a hypothalamic site(s) of action. Brain Res Bull. 1985;14:521–524. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 8.Pickel VM, Beck-Sickinger AG, Chan J, Weiland HA. Y1 receptors in the nucleus accumbens: Ultrastructural localization and association with neuropeptide Y. J Neurosci Res. 1998;52:54–68. doi: 10.1002/(SICI)1097-4547(19980401)52:1<54::AID-JNR6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- 10.Kishi T, Aschkenasi CJ, Choi BJ, Lopez ME, Lee CE, Liu H, et al. Neuropeptide Y Y1 receptor mRNA in rodent brain: Distribution and colocalization with melanocortin-4 receptor. J Comp Neurol. 2005;482:217–243. doi: 10.1002/cne.20432. [DOI] [PubMed] [Google Scholar]
- 11.Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: Regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- 12.Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, et al. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J Neurosci. 2010;30:8263–8273. doi: 10.1523/JNEUROSCI.5858-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci. 2010;30:4746–4756. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giesbrecht CJ, Mackay JP, Silveira HB, Urban JH, Colmers WF. Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors. J Neurosci. 2010;30:16970–16982. doi: 10.1523/JNEUROSCI.2306-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci. 2005;25:7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18:2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- 18.van de Giessen E, la Fleur SE, Eggels L, de Bruin K, van den Brink W, Booij J. High fat/carbohydrate ratio but not total energy intake induces lower striatal dopamine D2/3 receptor availability in diet-induced obesity. Int J Obes (Lond) 2013;37:754–757. doi: 10.1038/ijo.2012.128. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]
- 20.Zheng H, Townsend RL, Shin AC, Patterson LM, Phifer CB, Berthoud HR. High-fat intake induced by mu-opioid activation of the nucleus accumbens is inhibited by Y1R-blockade and MC3/4R-stimulation. Brain Res. 2010;1350:131–138. doi: 10.1016/j.brainres.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi CX, van der Vliet J, Dai J, Yin G, Ru L, Buijs RM. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology. 2006;147:283–294. doi: 10.1210/en.2005-1051. [DOI] [PubMed] [Google Scholar]
- 22.Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 23.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown CM, Fletcher PJ, Coscina DV. Neuropeptide Y-induced operant responding for sucrose is not mediated by dopamine. Peptides. 1998;19:1667–1673. doi: 10.1016/s0196-9781(98)00117-x. [DOI] [PubMed] [Google Scholar]
- 25.Brown CM, Coscina DV, Fletcher PJ. The rewarding properties of neuropeptide Y in perifornical hypothalamus vs. nucleus accumbens. Peptides. 2000;21:1279–1287. doi: 10.1016/s0196-9781(00)00270-9. [DOI] [PubMed] [Google Scholar]
- 26.van den Heuvel JK, Eggels L, Fliers E, Kalsbeek A, Adan RA, la Fleur SE. Differential modulation of arcuate nucleus and mesolimbic gene expression levels by central leptin in rats on short-term high-fat high-sugar diet. PLoS One. 2014;9:e87729. doi: 10.1371/journal.pone.0087729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faulconbridge LF, Grill HJ, Kaplan JM. Distinct forebrain and caudal brainstem contributions to the neuropeptide Y mediation of ghrelin hyperphagia. Diabetes. 2005;54:1985–1993. doi: 10.2337/diabetes.54.7.1985. [DOI] [PubMed] [Google Scholar]
- 28.Skibicka KP, Shirazi RH, Hansson C, Dickson SL. Ghrelin interacts with neuropeptide Y Y1 and opioid receptors to increase food reward. Endocrinology. 2012;153:1194–1205. doi: 10.1210/en.2011-1606. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: Rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: Map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- 31.Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolk SM, Whitman MC, Yun ME, Shete P, Donoghue MJ. A unique subpopulation of Tbr1-expressing deep layer neurons in the developing cerebral cortex. Mol Cell Neurosci. 2006;32:200–214. doi: 10.1016/j.mcn.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Will MJ, Vanderheyden WM, Kelley AE. Striatal opioid peptide gene expression differentially tracks short-term satiety but does not vary with negative energy balance in a manner opposite to hypothalamic NPY. Am J Physiol Regul Integr Comp Physiol. 2007;292:R217–R226. doi: 10.1152/ajpregu.00852.2005. [DOI] [PubMed] [Google Scholar]
- 34.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 35.Kirouac GJ, Ganguly PK. Topographical organization in the nucleus accumbens of afferents from the basolateral amygdala and efferents to the lateral hypothalamus. Neuroscience. 1995;67:625–630. doi: 10.1016/0306-4522(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 36.Christie MJ, Summers RJ, Stephenson JA, Cook CJ, Beart PM. Excitatory amino acid projections to the nucleus accumbens septi in the rat: A retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neuroscience. 1987;22:425–439. doi: 10.1016/0306-4522(87)90345-9. [DOI] [PubMed] [Google Scholar]
- 37.la Fleur SE, van Rozen AJ, Luijendijk MC, Groeneweg F, Adan RA. A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int J Obes (Lond) 2010;34:537–546. doi: 10.1038/ijo.2009.257. [DOI] [PubMed] [Google Scholar]
- 38.van den Heuvel JK, Eggels L, van Rozen AJ, Diepenbroek C, Kalsbeek A, Fliers E, et al. Neuropeptide Y sensitivity in an animal model of diet induced obesity. Appetite. 2011;57(suppl 1):S45. [Google Scholar]
- 39.la Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes (Lond) 2007;31:1286–1294. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- 40.Pandit R, Luijendijk MC, Vanderschuren LJ, la Fleur SE, Adan RA. Limbic substrates of the effects of neuropeptide Y on intake of and motivation for palatable food. Obesity (Silver Spring) 2014;22:1216–1219. doi: 10.1002/oby.20718. [DOI] [PubMed] [Google Scholar]
- 41.Smith BK, Berthoud HR, York DA, Bray GA. Differential effects of baseline macronutrient preferences on macronutrient selection after galanin, NPY, and an overnight fast. Peptides. 1997;18:207–211. doi: 10.1016/s0196-9781(96)00318-x. [DOI] [PubMed] [Google Scholar]
- 42.Katsuura Y, Taha SA. Modulation of feeding and locomotion through mu and delta opioid receptor signaling in the nucleus accumbens. Neuropeptides. 2010;44:225–232. doi: 10.1016/j.npep.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meredith GE, Pennartz CM, Groenewegen HJ. The cellular framework for chemical signalling in the nucleus accumbens. Prog Brain Res. 1993;99:3–24. doi: 10.1016/s0079-6123(08)61335-7. [DOI] [PubMed] [Google Scholar]
- 44.Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stratford TR, Swanson CJ, Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- 46.Stratford TR, Kelley AE, Simansky KJ. Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res. 1999;825:199–203. doi: 10.1016/s0006-8993(99)01239-1. [DOI] [PubMed] [Google Scholar]
- 47.Kelley AE. Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 48.de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system–II. Immunohistochemical analysis. Neuroscience. 1986;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- 49.Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: Appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1436–R1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- 50.Stratford TR, Wirtshafter D. NPY mediates the feeding elicited by muscimol injections into the nucleus accumbens shell. Neuroreport. 2004;15:2673–2676. doi: 10.1097/00001756-200412030-00024. [DOI] [PubMed] [Google Scholar]
- 51.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


